Abstract
Asthma is a chronic respiratory disease resulting from a complex interplay of genetic, environmental, and occupational factors. Key environmental risks include exposure to tobacco smoke and respiratory sensitizing agents, many of which are prevalent in workplace settings. In adults, asthma is associated with reduced employment, job instability, and work-related disability, resulting in significant social and economic consequences. This scoping review investigates the role of exposure to respiratory sensitizers in the onset and progression of asthma, considering data from the general population to occupational settings, with a focus on sex and gender as key modifiers of risk, disease severity, and occupational outcomes. Biological studies were also considered to clarify the mechanisms underlying observed sex/gender differences. Epidemiological data indicate that women are disproportionately affected by asthma, experiencing more severe symptoms, higher comorbidity rates, and increased exposure in certain professions such as healthcare, cleaning, and textile work. These disparities are attributed to both sex-related factors (e.g., hormonal influences) and gender-related factors (e.g., occupational roles, smoking habits). Although traditional job roles are changing, women continue to face greater occupational asthma risks. As roles evolve, physiological sex-based differences may become increasingly relevant in shaping asthma susceptibility. This review emphasizes the need for sex- and gender-sensitive strategies in asthma prevention, surveillance, and management, especially in occupational health contexts.
1. Introduction
It is well established that certain chemicals can induce allergic sensitization of the respiratory tract—a process in which the immune system becomes hypersensitive following initial exposure. With sufficient and typically repeated exposure, this sensitization may lead to respiratory allergic reactions and chronic conditions such as asthma, allergic rhinitis, or other respiratory disorders. Clinical manifestations can include coughing, wheezing, shortness of breath, chest tightness, and, in more severe cases, asthma attacks [1,2].
In Europe, the classification, labeling, and packaging of chemical substances and mixtures are regulated by Regulation (EC) No. 1272/2008 (CLP), which aligns with the United Nations Globally Harmonized System of Classification and Labeling of Chemicals (GHS). According to CLP Regulation, a substance must be classified as a respiratory sensitizer (Category 1) if (a) there is evidence in humans that the substance can induce specific respiratory hypersensitivity and/or (b) there are positive results from an appropriate animal test. From a physiological perspective, the first contact with a chemical sensitizer usually does not produce noticeable symptoms but instead primes the body through the development of specialized memory cells. Subsequent exposures to the same substance, even at low levels, elicit an abnormal and exaggerated immune response, resulting in symptoms of varying severity [1,2]. In contrast, although inhaled chemical irritants can cause a range of effects on the respiratory system, they induce direct tissue damage through localized inflammation at the site of contact without triggering an allergic reaction. Therefore, they should not be confused with respiratory chemical sensitizers, as their mechanism of action is different. According to the CLP, a substance that “may cause respiratory irritation” is classified as “specific target organ toxicity after single exposure” (Category 3), causing transient effects that temporarily impair human function but from which recovery occurs without lasting structural or functional damage.
The CLP definition of respiratory sensitizer and its regulatory approach are largely consistent with those in other jurisdictions and align with the harmonized criteria of GHS. Although the GHS itself is not legally binding, its provisions are implemented through national regulatory systems, with varying degrees of alignment depending on the adopted GHS revision, the hazard classes covered, and whether implementation is mandatory or voluntary (see Table 1).

Table 1.
Examples of national regulatory approaches to chemicals and their alignment with GHS harmonized criteria.
The European Chemicals Agency (ECHA) implements EU chemicals legislation and identifies respiratory sensitizers, with over 100 substances now harmonized within the EU [3]. ECHA also identifies Substances of Very High Concern (SVHCs), which include chemicals with serious or potentially irreversible health effects, and since 2012, the Member State Committee (MSC) has considered strong respiratory sensitizers equivalent to SVHCs [4,5]. Respiratory sensitizers are classified as high-risk because no reliable safe exposure threshold exists, and even low doses may trigger severe allergic respiratory effects. The inclusion of some sensitizers among SVHCs underscores their potency, associated health risks, and EU-level concerns, highlighting the relevance of implementing specific measures (e.g., restrictions or specific authorization for their use) to protect both the general and working populations.
Respiratory sensitizers are widely present in both living and occupational environments and represent a considerable burden on occupational health [6]. Occupational asthma is currently recognized as the most prevalent work-related respiratory disease in industrialized countries, a condition that can severely compromise workers’ quality of life and negatively impact productivity [7,8,9].
The ongoing introduction of new chemicals and industrial processes has broadened the range of environments in which individuals may be exposed to respiratory sensitizers, thereby making diagnosis and clinical management increasingly complex [10].
The main respiratory chemical sensitizers used or present in occupational environments include a wide range of substances, many of which are industrially synthesized (see Table 2). The information provided in Table 2 is indicative and not exhaustive.

Table 2.
The main respiratory chemical sensitizers used in occupational settings.
The occupational settings most frequently involved include the chemical and manufacturing industries, construction and carpentry, the wood and furniture industry, the textile sector, the automotive industry, the food and detergent industries, as well as healthcare and the pharmaceutical sector [24]. In addition to their deliberate use, respiratory sensitizers can also be present in the dust generated during certain specific material processing activities or from the deterioration of the material itself, adding a specific risk that requires dedicated assessment and the implementation of appropriate prevention and protection measures [25,26].
This highlights the widespread presence of respiratory sensitizers in the workplace and their potential indirect impact on living environments. Tobacco exposure should be recognized as an important potential confounding factor, as it is independently associated with airway sensitization, hyperresponsiveness, and inflammation, and may contribute to both atopic and non-atopic. While the effects of active or passive smoking are not the focus of this review, it should be considered alongside other potential risk factors (e.g., air pollution or comorbidities) when interpreting asthma outcomes [27,28,29].
In understanding the risk of developing occupational asthma related to exposure to chemical agents, it is crucial to consider the impact of sex and gender differences to enable more precise prevention strategies, particularly during workplace health surveillance [30]. According to the Committee on Understanding the Biology of Sex and Gender Differences of the US Institute of Medicine, sex refers to the biological classification of organisms, typically as male or female, based on reproductive anatomy and chromosomal composition. In contrast, gender pertains to an individual’s personal identification as male or female, along with the roles and expectations assigned by societal and institutional frameworks [31].
Incorporating sex and gender as key factors influencing diverse health outcomes presents a significant challenge across all medical disciplines [32]. Primarily, biological differences between sexes can lead to varied responses to chemical exposures in males and females, while gender differences also contribute to a better understanding of the observed disparities [33,34].
The role of sex and gender differences in the development of asthma induced by respiratory chemical sensitizers remains, to some extent, unclear. This scoping review aimed to elucidate this topic and systematically map the scientific evidence available in this field, considering data starting from general population to occupational settings. To better interpret the epidemiological evidence, we also explored the biological mechanisms underlying the allergic response, with particular attention to sex differences, as well as lifestyle factors that may exacerbate respiratory function impairment (e.g., tobacco smoke).
The specific objective of this review was to map the existing scientific evidence in a systematic manner. The formulated research question was: What does the scientific literature report regarding the role of sex and gender in the onset of asthma and its severity, particularly in occupational settings?
2. Materials and Methods
This scoping review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist [35]. The PRISMA checklist for scoping review is provided as a Supplementary File.
2.1. Information Sources and Literature Search
A bibliographic search was performed from March to May 2025 using public databases, in particular Scopus, PubMed, and Google Scholar, employing the keywords “sex” AND “gender”AND “asthma” or “respiratory sensitizer” AND “hormones” AND “occupational exposure”.
2.2. Inclusion Criteria
Papers were included if they evaluated, compared, described, or involved situations of exposure to chemical sensitizers and their effects on both male and female populations in everyday life and occupational settings. Eligible studies were required to identify the type of chemical involved and describe its effects on the onset or severity of asthma.
Studies published in languages other than English, Italian, French, or Spanish were excluded. Studies aimed at evaluating clinical treatments—regardless of sex differentiation—were not considered. Research on biological mechanisms of action, although not selected for formal inclusion in the scoping review, was used to illustrate the underlying mechanisms of observed effects and to support the interpretation of sex- and gender-stratified data. Gray literature was not included. Only studies published since 2000 were considered.
The scoping review focused primarily on epidemiological studies conducted in human populations, with particular emphasis on investigations addressing the effects of chemical sensitizer exposure on respiratory function and asthma. The review specifically targeted studies in which exposure to chemical substances was associated with occupational or specific environmental living conditions. For occupational exposure studies, eligible designs included cohort studies comparing exposed and unexposed groups, as well as case–control studies in which recruitment was based on asthma diagnosis and professional exposure was characterized retrospectively.
