Abstract
Atmospheric deposition through rainfall plays a significant role in transporting various anthropogenic contaminants to terrestrial and aquatic ecosystems. However, rainwater’s integrated ionic and molecular composition remains underexplored in semiurban environments. This study provides a comprehensive chemical characterization of rainwater collected during seven precipitation events from February to April 2025 in Athens, Georgia, USA. This semiurban area is characterized by substantial vehicular traffic, seasonal agricultural activities, and ongoing construction, while lacking significant industrial emissions. Targeted spectrophotometric analyses revealed heightened concentrations of nitrate (ranging from 2.0 to 4.3 mg/L), sulfate (17 to 26 mg/L), and phosphate (2.4 to 3.1 mg/L), with peak concentrations observed during high-intensity rainfall events. These findings are consistent with enhanced wet scavenging of atmospheric emissions. Concurrently, both targeted and non-targeted gas chromatography-mass spectrometry (GC-MS) analyses identified a diverse array of organic pollutants in the rainwater, including organophosphate, organochlorine, and triazine pesticides; polycyclic aromatic hydrocarbons (PAHs); plasticizers; flame retardants; surfactant degradation products; and industrial additives such as bisphenol A, triclosan, and nicotine. Furthermore, several legacy contaminants, such as organochlorines, were detected alongside currently utilized compounds, including glyphosate and its metabolite aminomethylphosphonic acid (AMPA). The concurrent presence of elevated anion and organic pollutant levels during significant storm events suggests that atmospheric washout can be the primary deposition mechanism. These findings underscore the capability of semiurban atmospheres to accumulate and redistribute complex mixtures of pollutants through rainfall, even in the absence of large-scale industrial activity. The study emphasizes the importance of integrated ionic and molecular analyses for uncovering concealed pollution sources. It highlights the potential of rainwater chemistry as a diagnostic tool for monitoring atmospheric contamination in urbanizing environments.
1. Introduction
With the continuous growth of the world population and the acceleration of global urbanization, increased emissions of air pollutants have been widely observed in urban regions [1]. Expanding cities experience complex air pollution problems driven by anthropogenic activities [2]. In particular, vehicular traffic and fuel combustion are major contributors to urban air contaminants, while processes like construction and road dust also add substantially to particulate pollution [3,4]. These pollution sources degrade air quality and pose health and environmental risks [3], underscoring the need to understand and mitigate urban atmospheric pollution at its sources [5]. Rapidly developing urban areas are not exempt: Athens, Georgia—a college town with a growing population—exemplifies how increased traffic, intensive college-related activity, and ongoing construction can elevate local emissions [6]. Such observations highlight the importance of identifying the underlying sources of pollutants in developing urban regions like Athens. Rainfall plays a crucial role in the atmospheric environment as a natural scavenger of pollutants [7]. Wet deposition is a significant sink for airborne contaminants such as sulfur, nitrogen oxides, and aerosols [8]. As a result, the chemical composition of rainwater can effectively indicate the sources of atmospheric pollutants [9]. The study of rainwater chemistry provides valuable insights into scavenging processes and pollutant origins [9]. Analyzing ions and compounds washed from the air provides an integrated snapshot of atmospheric deposition and can indicate the presence of both natural and anthropogenic inputs; however, distinguishing between local and remote sources requires complementary approaches such as air mass trajectory analysis, meteorological modeling, and emissions data [10]. Decades of precipitation chemistry research have shown that major inorganic ions in rainwater often reflect distinct sources: crustal dust adds calcium and other cations, and marine spray contributes sodium and chloride. Fossil fuel combustion produces sulfate and nitrate anions [11]. Source apportionment studies consistently find that rainwater nitrate (NO3−) and sulfate (SO42−) in urban areas derive overwhelmingly from anthropogenic activities such as vehicle and industrial NOₓ/SO2 emissions [12]. In one recent case, nearly all NO3− and SO42− in Beijing rainfall was traced to mobile transportation sources [9,13]. In fact, fossil fuel combustion has overtaken other sources, such as coal, in driving urban wet deposition in some regions [14].
