Optimizing Biochar for Heavy Metal Remediation: A Meta-Analysis of Modification Methods and Pyrolysis Conditions
Abstract
1. Introduction
2. Methods
2.1. Literature Survey and Eligibility Criteria
2.2. Collection of Data and Heavy Metals’ Calculations
2.3. Meta-Analyses
2.4. Statistical Analyses
3. Results and Discussions
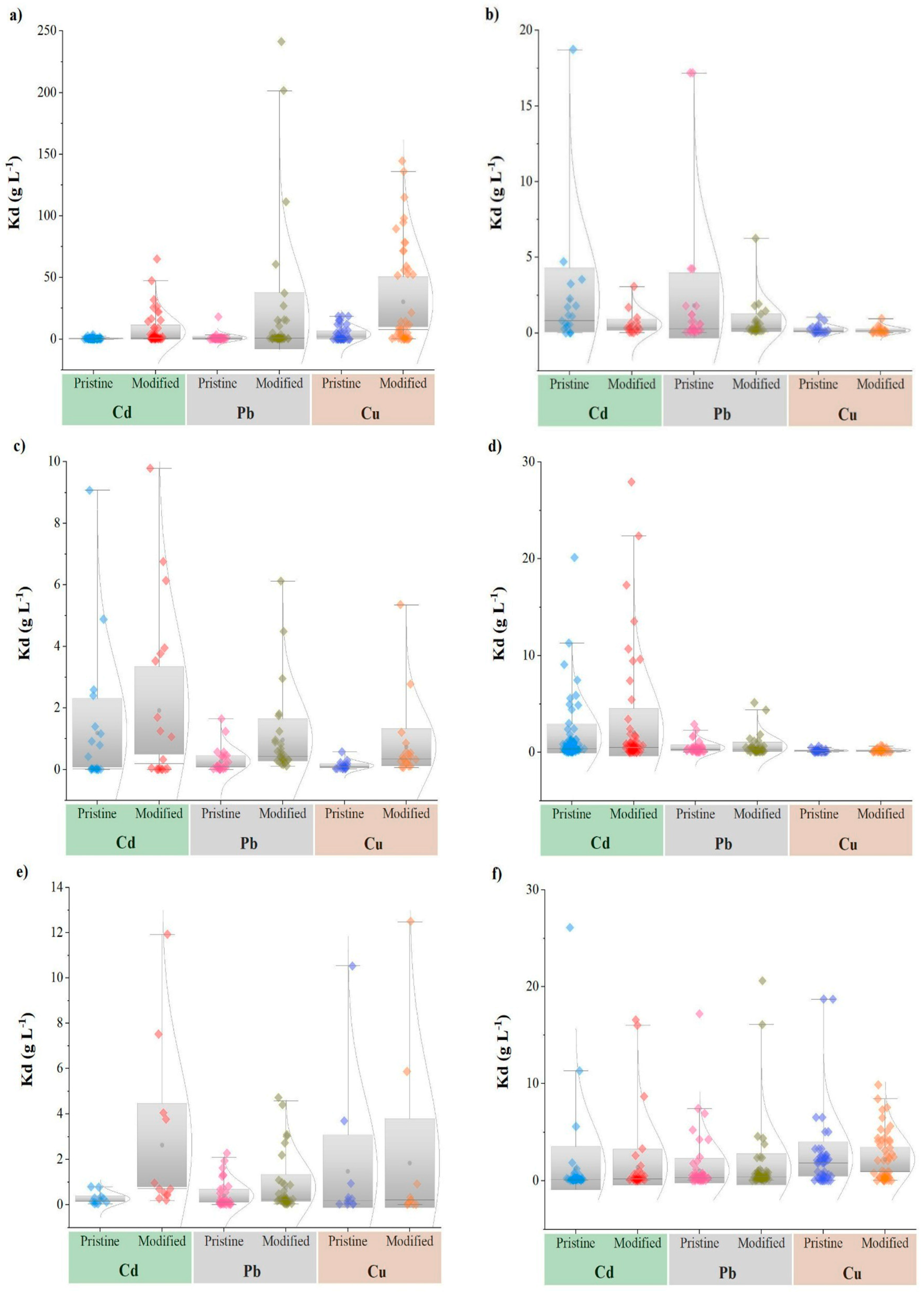
3.1. Effect of Biochar Modification Techniques on Heavy Metal Adsorption
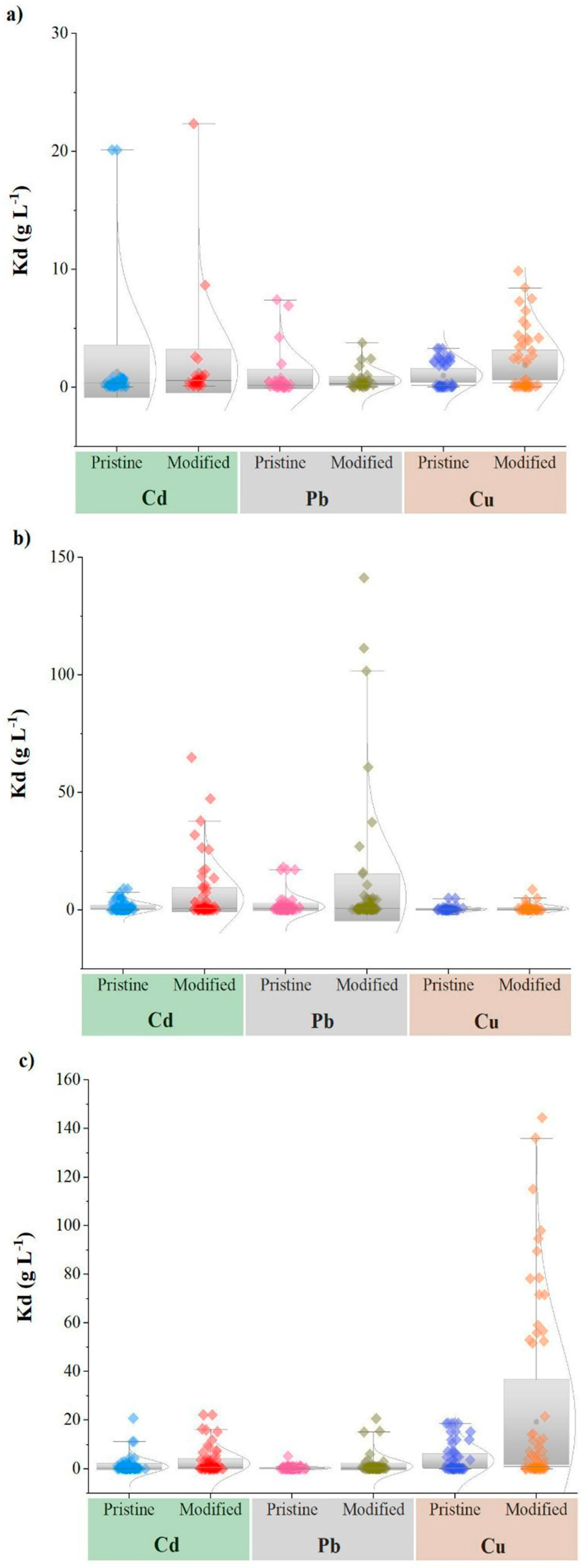
3.2. Effect of Pyrolysis Temperature on Heavy Metal Sorption
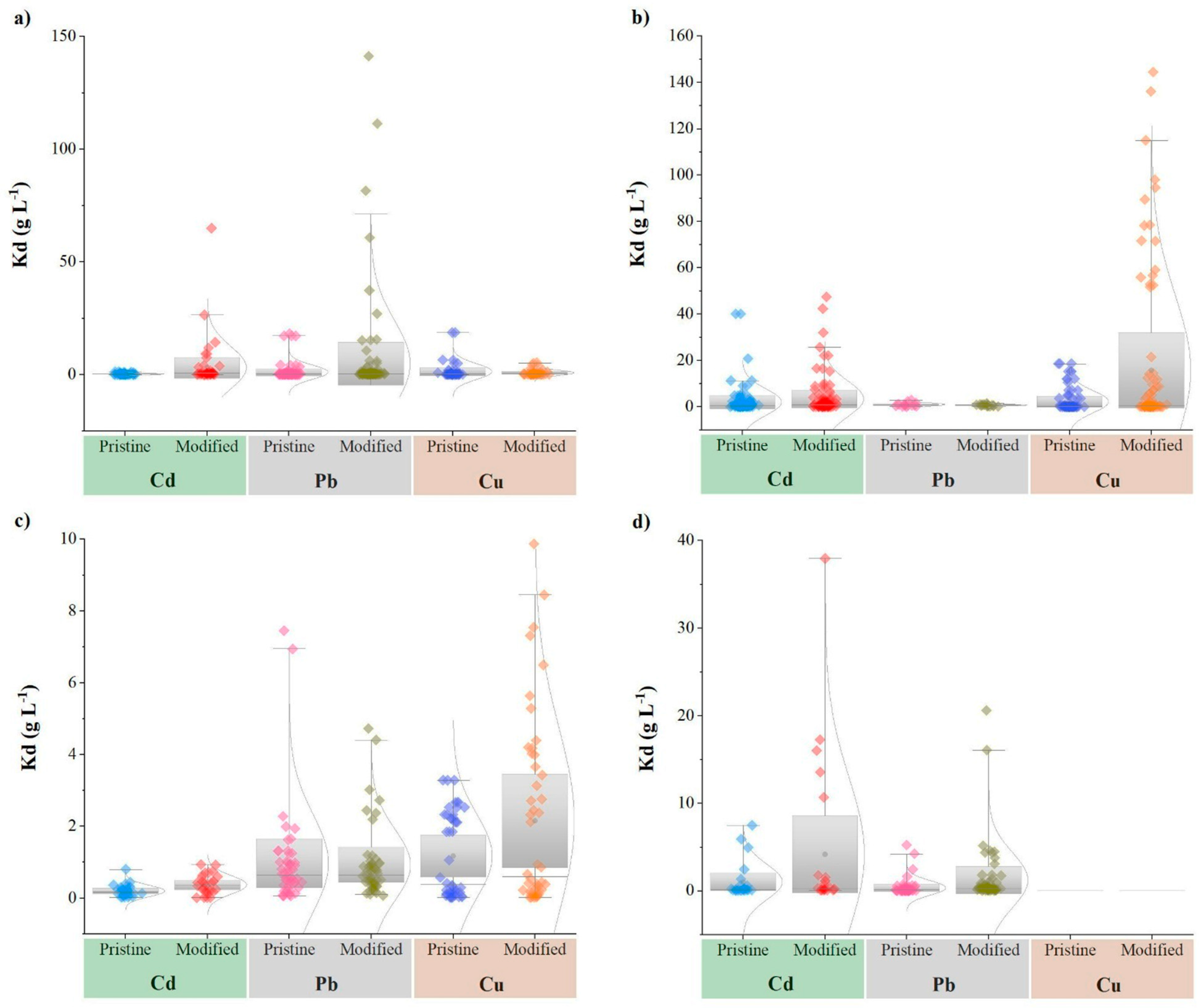
3.3. Effect of Feedstock on Heavy Metal Sorption
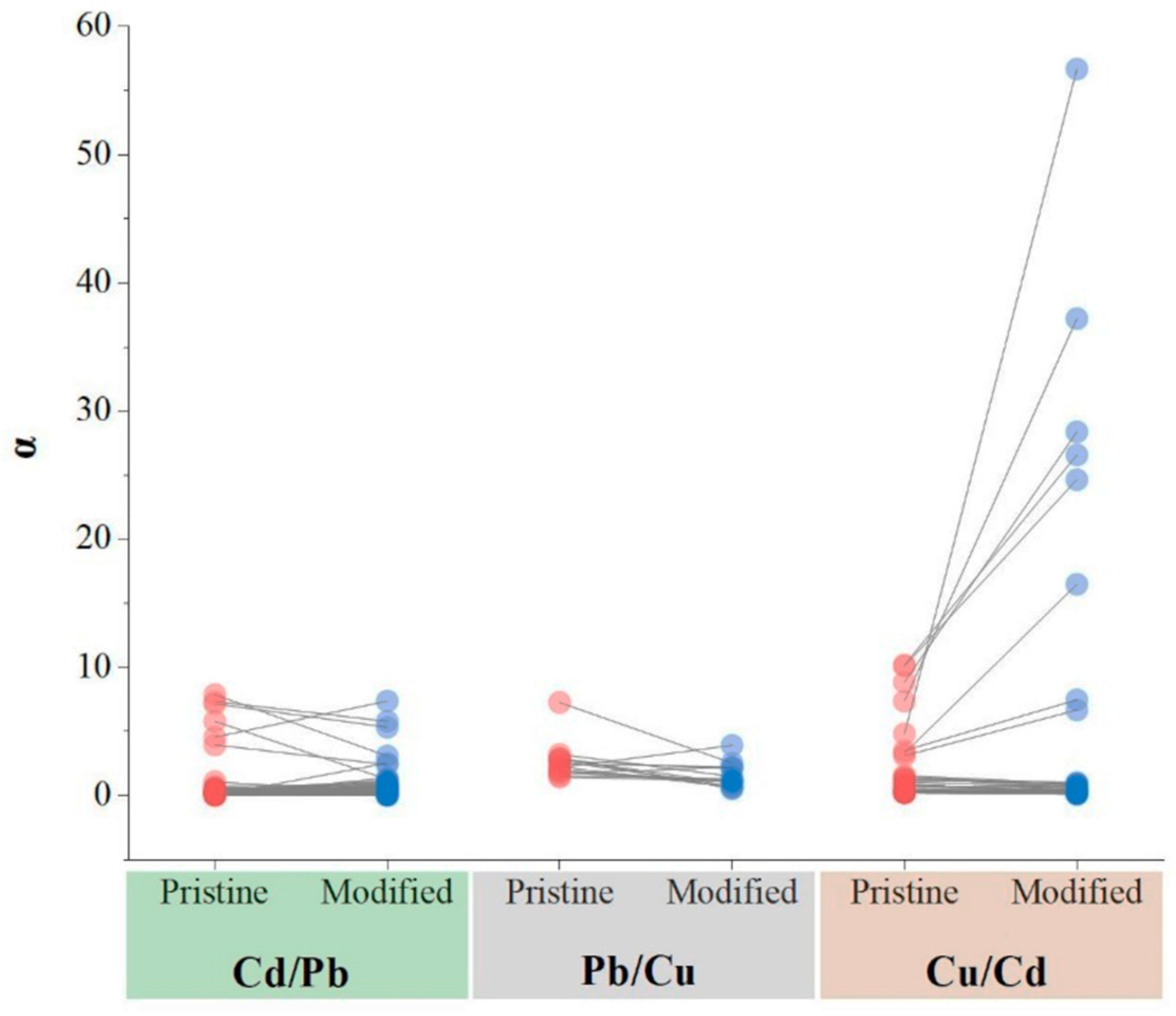
3.4. Biochar Preference in Heavy Metal Absorption
3.5. Study Limitations and Prospects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mai, X.; Tang, J.; Tang, J.; Zhu, X.; Yang, Z.; Liu, X.; Zhuang, X.; Feng, G.; Tang, L. Research Progress on the Environmental Risk Assessment and Remediation Technologies of Heavy Metal Pollution in Agricultural Soil. J. Environ. Sci. 2025, 149, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yang, S.; Han, H.; Bai, Y.; Luo, W.; Wang, Q. Is Biomagnetic Leaf Monitoring Still an Effective Method for Monitoring the Heavy Metal Pollution of Atmospheric Particulate Matter in Clean Cities? Sci. Total Environ. 2024, 906, 167564. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Han, Y.; Wu, X.; Wang, H.; Wang, S.; Zheng, J.; Ran, R.; Zhang, C. Review: Phytate Modification Serves as a Novel Adsorption Strategy for the Removal of Heavy Metal Pollution in Aqueous Environments. J. Environ. Chem. Eng. 2023, 11, 111440. [Google Scholar] [CrossRef]
- Li, F.; Yang, X.; Zhang, Z.; Jiang, Y.; Gong, Y. Behaviour, Ecological Impacts of Microplastics and Cadmium on Soil Systems: A Systematic Review. Environ. Technol. Innov. 2024, 35, 103637. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil Contamination with Cadmium, Consequences and Remediation Using Organic Amendments. Sci. Total Environ. 2017, 601–602, 1591–1605. [Google Scholar] [CrossRef]
- Wei, S.; Tao, Y.; Ma, M.; Tong, W.; Bi, F.; Wang, L.; Qu, J.; Zhang, Y. One-Step Microwave-Assisted Synthesis of MgO-Modified Magnetic Biochar for Enhanced Removal of Lead and Phosphate from Wastewater: Performance and Mechanisms. Sep. Purif. Technol. 2025, 354, 128936. [Google Scholar] [CrossRef]
- Sevak, P.I.; Pushkar, B.K.; Kapadne, P.N. Lead Pollution and Bacterial Bioremediation: A Review. Environ. Chem. Lett. 2021, 19, 4463–4488. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Guo, Z.; Xie, H.; Hu, Z.; Ran, H.; Li, C.; Jiang, Z. Spatial Heterogeneity and Source Apportionment of Soil Metal(Loid)s in an Abandoned Lead/Zinc Smelter. J. Environ. Sci. 2023, 127, 519–529. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Gu, H.; Lam, S.S.; Chen, X.; Sonne, C.; Peng, W. A Review of Phytoremediation of Environmental Lead (Pb) Contamination. Chemosphere 2024, 362, 142691. [Google Scholar] [CrossRef]
- Owumi, S.E.; Adedara, I.A.; Otunla, M.T.; Owoeye, O. Influence of Furan and Lead Co-Exposure at Environmentally Relevant Concentrations on Neurobehavioral Performance, Redox-Regulatory System and Apoptotic Responses in Rats. Environ. Toxicol. Pharmacol. 2023, 97, 104011. [Google Scholar] [CrossRef]
- Shao, C.; Fan, F.; Dai, Y. Lead Ions Removal from Water by Tartaric Acid Modified Biochar Materials: Equilibrium, Kinetic Studies and Mechanism. Desalination Water Treat. 2024, 320, 100601. [Google Scholar] [CrossRef]