2.3. Screening Process
The screening and data extraction processes were refined through calibration exercises in which all reviewers screened the same set of publications and discussed the results. Titles, abstracts, and full texts of the identified studies were assessed sequentially by four researchers working in pairs. Any disagreements regarding study selection or data extraction were resolved through discussion and, when necessary, with the involvement of additional reviewers to reach consensus.
A data-charting form was collaboratively developed by the four reviewers to define the variables to be extracted. Each researcher independently charted the data, after which the results were discussed collectively. The data-charting form was iteratively updated throughout the process to ensure consistency and comprehensiveness.
Extracted data included general study characteristics, with particular emphasis on the type of chemical exposure. Experimental studies—both in vitro and in vivo—were included to illustrate proposed biochemical mechanisms, although the scoping review primarily focused on epidemiological investigations in human populations.
A schematic overview of the search strategy and the selection of articles is presented in Figure 1.
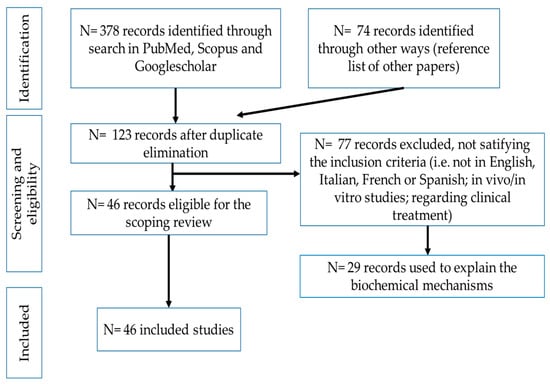
Figure 1.
Scheme of the bibliographic search.
3. Biological Mechanism Underlying Sex Differences in Asthma
3.1. Sex: Biological and Physiological Differences Linked with Possible Different Health Effects
The prevalence, severity, and susceptibility to a range of lung diseases, including asthma, has been observed to differ among men and women throughout life. Chronic airway inflammation is the main feature of asthma, which is a disease that can be categorized as heterogeneous [36]. According to the World Health Organization, more than 339 million people worldwide suffer from asthma, with the condition accounting for over 400,000 deaths annually [37].
The underlying causes of these sex-based differences in asthma development are not yet fully understood, although a substantial body of research has investigated the association between sexual dimorphism and asthma. Several studies have identified biological (sex-related) factors—such as airway anatomy and physiology, chromosomal differences, genetic and epigenetic influences, and the role of sex hormones—as potential contributors to the variation in disease occurrence and outcomes between males and females (Figure 2).
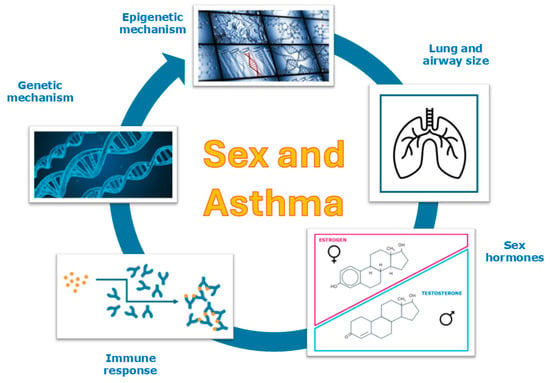
Figure 2.
Sex impact on asthma.
3.1.1. Sex and Anatomic Factor’s Role on Asthma
Sex differences in lung and airway development have been well documented. Females tend to have larger airways than males; however, the number of alveoli per unit area and alveolar size do not differ significantly between the sexes. Notably, boys exhibit higher age- and height-adjusted lung volumes compared to girls, potentially resulting in a greater alveolar surface area and a higher diffusion capacity for carbon monoxide (DLCO) in males [38,39,40].
According to the Centers for Disease Control and Prevention (CDC), in the United States, an estimated 8.3% of boys and 6.7% of girls under the age of 18 currently suffer from asthma. Interestingly, this pattern reverses after puberty: among adults, asthma prevalence is nearly twice as high in women compared to men (9.8% vs. 5.5%, respectively) [37]. This inversion in asthma prevalence across the lifespan is associated with changes in sex hormone levels during development, puberty, and key physiological events such as pregnancy and menopause, all of which influence lung function and respiratory health.
3.1.2. Sex Hormone’s Role on Asthma
Steroid hormones regulate and modulate a wide range of biological processes, including the functioning of the immune system, and represent key factors contributing to phenotypic differences between males and females. These hormones interact with their respective receptors, which are expressed in various cell types—including immune cells such as eosinophils, mast cells, and T lymphocytes. Estrogen, progesterone, and testosterone influence not only the immune response but also airway inflammation and bronchial hyperresponsiveness, thereby affecting asthma onset and severity at different stages of life.
In early life, female sex hormones appear to promote lung development and maturation, whereas androgens may exert inhibitory effects [41]. Estrogen and testosterone are widely recognized as contributing factors to asthma severity; however, the extent to which these hormones are primary drivers of disease pathogenesis remains uncertain [42].
Sex hormones modulate asthma pathophysiology through their effects on both the innate and adaptive immune responses. The immune response in asthma is primarily mediated through two distinct inflammatory mechanisms: Type 2 (T2-high) and non-Type 2 (T2-low) pathways [43]. T2-high inflammation is the predominant mechanism in bronchial asthma (see Figure 3 and Figure 4). Exposure to a variety of antigens—including viruses, bacteria, and chemical substances—can trigger both arms of the immune system Via interactions with the bronchial epithelium. Upon antigen exposure, epithelial cells in the airways release alarmins such as interleukin (IL)-25, IL-33, and thymic stromal lymphopoietin (TSLP). These alarmins activate innate lymphoid cells type 2 (ILC2), which in turn produce IL-5 and IL-13. Simultaneously, antigen-presenting cells (APCs) of the adaptive immune system—such as dendritic cells, macrophages, and mast cells—are activated and promote the differentiation of naïve T helper cells (Th0) into Th2 lymphocytes. Th2 cells migrate to the airways, where they secrete IL-4, IL-5, IL-9, and IL-13, which play critical roles in recruiting and activating additional immune cells, including eosinophils, basophils, and mast cells.
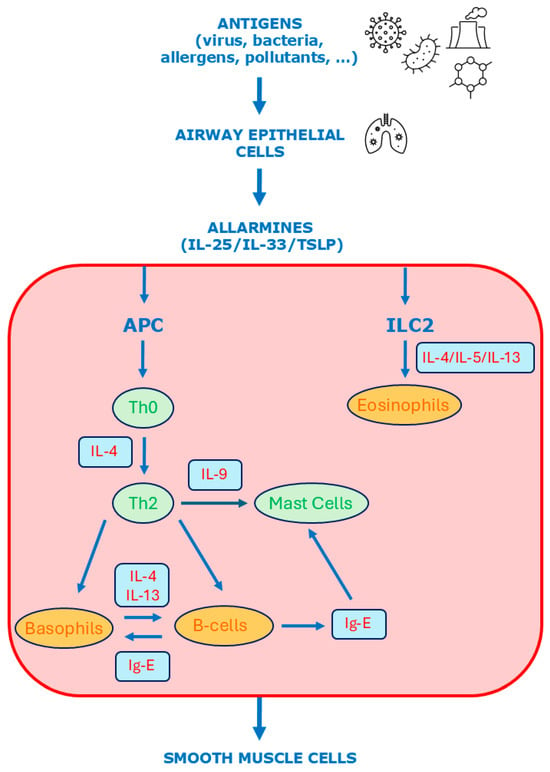
Figure 3.
Molecular mechanisms of type 2 inflammation in asthma. APC = antigen-presenting cells; ILC = innate lymphoid cells.
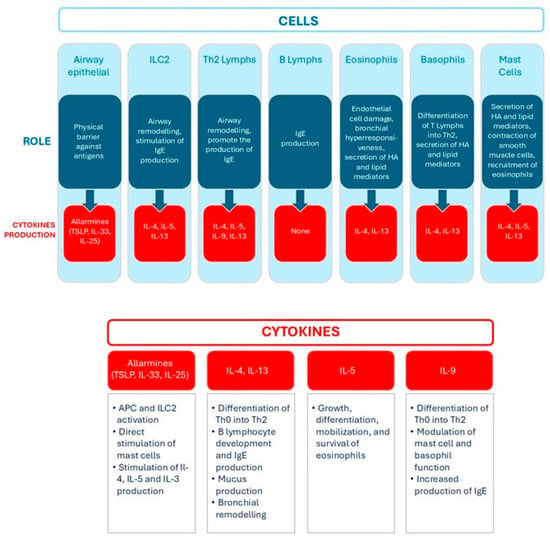
Figure 4.
