High Density of Microplastics in the Caddisfly Larvae Cases
Abstract
1. Introduction
2. Materials and Methods
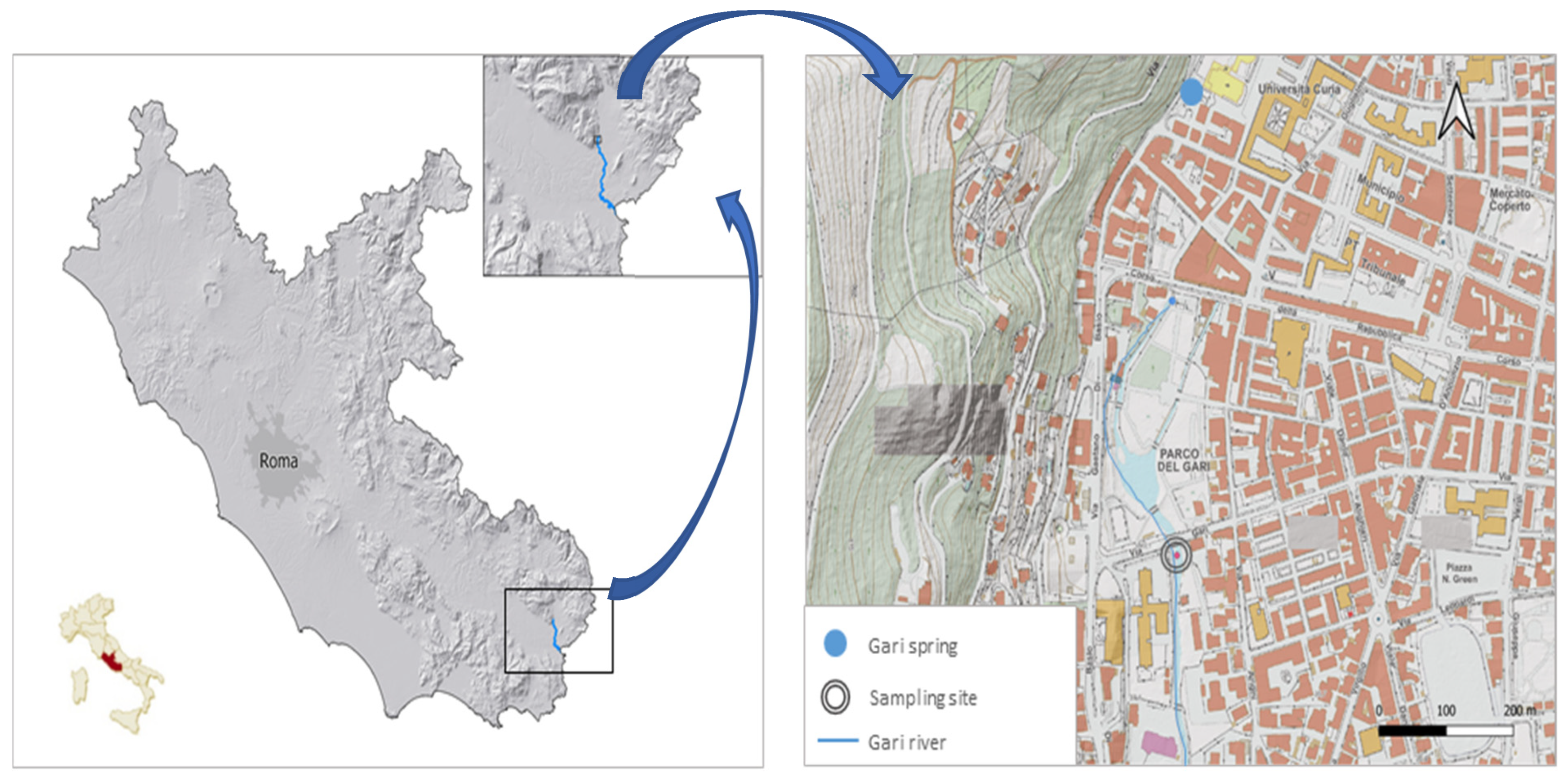
2.1. Study Area
2.2. Sample Collection
2.3. Laboratory Activities
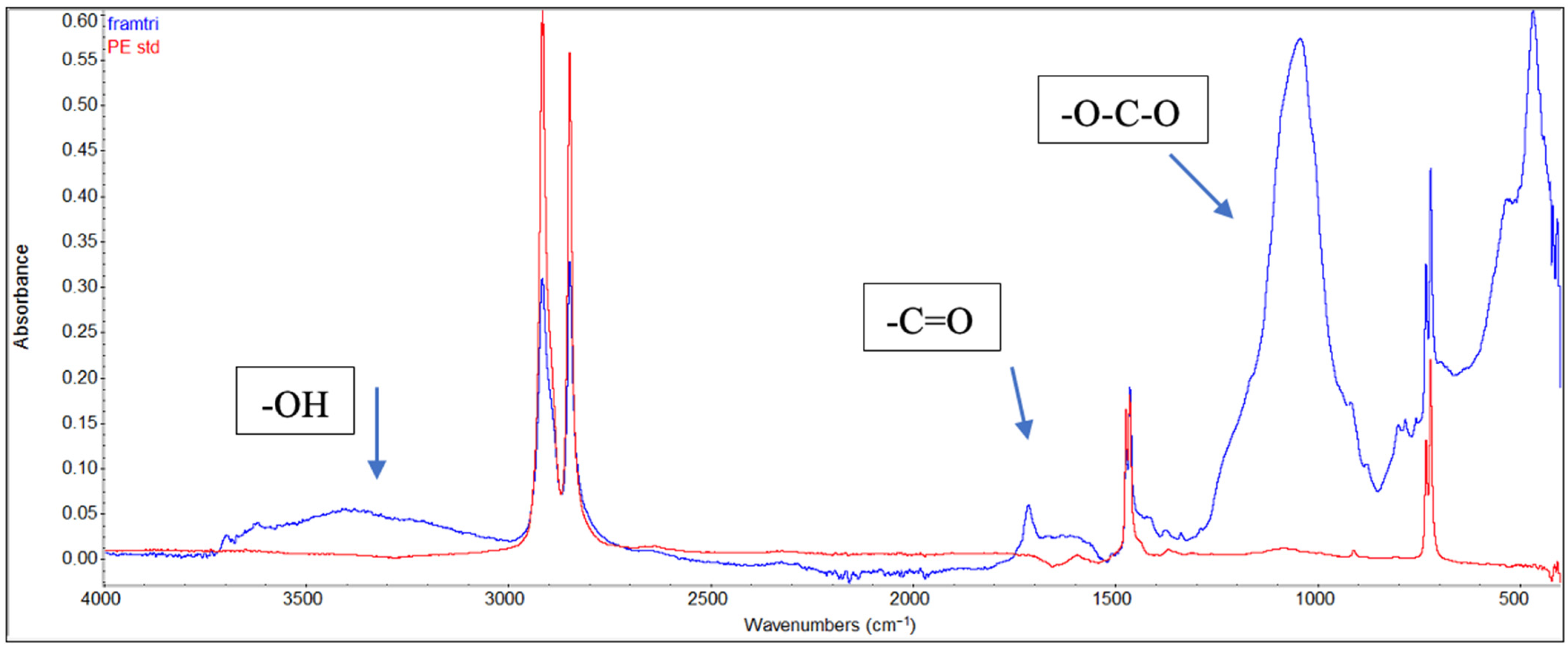
2.4. Polymer Characterisation
2.5. Quality and Contamination Control
2.6. Statistical Analyses
3. Results and Discussion
3.1. Water Quality
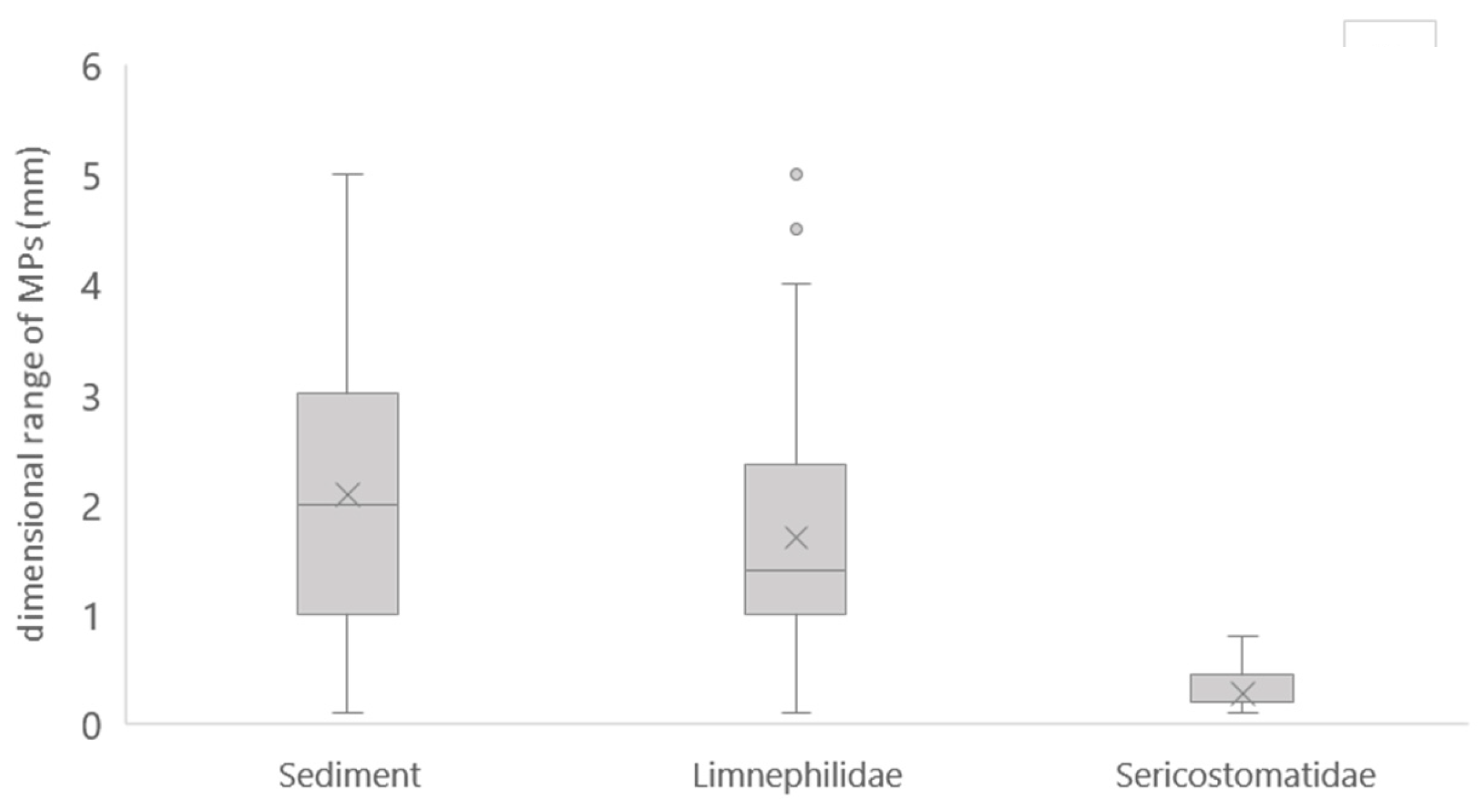
3.2. MP Analysis in Caddisfly Cases
3.3. Sediment Analyses
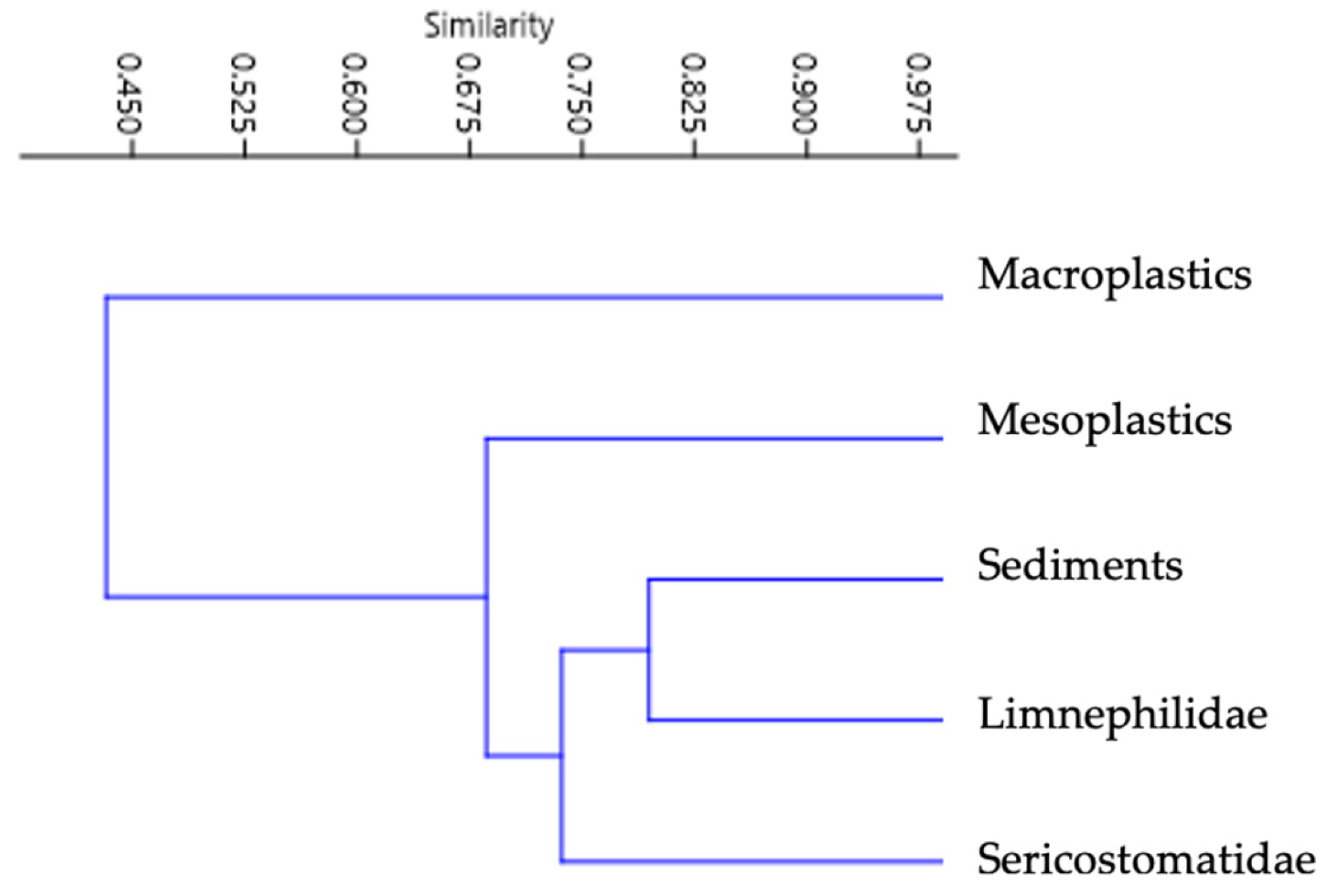
3.4. MPs Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philosophical trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Pietrelli, L. Fate of the biofilm chips overflowed from a wastewater treatment plant. Mar. Pollut. Bull. 2024, 200, 116142. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.J. Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci. Total Environ. 2020, 703, 134699. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives presents in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef]
- Chen, Q.; Allgeier, A.; Yin, D.; Hollert, H. Leaching of endocrine disrupting chemicals from marine microplastics and mesoplastics under common life stress conditions. Environ. Int. 2019, 130, 104938. [Google Scholar] [CrossRef]
- Okoye, C.O.; Addey, C.I.; Oderinde, O.; Okoro, J.O.; Uwamungu, J.Y.; Ikechukwu, C.K.; Okeke, E.S.; Ejeromedoghene, O.; Odii, E.C. Toxic chemicals and persistent organic pollutants associated with micro-and nanoplastics pollution. Chem. Eng. J. Adv. 2022, 11, 100310. [Google Scholar] [CrossRef]
