Abstract
The environmental fate of nickel (Ni) is dictated by its interaction with organic matter (OM), yet the specific roles of OM source and molecular size remain unclear. This study investigated the binding characteristics of Ni with size-fractionated dissolved OM (DOM) from the water column and alkaline-extractable OM (AEOM) from sediments in a tropical wetland. Using ultrafiltration and spectroscopy, we found that sedimentary AEOM was predominantly high-molecular-weight (HMW) and terrestrial compounds, whereas aquatic DOM was dominated by low-molecular-weight (LMW), microbial-derived compounds. Counterintuitively, the highest Ni binding affinity (NiBA) for both DOM and AEOM occurred in the smallest-molecular-weight fraction (<0.3 kDa). Predictive models confirmed this divergence: the model for the more chemically homogeneous AEOM was highly predictive (r = 0.89), while the model for the complex DOM was less robust (r = 0.70). Our findings demonstrate that LMW fractions are hotspots for Ni binding, challenging the common assumption that larger molecules are more reactive. We conclude that biogeochemical processing in sediments creates an OM pool that is chemically distinct and more predictable than that in the overlying water. This distinction is critical for accurately assessing Ni mobility and ecological risk in aquatic systems.
1. Introduction
Potentially toxic metals (PTMs) in aquatic environments pose significant risks to ecosystem stability and human health due to their toxicity, persistence, and potential for bioaccumulation through the food chain [1,2]. Sediments often act as the primary sink, accumulating a large proportion of PTMs from external inputs [3]. However, these sequestered metals are not permanently fixed; they can be remobilized into the overlying water and suspended particulate matter (SPM) via various biogeochemical processes, leading to secondary contamination [3,4,5]. Their interactions with organic matter fundamentally control the distribution, mobility, and ultimate fate of PTMs, broadly divided into dissolved (DOM) and particulate (POM) phases [4,6,7].
The binding of PTMs to organic matter occurs through complexation and electrostatic interactions with carboxyl and phenolic functional groups [8,9,10]. This interaction is critical as it dictates metal bioavailability and toxicity [8,11,12]. Crucially, the affinity of PTMs for organic matter is not uniform; it is highly dependent on the physicochemical properties of the organic matter itself, such as its source, aromaticity, and molecular weight distribution [13,14,15]. For instance, DOM originating from terrestrial sources is often more aromatic and compositionally different from microbially derived autochthonous DOM, leading to varied metal binding behaviors [7,8].
To probe these critical properties, researchers employ a combination of analytical techniques. Optical spectroscopy, including UV-Vis absorbance and fluorescence, offers a rapid and sensitive means to characterize DOM and POM [16,17]. Indices such as the specific UV absorbance at 254 nm (SUVA254) [18], absorbance ratios (e.g., A254/A204 [19,20]), and the fluorescence index (FI) [21] are widely used to infer aromaticity and the relative contributions of terrestrial versus microbial sources [17,22,23]. Concurrently, sequential ultrafiltration is a valuable tool for physically fractionating the organic matter continuum into different molecular weight classes, allowing for a detailed investigation of how metal binding varies across the size spectrum [24,25].
Previous studies have revealed complex and sometimes conflicting binding mechanisms, partly because DOM source and composition significantly influence binding, and the interpretation of optical surrogates is itself source-dependent [10,13,26,27], while others report stronger affinities with lower molecular weight (LMW) fractions [28,29]. Although nickel (Ni) has shown a preference for high-molecular-weight (HMW) fractions in some settings [30], these behaviors are highly sensitive to the environment. Crucially, most insights come from dynamic, high-flow riverine systems [24,25], which differ sharply from the low-flow wetland in our study [31]. In such wetlands, longer water residence times intensify biogeochemical processing at the sediment-water interface, creating a distinct environment where metal–organic matter interactions are poorly understood. Given its widespread presence and known sensitivity to the molecular size of organic ligands [8,10,32,33], Ni serves as an excellent model metal to probe these interactions. Its selection was further supported by its ambient concentration in the wetland, which was suitable for reliable quantification, and its stable recovery during the fractionation process. Understanding its partitioning is therefore critical for assessing ecological risk and can also provide insights relevant to other borderline transition metals (e.g., Cu, Zn), although each metal’s unique binding preferences necessitate specific investigation.
This study addresses this gap by investigating the binding characteristics of Ni with both DOM and AEOM from a low-flow wetland system, employing a combination of ultrafiltration for molecular weight fractionation and advanced spectroscopic techniques [24,30,34,35,36]. The specific objectives were to (1) characterize the molecular size distributions of DOM and AEOM, along with the associated Ni; (2) determine the Ni binding affinity (NiBA, [Ni]/[DOC] ratio) in each molecular weight fraction; (3) quantify the optical properties (A254/A204, SUVA254, FI) of the size-fractionated DOM and AEOM; and (4) evaluate the relationships between these optical indicators and NiBA to elucidate the key factors controlling Ni–organic matter interactions in a wetland system.
2. Materials and Methods
2.1. Study Site Description and Sampling
This study was conducted at Lungluan Wetland (LLW) located within Kenting National Park in southern Taiwan (21°59′ N, 120°44′ E). The wetland covers approximately 1.75 km2 with water depths ranging from 2 to 3 m. The site represents a unique tropical ecosystem characterized by distinct wet and dry seasons. The wetland’s hydrology is primarily maintained by monsoon rainfall and treated domestic wastewater from surrounding communities, with occasional intense precipitation during typhoon events in summer. The wetland serves multiple ecological functions, particularly as a critical stopover site for migratory birds along the East Asian-Australasian Flyway. It also provides essential ecosystem services including water purification and irrigation water for local agriculture.
Three water and sediment samples were collected near the LLW outlet using a stainless-steel sampler with an extended handle. Concurrently, water and sediment samples were collected in the 0–30 cm surface sediment. The overlying water was separated from sediment by gravity drainage as regarded DOM sample. The separated sediment samples were air-dried at room temperature and then sieved through a 2.0 mm mesh to remove coarse particles.
Prior to sampling, all sampling bottles were pre-rinsed with sample water. Basic water quality parameters were analyzed on the same day of collection, while the remaining water samples were stored at 4 °C for subsequent analyses.
The basic physicochemical properties of the water and sediment samples from LLW have been characterized in previous studies [24]. The overlying water is typically slightly alkaline, with pH values ranging from approximately 7.9 to 8.5, and contains dissolved organic carbon (DOC) concentrations in the range of 3.6–4.3 mg/L. The underlying sediments show significant spatial heterogeneity. The sediment pH is generally near-neutral (ranging from 6.3 to 7.8), and the Total Organic Carbon (TOC) content varies widely from 0.4% to 5.1%, indicating diverse depositional conditions across the wetland. The sediment texture is also highly variable, composed of a mixture of sand (21.7–82.5%), silt (10.3–55.6%), and clay (2.5–26.2%).
2.2. AEOM Extraction and Size Fractionation
2.2.1. AEOM Extraction
The dissolved organic matter (DOM) solution was obtained by filtering the overlying water samples through 0.45 μm membrane filters. The alkaline-extractable organic matter (AEOM) was extracted from the sediments following a standard procedure with minor modifications [24,25]. Specifically, 5 g of air-dried sediment was weighed into a centrifuge tube. To remove carbonates and soluble alkaline metals, 100 mL of 0.1 N HCl was added, and the mixture was agitated for 1 h. After centrifugation, the supernatant was discarded. Subsequently, 100 mL of 0.1 N NaOH solution was added to the residual sediment, achieving a solid-to-solution ratio of 1:20. The mixture was then shaken for 24 h in the dark at room temperature to extract the AEOM. Finally, the suspension was centrifuged at 4500 rpm for 30 min, and the resulting supernatant was collected as the bulk AEOM solution.