Rainwater is a carrier of inorganic species and can accumulate organic pollutants in the atmosphere. Semi-volatile organic compounds such as polycyclic aromatic hydrocarbons (PAHs), plasticizers, and certain pesticides are scavenged by rain droplets and delivered to the surface [15,16]. Detecting these organic molecules in precipitation provides a unique window into urban air quality and pollutant loadings. Notably, recent measurements have identified a wide suite of PAHs and organochlorine pesticides in rainwater from cities with ostensibly “clean” air [15], demonstrating that significant amounts of organic pollutants can deposit even in developing urban areas [17,18]. Such findings highlight the potential of rainwater chemistry as a complementary medium for characterizing atmospheric pollutants that may not be captured through routine air monitoring9. Despite the complementary information offered by inorganic ions and organic compounds in rain, many prior studies have examined these pollutant classes in isolation. Acid rain and deposition research, for instance, traditionally focuses on ions and nutrients [19], whereas studies of persistent organic pollutants often consider air and dry deposition but less frequently wet deposition [20]. A combined analysis of molecular (organic) and ionic constituents in rainwater can enhance our understanding of pollution dynamics by linking broad atmospheric chemistry with specific source markers. By simultaneously measuring organic molecules and major anions in the same rain events, source-specific tracers with general indicators of anthropogenic influence can be correlated. Motor vehicles emit nitrogen oxides and excess hydrocarbons byproducts, including PAHs; accordingly, the co-occurrence of elevated NO3− and PAH concentrations in rainwater would strongly implicate vehicular emissions as a pollution source [21,22]. Likewise, the presence of agricultural pesticides in rain, coupled with high nitrate or phosphate levels, might signal fertilizer use, runoff, or biomass burning in the region [23]. Integrating organic and inorganic rainwater signatures provides a more holistic fingerprint of atmospheric pollution, improving source identification and apportionment. Recent studies in the Oconee River watershed have shown that nitrate and sulfate ions can facilitate the degradation of organic contaminants such as malathion, highlighting the synergistic roles of rainwater constituents after deposition [22,23,24].
Given this background, there is a need to investigate rainwater chemistry in growing urban areas like Athens, GA, where multiple subtle pollution sources may be underlying the overall atmospheric mix. Athens is a semi-urban area (population ~130,000) centered around the University of Georgia (UGA), with no large industrial emitters but significant urban activity from traffic, seasonal population influx, and construction [22,23,24]. These conditions make it an ideal testbed for studying how everyday urban activities affect rainwater chemistry. By examining organic pollutants and inorganic ions in precipitation, we can unveil the contributing sources of atmospheric pollution in such an environment. The present study addresses this need by analyzing rainwater from six precipitation events in early 2025 in the UGA–Athens area, targeting a suite of organic contaminants (pesticides, plasticizers, PAHs) and key anions (nitrate, sulfate, phosphate). The primary goal of this study is to investigate the chemical signatures in rainwater over the UGA–Athens region and to elucidate their potential sources. To achieve this, we collected rainwater samples from six precipitation events between February and April 2025 and comprehensively analyzed organic and inorganic constituents. his study integrates the analysis of organic pollutants—including agricultural pesticides, plasticizers, and PAHs—using gas chromatography–mass spectrometry (GC–MS) [22,25], with spectrophotometric quantification of major anions (NO3−, SO42−, and PO43−) [24] to characterize the ionic and molecular composition of rainwater. Combining these datasets provides a representative snapshot of atmospheric deposition in Athens during late winter to spring 2025, a period influenced by diverse anthropogenic activities (e.g., residential heating, agricultural preparation) and seasonal meteorological conditions. Through this dual analytical approach, this study seeks to identify whether the rainwater chemistry reflects contributions from vehicular traffic (e.g., via PAH and nitrate signatures), ongoing construction and urban dust (e.g., via particulate-bound organics and crustal ions), or other sources such as local agriculture and biomass burning. The findings of this work will enhance understanding of how different pollution sources imprint rainwater in a developing urban environment. These insights contribute to broader environmental monitoring and source apportionment efforts, informing strategies to mitigate atmospheric pollution in Athens and similar growing semi-urban areas.
