The Effect of Acid Catalysis on Hydroxycinnamate Recovery from Corn Stover Using Hydrothermal and Organosolv Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection and Handling of Corn Stover (CS)
2.3. Hydrothermal and Organosolv Treatments
2.4. Kinetics of Polyphenol Recovery
2.5. Determination of Severity Factor
2.6. Response Surface-Based Treatment Optimization
2.7. Treatment Yield Determination and Antioxidant Assays
2.8. Chromatographic Analyses
2.9. Data Processing and Statistics
3. Results and Discussion
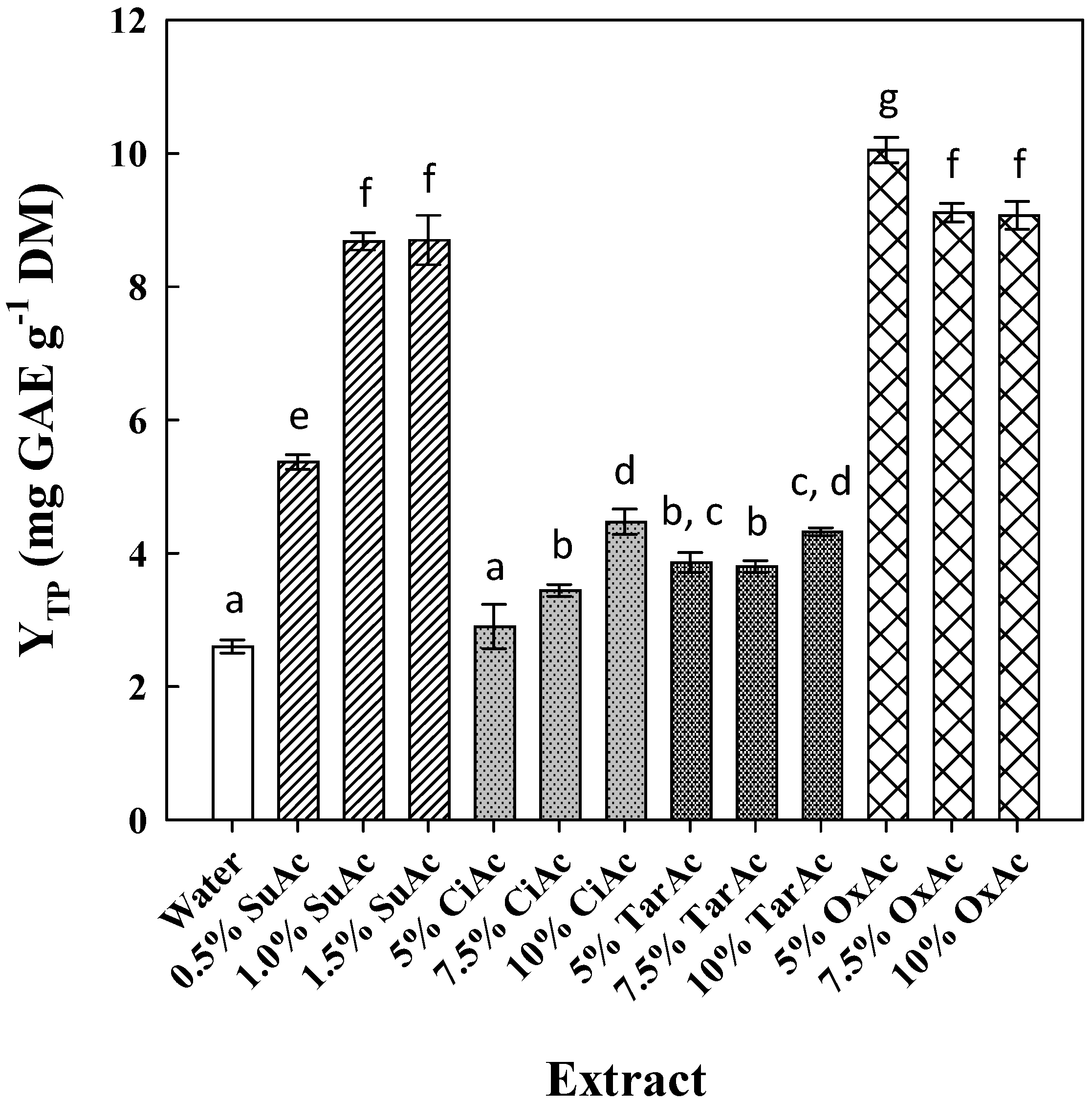
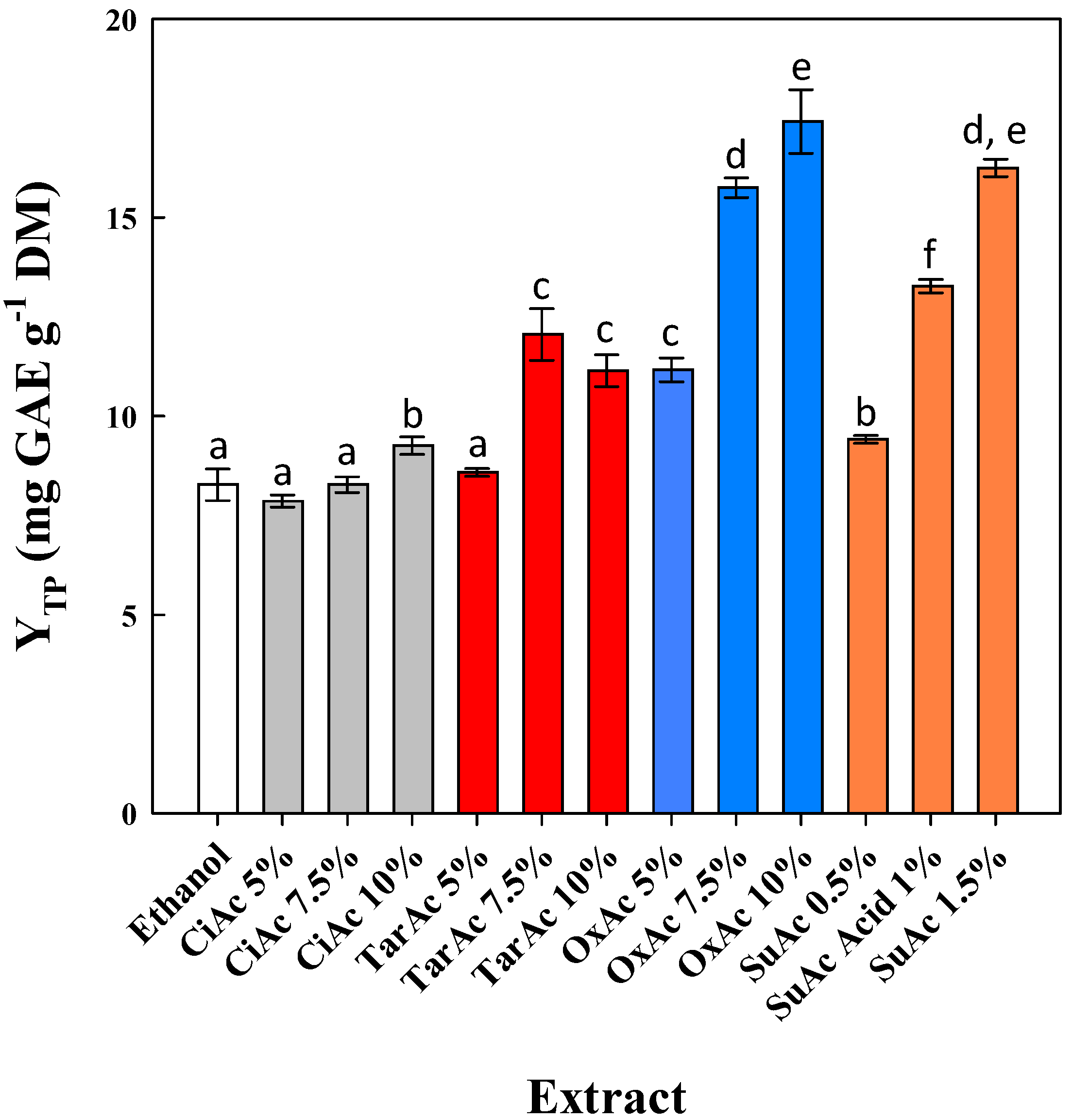
3.1. Effect of Acid and Ethanol Concentration
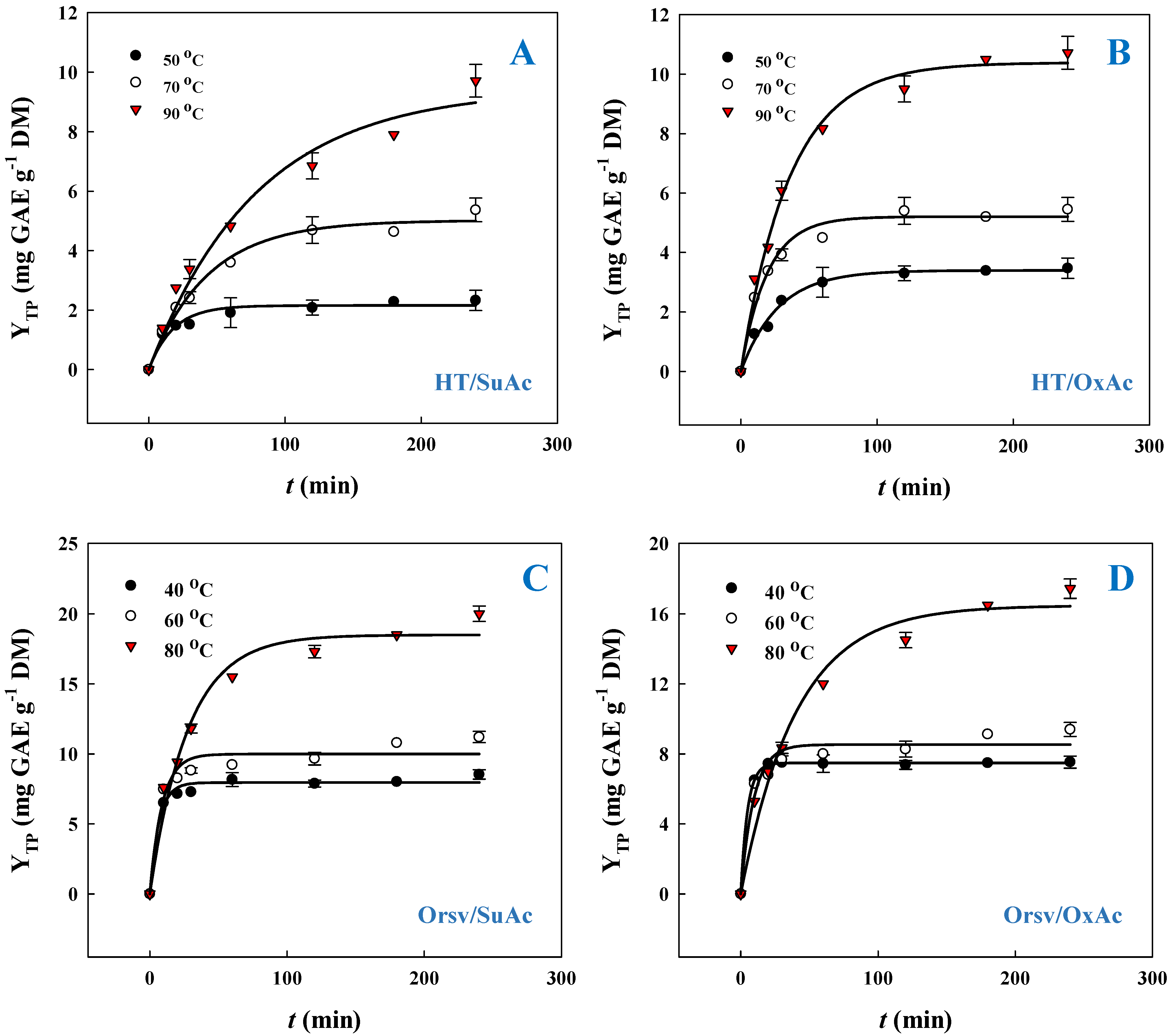
3.2. Polyphenol Recovery Kinetics
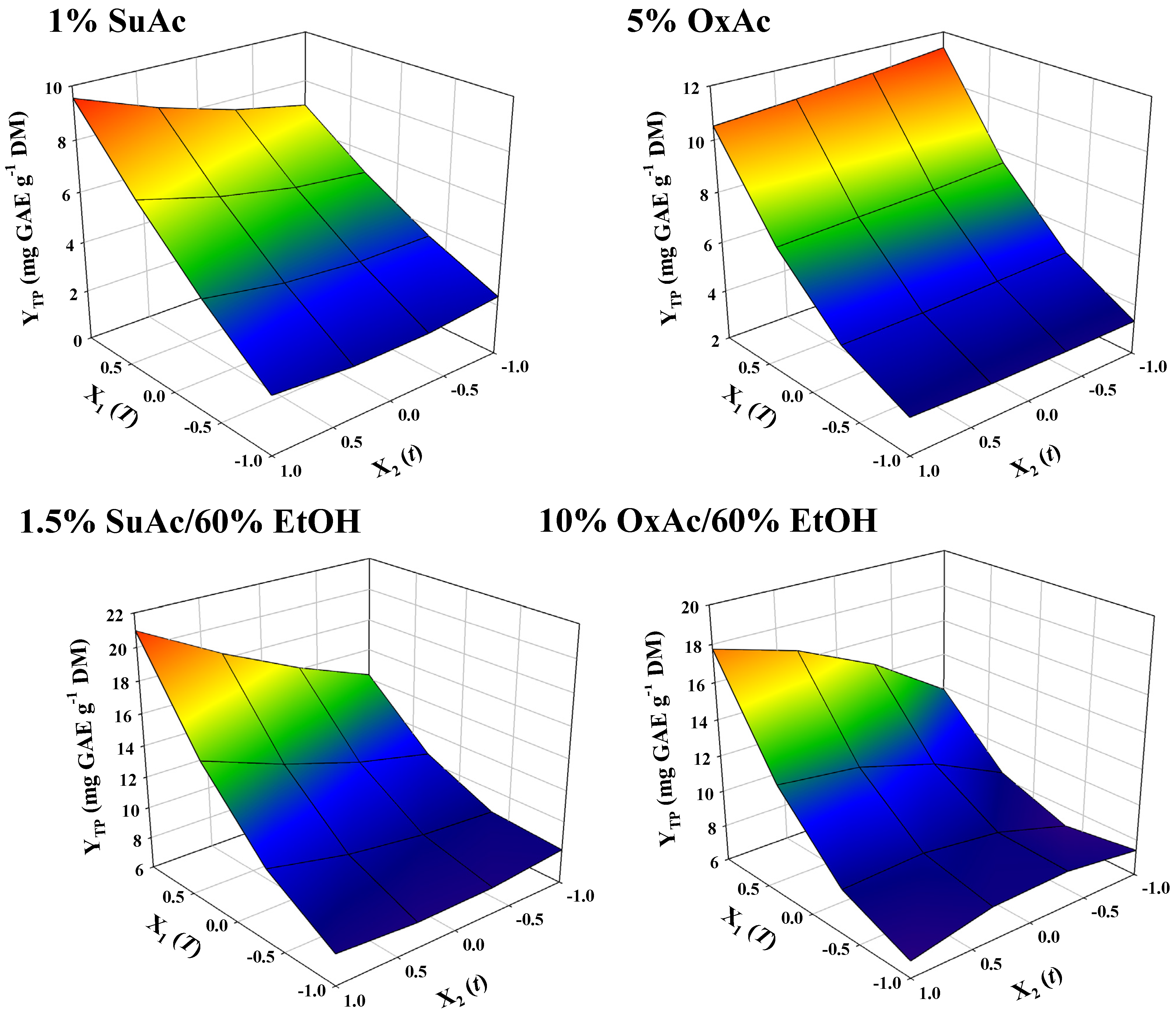
3.3. Treatment Optimization
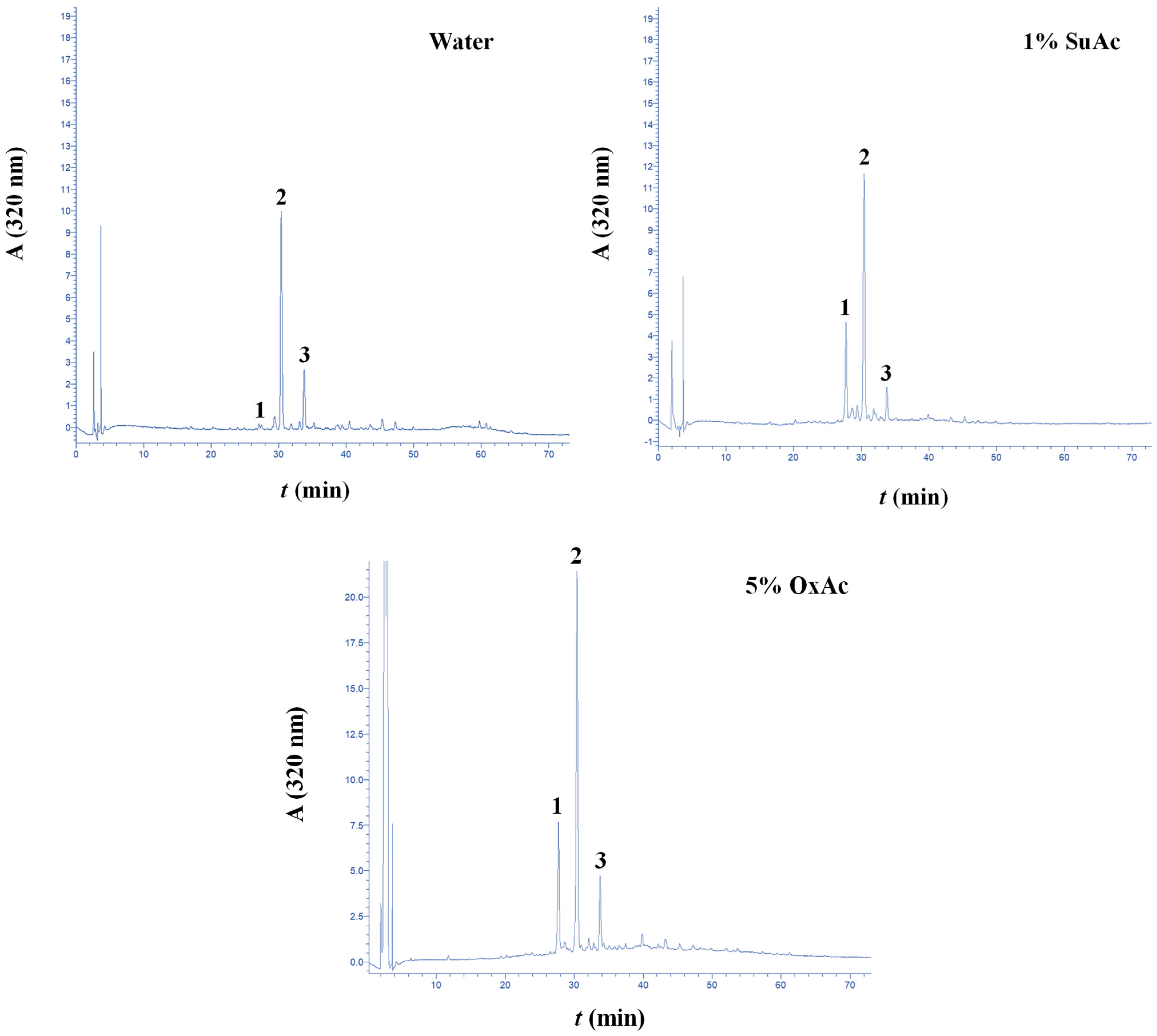
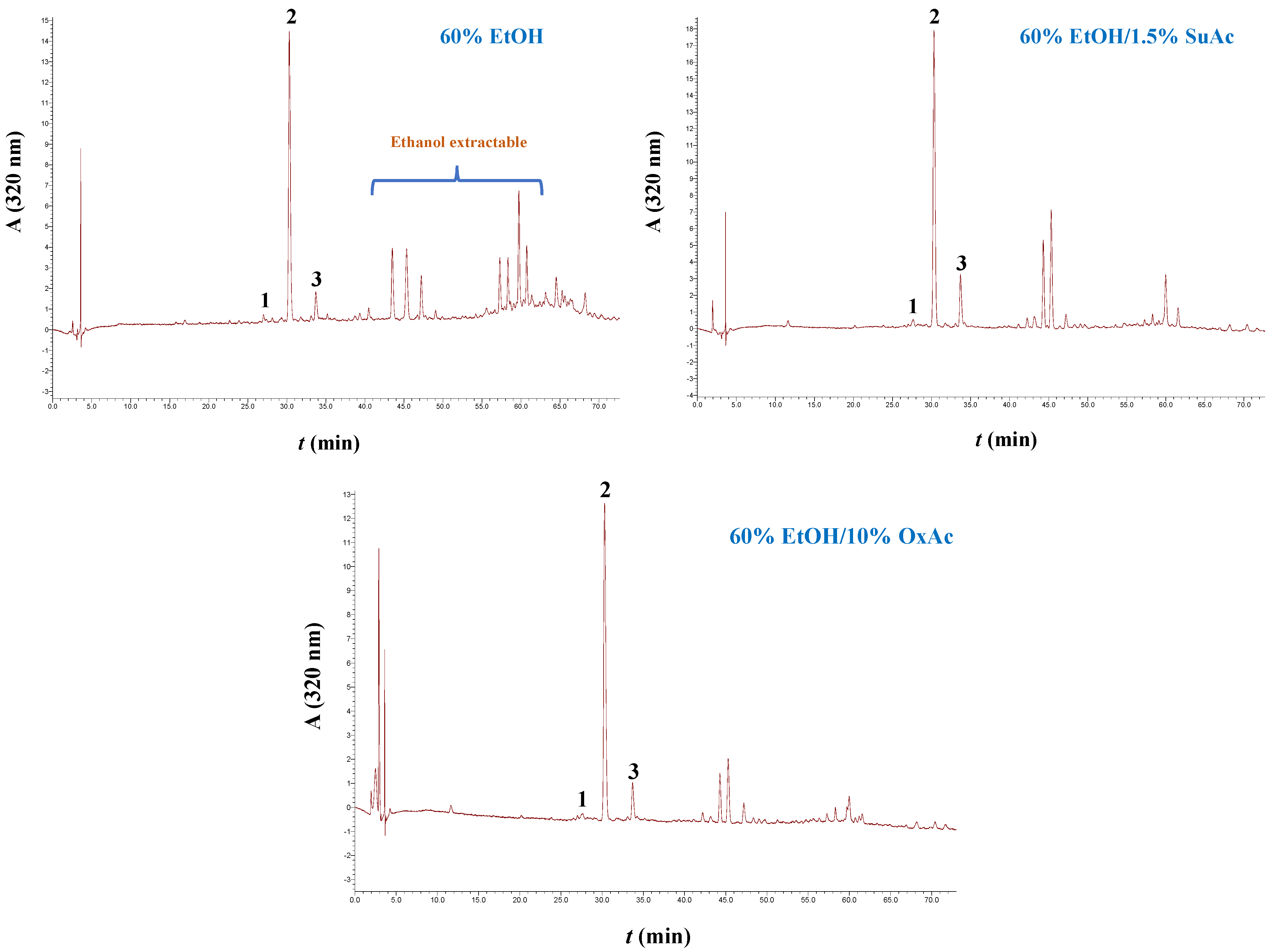
3.4. Treatment Effect on the Polyphenolic Composition
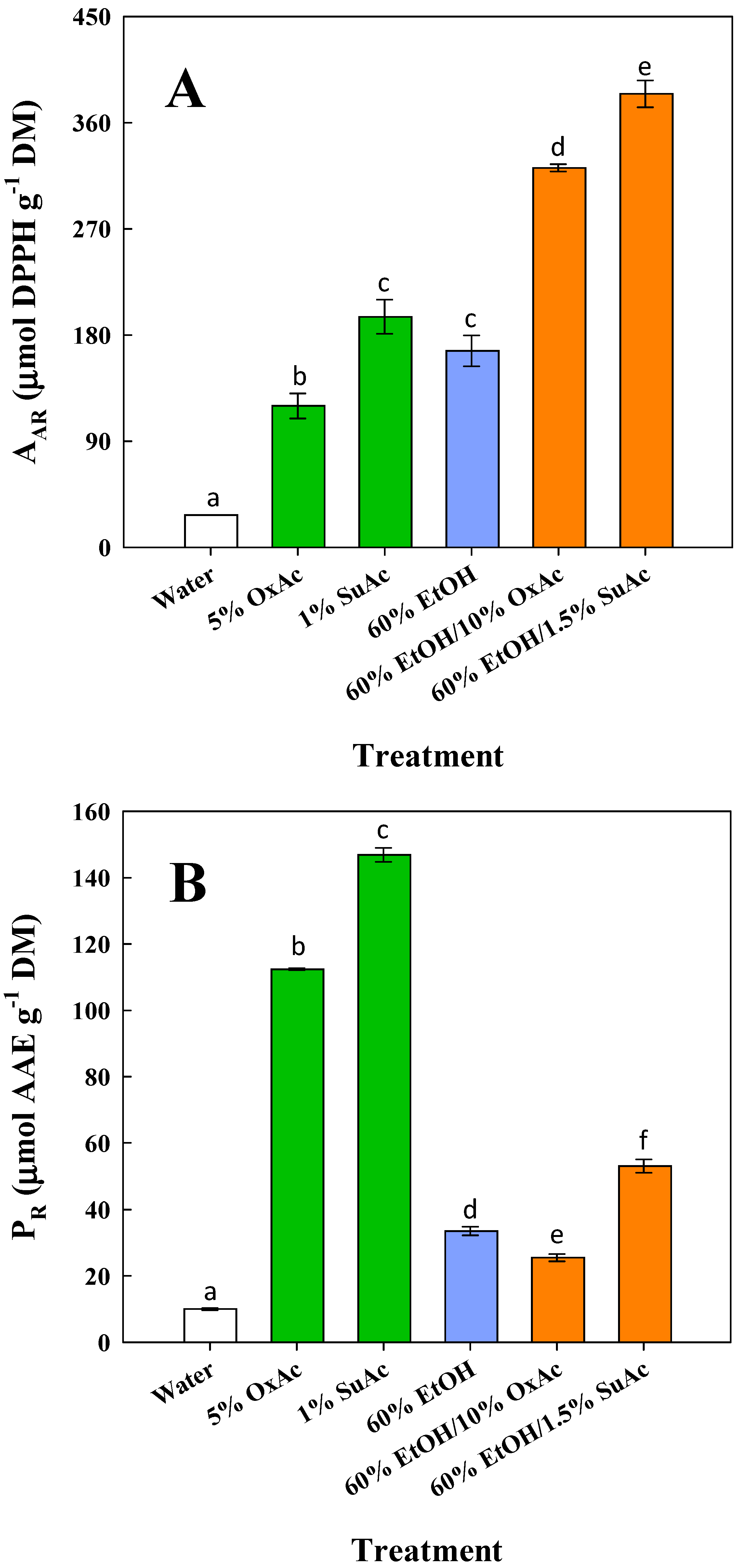
3.5. Antioxidant Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ouro-Salim, O.; Guarnieri, P. Circular economy of food waste: A literature review. Environ. Qual. Manag. 2022, 32, 225–242. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Bai, H.; Osman, A.I.; Eltohamy, K.M.; Chen, Z.; Younis, H.A.; Al-Fatesh, A.; Rooney, D.W.; Yap, P.-S. Recycling food and agriculture by-products to mitigate climate change: A review. Environ. Chem. Lett. 2023, 21, 3351–3375. [Google Scholar] [CrossRef]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The potential of selected agri-food loss and waste to contribute to a circular economy: Applications in the food, cosmetic and pharmaceutical industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- Paini, J.; Benedetti, V.; Ail, S.S.; Castaldi, M.J.; Baratieri, M.; Patuzzi, F. Valorization of wastes from the food production industry: A review towards an integrated agri-food processing biorefinery. Waste Biomass Valor. 2022, 13, 31–50. [Google Scholar] [CrossRef]