2.2.2. Size Fractionation
Prior to fractionation, the pH of the bulk DOM solution was confirmed to be at its ambient level (8.2 ± 0.3). The bulk AEOM solution, following alkaline extraction, was carefully neutralized with 0.1 N HCl to a pH of 8.0 ± 0.2 to mimic the environmental conditions of the overlying water and to ensure membrane integrity. Both the bulk DOM and AEOM solutions (initial volume for each: 10 L) were sequentially fractionated into five distinct molecular weight (MW) fractions using a cross-flow ultrafiltration system. The system was equipped with a series of ceramic membrane cartridges (TAMI Industries, Noyns, France) with nominal molecular weight cutoffs (NMWCs) of 10, 3, 1, and 0.3 kDa.
During the fractionation process, the feed flow rate was maintained at 1.7–2.0 L/min. The process was operated in concentration mode until the volume concentration factor (Cf), calculated using Equation (1), reached 10.
where Vpen and Vret are the volumes of the permeate (filtrate) and the retentate (concentrate), respectively. The filtrate from one filtration step served as the feed solution for the next smaller-pore-size membrane. This sequential process yielded five size fractions, designated as: MW-A (10 kDa–0.45 μm), MW-B (3–10 kDa), MW-C (1–3 kDa), MW-D (0.3–1.0 kDa), and MW-E (<0.3 kDa).
The mass balance recovery (R, %) for dissolved organic carbon (DOC) and the target metal (Ni) across all fractions was calculated to ensure procedural accuracy, using Equation (2). Recoveries were considered acceptable if they fell within the range of 75–125%, a standard criterion for such fractionation studies that accounts for the propagation of analytical uncertainty from multiple concentration and volume measurements [25,37].
The mass fraction (Mi, %) of DOC and Ni in each size fraction was calculated using Equation (3):
where [Cbulk] and Vbulk are the concentration and volume of the initial bulk solution, and [Ci] and Vi are the concentration and volume for each respective fraction i.
2.2.3. System Cleaning Protocol
To prevent cross-contamination between samples, the ultrafiltration membranes and the entire system were subjected to a rigorous cleaning protocol after each run. The cleaning sequence was as follows: (1) flushing with reverse osmosis (RO) water; (2) circulating a neutral detergent; (3) rinsing with RO water; (4) circulating a 0.5 N NaOH solution; and finally, (5) flushing with RO water until the permeate’s pH returned to neutral. This cleaning process was performed at an operating pressure of 2 kg/cm2 with a permeate flow rate of approximately 1 L/min. For long-term storage, the membrane cartridges were kept in a 0.5 N NaOH solution to maintain their integrity and prevent microbial growth.
2.3. Analysis of Dissolved Organic Carbon and Metals
DOC concentrations in both AEOM and DOM solutions were determined using a TOC-V analyzer (Shimadzu, Kyoto, Japan). Ni concentrations were analyzed using two different atomic absorption spectroscopy techniques: high concentration samples were measured using flame atomic absorption spectroscopy (FAAS) (Hitachi Z-2300, Tokyo, Japan), while low concentration samples were analyzed using graphite furnace atomic absorption spectroscopy (GFAAS) (Hitachi ZA-3000, Tokyo, Japan).
2.4. UV-Visible and Fluorescence Spectroscopy
The bulk and size-fractionated AEOM and DOM solutions were diluted with ultrapure water to final concentrations of 5.0 and 1.0 mg-C/L, respectively. UV-visible absorption spectra were recorded using a UV-visible spectrophotometer (Hitachi U-2900, Tokyo, Japan) over the wavelength range of 200–800 nm. Background correction was performed by subtracting the average absorbance value between 700 and 800 nm from the sample spectra [38].
Fluorescence measurements were conducted using a Hitachi F-7000 fluorescence spectrometer (Hitachi F-7000, Tokyo, Japan). Excitation-emission matrices (EEMs) were generated by recording emission spectra from 250 to 550 nm at 2.0 nm intervals, with excitation wavelengths ranging from 200 to 450 nm at 5 nm increments. The scanning rate was set at 2400 nm/min. All fluorescence data were blank-corrected by subtracting the EEM of ultrapure water. Since the UV absorbance values at 254 nm were below 0.2, inner filter effect corrections were not applied [21].
2.5. Calculation of Optical Indices and Nickel Binding Affinity
2.5.1. Optical Indices
To characterize the properties of dissolved and particulate organic matter, several spectroscopic indices were calculated from the UV-Vis absorbance and fluorescence measurements.
Specific UV Absorbance at 254 nm (SUVA254), an indicator of DOM aromaticity [18], was calculated from the decadic absorbance at 254 nm normalized by both optical pathlength and dissolved organic carbon concentration, as shown in Equation (4).
where SUVA254 (L/mg-C/m) is the specific UV absorbance at 254 nm, A254 is the raw decadic absorbance at 254 nm (dimensionless), l is the optical pathlength of the cuvette (m; 0.01 m for a 1 cm pathlength), and [DOC] is the dissolved organic carbon concentration (mg-C/L). All SUVA values were calculated using replicate measurements of A254 and DOC. Absorbance Ratio (A254/A204): This ratio, used to infer the relative proportion of hydrophobic to hydrophilic compounds, was calculated as the dimensionless ratio of absorbance values at 254 nm and 204 nm [39].
The fluorescence index (FI) used to distinguish between terrestrial and microbial sources of organic matter, was determined as the ratio of fluorescence emission intensities at 450 nm to 500 nm, under an excitation wavelength of 370 nm [21].
2.5.2. Nickel Binding Affinity (NiBA)
The nickel binding affinity (NiBA) was calculated to evaluate the amount of nickel associated with a given mass of organic carbon. It is expressed as the molar ratio of nickel concentration to the carbon concentration of DOC [10,24,25], as shown in Equation (5).
where NiBA is expressed in μmol-Ni/g-C; [Ni] (μmol/L) is the total nickel concentration in each size fraction, and [DOC] (g-C/L) is the dissolved organic carbon concentration. Because total Ni is used, NiBA represents an operational (apparent) index of Ni partitioning with organic carbon rather than a speciation-resolved measurement distinguishing free versus complexed forms. For fractions >0.3 kDa, the retained Ni is operationally treated as organically and/or colloidally associated (including mixed organic–inorganic colloids), recognizing that only a negligible proportion, if any, of truly free aquo Ni(II) would be present in these retentates. Only the <0.3 kDa permeate can contain a meaningful proportion of free or small inorganic Ni species. This limitation is acknowledged and further discussed in Section 4.
2.6. Statistical Analysis
All statistical analyses, unless otherwise noted, were performed using S-Plus software (Version 6.2), with a significance level set at p < 0.05.
To elucidate the key factors controlling Ni binding, stepwise multiple linear regression (MLR) was used to build explanatory models. These models quantify the relationship between NiBA (the dependent variable) and the measured optical properties (A254/A204, SUVA254, FI; the independent variables). The objective was to evaluate how well these properties could account for the variance in the measured NiBA data for both DOM and AEOM. The strength of each model was assessed by the correlation coefficient (r) between model-calculated and experimentally observed NiBA values.