2. Materials and Methods
2.1. Sample Collection
Rainwater samples were collected during six separate precipitation events near the Department of Geology at the University of Georgia, Athens, GA, on the following dates: 12 February 2025; 15 February 2025; 10 March 2025; 31 March 2025; 6 April 2025; and 7 April 2025. Sampling commenced immediately at the onset of rainfall on each date. Approximately 170 mL of rainwater was collected per event using a pre-baked (200 °C for 6 h) 250 mL wide-mouth glass bottle fitted with a clean polyethylene funnel (10 cm diam.; stem diam. 1 cm × L 6 cm) to facilitate collection. The bottles were placed in an open area, elevated above ground level to minimize splash contamination and interference from surrounding surfaces. Immediately after each collection, samples were transported to the laboratory, filtered through 0.45 µm membrane filters to remove particulate matter, and stored at 4 °C for a maximum of 48 h to preserve integrity prior to extraction and analysis. Key water quality parameters, pH, oxidation-reduction potential (ORP), conductivity, total dissolved solids (TDS), turbidity, and salinity, were measured before filtration using the HANNA HI9829 multiparameter probe (Hanna Instruments, Smithfield, RI, USA), as recorded in Table S1. The probe underwent calibration with a quick calibration solution before parameter recording to ensure accuracy.
2.2. Measurement of Major Anions in Rainwater Samples
Concentrations of nitrate, phosphate, and sulfate were measured with a visible-range spectrophotometer (Hanna Iris, Smithfield, RI, USA) using manufacturer-supplied reagents (HI93728-01 for nitrate, HI93713-01 for low-range phosphate, and HI93751-01 for sulfate). Calibration curves were generated individually for each analyte to ensure accuracy, and all anion measurements were performed in triplicate to confirm reproducibility.
2.3. Extraction of Rainwater Samples
Rainwater samples (150 mL) were processed following the extraction protocols described in previous studies [22,23,24]. An internal standard, 20 µL of Naphthalene-d8 (Millipore Sigma®, Burlington, VT, USA), was added to each filtered sample [22,24,25]. A total of 150 mL of the filtered rainwater was then extracted using Supelco solid-phase extraction cartridges (6 cc Vac Cartridge, 150 mg sorbent; Supelco, Bellefonte, PA, USA), which were preconditioned with 5 mL of methanol (GC-MS SupraSolv®, Supelco, Darmstadt, Germany). The cartridges were rinsed three times with 5 mL of Type 1 ultrapure water, and analytes were eluted with 5 mL of hexane (GC-MS SupraSolv®, Supelco). The eluate was transferred to a clean test tube containing 5 g of anhydrous sodium sulfate to remove residual moisture. The supernatant was concentrated to 200 µL under a gentle stream of nitrogen. From this, 100 µL was transferred to a 2 mL amber GC vial and reconstituted to a final volume of 1 mL with 100% hexane. Samples were stored at −20 °C until analysis.
2.4. Targeted and Non-Targeted Characterization of Organic Compounds
Targeted and non-targeted analyses were conducted using an 8860 Gas Chromatograph coupled with a 5977B Mass Spectrometer (GC–MS; Agilent Technologies®, Santa Clara, CA, USA) (Tables S2 and S3) [23]. Non-targeted qualitative screening was performed in full scan mode, while targeted analysis of the same sample extract was conducted in selected ion monitoring (SIM) mode for 24 analytes (Tables S2 and S3). Peak identification for the non-targeted data analysis described in Basapuram et al. (2024) and Stamm et al. (2025) was performed using the National Institute of Standards and Technology (NIST) Mass Spectral Library [22,24,26].