- Sun, N.; Shi, H.; Li, X.; Gao, C.; Liu, R. Combined toxicity of micro/nanoplastics loaded with environmental pollutants to organisms and cells: Role, effects, and mechanism. Environ. Int. 2023, 171, 107711. [Google Scholar] [CrossRef]
- D’Souza, J.M.; Windsor, F.M.; Santillo, D.; Ormerod, S.J. Food web transfer of plastics to an apex riverine predator. Glob. Chang. Biol. 2020, 26, 3846–3857. [Google Scholar] [CrossRef]
- Cera, A.; Scalici, M. Freshwater wild biota exposure to microplastics: A global perspective. Ecol. Evol. 2021, 11, 9904–9916. [Google Scholar] [CrossRef] [PubMed]
- Gallitelli, L.; Battisti, C.; Pietrelli, L.; Scalici, M. Anthropogenic particles in coypu (Myocastor coypus; Mammalia, Rodentia)’ faeces: First evidence and considerations about their use as track for detecting microplastic pollution. Environ. Sci. Pollut. Res. 2022, 29, 55293–55301. [Google Scholar] [CrossRef] [PubMed]
- Grgić, I.; Cetinić, K.A.; Karačić, Z.; Previši, A.; Rožman, M. Fate and effects of microplastics in combination with pharmaceuticals and endocrine disruptors in freshwaters: Insights from a microcosm experiment. Sci. Total Environ. 2023, 859, 160387. [Google Scholar] [CrossRef] [PubMed]
- Kögel, T.; Bjorøy, Ø.; Toto, B.; Bienfait, A.M.; Sanden, M. Micro-and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total Environ. 2020, 709, 136050. [Google Scholar] [CrossRef]