- Ruan, Z.; Wang, X.; Liu, Y.; Liao, W. Corn. In Integrated Processing Technologies for Food and Agricultural by-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 59–72. [Google Scholar]
- Postamentel, M.; Coman, C.-N. Analysis of the main technical indicators of corn, wheat and sunflower crops at the level of the European Union. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2022, 22, 591–600. [Google Scholar]
- Aghaei, S.; Alavijeh, M.K.; Shafiei, M.; Karimi, K. A comprehensive review on bioethanol production from corn stover: Worldwide potential, environmental importance, and perspectives. Biomass Bioenergy 2022, 161, 106447. [Google Scholar] [CrossRef]
- Sahayaraj, D.V.; Lusi, A.; Mitchell, E.M.; Bai, X.; Tessonnier, J.-P. Comparative study of the solvolytic deconstruction of corn stover lignin in batch and flow-through reactors. Green Chem. 2021, 23, 7731–7742. [Google Scholar] [CrossRef]
- Vazquez-Olivo, G.; López-Martínez, L.X.; Contreras-Angulo, L.; Heredia, J.B. Antioxidant capacity of lignin and phenolic compounds from corn stover. Waste Biomass Valor. 2019, 10, 95–102. [Google Scholar] [CrossRef]
- Bunzel, M. Chemistry and occurrence of hydroxycinnamate oligomers. Phytochem. Rev. 2010, 9, 47–64. [Google Scholar] [CrossRef]
- Shahidi, F.; Chandrasekara, A. Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochem. Rev. 2010, 9, 147–170. [Google Scholar] [CrossRef]
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic acid antioxidants: An electrochemical overview. BioMed Res. Int. 2013, 2013, 251754. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar]