- Hasani, Z.; Shahsavani, A.; Aladaghlo, Z.; Fakhari, A. Application of Magnetic Nanoparticles Modified with Poly(8-Hydroxyquinoline) as a Nanosorbent for Magnetic Dispersive Micro-Solid Phase Extraction of Copper in Vegetable, Water, and Soil Samples. J. Food Compos. Anal. 2024, 132, 106333. [Google Scholar] [CrossRef]
- Kim, H.-B.; Kim, J.-G.; Alessi, D.S.; Baek, K. Temporal Changes in the Mobility of As, Pb, Zn, and Cu Due to Differences in Biochar Stability Caused by Lignin Content. Chem. Eng. J. 2024, 493, 152567. [Google Scholar] [CrossRef]
- Yang, S.; Dong, Z.; Zhu, B.; Yan, X.; Huang, J.; Xie, X.; Chang, Z.; Tian, S.; Ning, P. Feasibility and Solidification Mechanism Study of Self-Sustaining Smoldering Remediation for Copper and Lead-Contaminated Soil. Environ. Res. 2024, 250, 118498. [Google Scholar] [CrossRef]
- Dong, J.; Yang, S.; Kou, Z.; Chen, Y.; Yang, T.; Gao, P.; Zhang, W.; Zhang, J.; Che, D.; Wang, A. Oenothera Biennis with Strong Copper Toxicity Resistance Enriches Trace Copper in Seeds under Copper Pollution Soil. Ecotoxicol. Environ. Saf. 2024, 277, 116382. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.-X. Modified-Biochar Adsorbents (MBAs) for Heavy-Metal Ions Adsorption: A Critical Review. J. Environ. Chem. Eng. 2022, 10, 107393. [Google Scholar] [CrossRef]
- Wang, W.; Chen, G.; Tian, Q.; Liu, C.; Chen, J. Biochar Remediates Cadmium and Lead Contaminated Soil by Stimulating Beneficial Fungus Aspergillus spp. Environ. Pollut. 2024, 359, 124601. [Google Scholar] [CrossRef]
- Pathy, A.; Pokharel, P.; Chen, X.; Balasubramanian, P.; Chang, S.X. Activation Methods Increase Biochar’s Potential for Heavy-Metal Adsorption and Environmental Remediation: A Global Meta-Analysis. Sci. Total Environ. 2023, 865, 161252. [Google Scholar] [CrossRef]
- Gong, H.; Zhao, L.; Rui, X.; Hu, J.; Zhu, N. A Review of Pristine and Modified Biochar Immobilizing Typical Heavy Metals in Soil: Applications and Challenges. J. Hazard. Mater. 2022, 432, 128668. [Google Scholar] [CrossRef]
- Li, S.; Wen, Y.; Wang, Y.; Liu, M.; Su, L.; Peng, Z.; Zhou, Z.; Zhou, N. Novel α-Amino Acid-like Structure Decorated Biochar for Heavy Metal Remediation in Acid Soil. J. Hazard. Mater. 2024, 462, 132740. [Google Scholar] [CrossRef]
- Lee, G.; Jang, S.-E.; Jeong, W.-G.; Tsang, Y.F.; Baek, K. Stabilization Mechanism and Long-Term Stability of Endogenous Heavy Metals in Manure-Derived Biochar. Sci. Total Environ. 2024, 948, 174801. [Google Scholar] [CrossRef]
- Li, X.; Lin, S.; Ouvrard, S.; Sirguey, C.; Qiu, R.; Wu, B. Environmental Remediation Potential of a Pioneer Plant (Miscanthus sp.) from Abandoned Mine into Biochar: Heavy Metal Stabilization and Environmental Application. J. Environ. Manag. 2024, 366, 121751. [Google Scholar] [CrossRef]
- Zhu, Q.; Liang, Y.; Liu, H.; Guo, Y.; Zhang, Z.; Wang, C.; Liu, C.; Sun, H. Application of a Novel Ball-Milled Tourmaline-Biochar Composite Materials for Remediation of Groundwater and Bottom Mud Polluted with Heavy Metals. Sep. Purif. Technol. 2025, 352, 128278. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Bernas, J.; Konvalina, P. Testing Biochar’s Ability to Moderate Extremely Acidic Soils in Tea-Growing Areas. Agronomy 2024, 14, 533. [Google Scholar] [CrossRef]