Role of main cells and cytokines involved in type 2 inflammation. Lymphs = lymphocytes, HA = histamine.
These immune cells release various mediators that contribute to the pathophysiology of asthma by inducing smooth muscle contraction, increasing vascular permeability, and influencing T lymphocyte differentiation. Notably, B lymphocytes are stimulated to produce immunoglobulin E (IgE) antibodies, which bind to receptors on the surface of mast cells and basophils, priming them for a more effective immune response upon subsequent exposure to the allergen or chemical sensitizers. When the allergen or chemical sensitizer reappears, it binds to the IgE antibodies already attached to the cells, triggering their degranulation and the release of pro-inflammatory mediators (histamine, prostaglandins, and leukotrienes) that contribute to bronchoconstriction, increased vascular permeability, and mucus secretion [44]. In asthma, IgE thus plays a central role in the immunological response leading to the airway inflammation and bronchoconstriction characteristic of the disease. IgE is also implicated in the late-phase response, chronic airway inflammation, and airway remodeling, making it a key target for therapies in severe allergic asthma.
Interleukin-13 (IL-13) plays a central role by stimulating airway epithelial cells to produce excess mucus, which can obstruct the bronchi and impair normal breathing. IL-13 also promotes the recruitment and infiltration of eosinophils, further amplifying the asthmatic inflammatory response.
On the other hand, T2-low inflammation is less well defined. It is characterized by neutrophilic infiltration mediated by Th1 and Th17 lymphocytes. Th1 cells secrete interferon-gamma (IFN-γ), which enhances macrophage activation and sustains a pro-inflammatory environment. In contrast, Th17 cells release IL-17, which is involved in the recruitment and activation of neutrophils. Neutrophilic activity in T2-low inflammation is associated with increased oxidative stress, epithelial damage, and mucus hypersecretion [45].
The sexual dimorphism observed in asthma prevalence is closely associated with the physiological production of sex hormones. In particular, Type 2 asthma is more prevalent in females than in males and is characterized by elevated levels of ovarian hormones and decreased testosterone levels [46]. Androgens, such as testosterone, generally suppress innate immune responses, whereas estrogen and progesterone enhance Th2-mediated inflammation.
Overall, estrogens are considered immune enhancers, promoting immune responses, while androgens act as immune suppressors by dampening these responses [47]. Several studies support this view, indicating that estrogens contribute to a pro-inflammatory environment, whereas androgens exhibit anti-inflammatory properties [48]. Beyond their reproductive functions, androgens regulate the structure and function of non-reproductive organs, further demonstrating their systemic influence. Moreover, elevated testosterone levels have been associated with a reduced risk of asthma in both males and females [49]. Testosterone can inhibit IL-13 production, thereby decreasing mucus secretion and alleviating airway obstruction [50].
The role of testosterone may explain the observed decrease in asthma prevalence among males after puberty and the generally lower frequency of asthma in adult men compared to women [45]. In contrast, estrogen enhances Th2-mediated inflammation, promoting eosinophilic infiltration, mucus production, and airway hyperreactivity [47], partly by upregulating IL-4 and IL-13 expression [51]. Additionally, estrogen has been shown to augment IL-17-mediated airway inflammation, which tends to be more pronounced in females [52]. However, some studies suggest that estrogen may play a dual role in asthma pathogenesis, with the capacity to either inhibit or activate inflammatory responses depending on the context; further research is needed to elucidate the molecular pathways underlying this dual effect [53]. Similarly, the impact of progesterone on asthma is complex, exhibiting both pro-inflammatory and anti-inflammatory effects [54].
In pre-pubertal populations, asthma prevalence is higher in males than females; however, this trend reverses after puberty, with females exhibiting higher prevalence rates [55]. These observations indicate that fluctuations in sex hormone levels may play a pivotal role in asthma pathogenesis. While estrogen and progesterone directly modulate immune pathways involved in asthma development, testosterone appears to exert a protective effect against the inflammatory processes associated with the disease, though several mechanisms remain unclear [55,56,57].
3.1.3. Genetic and Epigenetic Mechanism’s Role on Asthma
Genetic and epigenetic factors play a key role in sex-based differences in asthma. Both sex chromosomes and autosomal genes contribute to the regulation of immune responses and airway remodeling, while epigenetic mechanisms such as DNA methylation, histone modifications, and microRNA (miRNA) regulation further modulate these processes. The X chromosome contains numerous genes involved in immune regulation that may contribute to the higher prevalence and severity of asthma observed in females. Notably, the TLR7 and TLR8 genes, which encode toll-like receptors critical for innate immune activation, are expressed at higher levels in females, potentially amplifying inflammatory responses in the airways [58]. Similarly, genes such as ORMDL3, which is implicated in sphingolipid metabolism and airway hyperresponsiveness, and ADAM33, involved in airway remodeling, exhibit sex-dependent expression patterns likely influenced by hormonal regulation [59,60].
DNA methylation plays a crucial role in regulating gene expression, and studies have identified distinct methylation patterns in asthma-related genes between males and females, suggesting a possible influence on immune cell function, airway inflammation, and steroid responsiveness. MiRNAs are key post-transcriptional regulators that affect cytokine production, immune cell activation, and airway remodeling. Several miRNAs have been implicated in asthma pathogenesis, with recent evidence suggesting their involvement in mediating sex-based differences in disease susceptibility and severity [45]. For instance, the Let-7 miRNA family is downregulated in asthma and may contribute to sex disparities; Let-7 miRNAs are known to inhibit IL-13 expression, resulting in lower IL-13 levels in males compared to females [61].
4. Results and Discussion
4.1. Sex and Gender Differences: Epidemiological Evidence on General Population
The impact of sex and gender on asthma prevalence is well established [62], and research in this field is steadily increasing [63], although some controversies remain [64,65].
The role of sex hormones leads to clear epidemiological observations: male children show more frequent asthma onset, while after puberty the prevalence is higher in the female population [55].
The association between severe asthma and female sex hormones has also been proposed by some authors [57,66], who observed a worsening of symptoms during specific phases of the menstrual cycle and pregnancy. In fact, 11–45% of women report experiencing “premenstrual asthma,” which is likely related to elevated estrogen and progesterone levels during the luteal phase. Additional evidence supporting a link between sex hormones and asthma has been reported by Nwaru et al. [67,68], who found that the use of hormonal contraceptives appears to reduce both asthma exacerbations and the incidence of new-onset asthma.
Although a definitive biochemical mechanism has not been identified, epidemiological evidence suggests that controlling fluctuations in estrogen and progesterone levels during the menstrual cycle may improve asthma management. Women in the menopausal period have been shown to experience more severe asthma and an increased risk of new-onset asthma [69]. Similar findings have been reported in women undergoing hormone replacement therapy (HRT), although variations in asthma risk appear to be associated with the specific type of HRT used.
In a large case–control study involving 379,649 participants, Hansen et al. [70] reported an increased asthma risk with combined estrogen–progesterone HRT, whereas a decreased risk was observed with progesterone-only therapy. However, these findings have not been consistently confirmed in other studies [71].
In this context, a noteworthy large cross-sectional study by Morales Estrella et al. [72], involving a cohort of 50,009,780 individuals, reported a higher prevalence of asthma among male-to-female transgender individuals (n = 7210) compared to those whose sex at birth remained unchanged as male. Notably, an even greater prevalence was observed among those who had undergone gender-affirming surgery (n = 490). These findings further support the hypothesis that hormonal profiles play a central role in the development of asthma.
Moreover, sex-based differences are evident not only in the incidence but also in the severity of the disease. Women generally present with more severe asthma phenotypes, more frequent comorbidities, poorer quality of life, and higher rates of exacerbations and hospitalizations compared to men [73,74]. These differences have been primarily attributed to physiological factors, particularly hormonal influences [55]. However, some authors have also suggested a role for gender-related factors, such as behavioral differences and symptom perception, in shaping disease outcomes [75,76].
Several surveys have been conducted to investigate whether symptom reporting differs by gender, potentially influencing therapeutic decision-making. Evidence suggests that men and women may present with distinct symptom profiles. Notably, women tend to perceive their asthma as more severe and symptomatic compared to men, despite similar clinical findings [75]. Additionally, women have reported a lower asthma-related quality of life [77]. Some authors have proposed that societal expectations may influence male patients to downplay their symptoms, potentially leading to underreporting of disease severity [78].