- El-Seedi, H.R.; El-Said, A.M.; Khalifa, S.A.; Goransson, U.; Bohlin, L.; Borg-Karlson, A.-K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [PubMed]
- Sova, M.; Saso, L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef] [PubMed]
- Contardi, M.; Lenzuni, M.; Fiorentini, F.; Summa, M.; Bertorelli, R.; Suarato, G.; Athanassiou, A. Hydroxycinnamic acids and derivatives formulations for skin damages and disorders: A review. Pharmaceutics 2021, 13, 999. [Google Scholar] [CrossRef] [PubMed]
- Bento-Silva, A.; Patto, M.C.V.; do Rosário Bronze, M. Relevance, structure and analysis of ferulic acid in maize cell walls. Food Chem. 2018, 246, 360–378. [Google Scholar] [CrossRef]
- Papadaki, E.S.; Palaiogiannis, D.; Lalas, S.I.; Mitlianga, P.; Makris, D.P. Polyphenol release from wheat bran using ethanol-based organosolv treatment and acid/alkaline catalysis: Process modeling based on severity and response surface optimization. Antioxidants 2022, 11, 2457. [Google Scholar] [CrossRef]
- Papadaki, E.; Grigorakis, S.; Palaiogiannis, D.; Lalas, S.I.; Mitlianga, P. Hydrothermal treatment of wheat bran under mild acidic or alkaline conditions for enhanced polyphenol recovery and antioxidant activity. Molecules 2024, 29, 1193. [Google Scholar] [CrossRef]
- Harouna-Oumarou, H.A.; Fauduet, H.; Porte, C.; Ho, Y.-S. Comparison of kinetic models for the aqueous solid-liquid extraction of Tilia sapwood in a continuous stirred tank reactor. Chem. Eng. Commun. 2007, 194, 537–552. [Google Scholar]
- Pedersen, M.; Meyer, A.S. Lignocellulose pretreatment severity–relating pH to biomatrix opening. New Biotechnol. 2010, 27, 739–750. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar]
