Toxicological Effects of Poly(methyl methacrylate) Microplastics in Caenorhabditis elegans: Impairment of Development, Reproduction, and Stress Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Microplastic Suspension Preparation and C. elegans Growth Conditions
2.2. Lifespan and Healthspan Analysis
2.3. Body Length Measurement
2.4. Intestinal Permeability Assay Using Brilliant Blue FCF Staining
2.5. Fertility Assay
2.6. Measurement of Cytosolic and Mitochondrial Reactive Oxygen Species (ROS)
2.7. Quantitative Real Time PCR
2.8. Fluorescence Analysis of Transgenic Strains
2.9. FT-IR Microanalysis
2.10. Statistical Analysis
3. Results
3.1. MPs Affect Larval Development and Lifespan
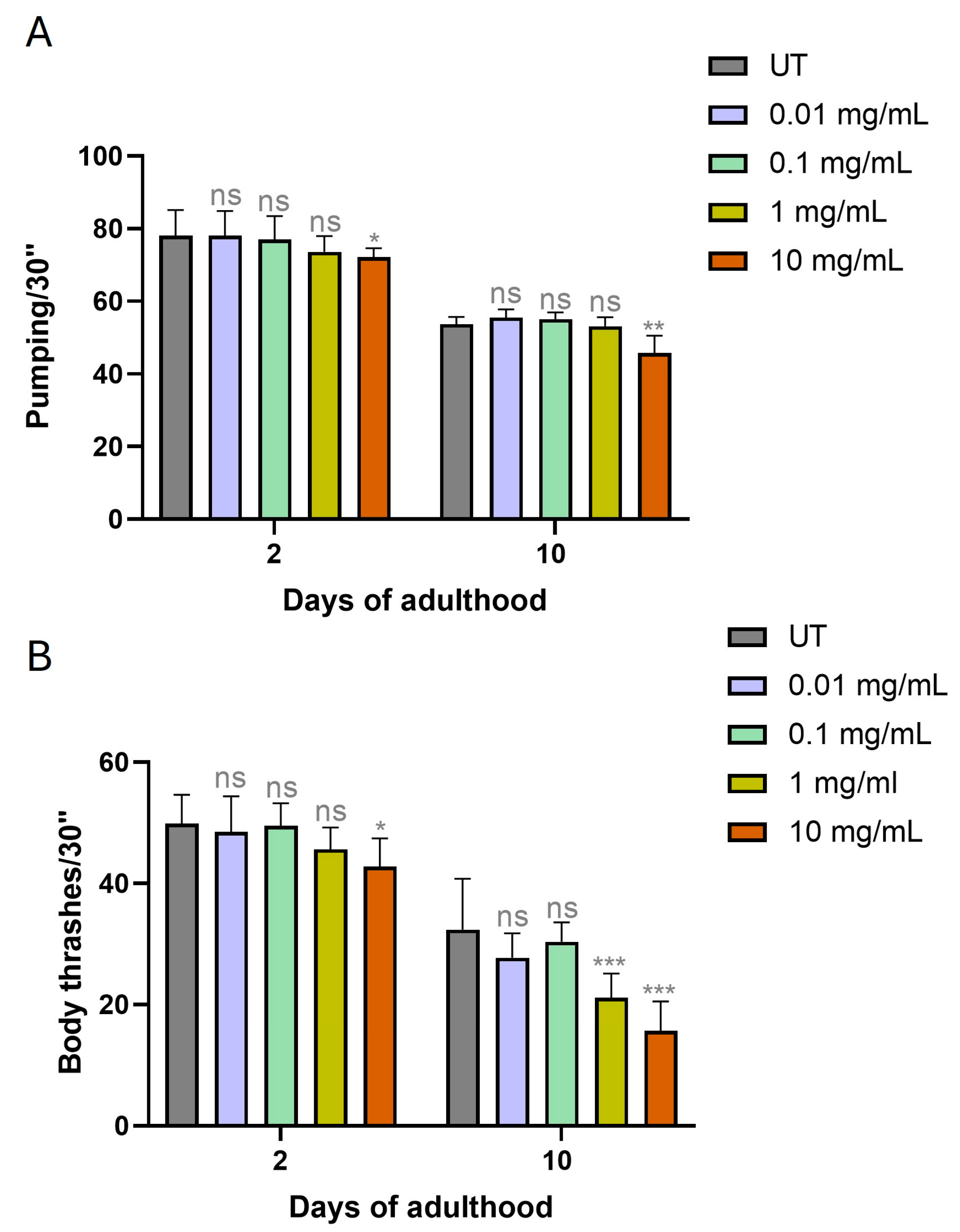
3.2. MP Supplementation Negatively Impacts Aging Markers
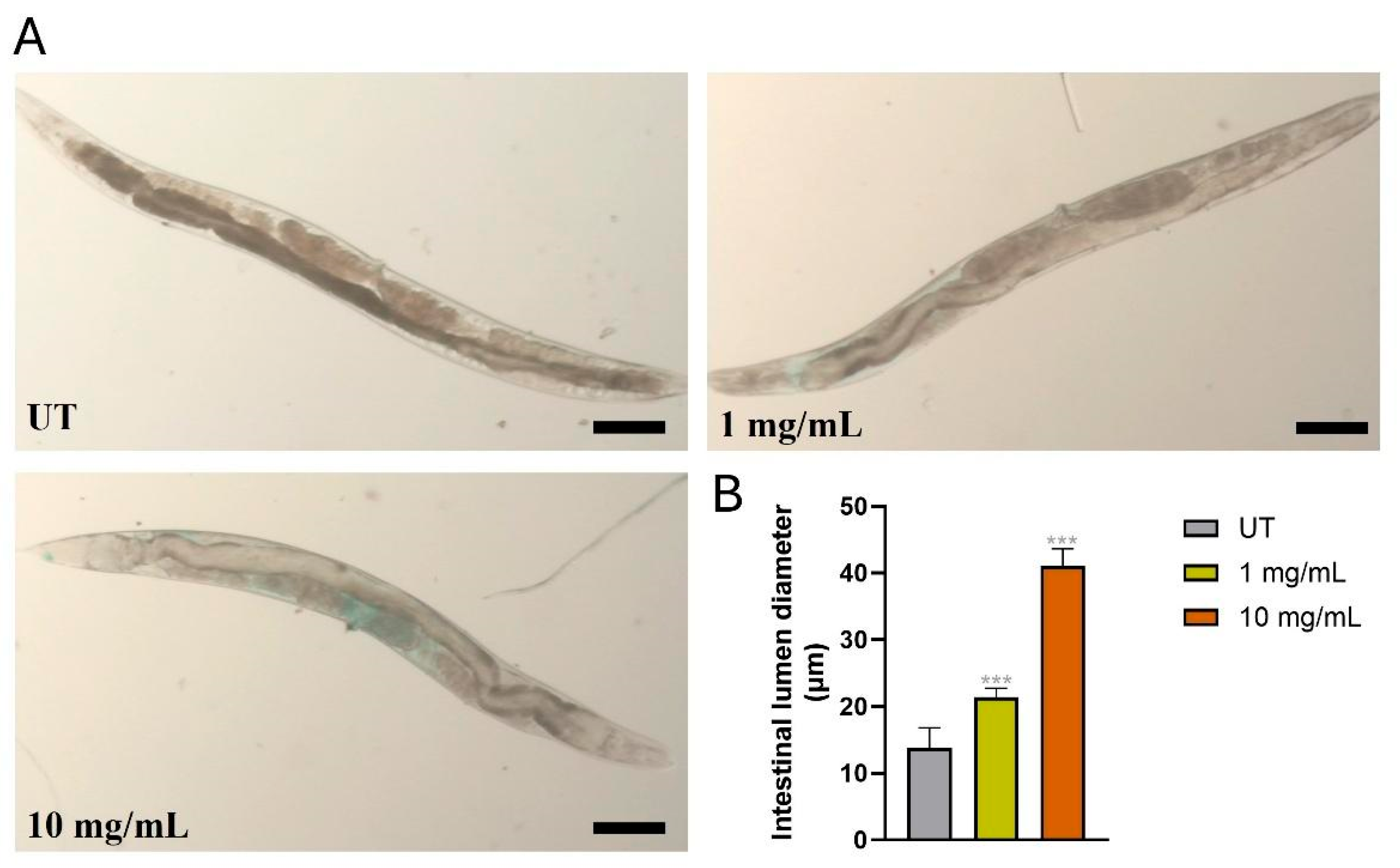
3.3. MPs Desegregate Intestinal Integrity in C. elegans
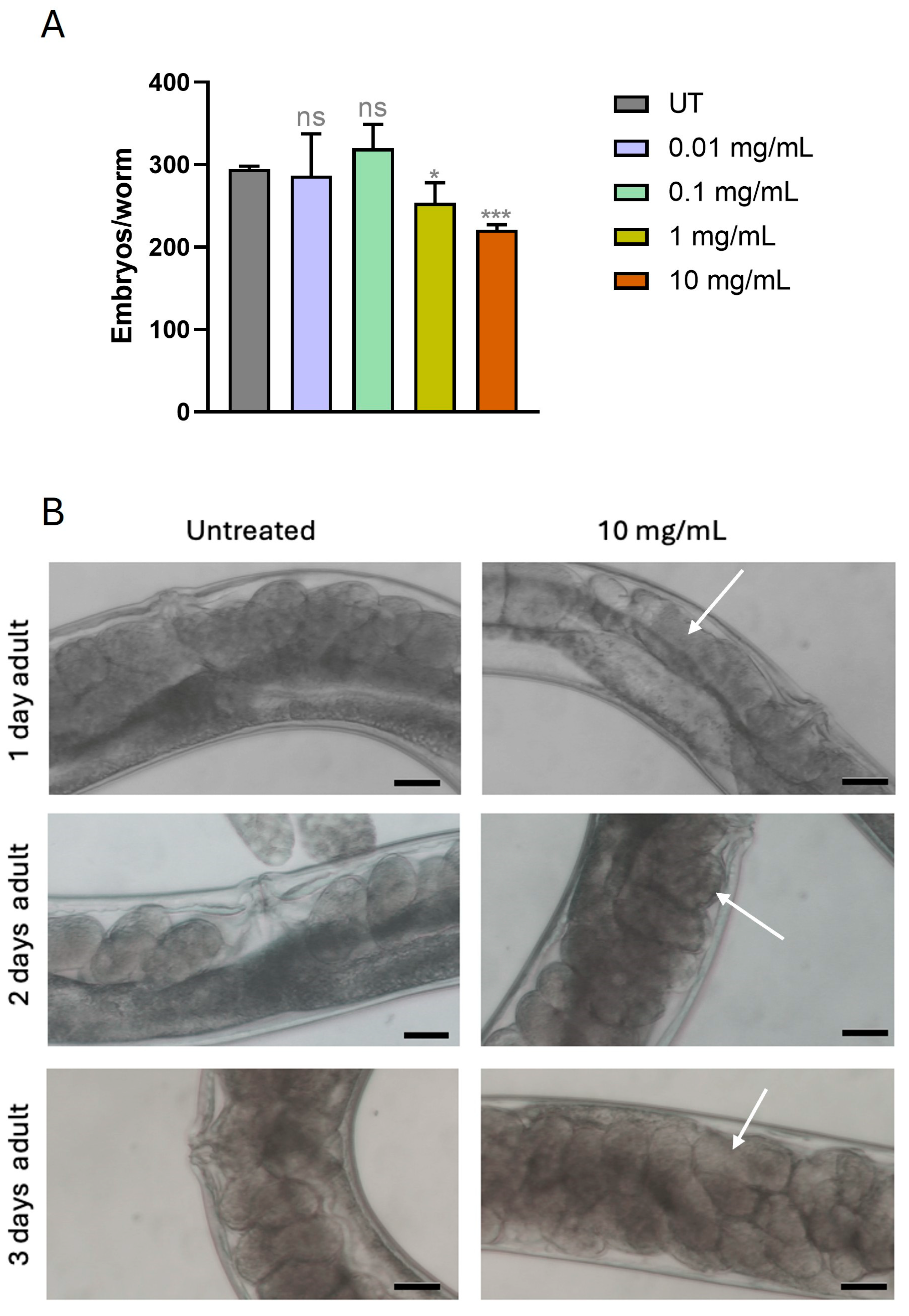
3.4. MPs Altered Fertility Rate and Embryos Formation
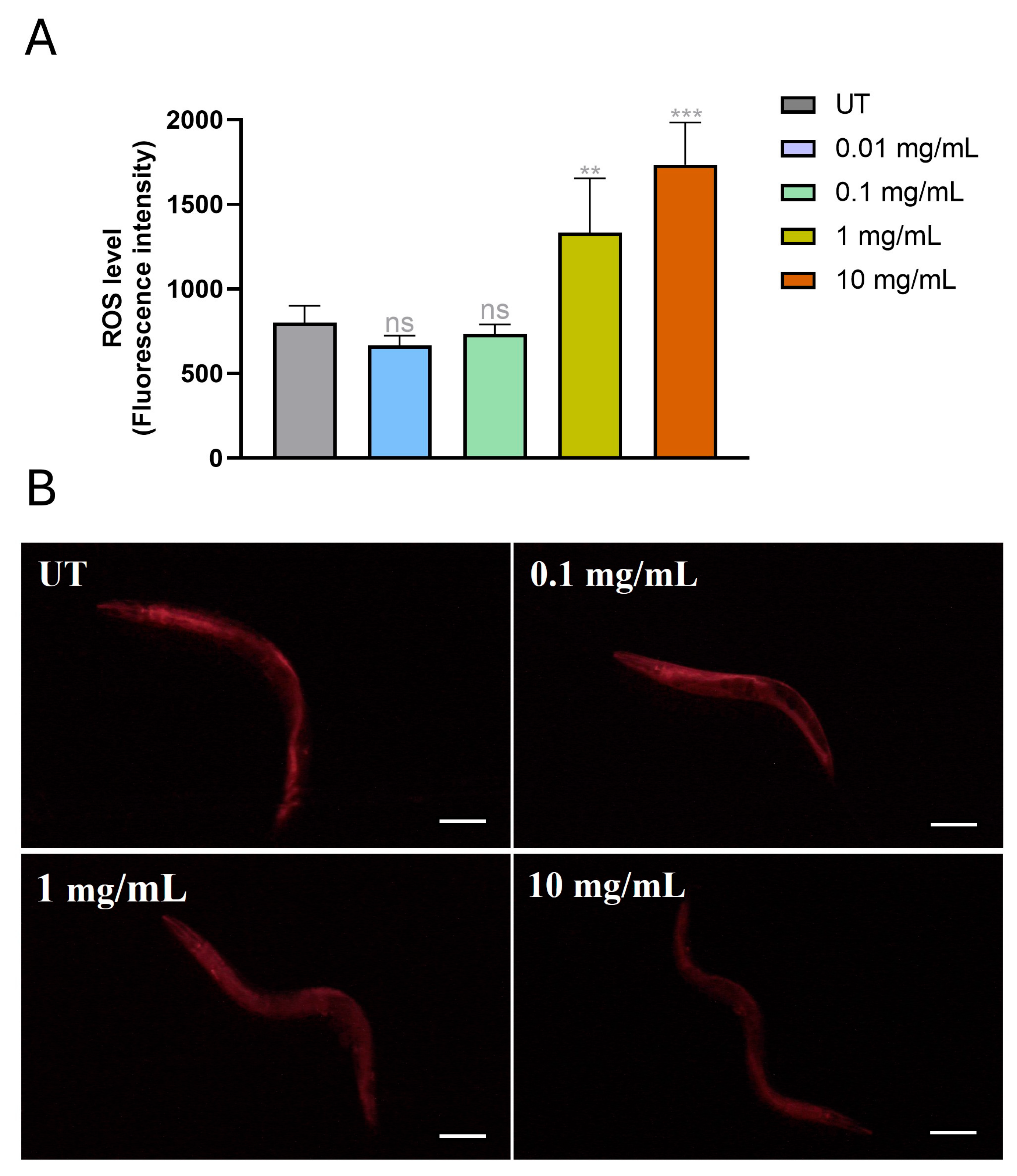
3.5. MP Supplementation Increase Reactive Oxygen Species (ROS) Production
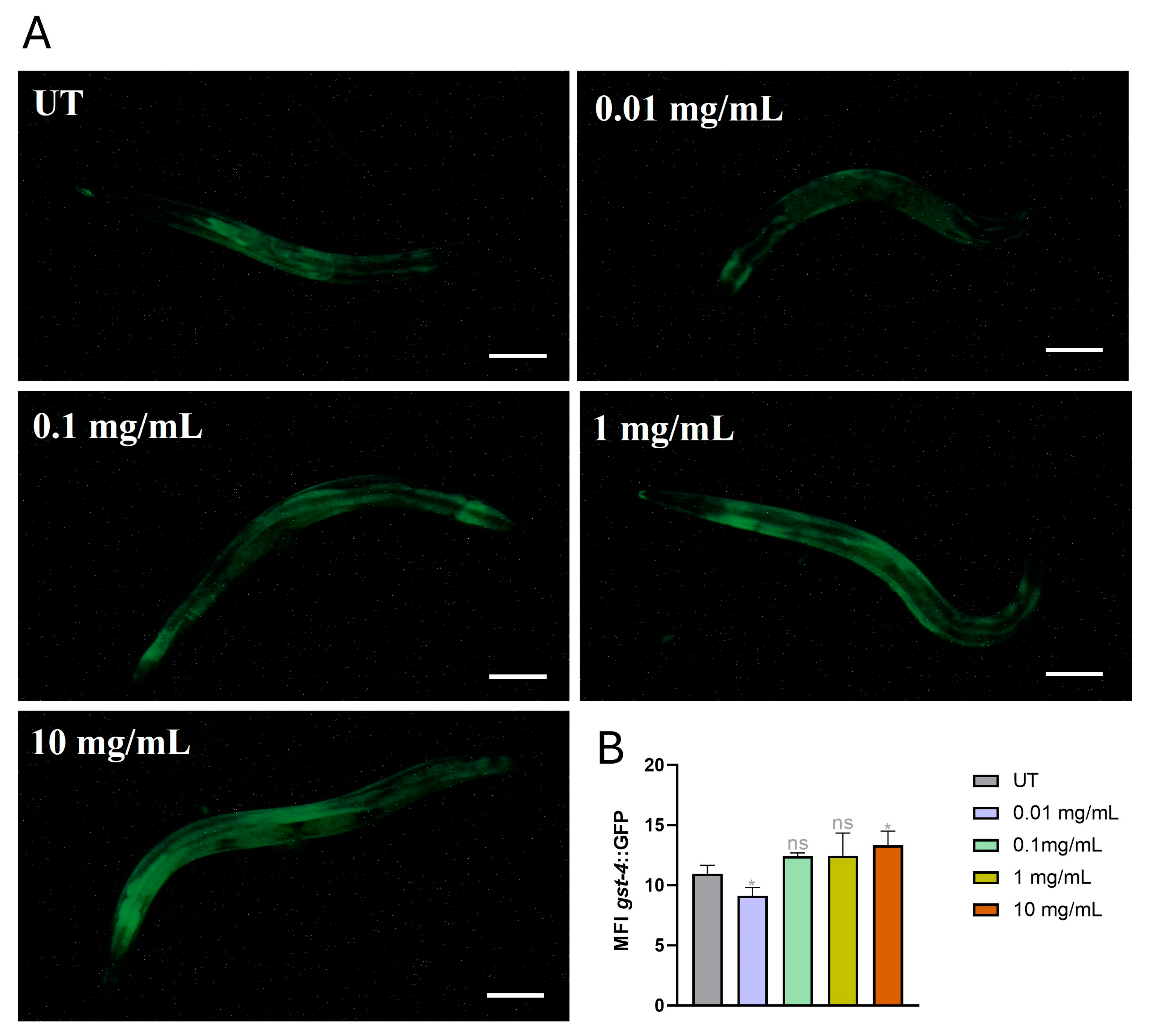
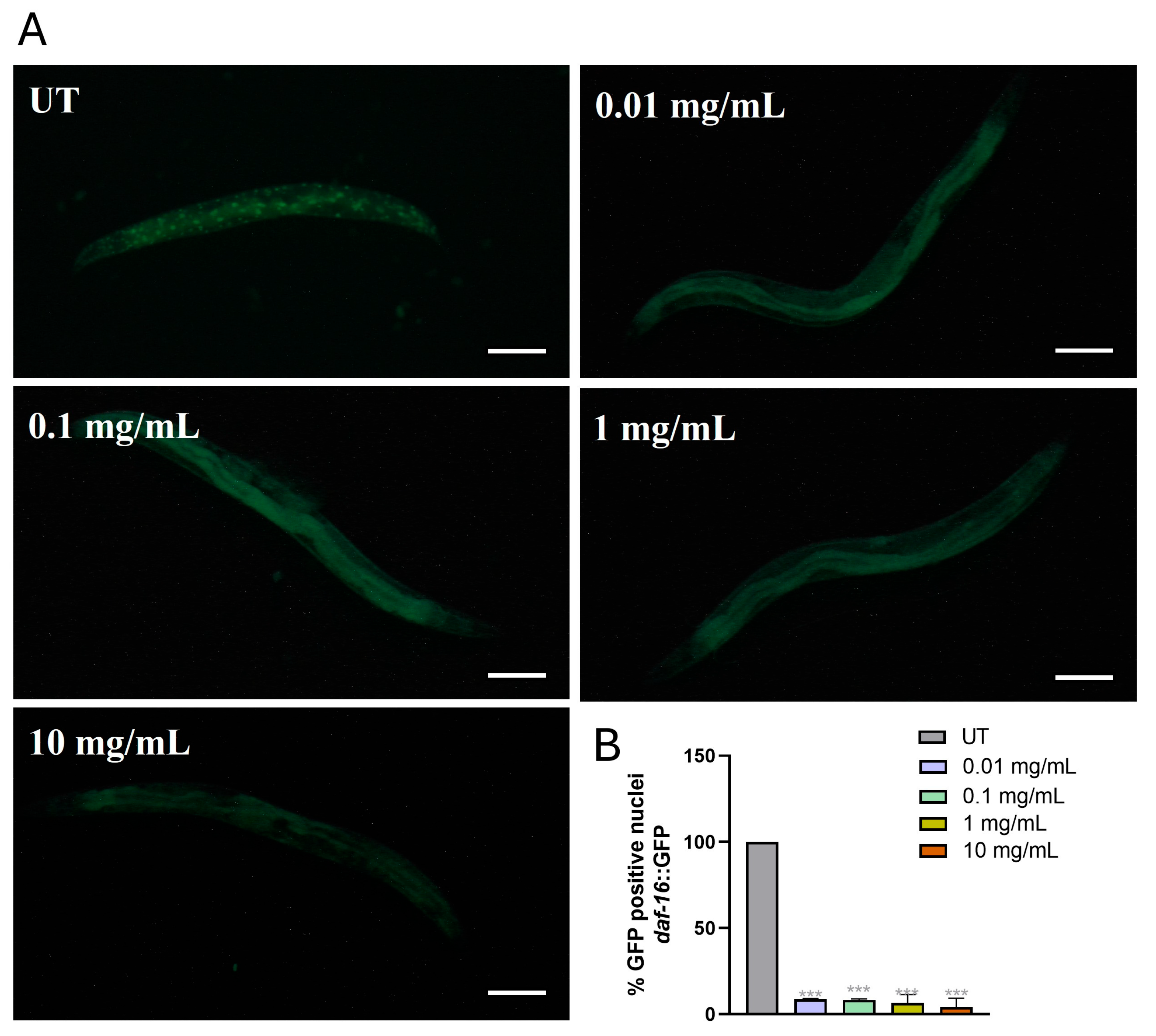
3.6. Activation of Oxidative Stress Machinery After MP Supplementation
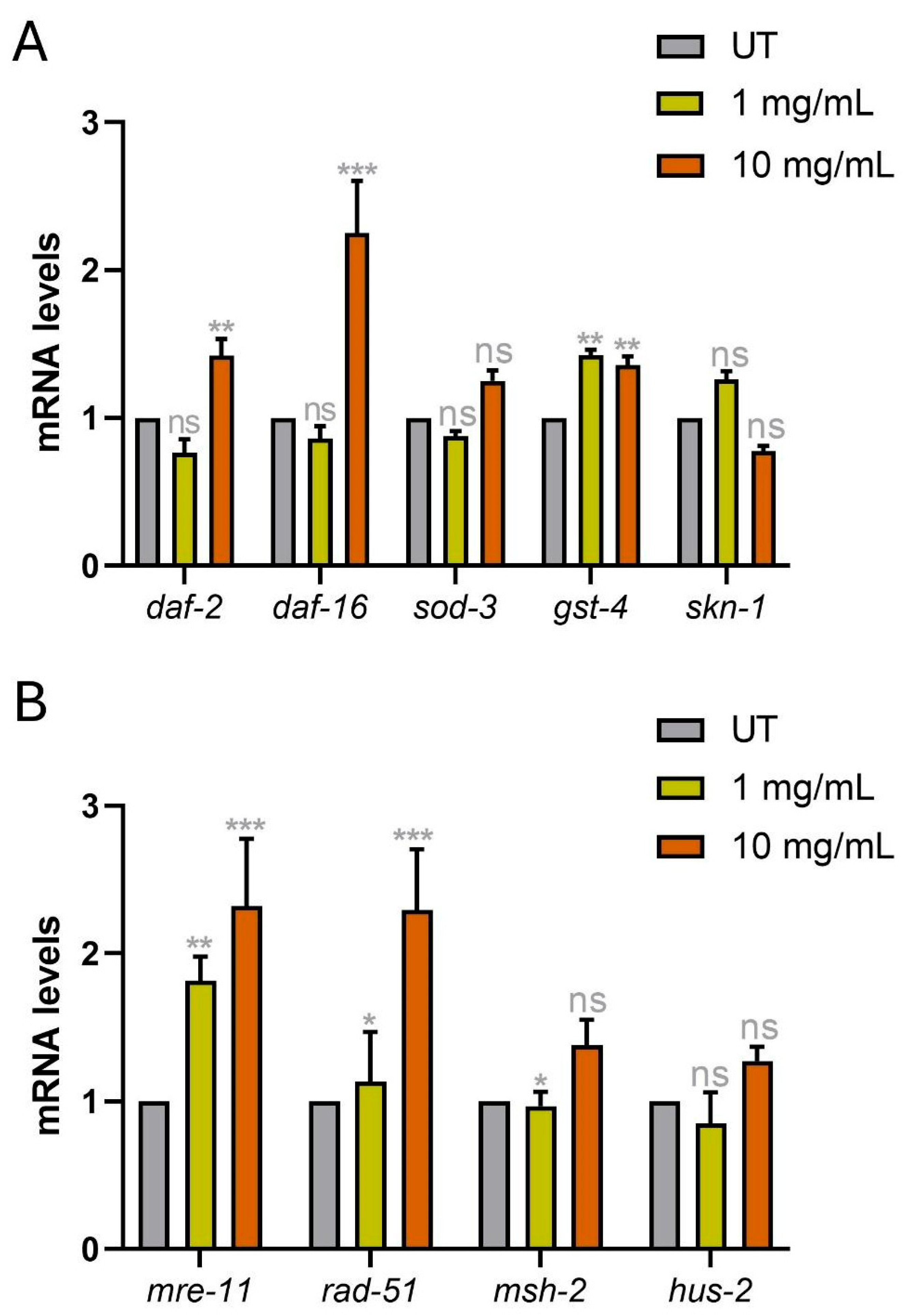