- Rochman, C.M. Microplastics research—From sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Kershaw, P.J.; GESAMP; IMO/FAO/UNES—COIOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection. Sources, fate and effects of microplastics in the marine environment: A global assessment. Rep. Stud. GESAMP 2015, 90, 96. [Google Scholar]
- Lambert, S.; Wagner, M. Microplastics are contaminants of emerging concern in freshwater environments: An overview. In Freshwater Microplastics; Wagner, M., Lambert, S., Eds.; The Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2018; Volume 58, pp. 1–23. [Google Scholar] [CrossRef]
- Meijer, L.J.; van Emmerik, T.; van der Ent, R.; Schmidt, C.; Lebreton, L. More than 1000 rivers account for 80% of global riverine plastic emissions into the ocean. Sci. Adv. 2021, 7, aaz5803. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cha, J.; Ha, K.; Viaroli, S. Microplastic pollution in groundwater: A systematic review. Environ. Pollut. Bioavailab. 2024, 36, 2299545. [Google Scholar] [CrossRef]
- Sighicelli, M.; Pietrelli, L.; Lecce, F.; Iannilli, V.; Falconieri, M.; Coscia, L.; Di Vito, S.; Nuglio, S.; Zampetti, G. Microplastic pollution in the surface waters of Italian Subalpine Lakes. Environ. Pollut. 2018, 236, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish, 2nd ed.; EPA, U.S. Environmental Protection Agency, Office of Water: Washington DC, USA, 1999. [Google Scholar]
- Morse, J.C.; Frandsen, P.B.; Graf, W.; Thomas, J.A. Diversity and ecosystem services of Trichoptera. Insects 2019, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Correa-Araneda, F.; Basaguren, A.; Abdala-Díaz, R.T.; Tonin, A.M.; Boyero, L. Resource-allocation tradeoffs in caddisflies facing multiple stressors. Ecol. Evol. 2017, 7, 5103–5110. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.; Svensson, B.S. Why do some caddis larvae in running waters construct heavy, bulky cases? Anim. Behav. 1995, 49, 473–478. [Google Scholar] [CrossRef]
- Holzenthal, R.W.; Thomson, R.E.; Ríos-Touma, B. Order Trichoptera. In Book Thorp and Covichs Freshwater Invertebrates, 4th ed.; Thorp, J.H., Rogers, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 965–1002. [Google Scholar] [CrossRef]
- Lenat, D.R. A biotic index for the southeastern United States: Derivation and list of tolerance values, with criteria for assigning water-quality ratings. J. N. Am. Benthol. Soc. 1993, 12, 279–290. [Google Scholar] [CrossRef]
- Bonada, N.; Zamora-Munoz, C.; Rieradevall, M.; Prat, N. Ecological profiles of caddisfly larvae in Mediterranean streams: Implications for bioassessment methods. Environ. Pollut. 2004, 132, 509–521. [Google Scholar] [CrossRef]
- Kalaninová, D.; Bulánková, E.; Šporka, F. Caddisflies (Trichoptera) as good indicators of environmental stress in mountain lotic ecosystems. Biologia 2014, 69, 1030–1045. [Google Scholar] [CrossRef]
- Ajiboye, M. The Role of Conspecific Damage-Released Alarm Cue and Plastic Pollution on Caddisfly Larvae Case Construction Behavior. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2021. [Google Scholar]
- Ehlers, S.M.; Al Najjar, T.; Taupp, T.; Koop, J.H. PVC and PET microplastics in caddisfly (Lepidostoma basale) cases reduce case stability. Environ. Sci. Pollut. Res. 2020, 27, 22380–22389. [Google Scholar] [CrossRef]