A cross-sectional survey conducted by Ricciardolo et al. [65] involving 499 asthmatic patients revealed a higher prevalence of asthma among women (301 females and 198 males). This study aimed to evaluate the association also with smoking habits and lung function. Specifically, asthmatic women were less likely to smoke compared to men (p < 0.0001), and they demonstrated significantly higher values of forced expiratory volume in one second (FEV1) (p = 0.0022) and forced vital capacity (FVC) (p = 0.0004). These findings suggest that in the sample studied, the severity of the pathology in the female population (although larger than the male population) was less problematic; this reminds us of the multifactorial nature of the etiology and the role of risk factors such as smoking.
In a large prospective cohort study conducted in Switzerland, Hansen et al. [79] followed 5128 asthma-free adults over a 20-year period. The incidence of asthma was 5.1% in men and 7.5% in women. After adjusting for confounding factors, the odds ratio for asthma incidence in females was 1.99 (95% CI: 1.54–2.57), confirming an approximately two-fold higher risk for women compared to men. The study also highlighted the role of age in asthma onset, reporting that asthma incidence in older women decreased and became more comparable to that in men—suggesting again a potential hormonal influence on disease development and/or progression. However, the role of age remains a subject of debate in the literature, with conflicting findings reported in previous studies [80,81,82].
Epidemiological studies in children support clinical trial findings indicating that, during the prepubertal period, males exhibit a higher prevalence and greater severity of asthma [82]. A notable example is a large population-based study conducted in China [83], in which 30,139 children aged 3–12 years (13,125 males and 12,879 females) were recruited to assess the impact of air pollution on respiratory symptoms. The results demonstrated that the effect of air pollution on asthma onset was more pronounced in male children, even in the absence of a pre-existing allergic predisposition.
4.2. Sex and Gender Differences: Epidemiological Evidence in Occupational Settings
When examining occupational environments, some preliminary considerations are necessary to understand the problem. Occupational exposure to respiratory sensitizers is a significant risk factor in the development of asthma. This is supported by a retrospective survey of 4468 adults, among whom 677 had a confirmed asthma diagnosis—75.9% of whom were women. The study identified occupational exposure to high molecular weight sensitizers (OR = 1.53, 95% CI: 1.18–2.00) and low molecular weight sensitizers (OR = 1.42, 95% CI: 1.09–1.87) as factors significantly associated with the onset of the disease [84].
Even within the same workplace, men and women may experience different levels of exposure or carry out different job tasks—factors that complicate the assessment of chemical exposure and its potential health effects [85,86]. Epidemiological evidence from various occupational settings suggests that female workers may be at greater risk for both the development and increased severity of occupational asthma. However, these data must be appropriately contextualized, considering not only differences in occupational exposure but also lifestyle habits that may predispose individuals to respiratory sensitization. This is particularly evident in laboratory environments [87] and in occupations involving frequent contact with cleaning agents, especially those containing chloramine [88].
Moreover, exposure to cleaning products is not limited to occupational settings. In daily life, women may experience higher exposure due to persistent gender-based divisions of domestic labor, with responsibilities such as household cleaning, childcare, and eldercare still disproportionately assigned to women. Both professional and domestic cleaning have been associated with work-related asthma [89].
Cleaning products encompass a wide array of chemicals designed to facilitate the removal of dust and dirt, as well as to disinfect surfaces. Most of these substances are low-molecular-weight agents. The main sensitizers found in cleaning products include disinfectants, quaternary ammonium compounds, amines, and fragrances—particularly those containing limonene and/or pinene [90]. However, new components are frequently introduced into commercial formulations.
Three key aspects should be considered regarding the development of asthma due to exposure to cleaning chemicals:
- The intrinsic sensitizing potential of the agent.
- The conditions of exposure (including the type, frequency, and duration of cleaning tasks, the application method—such as spraying vs. mopping—the volatility of the chemicals, and their concentration).
- Individual susceptibility [91].
Quirce et al. [90] proposed a list of the main sensitizers present in cleaning products (amine compounds, disinfectants, quaternary ammonium compounds, scents containing terpenes and/or eugenol, preservatives and others).
Prolonged exposure to moderate or low concentrations of respiratory sensitizers can lead to new-onset asthma and work-related asthma [92,93]. The use of cleaning sprays significantly increases inhalation exposure [94,95], and many low-molecular-weight agents found in these products are volatile organic solvents [92].
Occupations such as nursing and cleaning—where women typically represent more than 70% of the workforce [96]—show the highest relative risk for new-onset asthma. In a study involving 34,000 adults, health-related occupations were associated with an odds ratio (OR) of 1.77 (95% CI: 1.24–2.52), while cleaning occupations had an OR of 2.07 (95% CI: 1.25–3.42) for asthma [97].
In this context, both gender and sex differences contribute to the observed health disparities. Women not only have greater physiological susceptibility (sex-related factors) but also higher levels of exposure due to gender-related roles, leading to increased adverse health outcomes.
Furthermore, it is noteworthy that among nurses with asthma, the use of disinfectants for cleaning medical instruments was associated with more severe asthma symptoms (OR = 1.37, 95% CI: 1.05–1.79). This association was particularly strong in cases of exposure to formaldehyde, glutaraldehyde, hypochlorite bleach, hydrogen peroxide, and enzymatic cleaners [98].
A more recent study also confirmed a higher prevalence of occupational asthma among women working in the cleaning products industry. In a sample of 780 workers handling detergents and cleaning agents, several risk factors for asthma were identified, including female gender (OR = 1.397, 95% CI: 1.09–1.96), manual labor (OR = 3.067, 95% CI: 1.72–5.46), and a history of atopy (OR = 1.596, 95% CI: 1.09–2.33) [99].
By expanding their focus across various professional contexts, some authors have attempted to provide a broader overview of asthma prevalence among women [100]. Their findings highlight clear differences in occupational exposures between men and women, which are reflected in the varying incidence of the disease. Globally, women appear to be more occupationally exposed to cleaning chemicals and biomass fuels, while men are more commonly exposed to pyrolysis products, plant-based materials, isocyanates, and metals [76,101,102].
However, traditional gender roles in the workplace are evolving, suggesting that in the future, physiological differences (i.e., sex differences) may play a more prominent role than gender-related factors in influencing asthma risk. Additionally, daily habits such as smoking—once more prevalent among men—now show a reduced gender gap. Despite this shift, asthma symptoms associated with smoking tend to be more severe in women, pointing to a higher biological susceptibility [103].
Data from health surveillance activities in the United States [76] analyzed 8239 cases of occupational asthma, of which 60% involved female workers. When comparing asthma characteristics between the sexes in this workforce population, it was found that women experienced more severe forms of asthma (24.4% vs. 13.5%). The data also confirmed that a significant proportion of women with asthma were employed in social or medical care roles (28.7% vs. 5.2%), in clinical or technical positions (13.4% vs. 1.6%), as well as in office-based occupations (20.0% vs. 4.0%) and educational services (11.8% vs. 4.2%).
In the same study, the chemical agents most associated with the onset of occupational asthma differed by sex. Among women, the leading contributors were cleaning agents (15.3%), indoor pollutants (14.9%), and various chemical mixtures (20.3%). In contrast, for men, the primary exposures were inorganic mineral dusts (13.2%), pyrolysis products (12.7%), and mixed chemical agents (15.7%).
A French survey [104], aimed at examining the characteristics of occupational asthma using data from occupational medicine clinics, reported a predominance of female cases—60.81% out of 222 affected workers—confirming trends observed in the literature, particularly among workers in the textile sector (61.5%). One notable finding from this study was that 58% of women experienced a worsening of symptoms during the perimenstrual period, 75% during pregnancy, and 14% when using oral contraceptives. These results underscore the potential role of female hormones in influencing the severity of the disease. Regarding pregnant women, it should be added that physiological changes can lead to an increase in respiratory problems, which can contribute to the recording of more serious asthma symptoms [105].
Further insights were provided by Hsinet et al. [106], who investigated a cohort of 232 occupational asthma cases, focusing on the production context, specific job tasks, and the most frequently reported clinical symptoms. From this study, it emerged that women represented most cases (50.9%). Most of the affected workers lacked specific technical skills and were employed in predominantly manual labor (57.1%). The textile industry was again identified as a sector with a significant number of cases (10%), alongside the healthcare sector (10.9%). The main sensitizing agents included high molecular weight allergens, such as vegetable textile dust (9.9%), and low molecular weight agents like isocyanates (11.6%) and formaldehyde (11.2%). Notably, in 47.8% of cases, modifications to the workstation were required due to asthma symptoms.