For direct comparisons, Student’s t-tests were used to evaluate differences in parameters (e.g., concentrations, mass fractions, NiBA) between the two main groups (AEOM and DOM). One-way analysis of variance (ANOVA) was employed for comparisons across three or more groups, such as the different molecular weight fractions. Fluorescence indicators were computed separately using R scripts (Version 2.13.2) developed by Lapworth and Kinniburgh [40].
3. Results
3.1. DOC and Ni Concentrations and Distribution in Size-Fractioned DOM/AEOM
The DOC concentration indicates the abundance of organic matter in water and sediment [25,29,41]. Table 1 lists the DOC concentrations of bulk and size-fractioned DOM and AEOM. The bulk DOM DOC concentrations ranged from 3.1 to 3.8 mg/L, similar to the low-profile DOC concentrations found in lakes and rivers [24,42], since the present wetland is part of the Longluan Wetland watershed, receiving a mix of sources including treated domestic wastewater effluent, local agricultural runoff, and precipitation. The bulk AEOM DOC concentration was 5.94 g/kg based on sediment mass, with an average alkaline extracted organic carbon to total organic carbon ratio (AEOC/TOC) of 10.3%. This ratio was higher than that of alkaline-extracted organic carbon from soil (0.12–0.40%) [30] but lower than that extracted from river sediments (20.2%) [43].

Table 1.
The measured Ni and DOC concentrations (μg/L and mg/L, respectively) and mass balances of bulk and size-fractioned DOM and AEOM.
The average mass recoveries for OC (89 ± 2% for DOM, 107 ± 9% for AEOM) and Ni (111 ± 16% for DOM, 103 ± 9% for AEOM) fell within reasonable ranges (100 ± 25%) for such fractionation procedures, ensuring procedural accuracy [25,44]. The bulk DOM concentration of Ni (9.1 ± 0.2 μg/L) was similar to background levels in nearby river waters [24], while the bulk AEOM Ni concentration was 2.73 mg/kg.
The mass distributions of OC and Ni revealed a stark contrast between the two organic matter pools (Figure 1), directly reflecting their distinct origins and biogeochemical histories. For DOM, the mass was skewed towards the LMW fractions, with the truly dissolved phase (<1 kDa) accounting for 52.9% of the OC and a striking 77.8% of the Ni mass. In stark contrast, the AEOM pool was dominated by HMW compounds, with the colloidal fraction (>1 kDa) comprising 82.0% of the OC and 70.9% of the Ni mass.
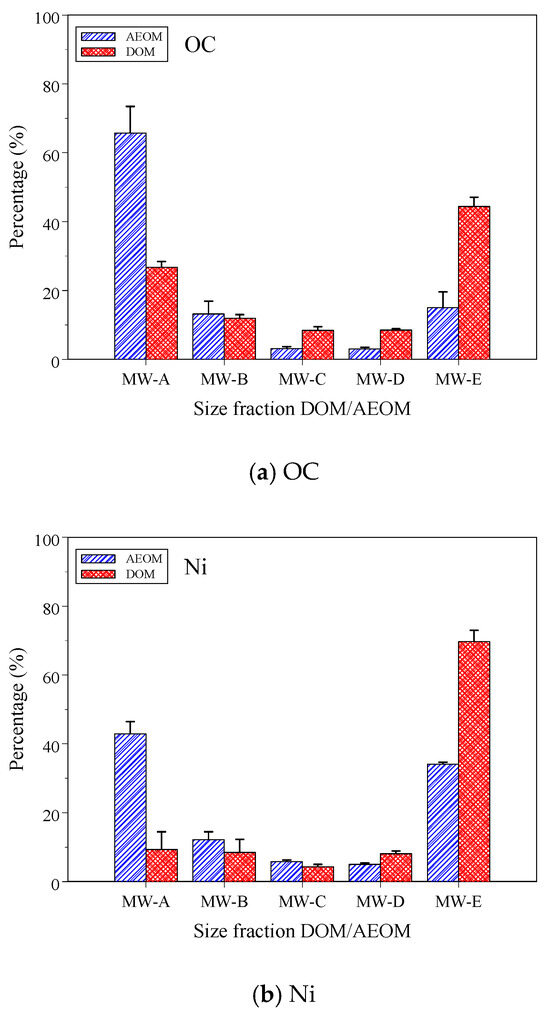
Figure 1.
The mass percentage of size-fraction DOM and AEOM (a) OC, and (b) Ni.
This contrasting distribution is explained by the fundamental roles of the water column and sediment. The sediment acts as a sink where labile, LMW compounds are preferentially decomposed, leading to the accumulation of large, structurally complex, and recalcitrant humic substances [3,45,46]. These HMW molecules, being the most abundant organic pool in the sediment, act as a vast reservoir of binding sites that sequester the majority of the Ni mass. Conversely, the water column is a dynamic system influenced by inputs of treated wastewater, in situ microbial production, and the release of LMW compounds from particulate organic matter decomposition [4,7,47]. Crucially, these microbially derived LMW compounds are not only abundant but also highly reactive, possessing a high density of potent N- and S-containing binding sites for Ni [29,48]. Therefore, the concentration of Ni mass in the LMW fraction of DOM is a result of a dual effect: this fraction is both the most abundant in mass and the most reactive, making it the primary carrier for Ni in the water column. This observation is consistent with a broader trend found in previous studies, which indicates that metals often associate with LMW DOM but preferentially bind to HMW organic matter extracted from soils and sediments [15,24,29,30,49,50].
3.2. Optical Properties of DOM and POM
Optical indicators are widely used to examine the chemical composition and structure of DOM and POM [16,20]. Figure 2a–c show the values of three selected indicators for size-fractionated DOM and AEOM.
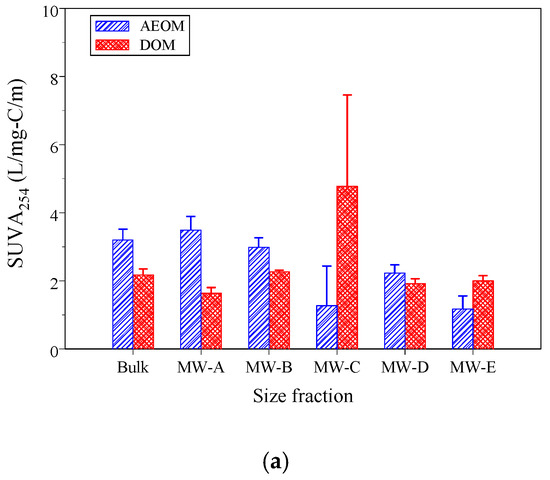
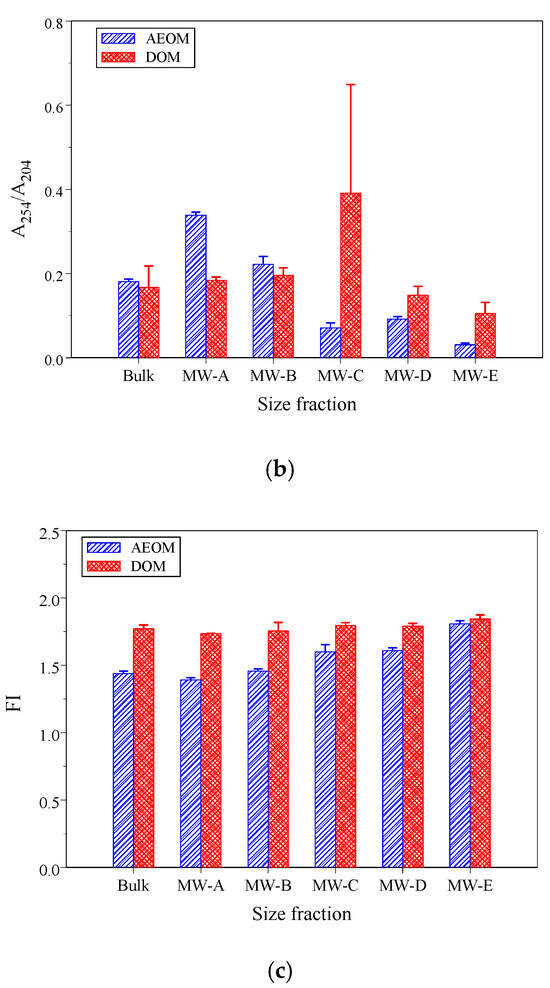
Figure 2.