2.5. Statistical Analysis
Heatmap and cluster analyses were performed in R using the vegan and pheatmap packages [27]. Prior to visualization, compound concentrations were standard-score normalized across samples to ensure comparability. Hierarchical clustering of rows (organic compounds) was performed using Bray–Curtis dissimilarity and the average linkage method, while column clustering (sampling time points) was based on Euclidean distance. A precipitation metadata track was included as an annotation to assess the influence of rainfall on organic contaminant profiles.
2.6. Quality Assurance and Quality Control (QA/QC)
External calibration curves were prepared using six standard concentrations for each analyte: 0.05, 1, 10, 50, 100, and 500 ng/mL. All calibration solutions included naphthalene-d8 (99%, Sigma-Aldrich, St. Louis, MO, USA) as an isotope-labeled internal standard. Average recoveries of the internal standard for each sample are reported in Supplementary Table S4. Analyte concentrations in rainwater samples were reported in ng/L. Reported concentrations represent the mean of duplicate analyses, with associated standard deviations provided in the Supplementary Information (Table S4). All glassware was pre-cleaned and baked at 200 °C for 6 h. Extracted samples were stored at −20 °C until analysis. Ultra-purified Type I water (18.2 MΩ·cm, ELGA® Water Purifying System, Woodridge, IL, USA) was used as the laboratory blank, and tap water at the Department of Geology was considered the field blank. A tap water field blank was collected on 6 April 2025. The tap water was sourced from the North Oconee River and treated at the Athens–Clarke County Water Treatment Plant. Due to logistical constraints during field collection, duplicate sample collection was not feasible; thus, analytical duplicates were used to assess measurement reproducibility.
2.7. Hazard Classification Based on Persistence, Bioaccumulation, and Toxicity (PBT)
To assess environmental risk and prioritize contaminants, each compound was assigned a PBT ranking based on criteria established by the European Union’s REACH regulation and the U.S. Environmental Protection Agency (EPA). Compounds were categorized as PBT (Persistent, Bioaccumulative, and Toxic), vPvB (very Persistent and very Bioaccumulative), PT (Persistent and Toxic), BT (Bioaccumulative and Toxic), or P (Persistent), as applicable. These classifications reflect regulatory hazard thresholds and were used to contextualize temporal concentration patterns and potential ecological impacts.
3. Results and Discussion
Rainwater samples collected during six precipitation events between February and April 2025 demonstrated slight acidity and relatively low concentrations of dissolved solids and salinity. These characteristics are indicative of atmospheric deposition in a semi-urban environment. The physicochemical parameters assessed included pH, oxidation-reduction potential (ORP), conductivity, total dissolved solids (TDS), turbidity, and salinity (Table S1). The pH values of the rainwater samples ranged from 5.38 to 5.70, reflecting slightly acidic conditions characteristic of unbuffered atmospheric precipitation. The lowest pH value of 5.38 was recorded on 6 April 2025, while the highest pH of 5.70 was noted on 15 February 2025. These values suggest the influence of atmospheric carbon dioxide and potential contributions from anthropogenic acidic pollutants. The ORP values were consistently elevated, ranging from 370.6 to 405.5 mV, indicating an oxidizing nature of the rainwater. The maximum ORP was also observed on 6 April 2025, which coincided with the minimum pH, suggesting a possible influx of reactive oxidants or acidic species during that particular event. Electrical conductivity exhibited modest variation, ranging from 20.4 to 29.5 µS/cm, with the highest reading on 6 April 2025. This trend was similarly reflected in TDS measurements, which spanned from 13.5 to 25.3 mg/L, indicating relatively clean rainwater with occasional enrichment of soluble ions, potentially resulting from local atmospheric inputs or resuspended particulates. Turbidity values remained consistently low across all samples, ranging from 0.7 to 1.1 NTU, with minor fluctuations that may indicate varying particulate loads during different rainfall intensities or changes in air mass conditions. Salinity values were negligible for all samples. This sharp increase corresponded with the highest conductivity and TDS measurements recorded. The results indicate that while the rainwater samples were predominantly clean and weakly acidic, episodic variations, particularly observed on 6 April 2025, may be attributed to changing atmospheric inputs, such as dust, aerosols, or combustion-related pollutants. The consistently low turbidity and salinity levels further support the conclusion that there is minimal contamination from terrestrial or marine sources during the sampled precipitation events.