3.7. Gene Expression Levels of Oxidative Stress-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thompson, R.C.; Courtene-Jones, W.; Boucher, J.; Pahl, S.; Raubenheimer, K.; Koelmans, A.A. Twenty Years of Microplastic Pollution Research—What Have We Learned? Science 2024, 386, eadl2746. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Zhou, N.; Chen, Y.; Ling, Z.; Xiang, P. Microplastics in the Human Body: A Comprehensive Review of Exposure, Distribution, Migration Mechanisms, and Toxicity. Sci. Total Environ. 2024, 946, 174215. [Google Scholar] [CrossRef]
- Pannetier, P.; Morin, B.; Le Bihanic, F.; Dubreil, L.; Clérandeau, C.; Chouvellon, F.; Van Arkel, K.; Danion, M.; Cachot, J. Environmental Samples of Microplastics Induce Significant Toxic Effects in Fish Larvae. Environ. Int. 2020, 134, 105047. [Google Scholar] [CrossRef] [PubMed]
- De Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an Emerging Threat to Terrestrial Ecosystems. Glob. Change Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss Floodplain Soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef]
- Fuller, S.; Gautam, A. A Procedure for Measuring Microplastics Using Pressurized Fluid Extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef]
- Hoang, V.-H.; Nguyen, M.-K.; Hoang, T.-D.; Ha, M.C.; Huyen, N.T.T.; Bui, V.K.H.; Pham, M.-T.; Nguyen, C.-M.; Chang, S.W.; Nguyen, D.D. Sources, Environmental Fate, and Impacts of Microplastic Contamination in Agricultural Soils: A Comprehensive Review. Sci. Total Environ. 2024, 950, 175276. [Google Scholar] [CrossRef]
- Adomako, M.O.; Wu, J.; Yu, F.-H. Ecological and Evolutionary Responses of Earthworm Holobionts to Environmental Changes. ISME J. 2025, 19, wraf044. [Google Scholar] [CrossRef]