Robin et al. [107] recently reported a seemingly contrasting trend: in a population of 8385 individuals with occupational asthma, 54.5% were men. However, this figure also included cases related to irritant exposures and non-allergic asthma, which accounted for 13.5% of male cases. This survey [107] also identified the work environments most frequently associated with occupational asthma. For women, these included hairdressing salons and healthcare settings—particularly due to exposure to quaternary ammonium compounds found in cleaning agents and disinfectants. For men, high-risk environments included bakeries, where exposure to flour dust is common, and construction sites, due to the presence of isocyanates and cyanates. In Table 3 it is summarized the occupational exposure published papers.

Table 3.
Summary of the evidence in workplaces exposure to sensitizer chemicals.
5. Conclusions
The main environmental risk factors for asthma include smoking and exposure to respiratory sensitizing chemical agents, many of which are commonly found in workplace settings. These exposures significantly contribute to the onset and progression of asthma, with substantial repercussions for public health. Particularly concerning is the burden of work-related disability among individuals with asthma, which results in considerable economic and social consequences, e.g., higher rates of unemployment, job instability, and sick leave.
This scoping review highlights that sex and gender differences play a crucial role in both the incidence and severity of asthma induced by respiratory chemical sensitizers. Biological differences, including hormonal influences and genetic/epigenetic mechanisms, can alter immune responses and airway inflammation, thereby impacting disease susceptibility and clinical outcomes. Data from population studies show that asthma is more frequently diagnosed in boys during childhood, but after puberty, it becomes more widespread and severe in women. This shift underscores the role of sex hormones in modulating immune responses and airway inflammation. Estrogen has been found to boost type 2 inflammatory processes and may lead to greater asthma severity in females, while testosterone seems to have a protective effect. Additionally, genetic and epigenetic mechanisms play a role in these differences, with variations in the expression of immune-related genes, DNA methylation patterns, and miRNA regulation contributing to distinct immune responses and airway remodeling in males and females. Current therapeutic approaches remain largely uniform for all patients; however, a better understanding of the specific sex-specific factors involved in asthma management could facilitate the development of more effective, personalized treatment strategies.
Some limitations are identified in our scoping review. Studies addressing biological mechanisms of action, using in vitro and in vivo surveys, were excluded by design. This was an intentional eligibility criterion, but it may result in the omission of valuable information on certain chemicals. Furthermore, the exclusion of publications not written in English, Italian, Spanish, or French limits our ability to capture evidence from studies published in other languages, potentially introducing a geographical bias. Lastly, we did not register our scoping review protocol. Although we do not consider this oversight a study limitation per se, protocol registration is regarded as best practice. Finally, some studies, while evaluating male and female working populations separately, do not properly investigate the differences in disease incidence between the two sexes. This should be acknowledged as a limitation of including these studies in the present discussion and further emphasizes the need for future research that considers the important issue of gender/sex effect.
Gender-related factors, including occupational roles and associated exposure levels, as well as behaviors such as smoking habits, further modulate asthma risk. Women appear to be disproportionately affected by occupational asthma due to both biological susceptibility and greater exposure to respiratory sensitizers, particularly in sectors such as healthcare, cleaning, and textile work. Moreover, hormonal fluctuations (e.g., during the menstrual cycle or pregnancy) may exacerbate asthma symptoms, highlighting the importance of considering hormonal status during clinical evaluations.
Integrating sex and gender perspectives into occupational health surveillance and asthma management is therefore essential to ensure more effective prevention, diagnosis, and treatment interventions.
Further investigations in different occupational settings are highly desirable in order to better characterize the risks associated with exposure to sensitizing substances in relation to sex and gender, especially considering that the female workforce is increasingly represented in roles and tasks that have traditionally been male-dominated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments12100382/s1, Table S1: Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist.
Author Contributions
Conceptualization, L.C.; methodology, L.C., S.D.R. and D.P.; investigation, L.C., S.D.R. and D.P.; data curation, L.C., S.D.R. and D.P.; writing—original draft preparation, L.C., S.D.R., E.P. and D.P.; writing—review and editing, L.C., S.D.R., D.C., P.T. and D.P.; visualization, E.P., D.C. and P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CLP | Classification, Labeling and Packaging |
| GHS | Globally Harmonized System |
| HCS | Hazard Communication Standard |
| ECHA | European Chemicals Agency |
| GB | Great Britain |
| OSHA | Occupational Safety and Health Agency |
| WHMIS | Workplace Hazardous Materials Information System |
| WHS | Workplace Health and Safety |
| MEM | Ministry of Emergency Management |
| SAMR | State Administration for Market Regulation |
| MHLW | Ministry of Health, Labor and Welfare |
| METI | Ministry of Economy, Trade and Industry |
| MOE | Ministry of the Environment |
| REACH | Registration, Evaluation and Authorization of Chemicals |
| SVHCs | Substances of very high concern |
| MSC | Member State Committee |
| TDI | Toluene diisocyanate |
| MDI | Methylene diphenyl diisocyanate |
| HDI | Hexamethylene diisocyanate |
| MHHPA | Methylhexahydrophthalic anhydride |
| HHPA | Hexahydrophthalic anhydride |
| HRT | Hormone Replacement Therapy |
| Hmws | High molecular weight sensitizers |
| Lmws | Low molecular weight sensitizers |
References
- Basketter, D.A.; Kimber, I. Assessing the potency of respiratory allergens: Uncertainties and challenges. Regulat. Toxicol. Pharmacol. 2011, 61, 365–372. [Google Scholar] [CrossRef]
- Kimber, I.; Basketter, D.A. Chemical allergens—What are the issues? Toxicol 2010, 268, 139–142. [Google Scholar] [CrossRef] [PubMed]
- 1272/2008-CLP; Regulation (EC), Annex VI—Table of Harmonised Entries. Publications Office of the European Union: Helsinki, Finland, 2008.
- ECHA. The Member State Committee Identifies the First Respiratory Sensitisers as Substances of very High Concern. Available online: https://echa.europa.eu/it/-/the-member-state-committee-identifies-the-first-respiratory-sensitisers-as-substances-of-very-high-concern (accessed on 31 May 2025).
- Minutes of the 25th Meeting of the Member State Committee (MSC-25) 19–21 September 2012. MSC/M/025/2012 Adopted at MSC-26. Available online: https://echa.europa.eu/documents/10162/17089/meet_minutes_msc_25_en.pdf/ (accessed on 31 May 2025).