- Gotore, O.; Itayama, T.; Dang, B.-T.; Nguyen, T.-D.; Ramaraj, R.; Osamu, N.; Shuji, T.; Maseda, H. Adsorption Analysis of Ciprofloxacin and Delafloxacin onto the Corn Cob Derived-Biochar under Different Pyrolysis Conditions. Biomass Convers. Biorefinery 2024, 14, 10373–10388. [Google Scholar] [CrossRef]
- Amirahmadi, E.; Mohammad Hojjati, S.; Kammann, C.; Ghorbani, M.; Biparva, P. The Potential Effectiveness of Biochar Application to Reduce Soil Cd Bioavailability and Encourage Oak Seedling Growth. Appl. Sci. 2020, 10, 3410. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Soja, G.; Bárta, J.; Chen, W.-H.; Amirahmadi, E. How Do Different Feedstocks and Pyrolysis Conditions Effectively Change Biochar Modification Scenarios? A Critical Analysis of Engineered Biochars under H2O2 Oxidation. Energy Convers. Manag. 2024, 300, 117924. [Google Scholar] [CrossRef]
- Lan, W.; Zhao, X.; Wang, Y.; Jin, X.; Ji, J.; Cheng, Z.; Yang, G.; Li, H.; Chen, G. Research Progress of Biochar Modification Technology and Its Application in Environmental Remediation. Biomass Bioenergy 2024, 184, 107178. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Shaikh, W.A.; Chakraborty, S.; Sarkar, D.; Biswas, J.K. Biochar Modification Methods for Augmenting Sorption of Contaminants. Curr. Pollut. Rep. 2022, 8, 519–555. [Google Scholar] [CrossRef]
- Tomczyk, A.; Kondracki, B.; Szewczuk-Karpisz, K. Chemical Modification of Biochars as a Method to Improve Its Surface Properties and Efficiency in Removing Xenobiotics from Aqueous Media. Chemosphere 2023, 312, 137238. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Zhang, A.; Li, X.; Xing, J.; Xu, G. Adsorption of Potentially Toxic Elements in Water by Modified Biochar: A Review. J. Environ. Chem. Eng. 2020, 8, 104196. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Ali, S.; Rizwan, M.; Abbas, F.; Bibi, I.; Riaz, M.; Khalil, U.; Niazi, N.K.; Rinklebe, J. A Review of Biochar-Based Sorbents for Separation of Heavy Metals from Water. Int. J. Phytoremediation 2020, 22, 111–126. [Google Scholar] [CrossRef]
- Rahim, H.U.; Akbar, W.A.; Alatalo, J.M. A Comprehensive Literature Review on Cadmium (Cd) Status in the Soil Environment and Its Immobilization by Biochar-Based Materials. Agronomy 2022, 12, 877. [Google Scholar] [CrossRef]
- Schommer, V.A.; Vanin, A.P.; Nazari, M.T.; Ferrari, V.; Dettmer, A.; Colla, L.M.; Piccin, J.S. Biochar-Immobilized Bacillus spp. for Heavy Metals Bioremediation: A Review on Immobilization Techniques, Bioremediation Mechanisms and Effects on Soil. Sci. Total Environ. 2023, 881, 163385. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified Biochar: Synthesis and Mechanism for Removal of Environmental Heavy Metals. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Tian, Y.; Li, P.; Chen, X.; He, J.; Tian, M.; Zheng, Z.; Hu, R.; Fu, Z.; Yi, Z.; Li, J. R3 Strain and Fe-Mn Modified Biochar Reduce Cd Absorption Capacity of Roots and Available Cd Content of Soil by Affecting Rice Rhizosphere and Endosphere Key Flora. Ecotoxicol. Environ. Saf. 2024, 278, 116418. [Google Scholar] [CrossRef]
- Xiang, D.; Wang, Z.; Rao, C.; Liu, X.; Fang, F.; Tang, W.; Bao, S.; Fang, T. Enhancing Cd (II) Immobilization with Thiol-Modified Low-Temperature Pyrolysis Biochar: Efficiency, Mechanism, and Applications. J. Environ. Chem. Eng. 2024, 12, 112387. [Google Scholar] [CrossRef]