- Burcea, A.; Bănățeanu, A.-M.; Poalelungi, C.-V.; Burcea, A.; Forna, N.; Cumpătă, C.N. Enhanced Properties and Multifaceted Applications of Polymethyl Methacrylate (PMMA) in Modern Medicine and Dentistry. RJOR 2024, 16, 108–123. [Google Scholar] [CrossRef]
- Edo, G.I.; Ndudi, W.; Ali, A.B.M.; Yousif, E.; Zainulabdeen, K.; Onyibe, P.N.; Akpoghelie, P.O.; Ekokotu, H.A.; Isoje, E.F.; Igbuku, U.A.; et al. An Updated Review on the Modifications, Recycling, Polymerization, and Applications of Polymethyl Methacrylate (PMMA). J. Mater. Sci. 2024, 59, 20496–20539. [Google Scholar] [CrossRef]
- Neves, B.; Oliveira, M.; Frazão, C.; Almeida, M.; Pinto, R.J.B.; Figueira, E.; Pires, A. The Role of Life Stages in the Sensitivity of Hediste Diversicolor to Nanoplastics: A Case Study with Poly(Methyl)Methacrylate (PMMA). Toxics 2024, 12, 352. [Google Scholar] [CrossRef]
- Yip, Y.J.; Sivananthan, G.D.; Lee, S.S.C.; Neo, M.L.; Teo, S.L.-M.; Valiyaveettil, S. Transfer of Poly(Methyl Methacrylate) Nanoparticles from Parents to Offspring and the Protection Mechanism in Two Marine Invertebrates. ACS Sustain. Chem. Eng. 2022, 10, 37–49. [Google Scholar] [CrossRef]
- Maupas, E. La mue et l’enkystement chez les Nématodes. Arch. Zool. Exper. Gén. Ser. 1899, 3, 7. [Google Scholar]
- Steinberg, C.E.W.; Stürzenbaum, S.R.; Menzel, R. Genes and Environment—Striking the Fine Balance between Sophisticated Biomonitoring and True Functional Environmental Genomics. Sci. Total Environ. 2008, 400, 142–161. [Google Scholar] [CrossRef] [PubMed]