- Bezerra, M.A.; Ferreira, S.L.C.; Novaes, C.G.; Dos Santos, A.M.P.; Valasques, G.S.; da Mata Cerqueira, U.M.F.; dos Santos Alves, J.P. Simultaneous optimization of multiple responses and its application in Analytical Chemistry—A review. Talanta 2019, 194, 941–959. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, G.; Zarnavalou, V.; Chatzimitakos, T.; Palaiogiannis, D.; Athanasiadis, V.; Lalas, S.I.; Makris, D.P. Comparison of sodium hydroxide and sodium carbonate as alkali catalysts in ethanol organosolv treatment of cotton stalks for the release of hydroxycinnamates. Recycling 2024, 9, 21. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Lakka, A.; Karageorgou, I.; Kaltsa, O.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D. Polyphenol extraction from Humulus lupulus (hop) using a neoteric glycerol/L-alanine deep eutectic solvent: Optimisation, kinetics and the effect of ultrasound-assisted pretreatment. AgriEngineering 2019, 1, 403–417. [Google Scholar] [CrossRef]
- Guenaoui, A.; Casasni, S.; Grigorakis, S.; Makris, D.P. Alkali-catalyzed organosolv treatment of oat bran for enhanced release of hydroxycinnamate antioxidants: Comparison of 1-and 2-propanol. Environments 2023, 10, 118. [Google Scholar] [CrossRef]
- Smyrnakis, G.; Stamoulis, G.; Palaiogiannis, D.; Chatzimitakos, T.; Athanasiadis, V.; Lalas, S.I.; Makris, D.P. Recovery of polyphenolic antioxidants from coffee silverskin using acid-catalyzed ethanol organosolv treatment. ChemEngineering 2023, 7, 72. [Google Scholar] [CrossRef]
- Geropoulou, M.; Yiagtzi, E.; Chatzimitakos, T.; Palaiogiannis, D.; Makris, D.P. Organosolv treatment of red grape pomace for effective recovery of antioxidant polyphenols and pigments using a ternary glycerol/ethanol/water system under mild acidic conditions. Molecules 2024, 29, 563. [Google Scholar] [CrossRef]