- Liu, S.; Ding, W.; Zhang, H.; Li, Z.; Tian, K.; Liu, C.; Geng, Z.; Xu, C. Magnetized Bentonite Modified Rice Straw Biochar: Qualitative and Quantitative Analysis of Cd(II) Adsorption Mechanism. Chemosphere 2024, 359, 142262. [Google Scholar] [CrossRef]
- Pei, X.; Li, T.; He, Y.; Wong, P.K.; Zeng, G.; Tang, Y.; Jia, X.; Peng, X. Adsorbed Copper on Urea Modified Activated Biochar Catalyzed H2O2 for Oxidative Degradation of sulfadiazine: Degradation Mechanism and Toxicity Assessment. J. Environ. Manag. 2023, 342, 118196. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, Q.; Guan, C.-Y.; Yang, X.; Jiang, X.; Wei, M.; Tan, J.; Li, X. Metal Oxide Modified Biochars for Fertile Soil Management: Effects on Soil Phosphorus Transformation, Enzyme Activity, Microbe Community, and Plant Growth. Environ. Res. 2023, 231, 116258. [Google Scholar] [CrossRef]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The Meta-Analysis of Response Ratios in Experimental Ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Harvest Impacts on Soil Carbon Storage in Temperate Forests. For. Ecol. Manag. 2010, 259, 857–866. [Google Scholar] [CrossRef]
- Jian, X.; Li, S.; Feng, Y.; Chen, X.; Kuang, R.; Li, B.; Sun, Y. Influence of Synthesis Methods on the High-Efficiency Removal of Cr(VI) from Aqueous Solution by Fe-Modified Magnetic Biochars. ACS Omega 2020, 5, 31234–31243. [Google Scholar] [CrossRef]
- Wu, J.; Huang, D.; Liu, X.; Meng, J.; Tang, C.; Xu, J. Remediation of As(III) and Cd(II) Co-Contamination and Its Mechanism in Aqueous Systems by a Novel Calcium-Based Magnetic Biochar. J. Hazard. Mater. 2018, 348, 10–19. [Google Scholar] [CrossRef]
- Jung, K.-W.; Lee, S.Y.; Lee, Y.J. Hydrothermal Synthesis of Hierarchically Structured Birnessite-Type MnO2/Biochar Composites for the Adsorptive Removal of Cu(II) from Aqueous Media. Bioresour. Technol. 2018, 260, 204–212. [Google Scholar] [CrossRef]
- Tan, X.; Wei, W.; Xu, C.; Meng, Y.; Bai, W.; Yang, W.; Lin, A. Manganese-Modified Biochar for Highly Efficient Sorption of Cadmium. Environ. Sci. Pollut. Res. 2020, 27, 9126–9134. [Google Scholar] [CrossRef]
- Cui, X.; Fang, S.; Yao, Y.; Li, T.; Ni, Q.; Yang, X.; He, Z. Potential Mechanisms of Cadmium Removal from Aqueous Solution by Canna Indica Derived Biochar. Sci. Total Environ. 2016, 562, 517–525. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, S.; Dong, C.; Meng, Z.; Wang, X. Competitive Adsorption Behaviour and Mechanisms of Cadmium, Nickel and Ammonium from Aqueous Solution by Fresh and Ageing Rice Straw Biochars. Bioresour. Technol. 2020, 303, 122853. [Google Scholar] [CrossRef]
- Park, J.-H.; Ok, Y.S.; Kim, S.-H.; Cho, J.-S.; Heo, J.-S.; Delaune, R.D.; Seo, D.-C. Competitive Adsorption of Heavy Metals onto Sesame Straw Biochar in Aqueous Solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Amirahmadi, E.; Cornelis, W.; Zoroufchi Benis, K. Understanding the Physicochemical Structure of Biochar Affected by Feedstock, Pyrolysis Conditions, and Post-Pyrolysis Modification Methods—A Meta-Analysis. J. Environ. Chem. Eng. 2024, 12, 114885. [Google Scholar] [CrossRef]
- Chen, J.P.; Wu. Acid/Base-Treated Activated Carbons: Characterization of Functional Groups and Metal Adsorptive Properties. Langmuir 2004, 20, 2233–2242. [Google Scholar] [CrossRef] [PubMed]
- Godwin, P.M.; Pan, Y.; Xiao, H.; Afzal, M.T. Progress in Preparation and Application of Modified Biochar for Improving Heavy Metal Ion Removal from Wastewater. J. Bioresour. Bioprod. 2019, 4, 31–42. [Google Scholar] [CrossRef]