The optical indicator values of the three selected indicators following size-fractioned DOM and AEOM (a) SUVA254, (b) A254/A204, and (c) FI.
The SUVA254 is a robust indicator of aromaticity [17,18]. SUVA254 values greater than 4 L/mg-C/m indicate a predominance of macromolecular, aromatic, hydrophobic compounds, while values below 3 L/mg-C/m suggest low molecular weight, hydrophilic compounds poor in aromatics [18]. A254/A204, an indicator for the ratio of hydrophobic to hydrophilic compounds, often correlates with SUVA254 [39]. Both SUVA254 and A254/A204 showed decreasing trends with decreasing molecular weight in AEOM fractions (Figure 2a,b), a dependency of optical properties on molecular size that is consistent with previous findings [27,34]. However, for DOM, these values were not significantly different across fractions, except for the much higher values in the MW-C fraction (1–3 kDa), suggesting this fraction may be enriched with humic- and fulvic-acid like matter [35].
Bulk A254/A204 values of DOM and AEOM were 0.17 ± 0.05 and 0.18 ± 0.01, respectively. The bulk SUVA254 value of AEOM was 3.20 ± 0.32 L/mg-C/m, while for DOM it was 2.17 ± 0.18 L/mg-C/m. The low SUVA254 of DOM reflects its origin from treated domestic wastewater, which typically has a higher content of microbially derived, less aromatic organic matter [39]. These SUVA254 values were comparable to sediments influenced by both allochthonous and autochthonous sources [35,42].
The FI value, a powerful tool for distinguishing DOM sources [22], differentiates between terrestrial (FI < 1.4) and microbial (FI > 1.9) origins [21,51]. The FI values (Figure 2c) of AEOM were significantly lower than those of DOM (p < 0.001), confirming that sedimentary and water-column organic matter pools possess distinct chemical characteristics [35]. This difference arises from the dynamic exchange processes between DOM and POM; the residual POM in sediment has undergone degradation, leaving it enriched in more terrestrial-like compounds (e.g., lignin phenols) compared to the water-column DOM [4]. Moreover, AEOM FI values increased as fraction size decreased, suggesting that lower molecular weight fractions had a greater microbial signature. The bulk AEOM and DOM FI values were 1.44 ± 0.02 (mixed source) and 1.77 ± 0.03 (strong microbial influence), respectively.
Table 2 shows the linear correlation coefficients of the three selected optical indicators. For AEOM, SUVA254, A254/A204, and FI showed significant correlations with each other, suggesting that the chemical properties within the size-fractionated AEOM were relatively uniform. In contrast, for DOM, only A254/A204 and SUVA254 were strongly correlated, while FI showed weak correlations with the aromaticity indicators. This suggests that the DOM, originating from complex sources and subject to various biochemical reactions, has more heterogeneous chemical characteristics, a challenge noted in studies evaluating mixed-source DOM [12].

Table 2.
Pearson correlation coefficients (r) of the selected optical indicators for size-fractionated AEOM (in bold, upper-right triangle) and DOM (in regular font, lower-left triangle).
3.3. Ni Binding Affinity to Size-Fractionated DOM and AEOM
It is crucial to consider the role of pH in the observed binding affinities. All experiments were conducted at a pH range of approximately 7.9–8.5, reflecting the natural conditions of the wetland. Within this range, the dominant free nickel species is the aquo-ion, [Ni2+], while major functional groups on the organic matter, such as carboxylic acids, are largely deprotonated and negatively charged. Therefore, the NiBA values reported herein primarily reflect the complexation between [Ni2+] and these available anionic binding sites on the DOM and AEOM fractions. The binding affinity of Ni (NiBA) to size-fractionated DOM and AEOM solutions exhibited distinct patterns (Figure 3). The DOM binding affinity for Ni was significantly higher than that of AEOM (p < 0.001). The bulk DOM showed a NiBA value of 48.3 ± 27.3 μmol/g-C, which was significantly higher than the bulk AEOM value of 10.9 ± 5.2 μmol/g-C (p = 0.03).
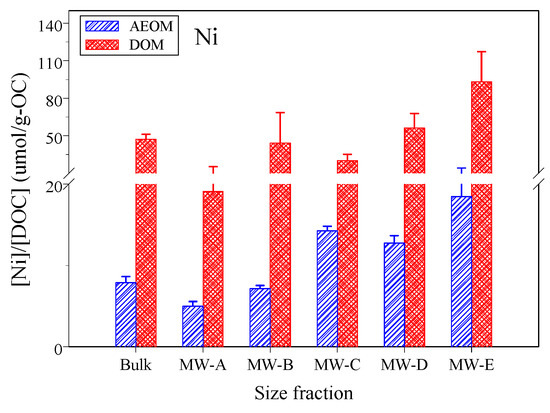
Figure 3.
The Ni binding affinity (NiBA) to size-fractioned DOM and AEOM solutions.
The DOM NiBA values were within ranges reported for natural waters (6–64 μmol/g-C) [52] but exceeded those found in other studies of natural waters (1.6–4.1 μmol/g-C) [10]. For AEOM, the bulk NiBA values exceeded previously reported soil AEOM values (5.81 ± 2.38 μmol/g-C) [30].
The binding affinity differences between DOM and AEOM can be attributed to their distinct molecular compositions and sources [4,7]. Given the wastewater influence in the study system, the DOM is expected to have different molecular characteristics compared to the more terrestrially derived AEOM from sediments; for instance, wastewater effluents are known to contribute DOM with unique molecular weight distributions that differ from those in natural waters [47]. A critical finding of this study is that this source-derived DOM exhibited a significantly higher Ni binding affinity despite having lower aromaticity (lower bulk SUVA254) and a stronger microbial signature (higher bulk FI) compared to AEOM (Section 3.2). This directly challenges the common assumption that metal binding capacity is primarily driven by aromatic, humic-like structures [10,29,53,54].
Instead, our results suggest that for Ni in this wastewater-influenced system, the binding mechanism is predominantly controlled by non-aromatic functional groups abundant in microbially derived organic matter. This observation is consistent with studies reporting that protein-like components and nitrogen- or sulfur-containing functional groups, which are prevalent in microbial DOM and wastewater effluents, are highly effective at complexing Ni2+ and other trace metals [55,56,57,58]. This counterintuitive relationship, where lower aromaticity correlates with higher binding affinity, is further substantiated by the statistical analysis in Section 3.4.