- Zhou, Z.; Lei, F.; Li, P.; Jiang, J. Lignocellulosic biomass to biofuels and biochemicals: A comprehensive review with a focus on ethanol organosolv pretreatment technology. Biotechnol. Bioeng. 2018, 115, 2683–2702. [Google Scholar] [CrossRef]
- Allerdings, E.; Ralph, J.; Steinhart, H.; Bunzel, M. Isolation and structural identification of complex feruloylated heteroxylan side-chains from maize bran. Phytochemistry 2006, 67, 1276–1286. [Google Scholar] [CrossRef]
- Eugene, A.; Lapierre, C.; Ralph, J. Improved analysis of arabinoxylan-bound hydroxycinnamate conjugates in grass cell walls. Biotechnol. Biofuels 2020, 13, 202. [Google Scholar] [CrossRef]
- Ralph, J.; Hatfield, R.D.; Quideau, S.; Helm, R.F.; Grabber, J.H.; Jung, H.-J.G. Pathway of p-coumaric acid incorporation into maize lignin as revealed by NMR. J. Am. Chem. Soc. 1994, 116, 9448–9456. [Google Scholar] [CrossRef]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef]
- Ralph, J. Hydroxycinnamates in lignification. Phytochem. Rev. 2010, 9, 65–83. [Google Scholar] [CrossRef]
- Sun, C.; Song, G.; Pan, Z.; Tu, M.; Kharaziha, M.; Zhang, X.; Show, P.-L.; Sun, F. Advances in organosolv modified components occurring during the organosolv pretreatment of lignocellulosic biomass. Bioresour. Technol. 2022, 368, 128356. [Google Scholar] [CrossRef] [PubMed]
- Linh, T.N.; Fujita, H.; Sakoda, A. Release kinetics of esterified p-coumaric acid and ferulic acid from rice straw in mild alkaline solution. Bioresour. Technol. 2017, 232, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.-Q.; Zhao, N.; Liu, Z.-Q.; Liao, C.-J.; Zheng, X.-Y.; Zheng, Y.-G. Enhanced production of xylose from corncob hydrolysis with oxalic acid as catalyst. Bioprocess Biosyst. Eng. 2018, 41, 57–64. [Google Scholar]