- Peng, H.; Gao, P.; Chu, G.; Pan, B.; Peng, J.; Xing, B. Enhanced Adsorption of Cu(II) and Cd(II) by Phosphoric Acid-Modified Biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar Surface Functional Groups as Affected by Biomass Feedstock, Biochar Composition and Pyrolysis Temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An Overview on Engineering the Surface Area and Porosity of Biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Sun, J.; He, F.; Pan, Y.; Zhang, Z. Effects of Pyrolysis Temperature and Residence Time on Physicochemical Properties of Different Biochar Types. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2017, 67, 12–22. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional Heterogeneity of Different Biochar: Effect of Pyrolysis Temperature and Feedstocks. J. Environ. Manag. 2021, 278, 111501. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Hu, S.; Xiang, J.; Sun, L.; Su, S.; Wang, J. Evaluation of the Porous Structure Development of Chars from Pyrolysis of Rice Straw: Effects of Pyrolysis Temperature and Heating Rate. J. Anal. Appl. Pyrolysis 2012, 98, 177–183. [Google Scholar] [CrossRef]
- Zeng, K.; Minh, D.P.; Gauthier, D.; Weiss-Hortala, E.; Nzihou, A.; Flamant, G. The Effect of Temperature and Heating Rate on Char Properties Obtained from Solar Pyrolysis of Beech Wood. Bioresour. Technol. 2015, 182, 114–119. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, S.; Liu, J.; Su, S.; Liang, Q.; Zeng, X.; Li, T. Study of the Effect of Pyrolysis Temperature on the Cd2+ Adsorption Characteristics of Biochar. Appl. Sci. 2018, 8, 1019. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, T.; Sun, X.; Bai, L.; Han, C.; Zhang, P. The Potential of Biochar and Lignin-Based Adsorbents for Wastewater Treatment: Comparison, Mechanism, and Application—A Review. Ind. Crops Prod. 2021, 166, 113473. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Oh, S.; Park, Y.-K. Overview of Biochar Production from Preservative-Treated Wood with Detailed Analysis of Biochar Characteristics, Heavy Metals Behaviors, and Their Ecotoxicity. J. Hazard. Mater. 2020, 384, 121356. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of Pyrolysis Temperature and Manure Source on Physicochemical Characteristics of Biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Oginni, O.; Singh, K. Influence of High Carbonization Temperatures on Microstructural and Physicochemical Characteristics of Herbaceous Biomass Derived Biochars. J. Environ. Chem. Eng. 2020, 8, 104169. [Google Scholar] [CrossRef]
- Lee, H.-S.; Shin, H.-S. Competitive Adsorption of Heavy Metals onto Modified Biochars: Comparison of Biochar Properties and Modification Methods. J. Environ. Manag. 2021, 299, 113651. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Mehmood, S.; Núñez-Delgado, A.; Ali, S.; Qaswar, M.; Shakoor, A.; Mahmood, M.; Chen, D.-Y. Enhanced Adsorption of Aqueous Pb(II) by Modified Biochar Produced through Pyrolysis of Watermelon Seeds. Sci. Total Environ. 2021, 784, 147136. [Google Scholar] [CrossRef]
- Chang, R.; Sohi, S.P.; Jing, F.; Liu, Y.; Chen, J. A Comparative Study on Biochar Properties and Cd Adsorption Behavior under Effects of Ageing Processes of Leaching, Acidification and Oxidation. Environ. Pollut. 2019, 254, 113123. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Wang, J.; Xu, H.; Liu, Y.; Zhang, Z.; Li, Y.; Zhang, Y. Adsorption of Cadmium and Lead Ions by Phosphoric Acid-Modified Biochar Generated from Chicken Feather: Selective Adsorption and Influence of Dissolved Organic Matter. Bioresour. Technol. 2019, 292, 121948. [Google Scholar] [CrossRef]