- Dotson, G.S.; Maier, A.; Siegel, P.D.; Anderson, S.E.; Green, B.J.; Stefaniak, A.B.; Codispoti, C.D.; Kimber, I. Setting Occupational Exposure Limits for Chemical Allergens—Understanding the Challenges. J. Occup. Environ. Hyg. 2015, 12, S82–S98. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.L.; Baelum, J.; Skadhauge, L.; Thomsen, G.; Omland, O.; Thilsing, T.; Dahl, S.; Sigsgaard, T.; Sherson, D. Consequences of asthma on job absenteeism and job retention. Scand. J. Public Health 2012, 40, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, P.; Vandenplas, O.; Bernstein, D. Assessment and Management of Occupational Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 3264–3275. [Google Scholar] [CrossRef]
- Cormier, M.; Lemière, C. Occupational asthma. Int. J. Tuberc. Lung Dis. 2020, 24, 8–21. [Google Scholar] [CrossRef]
- Rabatin, J.T.; Cowl, C.T. A guide to the diagnosis and treatment of occupational asthma. Mayo Clin. Proc. 2001, 76, 633–640. [Google Scholar] [CrossRef]
- Rother, D.; Schlüter, U. Occupational Exposure to Diisocyanates in the European Union. Ann. Work. Expo. Health 2021, 65, 893–907. [Google Scholar] [CrossRef]
- Verschoor, L.; Verschoor, A.H. Nonoccupational and occupational exposure to isocyanates. Curr. Opin. Pulm. Med. 2014, 20, 199–204. [Google Scholar] [CrossRef]
- Klusáčková, P.; Dušková, S.; Mráz, J.; Navrátil, T.; Vlčková, S.; Pelclová, D. Health effects of exposure to isocyanates in a car factory. Cent. Eur. J. Public Health 2022, 30, 32–36. [Google Scholar] [CrossRef]
- Zeiss, C.R. Advances in acid anhydride induced occupational asthma. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 89–92. [Google Scholar] [CrossRef]
- Heederik, D.; van Rooy, F. Update on occupational allergy, including asthma, to soluble platinum salts. Curr. Opin. Allergy Clin. Immunol. 2024, 24, 69–72. [Google Scholar] [CrossRef]
- Ibrahim, B.; Le Moual, N.; Sit, G.; Goldberg, M.; Leynaert, B.; Ribet, C.; Roche, N.; Varraso, R.; Zins, M.; Nadif, R.; et al. Occupational Exposure Patterns to Disinfectants and Cleaning Products and Its Association With Asthma Among French Healthcare Workers. Am. J. Ind. Med. 2025, 68, 516–530. [Google Scholar] [CrossRef]
- Hussein, H.; Mwanga, M.D.; Dumas, O.; Migueres, N.; Le Moual, N.; Jeebhay, M.F. Airway Diseases Related to the Use of Cleaning Agents in Occupational Settings. J. Allergy Clin. Immunol. 2024, 12, 1974–1986. [Google Scholar]
- Muñoz, X.; Clofent, D.; Cruz, M.J. Occupational respiratory allergy to reactive dyes. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.; Roberts, H. Methacrylate perspective in current dental practice. J. Esthet. Restor. Dent. 2020, 32, 673–680. [Google Scholar] [CrossRef]
- Walters, G.I.; Robertson, A.S.; Moore, V.C.; Burge, P.S. Occupational asthma caused by acrylic compounds from SHIELD surveillance (1989-2014). Occup. Med. 2017, 67, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, P. Occupational allergy to pharmaceutical products. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 101–106. [Google Scholar] [CrossRef]
- Baur, X.; Bakehe, P. Allergens causing occupational asthma: An evidence-based evaluation of the literature. Int. Arch. Occup. Environ. Health 2014, 87, 339–363. [Google Scholar] [CrossRef] [PubMed]
- Dalbøge, A.; Kolstad, H.A.; Jahn, A.; Ulrik, C.S.; Sherson, D.L.; Meyer, H.W.; Ebbehøj, N.; Sigsgaard, T.; Baur, X.; Schlünssen, V. A systematic review of the relation between ten potential occupational sensitizing exposures and asthma. Scand. J. Work. Environ. Health 2025, 51, 146–158. [Google Scholar] [CrossRef]
- Hargitai, R.; Parráková, L.; Szatmári, T.; Monfort-Lanzas, P.; Galbiati, V.; Audouze, K.; Jornod, F.; Staal, Y.C.M.; Burla, S.; Chary, A.; et al. Chemical respiratory sensitization—Current status of mechanistic understanding, knowledge gaps and possible identification methods of sensitizers. Front. Toxicol. 2024, 29, 1331803. [Google Scholar] [CrossRef]
- Unwin, J.; Coldwell, M.R.; Keen, C.; McAlinden, J.J. Airborne emissions of carcinogens and respiratory sensitizers during thermal processing of plastics. Ann. Occup. Hyg. 2013, 57, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Vangronsveld, E.; Berckmans, S.; Verbinnen, K.; Van Leeuw, C.; Bormans, C. Isocyanate and total inhalable particulate air measurements in the European wood panel industry. Int. J. Hyg. Environ. Health 2010, 213, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Pappas, R.S. Toxic Elements in Tobacco and in Cigarette Smoke: Inflammation and Sensitization. Metallomics 2011, 3, 1181–1198. [Google Scholar] [CrossRef]
- Lanckacker, E.A.; Tournoy, K.G.; Hammad, H.; Holtappels, G.; Lambrecht, B.N.; Joos, J.F.; Maes, T. Short cigarette smoke exposure facilitates sensitisation and asthma development in mice. Eur. Respir. J. 2013, 41, 1189–1199. [Google Scholar] [CrossRef]
- Shargorodsky, J.; Garcia-Esquinas, E.; Galán, I.; Navas-Acien, A.; Lin, S.Y. Allergic Sensitization, Rhinitis and Tobacco Smoke Exposure in US Adults. PLoS ONE 2015, 14, e0131957. [Google Scholar] [CrossRef]
- Poli, D.; Mozzoni, P.; Pinelli, S.; Cavallo, D.; Papaleo, B.; Caporossi, L. Sex Difference and Benzene Exposure: Does It Matter? Int. J. Environ. Res. Public. Health 2022, 19, 2339. [Google Scholar] [CrossRef]
- Wizemann, T.M.; Pardue, M.L. (Eds.) Exploring the Biological Contributions to Human Health: Does Sex Matter? Institute of Medicine: Washington, DC, USA, 2001. [Google Scholar]
- Ortona, E.; Delunardo, F.; Baggio, G.; Malorni, W. A sex and gender perspective in medicine: A new mandatory challenge for human health. Ann. Ist. Super. Sanita 2016, 52, 146–148. [Google Scholar]
- Messing, K.; Silverstein, B.A. Gender and occupational health. Scand. J. Work. Environ. Health 2009, 35, 81–83. [Google Scholar] [CrossRef]
- Franconi, F.; Brunelleschi, S.; Steardo, L.; Cuomo, V. Gender differences in drug responses. Pharmacol. Res. 2007, 55, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Silveyra, P.; Fuentes, N.; Rodriguez Bauza, D.E. Sex and Gender Differences in Lung Disease. Adv. Exp. Med. Biol. 2021, 1304, 227–258. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Most Recent Asthma Data. 2020. Available online: https://www.cdc.gov/asthma/most_recent_data.htm (accessed on 31 May 2025).
- Barre, S.F.; Haberthur, D.; Cremona, T.P.; Stampanoni, M.; Schittny, J.C. The total number of acini remains constant throughout postnatal rat lung development. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L1082–L1089. [Google Scholar] [CrossRef]
- Schwartz, J.; Katz, S.A.; Fegley, R.W.; Tockman, M.S. Sex and race differences in the development of lung function. Am. Rev. Respir. Dis. 1988, 138, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Thurlbeck, W.M. Postnatal human lung growth. Thorax 1982, 37, 564–571. [Google Scholar] [CrossRef]
- Silveyra, P. Chapter 9: Developmental Lung Disease. In Gender, Sex Hormones and Respiratory Disease. A Comprehensive Guide; Hemnes, A.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 243. [Google Scholar] [CrossRef]
- Reddy, K.D.; Oliver, B.G.G. Sexual dimorphism in chronic respiratory diseases. Cell Biosci. 2023, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Padem, N.; Saltoun, C. Classification of asthma. Allergy Asthma Proc. 2019, 40, 385–388. [Google Scholar] [CrossRef]
- Gutierrez-Brito, J.A.; Lomelí-Nieto, J.A.; Muñoz-Valle, J.F.; Oregon-Romero, E.; Corona-Angeles, J.A.; Hernández-Bello, J. Sex hormones and allergies: Exploring the gender differences in immune responses. Front. Allergy 2025, 5, 1483919. [Google Scholar] [CrossRef]
- Borrelli, R.; Brussino, L.; Lo Sardo, L.; Quinternetto, A.; Vitali, I.; Bagnasco, D.; Boem, M.; Corradi, F.; Badiu, I.; Negrini, S.; et al. Sex-Based Differences in Asthma: Pathophysiology, Hormonal Influence, and Genetic Mechanisms. Int. J. Mol. Sci. 2025, 26, 5288. [Google Scholar] [CrossRef]