- Polak, N.; Read, D.S.; Jurkschat, K.; Matzke, M.; Kelly, F.J.; Spurgeon, D.J.; Stürzenbaum, S.R. Metalloproteins and Phytochelatin Synthase May Confer Protection against Zinc Oxide Nanoparticle Induced Toxicity in Caenorhabditis Elegans. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 160, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Ficociello, G.; Gerardi, V.; Uccelletti, D.; Setini, A. Molecular and Cellular Responses to Short Exposure to Bisphenols A, F, and S and Eluates of Microplastics in C. Elegans. Environ. Sci. Pollut. Res. 2021, 28, 805–818. [Google Scholar] [CrossRef]
- Jewett, E.; Arnott, G.; Connolly, L.; Vasudevan, N.; Kevei, E. Microplastics and Their Impact on Reproduction—Can We Learn From the C. Elegans Model? Front. Toxicol. 2022, 4, 748912. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, M.; Gu, Y.; Jiang, Y.; Ding, P.; Wang, C.; Pan, R.; Shi, C.; Li, H. Microbial Colonization of Microplastics in Wastewater Accelerates the Aging Process Associated with Oxidative Stress and the Insulin/IGF1 Signaling Pathway. Environ. Pollut. 2023, 332, 121954. [Google Scholar] [CrossRef] [PubMed]
- Schifano, E.; Cicalini, I.; Pieragostino, D.; Heipieper, H.J.; Del Boccio, P.; Uccelletti, D. In Vitro and in Vivo Lipidomics as a Tool for Probiotics Evaluation. Appl. Microbiol. Biotechnol. 2020, 104, 8937–8948. [Google Scholar] [CrossRef] [PubMed]