- Cibati, A.; Foereid, B.; Bissessur, A.; Hapca, S. Assessment of Miscanthus × Giganteus Derived Biochar as Copper and Zinc Adsorbent: Study of the Effect of Pyrolysis Temperature, pH and Hydrogen Peroxide Modification. J. Clean. Prod. 2017, 162, 1285–1296. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, X.; Wan, Y.; Wang, S.; Gao, B. Removal of Lead, Copper, Cadmium, Zinc, and Nickel from Aqueous Solutions by Alkali-Modified Biochar: Batch and Column Tests. J. Ind. Eng. Chem. 2016, 33, 239–245. [Google Scholar] [CrossRef]
- Fan, J.; Cai, C.; Chi, H.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of Cadmium and Lead Polluted Soil Using Thiol-Modified Biochar. J. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef]
- Fan, Q.; Sun, J.; Chu, L.; Cui, L.; Quan, G.; Yan, J.; Hussain, Q.; Iqbal, M. Effects of Chemical Oxidation on Surface Oxygen-Containing Functional Groups and Adsorption Behavior of Biochar. Chemosphere 2018, 207, 33–40. [Google Scholar] [CrossRef]
- Gholami, L.; Rahimi, G.; Khademi Jolgeh Nezhad, A. Effect of Thiourea-Modified Biochar on Adsorption and Fractionation of Cadmium and Lead in Contaminated Acidic Soil. Int. J. Phytoremediation 2020, 22, 468–481. [Google Scholar] [CrossRef]
- Gholami, L.; Rahimi, G. Efficiency of CH4N2S−modified Biochar Derived from Potato Peel on the Adsorption and Fractionation of Cadmium, Zinc and Copper in Contaminated Acidic Soil. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100468. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, W.; Wu, J.; Huang, Z.; Dai, J. Mechanism of Cu(II) Adsorption Inhibition on Biochar by Its Aging Process. J. Environ. Sci. 2014, 26, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, T.; Xue, Q.; Zhou, Y.; Wang, H.; Bolan, N.S.; Jiang, R.; Tsang, D.C.W. Enhanced Adsorption of Cu(II) and Zn(II) from Aqueous Solution by Polyethyleneimine Modified Straw Hydrochar. Sci. Total Environ. 2021, 778, 146116. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, L.; Wang, C.; Zhang, Q.; Liu, Q.; Li, Y.; Xiao, R. Adsorption of Cd(II) from Aqueous Solutions by Rape Straw Biochar Derived from Different Modification Processes. Chemosphere 2017, 175, 332–340. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.; Gao, Y.; Li, A. Facile Synthesis of Nano ZnO/ZnS Modified Biochar by Directly Pyrolyzing of Zinc Contaminated Corn Stover for Pb(II), Cu(II) and Cr(VI) Removals. Waste Manag. 2018, 79, 625–637. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Zhang, Z.; Zhang, Y.; Guan, Y. Citrate-Modified Biochar for Simultaneous and Efficient Plant-Available Silicon Release and Copper Adsorption: Performance and Mechanisms. J. Environ. Manag. 2022, 301, 113819. [Google Scholar] [CrossRef]
- Mahdi, Z.; El Hanandeh, A.; Yu, Q.J. Preparation, Characterization and Application of Surface Modified Biochar from Date Seed for Improved Lead, Copper, and Nickel Removal from Aqueous Solutions. J. Environ. Chem. Eng. 2019, 7, 103379. [Google Scholar] [CrossRef]