A notable finding was that the truly dissolved phase (<1 kDa) exhibited higher metal binding affinity compared to the colloidal phase (>1 kDa) for both DOM and AEOM. For DOM, the truly dissolved phase showed a NiBA of 74.7 ± 26.3 μmol/g-C versus 31.1 ± 16.9 μmol/g-C for the colloidal phase (p = 0.007). Similarly for AEOM, the truly dissolved phase showed significantly higher binding affinity (15.6 ± 4.8 μmol/g-C) compared to the colloidal phase (8.8 ± 4.2 μmol/g-C) (p = 0.02). This observation aligns with recent studies showing that LMW DOM fractions can have higher metal binding capacities [29]. This enhanced reactivity in the LMW fraction is likely attributed to a higher density of accessible, N- and S-containing functional groups per unit of carbon and reduced steric hindrance, which facilitates the formation of stable metal complexes [29,48], rather than to aromatic content.
The highest metal binding affinity was observed in the smallest size fraction (MW-E, <0.3 kDa) for both DOM (93.1 ± 24.1 μmol/g-C) and AEOM (18.5 ± 5.8 μmol/g-C), while the lowest was in the largest fraction (MW-A, 10 kDa—0.45 μm). This pattern, emphasizing the reactivity of the LMW phase, highlights the complex nature of metal partitioning in wastewater-influenced systems. Our findings are consistent with other research that also observed a significant association of Ni with LMW fractions in such systems [59].
The enhanced metal binding in the truly dissolved phase is a key finding. Smaller molecules are fundamentally more reactive due to their higher surface area-to-volume ratio and greater accessibility of binding sites [53]. The distinct binding characteristics observed between DOM and AEOM also underscore how environmental processes, such as the exchange between dissolved and particulate pools, can significantly alter the fate and transport of metals in aquatic systems [24,25].
3.4. Modeling Nickel Binding Affinity: From Simple Correlations to Multiple Regression
To identify the key factors controlling metal-DOM complexation, correlations with optical indicators were analyzed. A clear distinction emerged between the behaviors of extracted AEOM and the whole DOM. For AEOM, NiBA correlated positively with the microbial signature (FI) but, interestingly, showed negative correlations with proxies for aromaticity and molecular size (SUVA254 and A254/A204, respectively) (Table 3). In stark contrast, for the whole DOM, these relationships with aromaticity and size disappeared, leaving only the moderate positive correlation with FI (r = 0.58, n = 18, p < 0.05). This divergence strongly indicates that in the complex, unaltered water matrix, the microbial signature of DOM, rather than its aromaticity, becomes the primary predictor of Ni binding affinity.

Table 3.
The correlation coefficients of DOM/AEOM NiBA values correlated with optical indicators including A254/A204, SUVA254, and FI.
This negative correlation between NiBA and aromaticity is particularly noteworthy, as it contrasts with the general trend of enhanced metal binding with more aromatic DOM reported in many studies [10,29,53]. The discrepancy can be explained by the heterogeneous nature of DOM and metal-specific binding mechanisms [4,60]. While aromatic structures contribute to binding, other factors such as the density of carboxylic sites and the presence of nitrogen or sulfur-containing functional groups, often associated with microbial processing, can play a dominant role, especially for Ni in wastewater-influenced systems [15,59]. Given the low SUVA254 values (<3) and high FI values observed in this study, it is plausible that our system’s DOM is dominated by hydrophilic, low-aromaticity structures where these non-aromatic, heteroatomic binding sites dictate the overall metal affinity.
The insufficiency of any single optical proxy to describe NiBA for whole DOM prompted the use of a more robust approach. To quantitatively assess whether a combination of these properties could yield a predictive relationship, we developed multiple linear regression (MLR) models [61]. Using a stepwise regression process, the most influential optical indicators were identified to construct predictive equations for both AEOM and DOM (Table 4).

Table 4.
The NiBA mutiple linear regression equations of size-fractioned DOM and AEOM correlated with optical indicators.
The results of the MLR analysis were revealing. For AEOM, the model demonstrated excellent predictive power (r = 0.89, p < 0.001), reinforcing that its binding behavior is well-described by these optical proxies. More importantly, for the whole DOM, the MLR model successfully established a strong, statistically significant relationship (r = 0.70, p = 0.006), despite the weakness of the individual correlations. This success demonstrates that while no single proxy can capture the complexity of Ni binding in the unaltered water, a holistic model incorporating multiple facets of DOM character can effectively predict its behavior [62,63,64]. The strong agreement between the values predicted by these models and the actual measured NiBA values is visually confirmed in Figure 4a,b, underscoring the models’ validity.
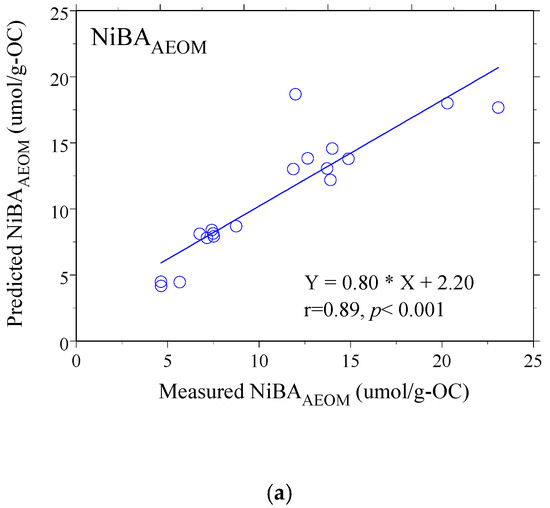
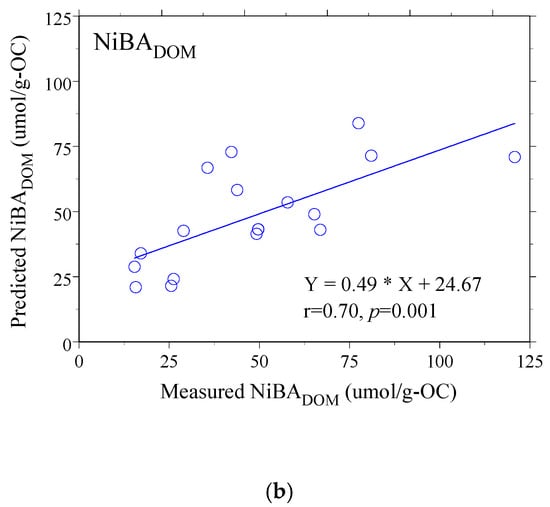
Figure 4.
The correlation between predicted and measured NiBA to size-fractioned (a) DOM and (b) AEOM solutions. The solid line in each panel indicates the linear regression fit. In the corresponding equations, Y and X denote the predicted and measured NiBA values, respectively.
4. Discussion
The strong affinity of nickel for organic matter observed in this study is rooted in its fundamental chemical properties. As a divalent transition metal ion (Ni(II)), it acts as an effective Lewis acid. According to the Hard and Soft Acids and Bases (HSAB) framework, Ni(II) is a borderline acid, enabling it to form relatively stable complexes with both “hard” oxygen-containing functional groups (e.g., carboxyl) and “softer” nitrogen- and sulfur-containing moieties. Its position in the Irving–Williams series (Mn(II) < Fe(II) < Co(II) < Ni(II) < Cu(II) > Zn(II)) further indicates that its complex stability is high, second only to Cu(II) among common divalent transition metals. This intrinsic coordination versatility explains why Ni functions as a sensitive probe for differentiating organic matter fractions and why its partitioning closely reflects the compositional attributes of DOM and AEOM.