3.1. Anion Composition and Variability in Rainwater
Rainwater samples collected between February and April 2025 revealed considerable concentrations of anions, likely influenced by precipitation-driven surface-atmospheric interactions. Nitrate (NO3−) concentrations ranged from 2.0 mg/L to 4.3 mg/L, with the highest level observed on 6 April 2025 (4.3 mg/L), coinciding with the significant precipitation event (2.05 inches) (Table 1, Figure 1). This suggests enhanced atmospheric deposition or washout of reactive nitrogen species during heavy rainfall. In contrast, the field blank (tap water) registered a nitrate concentration of just 0.5 mg/L, confirming minimal background contamination. Sulfate (SO42−) concentrations showed a similar trend, varying from 17 mg/L to 26 mg/L across rain events, peaking on April 6. Phosphate (PO43−) levels remained comparatively consistent (2.4–3.1 mg/L), with slight elevation during the April 6 event (Table 1). The field blank phosphate concentration was markedly lower (0.87 mg/L), further supporting the attribution of elevated anion levels to rainwater inputs rather than sampling artifacts. The data suggest that rainfall events significantly modulate anion fluxes, likely via atmospheric deposition, urban runoff, or interaction with surrounding land cover. A summary comparison of the present results with selected global studies is provided in Table S5.

Table 1.
Mean concentrations of major anions (mg/L), pesticides (ng/L), and polycyclic aromatic hydrocarbons (PAHs) (ng/L) in rainwater samples collected between February and April 2025, with tap water included as a reference matrix.
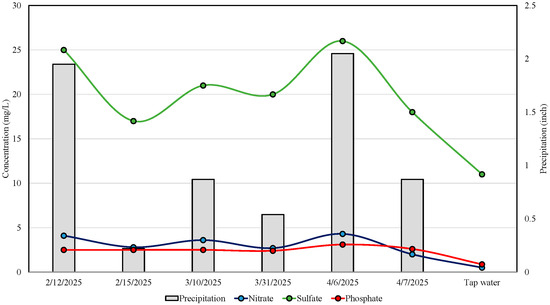
Figure 1.
Mean concentrations (mg/L) of nitrate, sulfate, and phosphate measured in rainwater samples collected during six precipitation events between February and April 2025, shown alongside corresponding daily precipitation data. Tap water concentrations are included as a reference baseline. Precipitation data were obtained from the National Oceanic and Atmospheric Administration (NOAA) weather database [28].
3.2. Profiles of Organic Contaminants Detected in Rainwater
Rainwater samples collected between February and April 2025 revealed a chemically diverse suite of organic contaminants, including contemporary-use and legacy pesticides as well as carcinogenic polycyclic aromatic hydrocarbons (PAHs) (Table 1, Figure 2). These compounds were consistently detected across sampling dates, with pronounced concentration elevation associated with high-precipitation events. The temporal distribution and compound-specific profiles suggest a combination of local atmospheric deposition, regional transport, and wet scavenging as dominant input mechanisms. In contrast, concentrations in the tap water field blank were negligible, supporting the integrity of the rainwater signal.
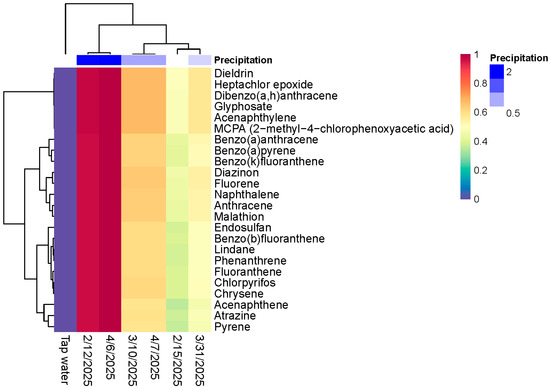
Figure 2.