- Gallitelli, L.; Cera, A.; Cesarini, G.; Pietrelli, L.; Scalici, M. Preliminary indoor evidence of microplastic effects on freshwater benthic macroinvertebrates. Sci. Rep. 2021, 11, 720. [Google Scholar] [CrossRef]
- Hiemstra, A.F.; van der Velden, I.; Ciliberti, P.; D’Alba, L.; Gravendeel, B.; Schilthuizen, M. Half a century of caddisfly casings (Trichoptera) with microplastic from natural history collections. Sci. Total Environ. 2025, 974, 178947. [Google Scholar] [CrossRef] [PubMed]
- Nel, H.A.; Froneman, P.W. Presence of microplastics in the tube structure of the reef-building polychaete Gunnarea gaimardi (Quatrefages 1848). Afr. J. Mar. Sci. 2018, 40, 87–89. [Google Scholar] [CrossRef]
- Lo Bue, G.; Marchini, A.; Musa, M.; Croce, A.; Gatti, G.; Riccardi, M.P.; Lisco, S.; Mancin, N. First attempt to quantify microplastics in Mediterranean Sabellaria spinulosa (Annelida, Polychaeta) bioconstructions. Mar. Pollut. Bull. 2023, 196, 115659. [Google Scholar] [CrossRef] [PubMed]
- Celico, P.B. Idrogeologia dei Massicci Carbonatici, Delle Piane Quaternarie e Delle Aree Vulcaniche Dell’italia Centro-Meridionale: Marche e Lazio Meridionali, Abruzzo, Molise e Campania; Cassa per il Mezzogiorno Ed.: Rome, Italy, 1983; p. 221. [Google Scholar]
- EEA, European Environment Agency. Water Framework Directive (WFD) 2000/60/EC. 2000. Available online: https://www.eea.europa.eu/policy-documents/water-framework-directive-wfd-2000 (accessed on 15 March 2025).