- Meng, Z.; Xu, T.; Huang, S.; Ge, H.; Mu, W.; Lin, Z. Effects of Competitive Adsorption with Ni(II) and Cu(II) on the Adsorption of Cd(II) by Modified Biochar Co-Aged with Acidic Soil. Chemosphere 2022, 293, 133621. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Rahimi, G.; Khademi Jolgeh Nezhad, A. Effectiveness of Native and Citric Acid-Enriched Biochar of Chickpea Straw in Cd and Pb Sorption in an Acidic Soil. J. Environ. Chem. Eng. 2019, 7, 103064. [Google Scholar] [CrossRef]
- Nie, T.; Hao, P.; Zhao, Z.; Zhou, W.; Zhu, L. Effect of Oxidation-Induced Aging on the Adsorption and Co-Adsorption of Tetracycline and Cu2+ onto Biochar. Sci. Total Environ. 2019, 673, 522–532. [Google Scholar] [CrossRef]
- Rechberger, M.V.; Kloss, S.; Wang, S.-L.; Lehmann, J.; Rennhofer, H.; Ottner, F.; Wriessnig, K.; Daudin, G.; Lichtenegger, H.; Soja, G.; et al. Enhanced Cu and Cd Sorption after Soil Aging of Woodchip-Derived Biochar: What Were the Driving Factors? Chemosphere 2019, 216, 463–471. [Google Scholar] [CrossRef]
- Shim, T.; Yoo, J.; Ryu, C.; Park, Y.-K.; Jung, J. Effect of Steam Activation of Biochar Produced from a Giant Miscanthus on Copper Sorption and Toxicity. Bioresour. Technol. 2015, 197, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Lian, F.; Yu, Z.; Zhu, L.; Xing, B.; Qiu, W. Synthesis and Characterization of a Novel MnOx-Loaded Biochar and Its Adsorption Properties for Cu2+ in Aqueous Solution. Chem. Eng. J. 2014, 242, 36–42. [Google Scholar] [CrossRef]
- Tan, L.; Ma, Z.; Yang, K.; Cui, Q.; Wang, K.; Wang, T.; Wu, G.-L.; Zheng, J. Effect of Three Artificial Aging Techniques on Physicochemical Properties and Pb Adsorption Capacities of Different Biochars. Sci. Total Environ. 2020, 699, 134223. [Google Scholar] [CrossRef]
- Trakal, L.; Veselská, V.; Šafařík, I.; Vítková, M.; Číhalová, S.; Komárek, M. Lead and Cadmium Sorption Mechanisms on Magnetically Modified Biochars. Bioresour. Technol. 2016, 203, 318–324. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Cao, Y. Pb(II) Sorption by Biochar Derived from Cinnamomum Camphora and Its Improvement with Ultrasound-Assisted Alkali Activation. Colloids Surf. Physicochem. Eng. Asp. 2018, 556, 177–184. [Google Scholar] [CrossRef]
- Wang, F.; Jin, L.; Guo, C.; Min, L.; Zhang, P.; Sun, H.; Zhu, H.; Zhang, C. Enhanced Heavy Metals Sorption by Modified Biochars Derived from Pig Manure. Sci. Total Environ. 2021, 786, 147595. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of Arsenic by Magnetic Biochar Prepared from Pinewood and Natural Hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Mosa, A.; Zimmerman, A.R.; Ma, L.Q.; Harris, W.G.; Migliaccio, K.W. Manganese Oxide-Modified Biochars: Preparation, Characterization, and Sorption of Arsenate and Lead. Bioresour. Technol. 2015, 181, 13–17. [Google Scholar] [CrossRef]
- Wongrod, S.; Simon, S.; Guibaud, G.; Lens, P.N.L.; Pechaud, Y.; Huguenot, D.; Van Hullebusch, E.D. Lead Sorption by Biochar Produced from Digestates: Consequences of Chemical Modification and Washing. J. Environ. Manag. 2018, 219, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, T.; Wang, J.; Zhang, Y.; Pan, W.-P. A Novel Modified Method for the Efficient Removal of Pb and Cd from Wastewater by Biochar: Enhanced the Ion Exchange and Precipitation Capacity. Sci. Total Environ. 2021, 754, 142150. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, J.; Lan, T.; Müller, K.; Niazi, N.K.; Chen, X.; Xu, S.; Zheng, L.; Chu, Y.; Li, J.; et al. Unraveling Sorption of Lead in Aqueous Solutions by Chemically Modified Biochar Derived from Coconut Fiber: A Microscopic and Spectroscopic Investigation. Sci. Total Environ. 