The dynamics between dissolved organic matter (DOM) and particulate- or sediment-associated organic phases (represented here by AEOM as a proxy for particulate/sedimentary organic matter) are central to regulating heavy metal distribution and binding affinity. By examining size-fractionated organic matter from both the water column (DOM) and sediment (AEOM), this work provides finer resolution of cross-compartment exchange processes than prior bulk-only approaches [4,6,7].
A widely accepted conceptual pathway posits that biogenic DOM progressively aggregates and settles; during this vertical and early diagenetic processing, labile components are preferentially mineralized, enriching the residual material in more recalcitrant, terrestrially influenced structures [3,4,5,45,46]. Our observations are consistent with this mechanism. Spectroscopic contrasts between water-column DOM and sediment-derived AEOM are in agreement with previously documented optical differentials [35]. Higher mass fractions of HMW organic carbon and nickel in AEOM, together with lower (more terrestrially influenced) fluorescence index (FI) values, indicate selective accumulation and transformation. In contrast, the DOM pool is dominated by LMW fractions with a stronger microbial signature, reflecting divergent source contributions and processing histories. These compositional contrasts highlight the need to interpret the nickel binding index (NiBA) within its operational constraints.
The nickel binding affinity (NiBA) reported here is an operational, apparent index because it uses total Ni in each size fraction. Only the <0.3 kDa fraction can contain a non-negligible proportion of free or small inorganic Ni(II) species under the observed conditions (pH 7.9–8.5; DOC 3.6–4.3 mg/L). Consequently, the elevated NiBA in this fraction, even if potentially overestimating the intrinsic complexation capacity due to the inclusion of free Ni(II), highlights the disproportionate role of LMW components in sequestering and partitioning Ni across the molecular size continuum. This reinforces the interpretation that this fraction is a critical domain for Ni’s mobility and bioavailability. This supports the interpretation that small, functionally active organic components disproportionately influence Ni partitioning across the molecular size continuum.
Building on this clarified limitation, one of the strongest lines of evidence for the divergent behavior of DOM and AEOM arises from the predictive NiBA models (Table 4; Figure 4). For AEOM, its comparatively narrower compositional variability enabled development of a robust model (r = 0.89). The coefficient structure indicates that lower relative hydrophobicity (negative A254/A204 coefficient, where A254/A204 serves as a proxy for bulk aromatic/structural absorption balance) together with higher microbial character (positive FI) enhances Ni binding. This implies that, within the comparatively buffered sedimentary environment, mechanistic drivers of Ni association are consistent and predictable.
In contrast, modeling NiBA in the compositionally heterogeneous DOM pool required accommodating multi-parameter interactions among optical descriptors. A significant model (r = 0.70) emerged only when this complexity was retained: while higher overall aromaticity (positive SUVA254) promotes Ni binding, the effect is modulated by structural heterogeneity reflected in the large negative coefficient for A254/A204. This pattern reflects DOM’s mixed provenance (e.g., wastewater inputs, in situ production, and degradation products of particulate/sedimentary organic matter) and dynamic processing.
These results confirm that single optical indices alone are often insufficient to represent metal-binding dynamics in mixed-source DOM [6,65]. The internal controls on Ni complexation within DOM are demonstrably more intricate than in the relatively more compositionally constrained AEOM. Recognizing this fundamental difference is essential for accurate modeling of metal fate, transport, and bioavailability in aquatic systems. The findings align with site-specific studies on heavy metal partitioning in riverine environments [24,25], underscoring the value of integrating size fractionation with multi-parameter optical characterization.
5. Conclusions
This study successfully elucidated the contrasting mechanisms controlling nickel (Ni) binding to dissolved (DOM) and sediment-derived (AEOM) organic matter in a wetland ecosystem. Our findings reveal a fundamental divergence shaped by biogeochemical processing: sediment AEOM is dominated by high-molecular-weight, terrestrial-like compounds, while water-column DOM is characterized by low-molecular-weight, microbially derived substances. Critically, we discovered that the low-molecular-weight, truly dissolved fractions (<1 kDa) are the primary hotspots for Ni binding in both pools, challenging the common assumption that larger, more aromatic molecules have higher binding capacity. This distinction in chemical nature directly translated to their predictability. The relative homogeneity of AEOM permitted a highly accurate predictive model for NiBA (r = 0.89), whereas the complex nature of DOM yielded a less robust model (r = 0.70).
In summary, our research demonstrates that assessing the ecological risk of Ni requires moving beyond bulk measurements to consider the distinct roles of different organic matter pools. This implies that environmental fate models must treat DOM and AEOM as separate, non-interchangeable compartments with distinct parameters. From a practical standpoint, the optical indices used, particularly the fluorescence index, offer a rapid tool for monitoring OM quality and its potential metal reactivity, informing more accurate risk assessments. The high reactivity of the LMW fraction is a key factor for future models and warrants deeper investigation. Future work should focus on the precise molecular-level identification of these LMW ligands (e.g., using FT-ICR-MS) and expand this comparative approach to other metals and ecosystems to verify the broader applicability of these findings.
Author Contributions
Conceptualization, K.-H.Y. and T.-C.C.; methodology, K.-H.Y., H.-C.T. and W.-H.H.; formal analysis and investigation, K.-H.Y., W.-H.H. and H.-C.T.; writing—original draft preparation, review and editing, K.-H.Y., L.-F.H. and T.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available through the request to the corresponding author.