Heatmap illustrating the normalized concentrations of the identified organic compounds in rainwater samples across all sampling events.
3.2.1. Organophosphate Pesticides
Malathion, chlorpyrifos, and diazinon—three commonly applied organophosphate insecticides—were consistently detected in rainwater samples. Malathion concentrations ranged from 10.29 to 21 ng/L, peaking on April 6 (21 ng/L), likely reflecting enhanced atmospheric loading during the high-rainfall event. Chlorpyrifos was detected at concentrations ranging from 6.86 to 14 ng/L, while diazinon ranged from 5.49 to 11.2 ng/L (Table 1, Figure 2). These findings are consistent with volatilization from agricultural or urban sources, followed by wet deposition. Tap water values for these compounds were minimal, confirming low background contamination.
3.2.2. Triazine and Phenoxy Herbicides
Atrazine, a widely used triazine herbicide, was detected at consistently high concentrations (20.6–42 ng/L), with a peak concentration on April 6. MCPA (2-methyl-4-chlorophenoxyacetic acid), a phenoxy acid herbicide, followed a similar temporal trend, with concentrations ranging from 9.6 to 19.6 ng/L. Glyphosate, a non-selective herbicide with extensive global application, was found at exceptionally high levels in all samples (1028 to 2100 ng/L), also peaking on April 6. Its high solubility and resistance to degradation likely account for its persistence and concentration in rainwater (Table 1, Figure 2). Glyphosate was absent in the field blank.
3.2.3. Organochlorine Pesticides (OCPs)
Legacy OCPs, including heptachlor epoxide, dieldrin, lindane (γ-HCH), and endosulfan, were detected in all rainwater samples at sub- to low-ng/L levels. Lindane concentrations ranged from 0.686 to 1.4 ng/L, while endosulfan reached up to 2.8 ng/L on April 6 (Table 1, Figure 2). These compounds, though largely restricted, continue to persist in the environment due to their high stability and propensity for long-range atmospheric transport [29,30]. The observed profiles suggest re-emission from soils or secondary sources and subsequent atmospheric deposition.
3.2.4. Polycyclic Aromatic Hydrocarbons (PAHs)
A complete suite of 16 priority PAHs—including low-, medium-, and high-molecular-weight compounds—was detected in all rainwater samples. Their concentrations peaked during rainfall events, especially on April 6, consistent with atmospheric washout processes. Key PAHs include: Naphthalene (LPAH): 13.7–28 ng/L; Fluorene and phenanthrene (LPAHs): up to 21 ng/L and 28 ng/L, respectively; Fluoranthene and pyrene (MPAHs): 17–35 ng/L and 15–30.8 ng/L; Benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene (HPAHs): 6.85–16.8 ng/L; Benzo[a]pyrene and dibenzo[a,h]anthracene (carcinogenic PAHs): 2.74–5.6 ng/L and 1.03–2.1 ng/L (Table 1, Figure 2). Both low- and high-weight PAHs suggest mixed sources, including vehicular emissions, fossil fuel combustion, and biomass burning. The elevated concentrations during rainfall highlight the importance of wet deposition in PAHs cycling. Tap water concentrations were uniformly low, ruling out procedural contamination.
3.3. Non-Targeted Identification and Environmental Hazard Assessment of Organic Compounds in Rainwater
Non-targeted chemical analysis of rainwater samples collected between February and April 2025 revealed the presence of 43 distinct organic contaminants across various chemical classes. These included pesticides, herbicides, polycyclic aromatic hydrocarbons, plasticizers, surfactants, and tobacco-related alkaloids. Each compound was evaluated based on its environmental persistence, bioaccumulation potential, toxicity as indicated by LC50 values, and its most probable source [31,32]. A large number of pesticides were detected, particularly organophosphate insecticides such as chlorpyrifos, methidathion, diazinon, and parathion. These compounds are widely applied in agricultural pest control and are known for their acute aquatic toxicity, with LC50 values ranging from 0.09 to 1.8 µg/L. All four compounds meet the persistence, bioaccumulation, and toxicity criteria, classifying them as PBT substances with high ecological concern [33,34]. Bromophos, while not bioaccumulative, was identified as persistent and toxic [35]. In addition, several herbicides, including atrazine, simazine, MCPA, and mecoprop, were frequently observed. These compounds were also classified as persistent and toxic and are commonly introduced to aquatic systems through surface runoff from agricultural land [36] (Table 2).