- Jeayeng, S.; Thongsroy, J.; Chuaijit, S. Caenorhabditis Elegans as a Model to Study Aging and Photoaging. Biomolecules 2024, 14, 1235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, W. Automated Recognition and Analysis of Body Bending Behavior in C. Elegans. BMC Bioinform. 2023, 24, 175. [Google Scholar] [CrossRef] [PubMed]
- Armeli, F.; Mengoni, B.; Schifano, E.; Lenz, T.; Archer, T.; Uccelletti, D.; Businaro, R. The Probiotic Yeast, Milmed, Promotes Autophagy and Antioxidant Pathways in BV-2 Microglia Cells and C. Elegans. Antioxidants 2025, 14, 393. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Ośko, J.; Knez, E.; Grembecka, M. Microplastics and Oxidative Stress—Current Problems and Prospects. Antioxidants 2024, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Dubey, K.A.; Bhardwaj, Y.K.; Jain, D.; Choudhury, S.; Tyagi, A.K. UV-Shielding Transparent PMMA/In2O3 Nanocomposite Films Based on In2O3 Nanoparticles. RSC Adv. 2013, 3, 20913. [Google Scholar] [CrossRef]
- Bouyanfif, A.; Liyanage, S.; Hequet, E.; Moustaid-Moussa, N.; Abidi, N. Review of FTIR Microspectroscopy Applications to Investigate Biochemical Changes in C. Elegans. Vib. Spectrosc. 2018, 96, 74–82. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Heidari, S.; Sepahvand, P.; Afkhami, H.; Kheradjoo, H. Microplastics and Environmental Effects: Investigating the Effects of Microplastics on Aquatic Habitats and Their Impact on Human Health. Front. Public Health 2024, 12, 1411389. [Google Scholar] [CrossRef]