- Neenu, K.; Dominic, C.M.; Begum, P.S.; Parameswaranpillai, J.; Kanoth, B.P.; David, D.A.; Sajadi, S.M.; Dhanyasree, P.; Ajithkumar, T.; Badawi, M. Effect of oxalic acid and sulphuric acid hydrolysis on the preparation and properties of pineapple pomace derived cellulose nanofibers and nanopapers. Int. J. Biol. Macromol. 2022, 209, 1745–1759. [Google Scholar] [CrossRef]
- Huang, J.; Ren, J.; Tao, G.; Chen, Y.; Yao, S.; Han, D.; Qiu, R. Maize bran feruloylated oligosaccharides inhibited AGEs formation in glucose/amino acids and glucose/BSA models. Food Res. Int. 2019, 122, 443–449. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, Y.; Zargar, S.; Wu, J.; Oguzlu, H.; Baldelli, A.; Yu, Z.; Saddler, J.; Sun, R.; Tu, Q. Rapid, high-yield production of lignin-containing cellulose nanocrystals using recyclable oxalic acid dihydrate. Ind. Crops Prod. 2021, 173, 114148. [Google Scholar] [CrossRef]
- Lindsay, A.C.; Kudo, S.; Sperry, J. Cleavage of lignin model compounds and lignin ox using aqueous oxalic acid. Org. Biomol. Chem. 2019, 17, 7408–7415. [Google Scholar] [CrossRef] [PubMed]
- Bozinou, E.; Palaiogiannis, D.; Mourtzinos, I.; Chatzilazarou, A.; Gkatzionis, C.; Makris, D.P. The use of tailor-made acidic deep eutectic solvents for preparation of quercetin-enriched extracts from onion solid wastes. Environ. Qual. Manag. 2023, 33, 147–155. [Google Scholar] [CrossRef]
- Mateo Anson, N.; van den Berg, R.; Havenaar, R.; Bast, A.; RMM Haenen, G. Ferulic acid from aleurone determines the antioxidant potency of wheat grain (Triticum aestivum L.). J. Agric. Food Chem. 2008, 56, 5589–5594. [Google Scholar] [CrossRef] [PubMed]
- Verma, B.; Hucl, P.; Chibbar, R. Phenolic acid composition and antioxidant capacity of acid and alkali hydrolysed wheat bran fractions. Food Chem. 2009, 116, 947–954. [Google Scholar] [CrossRef]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