2017, 576, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhou, M.; Qu, C.; Yu, D.; Chen, C.; Wang, M.; Tan, W. Quantitative Analysis of Pb Adsorption on Sulfhydryl-Modified Biochar. Biochar 2021, 3, 37–49. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen Peroxide Modification Enhances the Ability of Biochar (Hydrochar) Produced from Hydrothermal Carbonization of Peanut Hull to Remove Aqueous Heavy Metals: Batch and Column Tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, Y.; Liu, S.; Jiang, L.; Tan, X.; Zeng, G.; Li, M.; Liu, S.; Tian, S.; Fang, Y. Activated Magnetic Biochar by One-Step Synthesis: Enhanced Adsorption and Coadsorption for 17β-Estradiol and Copper. Sci. Total Environ. 2018, 639, 1530–1542. [Google Scholar] [CrossRef]
- Yuan, S.; Tan, Z. Effect and Mechanism of Changes in Physical Structure and Chemical Composition of New Biochar on Cu(II) Adsorption in an Aqueous Solution. Soil Ecol. Lett. 2022, 4, 237–253. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, J.; Jin, Q.; Zhang, X.; Yang, H.; Chen, Y.; Zhang, S.; Chen, H. Effect of Deashing on Activation Process and Lead Adsorption Capacities of Sludge-Based Biochar. Sci. Total Environ. 2020, 716, 137016. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, J.; Jin, Q.; Li, Z.; Zhang, X.; Chen, Y.; Zhang, S.; Chen, H. Sludge-Based Biochar Activation to Enhance Pb(II) Adsorption. Fuel 2019, 252, 101–108. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Cai, J.; Zhang, X.; Zhang, J.; Shao, J. Evaluation and Prediction of Cadmium Removal from Aqueous Solution by Phosphate-Modified Activated Bamboo Biochar. Energy Fuels 2018, 32, 4469–4477. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, B.; Lin, L.; Qiu, W.; Song, Z. Adsorption of Cu(II) and Cd(II) from Aqueous Solutions by Ferromanganese Binary Oxide–Biochar Composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef]
- Zhu, L.; Tong, L.; Zhao, N.; Wang, X.; Yang, X.; Lv, Y. Key Factors and Microscopic Mechanisms Controlling Adsorption of Cadmium by Surface Oxidized and Aminated Biochars. J. Hazard. Mater. 2020, 382, 121002. [Google Scholar] [CrossRef]
- Zuo, X.; Liu, Z.; Chen, M. Effect of H2O2 Concentrations on Copper Removal Using the Modified Hydrothermal Biochar. Bioresour. Technol. 2016, 207, 262–267. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghorbani, M.; Amirahmadi, E. Optimizing Biochar for Heavy Metal Remediation: A Meta-Analysis of Modification Methods and Pyrolysis Conditions. Environments 2025, 12, 399. https://doi.org/10.3390/environments12110399
Ghorbani M, Amirahmadi E. Optimizing Biochar for Heavy Metal Remediation: A Meta-Analysis of Modification Methods and Pyrolysis Conditions. Environments. 2025; 12(11):399. https://doi.org/10.3390/environments12110399
Chicago/Turabian StyleGhorbani, Mohammad, and Elnaz Amirahmadi. 2025. "Optimizing Biochar for Heavy Metal Remediation: A Meta-Analysis of Modification Methods and Pyrolysis Conditions" Environments 12, no. 11: 399. https://doi.org/10.3390/environments12110399
APA StyleGhorbani, M., & Amirahmadi, E. (2025). Optimizing Biochar for Heavy Metal Remediation: A Meta-Analysis of Modification Methods and Pyrolysis Conditions. Environments, 12(11), 399. https://doi.org/10.3390/environments12110399