- Buffagni, A.; Erba, S. Macroinvertebrati acquatici e Direttiva 2000/60/EC (WFD)—Parte A. Metodo di campionamento per i fiumi guadabili. IRSA-CNR Not. Metod. Anal. 2007, 1, 2–27. [Google Scholar]
- ISPRA. Linee Guida per la valutazione della componente macrobentonica fluviale ai sensi del DM 260/2010. ISPRA Manuali E Linee Guid. 2014, 107, 44–45, 50–51. [Google Scholar]
- Tibbetts, J.; Krause, S.; Lynch, I.; Smith, G.H.S. Abundance, distribution, and drivers of microplastic contamination in urban river environments. Water 2018, 10, 1597. [Google Scholar] [CrossRef]
- van Emmerik, T.; Kieu-Le, T.C.; Loozen, M.; van Oeveren, K.; Strady, E.; Bui, X.T.; Egger, M.; Gasperi, J.; Lebreton, L.; Nguyen, P.D.; et al. A Methodology to Characterize Riverine Macroplastic Emission into the Ocean. Front. Mar. Sci. 2018, 5, 3404. [Google Scholar] [CrossRef]
- Gallitelli, L.; Cesarini, G.; Cera, A.; Sighicelli, M.; Lecce, F.; Menegoni, P.; Scalici, M. Transport and deposition of microplastics and mesoplastics along the river course: A case study of a small river in Central Italy. Hydrology 2020, 7, 90. [Google Scholar] [CrossRef]
- Alvarez-Troncoso, R.; Gutiérrez, D.; Villar, I.; Ehlers, S.E.; Soto, B.; Mato, S.; Garrido, J. Microplastics in water, sediments and macroinvertebrates in a small river of NW Spain. Limnetica 2022, 43, 199–212. [Google Scholar] [CrossRef]
- Roy, P.K.; Surekha, P.; Rajagopal, C.; Chatterjee, S.N.; Choudhary, V. Studies on the photo-oxidative degradation of LDPE films in the presence of oxidized Polyethylene. Polym. Degrad. Stab. 2007, 92, 1151–1160. [Google Scholar] [CrossRef]
- Ehlers, S.M.; Manz, W.; Koop, J.H. Microplastics of different characteristics are incorporated into the larval cases of the freshwater caddisfly Lepidostoma basale. Aquat. Biol. 2019, 28, 67–77. [Google Scholar] [CrossRef]
- Cera, A.; Cesarini, G.; Scalici, M. Microplastics in freshwater: What is the news from the world? Diversity 2020, 12, 276. [Google Scholar] [CrossRef]
- Windsor, F.M.; Tilley, R.M.; Tyler, C.R.; Ormerod, S.J. Microplastic ingestion by riverine macroinvertebrates. Sci. Total Environ. 2019, 646, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Simmerman, C.B.; Wasik, C.J.K. The effect of urban point source contamination on microplastic levels in water and organisms in a cold-water stream. Limnol. Ocean. Lett. 2020, 5, 137–146. [Google Scholar] [CrossRef]
- Parker, B.; Andreou, D.; Pabortsava, K.; Barrow, M.; Green, I.D.; Britton, J.R. Microplastic loads within riverine fishes and macroinvertebrates are not predictable from ecological or morphological characteristics. Sci. Total Environ. 2022, 839, 156321. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, M.; Pastorino, P.; Lesa, D.; Renzi, P.; Anselmi, S.; Prearo, M.; Pizzul, E. Microplastics accumulation in functional feeding guilds and functional habit groups of freshwater macrobenthic invertebrates: Novel insights in a riverine ecosystem. Sci. Total Environ. 2022, 804, 150207. [Google Scholar] [CrossRef]
- Valentine, K.; Cross, R.; Cox, R.; Woodmancy, G.; Boxall Aba, A. Caddisfly Larvae are a Driver of Plastic Litter Breakdown and Microplastic Formation in Freshwater Environments. Environ. Toxicol. Chem. 2022, 41, 3058–3069. [Google Scholar] [CrossRef]
- Fritz, S.F.; Albertson, L.K.; Hobgood, J.L.; Mohr, E.J.; Oakland, H.C.; Poole, G.C. Macroinvertebrate ecosystem engineering affects streambed retention of microplastics. Freshw. Sci. 2023, 42, 133–145. [Google Scholar] [CrossRef]
- Mat Zain, N.; Fauzi, N.; Subki, N.S.; Ghazali, Z.Z. Occurrence of Microplastics in immature aquatic insects of Gua Musang Tributaries in Kelantan. 4th Intern. Conf. Tropical Resources and Sustainable Sciences 2022. IOP Conf. Ser. Earth Environ. Sci. 2022, 1102, 012047. [Google Scholar] [CrossRef]