| Process Variables | Codes | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| t (min) | X1 | 60 | 180 | 300 |
| T (°C) (hydrothermal treatment) | X2 | 50 | 70 | 90 |
| T (°C) (organosolv treatment) | 40 | 60 | 80 | |
| Treatment | T (°C) | R2 * | k (min−1) × 10−3 | YTP(s) (mg GAE g−1 DM) |
|---|---|---|---|---|
| Hydrothermal treatment | ||||
| 1% SuAc | 50 | 0.949 | 56.4 | 2.2 ± 0.1 a |
| 70 | 0.984 | 23.3 | 5.0 ± 0.2 b | |
| 90 | 0.977 | 12.6 | 9.4 ± 0.5 c | |
| 5% OxAc | 50 | 0.987 | 36.5 | 3.4 ± 0.1 d |
| 70 | 0.979 | 51.8 | 5.2 ± 0.2 b | |
| 90 | 0.991 | 27.7 | 10.4 ± 0.7 c | |
| Organosolv treatment | ||||
| 60% ethanol/1.5% SuAc | 40 | 0.982 | 153.4 | 7.9 ± 0.3 e,g |
| 60 | 0.949 | 112.8 | 10.0 ± 0.6 c | |
| 80 | 0.974 | 36.4 | 18.5 ± 1.2 f | |
| 60% ethanol/10% OxAc | 40 | 0.999 | 205.2 | 7.5 ± 0.2 e |
| 60 | 0.957 | 107.0 | 8.5 ± 0.3 g | |
| 80 | 0.976 | 24.7 | 16.5 ± 0.9 f |
| Treatment | Equation (Model) | R2 | p |
|---|---|---|---|
| Hydrothermal (5% OxAc) | YTP = 5.6 + 3.8X1 + 1.4X12 | 0.99 | <0.0001 |
| Hydrothermal (1% SuAc) | YTP = 4.6 + 3X1 + 0.6X2 + 0.7X1X2 | 0.98 | 0.0002 |
| Organosolv (60% EtOH/10% OxAc) | YTP = 9.5 + 3.8X1 + 1.4X2 + 1.6X1X2 + 2.4X12 | 0.96 | 0.0016 |
| Organosolv (60% EtOH/1.5% SuAc) | YTP = 10.4 + 4.8X1 + 1.6X2 + 1.7X1X2 + 2X12 | 0.98 | 0.0003 |
| Treatment | Optimal T (°C) | Optimal t (min) | Maximum Predicted YTP (mg GAE g−1 DM) | Severity (CSF’) |
|---|---|---|---|---|
| Hydrothermal (5% OxAc) | 90 | 120 | 11.4 ± 0.9 a | 7.03 |
| Hydrothermal (1% SuAc) | 90 | 240 | 9.5 ± 1.3 a | 8.23 |
| Organosolv (60% EtOH/10% OxAc) | 80 | 240 | 17.8 ± 2.3 b | 8.63 |
| Organosolv (60% EtOH/1.5% SuAc) | 80 | 240 | 21.0 ± 2.0 b | 8.73 |
| Treatment | Y (mg g−1 DM) | ||||