- Liu, P.; Zhan, X.; Wu, X.; Li, J.; Wang, H.; Gao, S. Effect of Weathering on Environmental Behavior of Microplastics: Properties, Sorption and Potential Risks. Chemosphere 2020, 242, 125193. [Google Scholar] [CrossRef]
- Hunt, P.R. The C. elegans Model in Toxicity Testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Q.; Li, Y.; Wang, D. Translocation, Transfer, and in Vivo Safety Evaluation of Engineered Nanomaterials in the Non-Mammalian Alternative Toxicity Assay Model of Nematode Caenorhabditis Elegans. RSC Adv. 2013, 3, 5741. [Google Scholar] [CrossRef]
- Diomede, L.; Cassata, G.; Fiordaliso, F.; Salio, M.; Ami, D.; Natalello, A.; Doglia, S.M.; De Luigi, A.; Salmona, M. Tetracycline and Its Analogues Protect Caenorhabditis Elegans from β Amyloid-Induced Toxicity by Targeting Oligomers. Neurobiol. Dis. 2010, 40, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Lee, D.-K.; Long, N.P.; Kwon, S.W.; Park, J.H. Uptake of Nanopolystyrene Particles Induces Distinct Metabolic Profiles and Toxic Effects in Caenorhabditis Elegans. Environ. Pollut. 2019, 246, 578–586. [Google Scholar] [CrossRef]
- Wu, T.; He, J.; Ye, Z.; Xia, J.; Chen, M.; Chen, S.; Liu, K.; Xing, P.; Yang, J.; Qian, Y.; et al. Aged Biodegradable Nanoplastics Enhance Body Accumulation Associated with Worse Neuronal Damage in Caenorhabditis elegans. Environ. Sci. Technol. 2025, 59, 4352–4363. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; McCracken, A.; Simons, M.; Henriques, C.; Rera, M. How to Catch a Smurf?—Ageing and Beyond…In Vivo Assessment of Intestinal Permeability in Multiple Model Organisms. BIO-Protoc. 