Acknowledgments
All authors gratefully acknowledge the Department of Environmental Science and Engineering at NPUST for providing access to sophisticated analytical instruments used in this research, including UV-vis spectrophotometer, fluorescence spectroscopy analyzer, graphite furnace and flame atomic absorption spectrometers for Ni analysis, as well as equipment for the separation of DOM/AEOM fractions with different molecular weights.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxic. 2012, 3, 133–164. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Burdige, D.J.; Komada, T. Sediment pore waters. In Biogeochemistry of Marine Dissolved Organic Matter, 2nd ed.; Academic Press: Burlington, VT, USA, 2015; Chapter 12; pp. 535–577. [Google Scholar]
- He, W.; Chen, M.; Schlautman, M.A.; Hur, J. Dynamic exchanges between DOM and POM pools in coastal and inland aquatic ecosystems: A review. Sci. Total Environ. 2016, 551, 415–428. [Google Scholar] [CrossRef]
- Li, X.-M.; Sun, G.-X.; Chen, S.-C.; Fang, Z.; Yuan, H.-Y.; Shi, Q.; Zhu, Y.-G. Molecular chemodiversity of dissolved organic matter in paddy soils. Environ. Sci. Technol. 2018, 52, 963–971. [Google Scholar] [CrossRef]
- Ward, N.D.; Bianchi, T.S.; Medeiros, P.M.; Seidel, M.; Richey, J.E.; Keil, R.G.; Sawakuchi, H.O. Where carbon goes when water flows: Carbon cycling across the aquatic continuum. Front. Mar. Sci. 2017, 4, 7. [Google Scholar] [CrossRef]
- Cai, S.; Wang, C.; Zhu, Q.; Lao, Q. Source, distribution, and transformation of dissolved and particulate organic matters in Qinzhou Bay, Northern Beibu Gulf. Front. Mar. Sci. 2023, 10, 1163899. [Google Scholar] [CrossRef]
- Aiken, G.R.; Hsu-Kim, H.; Ryan, J.N. Influence of dissolved organic matter on the environmental fate of metals, nanoparticles, and colloids. Environ. Sci. Technol. 2011, 45, 3196–3201. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, K.; Zhou, W.; Li, Y.; Zou, G.; Wang, Z. Occurrence, risk, and source of heavy metals in lake water columns and sediment cores in Jianghan Plain, Central China. Int. J. Environ. Res. Public Health 2023, 20, 3676. [Google Scholar] [CrossRef]
- Baken, S.; Degryse, F.; Verheyen, L.; Merckx, R.; Smolders, E. Metal complexation properties of freshwater dissolved organic matter are explained by its aromaticity and by anthropogenic ligands. Environ. Sci. Technol. 2011, 45, 2584–2590. [Google Scholar] [CrossRef] [PubMed]
- Lian, G.; Lee, X. Concentrations, distribution, and pollution assessment of metals in river sediments in China. Int. J. Environ. Res. Public Health 2021, 18, 6908. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, M.; Yu, K.; Gin, K.Y.-H.; Hassan, M.; He, Y. Heavy metals in a typical city-river-reservoir system of East China: Multi-phase distribution, microbial response and ecological risk. J. Environ. Sci. 2022, 112, 343–354. [Google Scholar] [CrossRef]
- Hoffmann, S.R.; Shafer, M.M.; Armstrong, D.E. Strong colloidal and dissolved organic ligands binding copper and zinc in rivers. Environ. Sci. Technol. 2007, 41, 6996–7002. [Google Scholar] [CrossRef] [PubMed]
- Thanh-Nho, N.; Strady, E.; Nhu-Trang, T.T.; David, F.; Marchand, C. Trace metals partitioning between particulate and dissolved phases along a tropical mangrove estuary (Can Gio, Vietnam). Chemosphere 2018, 196, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Worms, I.A.; Szigeti, Z.A.-G.; Dubascoux, S.; Lespes, G.; Traber, J.; Sigg, L.; Slaveykova, V.I. Colloidal organic matter from wastewater treatment plant effluents: Characterization and role in metal distribution. Water Res. 2010, 44, 340–350. [Google Scholar] [CrossRef]
- Matilainen, A.; Gjessing, E.T.; Lahtinen, T.; Hed, L.; Bhatnagar, A.; Sillanpää, M. An overview of the methods used in the characterisation of natural organic matter (NOM) in relation to drinking water treatment. Chemosphere 2011, 83, 1431–1442. [Google Scholar] [CrossRef]
- Korak, J.A.; McKay, G. Meta-Analysis of Optical Surrogates for the Characterization of Dissolved Organic Matter. Environ. Sci. Technol. 2024, 58, 7380–7392. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Korshin, G.V.; Li, C.-W.; Benjamin, M.M. Monitoring the properties of natural organic matter through UV spectroscopy: A consistent theory. Water Res. 1997, 31, 1787–1795. [Google Scholar] [CrossRef]
- Zeng, R.; Mannaerts, C.M.; Lievens, C. Assessment of UV-VIS spectra analysis methods for quantifying the absorption properties of chromophoric dissolved organic matter (CDOM). Front. Environ. Sci. 2023, 11, 1152536. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Fellman, J.B.; Spencer, R.G.; Hernes, P.J.; Edwards, R.T.; D’Amore, D.V.; Hood, E. The impact of glacier runoff on the biodegradability and biochemical composition of terrigenous dissolved organic matter in near-shore marine ecosystems. Mar. Chem. 2010, 121, 112–122. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.-M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Chuang, C.-W.; Hsu, L.-F.; Tsai, H.-C.; Liu, Y.-Y.; Huang, W.-S.; Chen, T.-C. Nickel Binding Affinity with Size-Fractioned Sediment Dissolved and Particulate Organic Matter and Correlation with Optical Indicators. Appl. Sci. 2020, 10, 8995. [Google Scholar] [CrossRef]
- Hung, M.-Y.; Huang, W.-H.; Tsai, H.-C.; Hsieh, C.-Y.; Chen, T.-C. Copper distribution and binding affinity to size-fractioned dissolved and particulate organic matter in river sediment. Environments 2024, 11, 129. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, X.; Wang, Y.; Ji, J.; Wang, D.; Hou, Q.; Yu, T. Dissolved and particulate partitioning of trace elements and their spatial–temporal distribution in the Changjiang River. J. Geochem. Explor. 2014, 145, 114–123. [Google Scholar] [CrossRef]
- Korak, J.A.; McKay, G. Critical review of fluorescence and absorbance measurements as surrogates for the molecular weight and aromaticity of dissolved organic matter. Environ. Sci. Process. Impacts 2024, 26, 1663–1702. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, J.H.; Kang, S.Y.; Kim, S.Y. Hydroclimatic controls on dissolved organic matter (DOM) characteristics and implications for trace metal transport in Hwangryong River Watershed, Korea, during a summer monsoon period. Hydrol. Process. 2007, 21, 3025–3034. [Google Scholar] [CrossRef]
- Xu, H.; Zou, L.; Guan, D.; Li, W.; Jiang, H. Molecular weight-dependent spectral and metal binding properties of sediment dissolved organic matter from different origins. Sci. Total Environ. 2019, 665, 828–835. [Google Scholar] [CrossRef]
- Hsieh, S.-H.; Chiu, T.-P.; Huang, W.-S.; Chen, T.-C.; Yeh, Y.-L. Cadmium (Cd) and Nickel (Ni) Distribution on Size-Fractioned Soil Humic Substance (SHS). Int. J. Environ. Res. Public Health 2019, 16, 3398. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Choi, I.; Lee, J.-J.; Hur, J. Coupling effects of abiotic and biotic factors on molecular composition of dissolved organic matter in a freshwater wetland. Sci. Total Environ. 2016, 544, 525–534. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Saravanan, P.; Saravanan, V.; Rajeshkannan, R.; Arnica, G.; Rajasimman, M.; Baskar, G.; Pugazhendhi, A. Comprehensive review on toxic heavy metals in the aquatic system: Sources, identification, treatment strategies, and health risk assessment. Environ. Res. 2024, 258, 119440. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-Y.; Zhou, T.-H.; Du, Y.; Ye, B.; Wang, W.-L.; Hu, H.-Y. Characterizing the molecular weight distribution of dissolved organic matter by measuring the contents of electron-donating moieties, UV absorbance, and fluorescence intensity. Environ. Int. 2020, 137, 105570. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Jung, H.; Lee, J.-H.; Hur, J. Differences in spectroscopic characteristics between dissolved and particulate organic matters in sediments: Insight into distribution behavior of sediment organic matter. Sci. Total Environ. 2016, 547, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.-S.; Huang, W.-S.; Hsu, L.-F.; Yeh, Y.-L.; Chen, T.-C. Fluorescence of Size-Fractioned Humic Substance Extracted from Sediment and Its Effect on the Sorption of Phenanthrene. Int. J. Environ. Res. Public Health 2019, 16, 5087. [Google Scholar] [CrossRef]