- Yung, J.A.; Fuseini, H.; Newcomb, D.C. Hormones, sex, and asthma. Ann. Allergy Asthma Immunol. 2018, 120, 488–494. [Google Scholar] [CrossRef]
- Radzikowska, U.; Golebski, K. Sex hormones and asthma: The role of estrogen in asthma development and severity. Allergy 2023, 78, 620–622. [Google Scholar] [CrossRef]
- LoMauro, A.; Aliverti, A. Sex and gender in respiratory physiology. Eur. Respir. Rev. 2021, 30, 210038. [Google Scholar] [CrossRef]
- Bulkhi, A.A.; Shepard, K.V.; Casale, T.B.; Cardet, J.C. Elevated Testosterone Is Associated with Decreased Likelihood of Current Asthma Regardless of Sex. J. Allergy Clin. Immunol. Pract. 2020, 8, 3029–3035.e4. [Google Scholar] [CrossRef] [PubMed]
- Cephus, J.Y.; Stier, M.T.; Fuseini, H.; Yung, J.A.; Toki, S.; Bloodworth, M.H.; Zhou, W.; Goleniewska, K.; Zhang, J.; Garon, S.L.; et al. Testosterone Attenuates Group 2 Innate Lymphoid Cell-Mediated Airway Inflammation. Cell Rep. 2017, 21, 2487–2499. [Google Scholar] [CrossRef] [PubMed]
- Lauzon-Joset, J.F.; Mincham, K.T.; Abad, A.P.; Short, B.P.; Holt, P.G.; Strickland, D.H.; Leffler, J. Oestrogen amplifies pre-existing atopy-associated Th2 bias in an experimental asthma model. Clin. Exp. Allergy 2020, 50, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Fuseini, H.; Cephus, J.Y.; Wu, P.; Davis, J.B.; Contreras, D.C.; Gandhi, V.D.; Rathmell, J.C.; Newcomb, D.C. ERα Signaling Increased IL-17A Production in Th17 Cells by Upregulating IL-23R Expression, Mitochondrial Respiration, and Proliferation. Front. Immunol. 2019, 10, 2740. [Google Scholar] [CrossRef]
- Dong, W.; Peng, Q.; Liu, Z.; Xie, Z.; Guo, X.; Li, Y.; Chen, C. Estrogen plays an important role by influencing the NLRP3 inflammasome. Biomed. Pharmacother. 2023, 167, 115554. [Google Scholar] [CrossRef]
- Trivedi, S.; Deering-Rice, C.E.; Aamodt, S.E.; Huecksteadt, T.P.; Myers, E.J.; Sanders, K.A.; Paine, R.; Warren, K.J. Progesterone amplifies allergic inflammation and airway pathology in association with higher lung ILC2 responses. Am. J. Physiol. Lung Cell Mol. Physiol. 2024, 327, L65–L78. [Google Scholar] [CrossRef]
- Fuseini, H.; Newcomb, D.C. Mechanisms driving gender differences in asthma. Curr. Allergy Asthma 2017, 17, 19. [Google Scholar] [CrossRef]
- Jenkins, C.R.; Boulet, L.P.; Lavoie, K.L.; Raherison-Semjen, C.; Singh, D. Personalized treatment of asthma: The importance of sex and gender differences. J. Allergy Clin. Immunol. Pract. 2022, 4, 963–971. [Google Scholar] [CrossRef]
- Sanchez-Ramos, J.L.; Pereira-Vega, A.R.; Alvarado-Gomez, F.; Maldonado-Perez, J.A.; Svanes, C.; Gomez-Real, F. Risk factors for premenstrual asthma: A systematic review and metanalysis. Expert. Rev. Respir. Med. 2017, 11, 57–72. [Google Scholar] [CrossRef]
- Murray, L.M.; Yerkovich, S.T.; Ferreira, M.A.; Upham, J.W. Risks for cold frequency vary by sex: Role of asthma, age, TLR7 and leukocyte subsets. Eur. Respir. J. 2020, 56, 1902453. [Google Scholar] [CrossRef] [PubMed]
- James, B.; Milstien, S.; Spiegel, S. ORMDL3 and allergic asthma: From physiology to pathology. J. Allergy Clin. Immunol. 2019, 144, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Sleziak, J.; Gawor, A.; Błazejewska, M.; Antosz, K.; Gomułka, K. ADAM33′ s Role in Asthma Pathogenesis: An Overview. Int. J. Mol. Sci. 2024, 25, 2318. [Google Scholar] [CrossRef]
- Malmhäll, C.; Calvén, J.; Weidner, J.; Johansson, K.; Ramos-Ramirez, P.; Boberg, E.; Ekerljung, L.; Mincheva, R.; Nwaru, B.; Kankaanranta, H.; et al. Potential role of Let-7 family microRNAs in sex disparity in asthma. Eur. Respir. J. 2024, 64 (Suppl. S68), OA2004. [Google Scholar] [CrossRef]
- Boulet, L.P.; Lavoie, K.L.; Raherison-Semjen, C.; Kaplan, A.; Singh, D.; Jenkins, C.R. Addressing sex and gender to improve asthma management. Prim. Care Respir. Med. 2022, 32, 56. [Google Scholar] [CrossRef]
- Raghavan, D.; Jain, R. Increasing awareness of sex differences in airway disease. Respirology 2016, 21, 449–459. [Google Scholar] [CrossRef]
- Raherison, C.; Janson, C.; Jarvis, D.; Burney, P.; Cazzoletti, L.; De Marco, R.; Neukirch, F.; Leynaert, B. Evolution of asthma severity in a cohort of young adults: Is there any gender difference? PLoS ONE 2009, 4, e7146. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Levra, S.; Sprio, A.E.; Bertolini, F.; Carriero, V.; Gallo, F.; Ciprandi, G. Asthma in the real world: The relevance of gender. Int. Arch. Allergy Immunol. 2020, 181, 462–466. [Google Scholar] [CrossRef]
- Global Initiative for Asthma 2021. GINA Report, Global Strategy for Asthma Management and Prevention. Available online: https://ginasthma.org/gina-reports/ (accessed on 18 February 2022).
- Nwaru, B.I.; Sheikh, A. Hormonal contraceptives and asthma in women of reproductive age: Analysis of data from serial national Scottish Health Surveys. J. R. Soc. Med. 2015, 108, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Ekström, M.; Hasvold, P.; Wiklund, F.; Telg, G.; Janson, C. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: A nationwide cohort study of the global SABINA programme. Eur. Respir. J. 2020, 55, 1901872. [Google Scholar] [CrossRef] [PubMed]
- Triebner, K.; Johannensenn, A.; Puggini, L.; Benediktsdottir, B.; Bertelsen, R.J.; Bifulco, E.; Dharmage, S.C.; Dratva, J.; Franklin, K.A.; Gíslason, T.; et al. Menopause as a predictor of new onset asthma: A longitudinal northern European population study. J. Allergy Clin. Immunol. 2016, 137, 50–57.e6. [Google Scholar] [CrossRef]
- Hansen, E.S.H.; Aasbjerg, K.; Moeller, A.L.; Gade, E.J.; Torp-Pedersen, C.; Backer, V. Hormone replacement therapy and development of new asthma. Chest 2021, 160, 45–52. [Google Scholar] [CrossRef]
- Shah, S.A.; Tibble, H.; Pillinger, R.; McLean, S.; Ryan, D.; Critchley, H.; Price, D.; Hawrylowicz, C.M.; Simpson, C.R.; Soyiri, I.N.; et al. Hormone replacement therapy and asthma onset in menopausal women: National cohort study. J. Allerg. Clin. Immunol. 2021, 147, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Morales-Estrella, J.L.; Boyle, M.; Zein, J.G. Transgender status is associated with higher risk of lifetime asthma. In A34 Asthma Clinical Studies I. American Thoracic society, 2018. In Proceedings of the A1371- Thematic Poster Session Presentation at the American Thoracic Society International Conference, San Diego, CA, USA, 18–23 May 2018. [Google Scholar]
- Mattiuzzi, C.; Lippi, G. Worldwide asthma epidemiology: Insights from the Global Health Data Exchange database. Int. Forum Allergy Rhinol. 2020, 10, 75–80. [Google Scholar] [CrossRef]
- Senna, G.; Latorre, M.; Bugiani, M.; Caminati, M.; Heffler, E.; Morrone, D.; Paoletti, G.; Parronchi, P.; Puggioni, F.; Blasi, F.; et al. Sex differences in severe asthma: Results from severe asthma network in Italy-SANI. Allergy Asthma Immunol. Res. 2021, 13, 219–228. [Google Scholar] [CrossRef]
- Colombo, D.; Zagni, E.; Ferri, F.; Canonina, G.W. Gender differences in asthma perception and its impact on quality of life: A post hoc analysis of the PROXIMA (Patient Reported Outcomes and Xolair in the Management of Asthma) study. Allergy Asthma Clin. Immunol. 2019, 15, 65. [Google Scholar] [CrossRef]
- White, G.E.; Seaman, C.; Filios, M.S.; Mazurek, J.M.; Flattery, J.; Harrison, R.J.; Reilly, M.J.; Rosenman, K.D.; Lumia, M.E.; Stephens, A.C.; et al. Gender differences in work related asthma surveillance data from California, Massachusetts, Michigan and New Jearsey, 1993–2008. J. Asthma 2014, 51, 691–702. [Google Scholar] [CrossRef]
- Borges, R.C.; Alith, M.B.; Nascimento, O.A.; Jardim, J.R. Gender differences in the perception of asthma respiratory symptoms in five Latin American countries. J. Asthma 2022, 59, 1030–1040. [Google Scholar] [CrossRef]