- Karnaukhov, D.Y.; Lavnikova, A.V.; Nepokrytykh, A.V.; Zhdanov, I.A.; Salovarov, K.V.; Guliguev, A.T.; Osadchy, B.V.; Biritskaya, S.A.; Ermolaeva, Y.K.; Maslennikova, M.A.; et al. Baikal endemic and Palearctic species of caddisflies (Trichoptera) build cases from microplastics. Acta Biol. Sibirica 2024, 10, 649–659. [Google Scholar] [CrossRef]
- Pfister, H.; D’Avignon, G.; Wang, A.; Felismino, M.E.L.; Ricciardi, A. Caddisfly larvae (Trichoptera: Limnephilidae) facilitate the uptake of microplastics by a freshwater fish (Ameiurus nebulosus). 2025; in press. [Google Scholar] [CrossRef]
- Arias-Paco, A.; Springer, M. Patterns of microplastic incorporation in larval and pupal cases of Limnephilus hamifer (Trichoptera: Limnephilidae). Rev. Biol. Trop. 2025, 73, e63696. [Google Scholar] [CrossRef]
- Vaughan, R.; Turner, S.D.; Rose, N.L. Microplastics in the sediments of a UK urban lake. Environ. Pollut. 2017, 229, 10–18. [Google Scholar] [CrossRef]
- Ghetti, P.F.; Sansoni, G.; Scientific Accomplishment of with the Contribution of the Italian Center Environmental Study of Biology. Atlante per il Riconoscimento dei Macroinvertebrati dei Corsi D’acqua Italiani, Realizzazione Scientifica di Giuseppe Sansoni, Con il Contributo del Centro Italiano Studi di Biologia Ambientale. (Atlas for the Recognition of the Macroinvertebrates of the Running Italian Water); APR & B; Agenzia Provinciale per la Protezione Dell’ambiente (Trento): Trento, Italia, 1998. (In Italian) [Google Scholar]
- Holzenthal, R.W.; Blahnik, R.J.; Prather, A.L.; Kjer, K.M. Order Trichoptera kirby, 1813 (insecta), caddisflies. Zootaxa 2007, 1668, 639–698. [Google Scholar] [CrossRef]
- Otto, C.; Svensson, B.S. The Significance of Case Material Selection for the Survival of Caddis Larvae. J. Anim. Ecol. 1980, 49, 855–865. [Google Scholar] [CrossRef]
- Prestidge, R.A. Case-building behaviour of Pycnocentrodes aeris (Trichoptera: Sericostomatidae). N. Zealand Entomol. 1977, 6, 296–301. [Google Scholar] [CrossRef]
- Koutsikos, N.; Koi, A.M.; Zeri, C.; Tsangaris, C.; Dimitriou, E.; Kalantzi, O.I. Exploring microplastic pollution in a Mediterranean river: The role of introduced species as bioindicators. Heliyon 2023, 9, e15069. [Google Scholar] [CrossRef]
- Thacharodi, A.; Meenatchi, R.; Hassan, S.; Hussain, N.; Bhat, A.M.; Arockiaraj, J.; Ngo, H.H.; Le, Q.H.; Pugazhendhi, A. Microplastics in the environment: A critical overview on its fate, toxicity, implications, management, and bioremediation strategies. J. Environ. Manag. 2024, 349, 119433. [Google Scholar] [CrossRef]
- Sutkar, P.R.; Gadewar, R.D.; Dhulap, V.P. Recent trends in degradation of microplastics in the environment: A state-of-the-art review. J. Hazard. Mater. Adv. 2023, 11, 100343. [Google Scholar] [CrossRef]
- Yan, M.; Nie, H.; Xu, K.; He, Y.; Hu, Y.; Huang, Y.; Wang, J. Microplastic abundance, distribution and composition in the Pearl River along Guangzhou city and Pearl River estuary, China. Chemosphere 2019, 217, 879–886. [Google Scholar] [CrossRef]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic pollution in table salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef]
- Stanković, J.; Milošević, D.; Paunović, M.; Jovanović, B.; Popović, N.; Tomović, J.; Atanacković, A.; Radulović, K.; Lončarević, D.; Raković, M. Microplastics in the Danube River and Its Main Tributaries—Ingestion by Freshwater Macroinvertebrates. Water 2024, 16, 962. [Google Scholar] [CrossRef]
- McCormick, A.R.; Hoellein, T.J.; London, M.G.; Hittie, J.; Scott, J.W.; Kelly, J.J. Microplastic in surface waters of urban rivers: Concentration, sources, and associated bacterial assemblages. Ecosphere 2016, 7, e01556. [Google Scholar] [CrossRef]