- Dabrin, A.; Roulier, J.-L.; Coquery, M. Colloidal and truly dissolved metal (oid) fractionation in sediment pore waters using tangential flow filtration. Appl. Geochem. 2013, 31, 25–34. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Hudson, N.; Baker, A.; Reynolds, D. Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters—A review. River Res. Appl. 2007, 23, 631–649. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Kinniburgh, D. An R script for visualising and analysing fluorescence excitation–emission matrices (EEMs). Comput. Geosci. 2009, 35, 2160–2163. [Google Scholar] [CrossRef]
- Chen, M.; Hur, J. Pre-treatments, characteristics, and biogeochemical dynamics of dissolved organic matter in sediments: A review. Water Res. 2015, 79, 10–25. [Google Scholar] [CrossRef]
- Hung, M.-Y.; Huang, W.-H.; Hsu, L.-F.; Hsieh, C.-Y.; Chen, T.-C. Investigation of the Distribution and Binding Affinity of Copper to Size-Fractioned Dissolved Organic Matter (DOM) in a Constructed Wetland. Separations 2024, 11, 191. [Google Scholar] [CrossRef]
- Huang, W.-H.; Lin, T.-C.; Huang, C.-M.; Chen, T.-C.; Yeh, Y.-L. Copper distribution and binding affinity of size-fractioned humic substances taken from paddy soil and correlation with optical characteristics. Agronomy 2022, 12, 1689. [Google Scholar] [CrossRef]
- Ilina, S.M.; Lapitskiy, S.A.; Alekhin, Y.V.; Viers, J.; Benedetti, M.; Pokrovsky, O.S. Speciation, size fractionation and transport of trace elements in the continuum soil water–mire–humic lake–river–large oligotrophic lake of a Subarctic watershed. Aquat. Geochem. 2016, 22, 65–95. [Google Scholar] [CrossRef]
- Fettweis, M.; Schartau, M.; Desmit, X.; Lee, B.J.; Terseleer, N.; Van der Zande, D.; Parmentier, K.; Riethmüller, R. Organic matter composition of biomineral flocs and its influence on suspended particulate matter dynamics along a nearshore to offshore transect. J. Geophys. Res. Biogeosci. 2022, 127, e2021JG006332. [Google Scholar] [CrossRef]
- Weiwei, L.; Xin, Y.; Keqiang, S.; Baohua, Z.; Guang, G. Unraveling the sources and fluorescence compositions of dissolved and particulate organic matter (DOM and POM) in Lake Taihu, China. Environ. Sci. Pollut. Res. 2019, 26, 4027–4040. [Google Scholar] [CrossRef]
- Chon, K.; Chon, K.; Cho, J. Characterization of size fractionated dissolved organic matter from river water and wastewater effluent using preparative high performance size exclusion chromatography. Org. Geochem. 2017, 103, 105–112. [Google Scholar] [CrossRef]
- Pontoni, L.; Van Hullebusch, E.D.; Pechaud, Y.; Fabbricino, M.; Esposito, G.; Pirozzi, F. Colloidal mobilization and fate of trace heavy metals in semi-saturated artificial soil (OECD) irrigated with treated wastewater. Sustainability 2016, 8, 1257. [Google Scholar] [CrossRef]
- Wu, F.; Tanoue, E. Molecular mass distribution and fluorescence characteristics of dissolved organic ligands for copper (II) in Lake Biwa, Japan. Org. Geochem. 2001, 32, 11–20. [Google Scholar] [CrossRef]
- Luan, H.; Vadas, T.M. Size characterization of dissolved metals and organic matter in source waters to streams in developed landscapes. Environ. Pollut. 2015, 197, 76–83. [Google Scholar] [CrossRef]
- Henderson, R.K.; Baker, A.; Murphy, K.; Hambly, A.; Stuetz, R.; Khan, S. Fluorescence as a potential monitoring tool for recycled water systems: A review. Water Res. 2009, 43, 863–881. [Google Scholar] [CrossRef]
- Wang, W.; Chen, M.; Guo, L.; Wang, W.-X. Size partitioning and mixing behavior of trace metals and dissolved organic matter in a South China estuary. Sci. Total Environ. 2017, 603, 434–444. [Google Scholar] [CrossRef]
- Martin, J.M.; Dai, M.H.; Cauwet, G. Significance of colloids in the biogeochemical cycling of organic carbon and trace metals in the Venice Lagoon (Italy). Limnol. Oceanogr. 1995, 40, 119–131. [Google Scholar] [CrossRef]
- Kikuchi, T.; Fujii, M.; Terao, K.; Jiwei, R.; Lee, Y.P.; Yoshimura, C. Correlations between aromaticity of dissolved organic matter and trace metal concentrations in natural and effluent waters: A case study in the Sagami River Basin, Japan. Sci. Total Environ. 2017, 576, 36–45. [Google Scholar] [CrossRef]
- Jackson, B.P.; Ranville, J.F.; Bertsch, P.M.; Sowder, A.G. Characterization of colloidal and humic-bound Ni and U in the “dissolved” fraction of contaminated sediment extracts. Environ. Sci. Technol. 2005, 39, 2478–2485. [Google Scholar] [CrossRef]
- Guo, X.-J.; He, X.-S.; Li, C.-W.; Li, N.-X. The binding properties of copper and lead onto compost-derived DOM using Fourier-transform infrared, UV–vis and fluorescence spectra combined with two-dimensional correlation analysis. J. Hazard. Mater. 2019, 365, 457–466. [Google Scholar] [CrossRef]
- Wei, D.; Li, M.; Wang, X.; Han, F.; Li, L.; Guo, J.; Ai, L.; Fang, L.; Liu, L.; Du, B. Extracellular polymeric substances for Zn (II) binding during its sorption process onto aerobic granular sludge. J. Hazard. Mater. 2016, 301, 407–415. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Shao, L.-M.; He, P.-J. Fluorescent characteristics and metal binding properties of individual molecular weight fractions in municipal solid waste leachate. Environ. Pollut. 2012, 162, 63–71. [Google Scholar] [CrossRef]
- Li, R.; Yue, D.; Liu, J.; Nie, Y. Size fractionation of organic matter and heavy metals in raw and treated leachate. Waste Manag. 2009, 29, 2527–2533. [Google Scholar] [CrossRef]
- Huang, X.; Liang, Y.; Ye, Q.; Ding, Z.; Liu, F.; Shi, Z. Theoretical modeling of molecular fractionation of dissolved organic matter on ferrihydrite and its impact on proton and metal binding properties. Sci. Total Environ. 2023, 888, 164276. [Google Scholar] [CrossRef]
- Brix, K.V.; DeForest, D.K.; Tear, L.; Grosell, M.; Adams, W.J. Use of multiple linear regression models for setting water quality criteria for copper: A complementary approach to the biotic ligand model. Environ. Sci. Technol. 2017, 51, 5182–5192. [Google Scholar] [CrossRef]
- Brix, K.V.; Tear, L.; Santore, R.C.; Croteau, K.; DeForest, D.K. Comparative performance of multiple linear regression and biotic ligand models for estimating the bioavailability of copper in freshwater. Environ. Toxicol. Chem. 2021, 40, 1649–1661. [Google Scholar] [CrossRef] [PubMed]
- DeForest, D.K.; Ryan, A.C.; Tear, L.M.; Brix, K.V. Comparison of multiple linear regression and biotic ligand models for predicting acute and chronic zinc toxicity to freshwater organisms. Environ. Toxicol. Chem. 2023, 42, 393–413. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, Z.; Zhao, Y.; Luo, Y.; He, S. Multivariate Linear Regression Models to Predict Monomer Poisoning Effect in Ethylene/Polar Monomer Copolymerization Catalyzed by Late Transition Metals. Inorganics 2022, 10, 26. [Google Scholar] [CrossRef]
- McCallister, S.L.; Bauer, J.E.; Ducklow, H.W.; Canuel, E.A. Sources of estuarine dissolved and particulate organic matter: A multi-tracer approach. Org. Geochem. 2006, 37, 454–468. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).