2018, 8, e2722. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Hua, X.; Dang, Y.; Han, Y.; Yu, Z.; Chen, X.; Ding, P.; Li, H. Polystyrene Microplastics (PS-MPs) Toxicity Induced Oxidative Stress and Intestinal Injury in Nematode Caenorhabditis Elegans. Sci. Total Environ. 2020, 726, 138679. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tian, L.; Wang, S.; Wang, D. Size-Dependent Transgenerational Toxicity Induced by Nanoplastics in Nematode Caenorhabditis Elegans. Sci. Total Environ. 2021, 790, 148217. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Virgolini, M.B.; Ávila, D.S.; Scharf, P.; Li, J.; Tinkov, A.A.; Skalny, A.V.; Bowman, A.B.; Rocha, J.B.T.; Aschner, M. Mitochondria in the Spotlight: C. Elegans as a Model Organism to Evaluate Xenobiotic-Induced Dysfunction. Cells 2023, 12, 2124. [Google Scholar] [CrossRef] [PubMed]
- Dilberger, B.; Baumanns, S.; Schmitt, F.; Schmiedl, T.; Hardt, M.; Wenzel, U.; Eckert, G.P. Mitochondrial Oxidative Stress Impairs Energy Metabolism and Reduces Stress Resistance and Longevity of C. elegans. Oxidative Med. Cell. Longev. 2019, 2019, 6840540. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Tan, X.; Peng, C.; Naz, I.; Zhang, Y.; Hernández, A.; Marcos, R.; Pervez, R.; Duan, Z.; Ruan, Y. Eco- and Bio-Corona-Based Microplastics and Nanoplastics Complexes in the Environment: Modulations in the Toxicological Behavior of Plastic Particles and Factors Affecting. Process Saf. Environ. Prot. 2024, 187, 356–375. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortuna, S.; Sonaglia, E.; Tacconi, S.; Sharbaf, M.; Uccelletti, D.; Dini, L.; Schifano, E.; Santarelli, M.L. Toxicological Effects of Poly(methyl methacrylate) Microplastics in Caenorhabditis elegans: Impairment of Development, Reproduction, and Stress Responses. Environments 2025, 12, 353. https://doi.org/10.3390/environments12100353
Fortuna S, Sonaglia E, Tacconi S, Sharbaf M, Uccelletti D, Dini L, Schifano E, Santarelli ML. Toxicological Effects of Poly(methyl methacrylate) Microplastics in Caenorhabditis elegans: Impairment of Development, Reproduction, and Stress Responses. Environments. 2025; 12(10):353. https://doi.org/10.3390/environments12100353
Chicago/Turabian StyleFortuna, Stefano, Erica Sonaglia, Stefano Tacconi, Mohammad Sharbaf, Daniela Uccelletti, Luciana Dini, Emily Schifano, and Maria Laura Santarelli. 2025. "Toxicological Effects of Poly(methyl methacrylate) Microplastics in Caenorhabditis elegans: Impairment of Development, Reproduction, and Stress Responses" Environments 12, no. 10: 353. https://doi.org/10.3390/environments12100353
APA StyleFortuna, S., Sonaglia, E., Tacconi, S., Sharbaf, M., Uccelletti, D., Dini, L., Schifano, E., & Santarelli, M. L. (2025). Toxicological Effects of Poly(methyl methacrylate) Microplastics in Caenorhabditis elegans: Impairment of Development, Reproduction, and Stress Responses. Environments, 12(10), 353. https://doi.org/10.3390/environments12100353