- Lagarde, F.; Olivier, O.; Zanella, M.; Daniel, P.; Hiard, S.; Caruso, A. Microplastic interactions with freshwater microalgae: Hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ. Pollut. 2016, 215, 331–339. [Google Scholar] [CrossRef]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]


| River, Lake (Country) | Caddisflies | MPs Pres. | Polymers | Abundance | Ref. |
|---|---|---|---|---|---|
| Tame (UK) | n.d. | LC | n.d. | n.d. | [42] |
| Saynbach (D) | Lepidostomatidae | LC | PP, PA, ABS, PU, VE, PE, PVC, PES | 1.14 MPs/case | [47] |
| Gafos (E) | Lepidostomatidae Limnephilidae | LC | PVC, PET, PE, PP, PES, PSA | n.d. | [45] |
| Taff, Usk, Wye (UK) | Hydropsychidae | LT | PP | 20–30 MPs/g | [49] |
| Kinnickinnic (USA) | Hydropsychidae | LT | n.d. | 5–15 MPs/g | [50] |
| Lab. study | Lepidostomatidae | LC | PVC, PET | n.d. | [33] |
| Lab. study | Odontocerum albicorne | LC | ABS, PET, PP, PS, PVDF | 17 MPs/case | [34] |
| Stour (UK) | Ord. Trichoptera | LT | PA, PS, PES, PE, PP | 0.62 MPs/case | [51] |
| Vipacco (I) | Hydropsychidae Lepidostomatidae | LT | PES | 0.003 MPs/case | [52] |
| Lab. study | Agrynia sp. | LC | PLA | n.d. | [53] |
| Lab. study | Hydropsychidae | LC, LT | PVC | n.d. | [54] |
| Sungai Chegeh and Sungai Galas (Malaysia) | n.d. | LT | CE | n.d. | [55] |
| Gafos River (Spain) | Leptoceridae Limnephilidae | LC | PE | n.d. | [45] |
| Baikal Lake (Russia) | Baicalina thamastoides Hydatophylax nigrovittatus | LC | PVA, PVC | n.d. | [56] |
| Lab. study | Limnephilidae | LC, LT | PET | n.d. | [57] |
| Lab. study | Limnephilus hamifer | LC | PET | 16.4–28.7/case | [58] |
| Gari (I) | Limnephilidae Sericostomatidae Goeridae | LC | PP, PE, PA, PVC, PET, PVA, Nylon, PAN, CE | 0–4 MPs/case | Present study |
| ASPT (*) | 5.333 | Shannon Index | 2.555 | EPT Families | 6 |
| Total families | 18 | log (SelEPTD+1) | 1.898 | Star_ICMi index | 0.797 |
| 1-GOLD | 0.736 | ||||
| Type | A | B | C | D | Colours | A | B | C | D |
|---|---|---|---|---|---|---|---|---|---|
| Fibres | - | 6 | - | 37.5 | White/yellow | 8 | 31 | 34 | - |
| Film | 18 | 1 | 25.0 | Blue | 28 | 20 | 24 | 25.0 | |
| Fragment | 78 | 93 | 100 | Black/brown | 8 | 7 | - | 12.5 | |
| Foam | - | - | - | 37.5 | Green | 19 | 10 | 3 | 37.5 |
| Pellet | 1 | Red | 27 | 20 | 31 | 25.0 | |||
| Sphere | 2 | Transparent | - | 6 | - | - | |||
| Grain | 1 | Others | 9 | 6 | 7 | - | |||
| TOTAL | 352 | 55 | 18 | 8 | TOTAL | 352 | 55 | 18 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barra, E.; Cicero, F.; Magliocchetti, I.; Menegoni, P.; Sighicelli, M.; Di Ludovico, A.; Le Foche, M.; Pietrelli, L. High Density of Microplastics in the Caddisfly Larvae Cases. Environments 2025, 12, 368. https://doi.org/10.3390/environments12100368
Barra E, Cicero F, Magliocchetti I, Menegoni P, Sighicelli M, Di Ludovico A, Le Foche M, Pietrelli L. High Density of Microplastics in the Caddisfly Larvae Cases. Environments. 2025; 12(10):368. https://doi.org/10.3390/environments12100368
Chicago/Turabian StyleBarra, Eliana, Francesco Cicero, Irene Magliocchetti, Patrizia Menegoni, Maria Sighicelli, Alberto Di Ludovico, Marco Le Foche, and Loris Pietrelli. 2025. "High Density of Microplastics in the Caddisfly Larvae Cases" Environments 12, no. 10: 368. https://doi.org/10.3390/environments12100368
APA StyleBarra, E., Cicero, F., Magliocchetti, I., Menegoni, P., Sighicelli, M., Di Ludovico, A., Le Foche, M., & Pietrelli, L. (2025). High Density of Microplastics in the Caddisfly Larvae Cases. Environments, 12(10), 368. https://doi.org/10.3390/environments12100368