|---|---|---|---|---|---|
| CouE | CouA | FA | CouA/FA Ratio | Total | |
| Water | <0.01 a | 0.57 ± 0.03 a | 0.23 ± 0.02 a | 2.5 | 0.80 |
| Hydrothermal (5% OxAc) | 0.48 ± 0.03 b | 1.18 ± 0.06 b | 0.33 ± 0.01 b | 3.6 | 1.99 |
| Hydrothermal (1% SuAc) | 0.45 ± 0.02 b | 0.67 ± 0.04 c | 0.19 ± 0.02 a,c | 3.5 | 1.31 |
| 60% EtOH | <0.01 a | 0.98 ± 0.03 d | 0.18 ± 0.01 c | 5.4 | 1.16 |
| Organosolv (60% EtOH/10% OxAc) | <0.01 a | 2.21 ± 0.09 e | 0.46 ± 0.02 d | 4.8 | 2.67 |
| Organosolv (60% EtOH/1.5% SuAc) | <0.01 a | 2.96 ± 0.07 f | 0.70 ± 0.02 e | 4.2 | 3.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantidou, A.; Sarris, A.; Tsaousi, I.; Tsela, M.; Chatzimitakos, T.; Makris, D.P. The Effect of Acid Catalysis on Hydroxycinnamate Recovery from Corn Stover Using Hydrothermal and Organosolv Treatments. Environments 2025, 12, 379. https://doi.org/10.3390/environments12100379
Fantidou A, Sarris A, Tsaousi I, Tsela M, Chatzimitakos T, Makris DP. The Effect of Acid Catalysis on Hydroxycinnamate Recovery from Corn Stover Using Hydrothermal and Organosolv Treatments. Environments. 2025; 12(10):379. https://doi.org/10.3390/environments12100379
Chicago/Turabian StyleFantidou, Anna, Antony Sarris, Ioanna Tsaousi, Maria Tsela, Theodoros Chatzimitakos, and Dimitris P. Makris. 2025. "The Effect of Acid Catalysis on Hydroxycinnamate Recovery from Corn Stover Using Hydrothermal and Organosolv Treatments" Environments 12, no. 10: 379. https://doi.org/10.3390/environments12100379
APA StyleFantidou, A., Sarris, A., Tsaousi, I., Tsela, M., Chatzimitakos, T., & Makris, D. P. (2025). The Effect of Acid Catalysis on Hydroxycinnamate Recovery from Corn Stover Using Hydrothermal and Organosolv Treatments. Environments, 12(10), 379. https://doi.org/10.3390/environments12100379