- Vitulano, L.A. Psychosocial issues for children and adolescents with chronic illness: Self-esteem, school functioning and sports participation. Child. Adolesc. Psychiatr. Clin. N. Am. 2003, 12, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.; Probst-Hensch, N.; Keidel, D.; Dratva, J.; Bettschart, R.; Pons, M.; Burdet, L.; Bridevaux, P.O.; Schikowski, T.; Schindler, C.; et al. Gender differences in adult-onset asthma: Results from the Swiss SAPALDIA cohort study. Eur. Respir. J. 2015, 46, 1011–1020. [Google Scholar] [CrossRef]
- Eagan, T.M.; Brøgger, J.C.; Eide, G.E.; Bakke, P.S. The incidence of adult asthma: A review. Int. J. Tuberc. Lung Dis. 2005, 9, 603–612. [Google Scholar]
- Rönmark, E.; Lundbäck, B.; Jönsson, E.; Jonsson, A.C.; Lindström, M.; Sandström, T. Incidence of asthma in adults: Report from the Obstructive Lung Disease in Norther Swedish study. Allergy 1997, 52, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Lundbäck, B.; Rönmark, E.; Jönsson, E.; Larsson, K.; Sandström, T. Incidence of physician diagnosed asthma in adults—A real incidence or a result of increased awareness? Report from the Obstructive Lung Disease in Norther Swedish studies. Respir. Med. 2001, 95, 685–692. [Google Scholar] [CrossRef]
- Dong, G.H.; Chen, T.; Liu, M.M.; Wang, D.; Ma, Y.N.; Ren, W.H.; Lee, Y.L.; Zhao, Y.D.; He, Q.C. Gender differences and effect of air pollution on asthma in children with and without allergic predisposition: Northeast Chinese children health study. PLoS ONE 2011, 6, e22470. [Google Scholar] [CrossRef]
- Sit, G.; Varraso, R.; Fezeu, L.K.; Galan, P.; Orsi, F.; Da Silva, E.P.; Touvier, M.; Hercberg, S.; Paris, C.; Le Moual, N.; et al. Occupational exposure to Irritants and sensitizers, Asthma and Asthma control in the Nutrinet-Santé Cohort. J. Allergy Clin. Immunol. Pract. 2022, 10, 3220–3227.e7. [Google Scholar] [CrossRef] [PubMed]
- Howse, D.; Gautrin, D.; Neis, B.; Cartier, A. Gender and snow crab occupational asthma in Newfoundland and Labrador. Canada. Environ. Res. 2006, 101, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Doyen, V.; Gautrin, D.; Vandenplas, O.; Malo, J.L. Comparison of high and low molecular weight sensitizing agents causing occupational asthma: An evidence-based insight. Expert. Rev. Clin. Immunol. 2024, 20, 635–653. [Google Scholar] [CrossRef] [PubMed]
- Elliott, L.; Heederik, D.; Marshall, S.; Peden, D.; Loomis, D. Progression of self-reported symptoms in laboratory animal allergy. J. Allergy Clin. Immunol. 2005, 116, 127–132. [Google Scholar] [CrossRef]
- Arif, A.A.; Delclos, G.L. Association between cleaning-related chemicals and work-related asthma and asthma symptoms among healthcare professionals. Occup. Environ. Med. 2012, 69, 35–40. [Google Scholar] [CrossRef]
- Ederle, C.; Donnay, C.; Khayath, N.; Mielcarek, M.; de Blay, F. Asthma and cleaning: What’s new? Curr. Treat. Options Allergy 2018, 5, 29–40. [Google Scholar] [CrossRef]
- Quirce, S.; Barranco, P. Cleaning agents and asthma. J. Investig. Allergol. Clin. Immunol. 2010, 20, 542–550. [Google Scholar]
- Le Moual, N.; Varraso, R.; Siroux, V.; Dumas, O.; Nadif, R.; Pin, I.; Zock, J.P.; Kauffmann, F. Domestic use of cleaning sprays and asthma activity in females. Eur. Repir. J. 2012, 40, 1381–1389. [Google Scholar] [CrossRef]
- Melchior Gerster, F.; Brenna Hopf, N.; Pierre Wild, P.; Vernez, D. Airborne exposures to monoethanolamine, glycol ethers, and benzyl alcohol during professional cleaning: A pilot study. Ann. Occup. Hyg. 2014, 58, 846–859. [Google Scholar] [CrossRef]
- Dumas, O.; Siroux, V.; Luu, F.; Nadif, R.; Zock, J.P.; Kauffmann, F.; Le Moual, N. Cleaning and asthma characteristics in women. Am. J. Ind. Med. 2014, 57, 303–311. [Google Scholar] [CrossRef]
- Zock, J.P.; Plana, E.; Jarvis, D.; Antó, J.M.; Kromhout, H.; Kennedy, S.M.; Künzli, N.; Villani, S.; Olivieri, M.; Torén, K.; et al. The use of household cleaning sprays and adult asthma. Am. J. Respir. Crit. Care Med. 2007, 176, 735–741. [Google Scholar] [CrossRef]
- Vizcaya, D.; Mirabelli, M.C.; Gimeno, D.; Antó, J.M.; Delclos, G.I.; Rivera, M.; Orriols, R.; Arjona, L.; Burgos, F.; Zock, J.P. Cleaning products and short term respiratory effects among female cleaners with asthma. Occup. Enviorn. Med. 2015, 72, 757–763. [Google Scholar] [CrossRef]
- Brun, E. The Occupational Safety and Health of Cleaning Workers; EU-OSHA, Luxembourg/Office for Official Publications of the European Communities: Luxembourg, 2009. [Google Scholar] [CrossRef]
- Dumas, O.; Laurent, E.; Bousquet, J.; Metspalu, A.; Milani, L.; Kauffmann, F.; Le Moual, N. Occupational irritants and asthma: An Estonian cross-sectional study of 34 000 adults. Eur. Respir. J. 2014, 44, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Dumas, O.; Wiley, A.S.; Quinot, C.; Varraso, R.; Zock, J.P.; Henneberger, P.K.; Speizer, F.E.; Moual, N.; Camargo, C.A.J. Occupational exposure to disinfectants and asthma control in US nurses. Eur. Respir. J. 2017, 50, 1700237. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Ibrahim, D.A.; Hassan, T.H.; Abd-El-Azem, W.G. Prevalence and predictors of occupational asthma among workers in detergent and cleaning products industry and its impact on quality of life in El Asher Men Ramadan, Egypt. Environ. Sci. Pollut. Res. 2022, 29, 33901–33908. [Google Scholar] [CrossRef] [PubMed]
- Fix, J.; Annesi-Maesano, I.; Baldi, I.; Boulanger, M.; Cheng, S.; Cortes, S.; Dalphin, J.C.; Dalvie, M.A.; Degano, B.; Douwes, J.; et al. Gender differences in respiratory health outcomes among farming cohorts around the globe: Findings from AGRICOH consortium. J. Agromed. 2021, 26, 97–108. [Google Scholar] [CrossRef]
- Okello, G.; Devereux, G.; Semple, S. Women and girls in resource poor countries experience much greater exposure to household air pollutants than men: Results from Uganda and Ethiopia. Environ. Int. 2018, 119, 429–437. [Google Scholar] [CrossRef]
- Svanes, O.; Bertelsen, R.J.; Lygre, S.H.L.; Carsin, A.E.; Antó, J.M.; Forsberg, B.; García-García, J.M.; Gullón, A.G.; Heinrich, J.; Holm, M.; et al. Cleaning at home and at work in relation to lung function decline and airway obstruction. Am. J. Respir. Crit. Care Med. 2018, 197, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, A.; Johnsen, R.; Holmen, J.; Gulsvik, A.; Bjermer, L. Cigarette smoking gives more respiratory symptoms among women than among men. The Nord-Trondelag Health Study (HUNT). J. Epidemiol. Community Health 2000, 54, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Aloui, A.; El Maalel, O.; Maoua, M.; Kacem, I.; Hani, Z.; Aroui, H.; El Guedri, S.; Brahem, A.; Kalboussi, H.; Chatti, S.; et al. Spéecificités de l’asthme professionnel chez les femmes et son interaction avec le statut hormonal. Rev. Pneumol. Clin. 2018, 74, 483–491. [Google Scholar] [CrossRef]
- Schwaiberger, D.; Karcz, M.; Menk, M.; Papadakos, P.J.; Dantoni, S.E. Respiratory Failure and Mechanical Ventilation in the Pregnant Patient. Crit. Care Clin. 2016, 32, 85–95. [Google Scholar] [CrossRef]
- Hsinet, J.; Dallagi, A.; Lâaroussi, R.; Ismail, S.; Khouja, N.; Baraketi, E.; Bousselmi, S.; Chemingui, S.; Aissa, I.; Benzarti Mezni, A.; et al. L’asthme professionnel et l’asthme aggravé par le travail. Quelles differences en termes de facteurs de risque et d’aptitude au travail? Rev. Française d’Allergologie 2022, 62, 462–469. [Google Scholar] [CrossRef]
- Robin, C.; Vongmany, N.; Dewitte, J.D.; Lodde, B.; Larabi, L.; Lucas, D. Asthmes en relation avec le travail chez la femme: Comparison aux données, asculines. Étude retrospective des données issues du Réseau National de Vigilance et de Prévention des Pathologies Professionnelles (RNV3P). Arch. Mal. Prof. L’Environ. 2022, 83, 181–192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).