Table 2.
Environmental hazard classification, primary sources, aquatic toxicity, and peak areas of organic contaminants detected in rainwater samples via non-targeted analysis.
In rainwater, there were also organochlorine insecticides and their degradation products, such as dieldrin, lindane, endosulfan, and heptachlor epoxide. These substances are no longer widely used due to regulatory restrictions, but persist in the environment due to their chemical stability [25,37]. All were classified as very persistent and bioaccumulative, with LC50 values typically below 1.5 µg/L. Their presence underscores the lasting impact of legacy pesticide use, especially in areas with a history of intensive agricultural practices. Combustion-derived pollutants were also prevalent, with a wide range of polycyclic aromatic hydrocarbons detected. These included naphthalene, acenaphthene, anthracene, pyrene, benzo(a) pyrene, and chrysene. Many of the polycyclic aromatic hydrocarbons identified in the samples meet the criteria for PBT classification, including benzo(a) pyrene, Indeno[1,2,3-cd]pyrene, and dibenzo(a,h)anthracene. These compounds are environmentally persistent and have low LC50 values, often below 1 µg/L, indicating a high ecological risk level. Their likely sources include vehicle exhaust, industrial activities, and atmospheric deposition onto impervious urban surfaces [38].
Plasticizers were also commonly found in the rainwater samples. These included phthalates such as di(2-ethylhexyl) phthalate, dibutyl phthalate, diisononyl phthalate, and benzyl butyl phthalate. All were classified as very persistent and bioaccumulative and are typically associated with plastic waste, construction materials, and leachate from urban infrastructure. Surfactant-related compounds, including nonylphenol, nonylphenol ethoxylates, bisphenol A, alkylphenols, and triclosan, were also prevalent. Nonylphenol and triclosan were classified as PBT substances due to their environmental stability and low LC50 values, while bisphenol A and alkylphenol ethoxylates were categorized as persistent and toxic. These contaminants are commonly released into the environment through household wastewater, industrial discharges, and the breakdown of consumer products [39,40]. Nicotine, a marker of tobacco product waste, was consistently present in rainwater samples and was also detected in tap water, albeit at lower concentrations. Although nicotine is not environmentally persistent or bioaccumulative, it exhibits high toxicity to aquatic organisms with an LC50 value of 4.9 µg/L, qualifying it as a bioactive and toxic substance. Its presence is likely due to urban littering and atmospheric transport of tobacco-related waste (Table 2).
The compounds detected in this study reflect a combination of legacy pollutants and contemporary contaminants introduced through various sources, including agriculture, fossil fuel combustion, plastic waste, and urban runoff. The widespread occurrence of substances classified as PBT, which are very persistent and bioaccumulative, highlights the long-term risks these contaminants pose to aquatic ecosystems [32]. Their absence in treated tap water suggests contamination is primarily linked to surface runoff and atmospheric inputs rather than municipal water supply systems. These results emphasize the need for improved rainwater storage, management, and pollution prevention strategies that address complex mixtures of hazardous organic compounds in urban environments.
The consistent detection of various contaminants, often peaking with higher rainfall, highlights the efficiency of wet scavenging as a dominant input mechanism, alongside local atmospheric deposition and regional transport. The analysis of anion composition revealed substantial concentrations of nitrate (NO3−), sulfate (SO42−), and phosphate (PO43−), with a notable increase during the largest precipitation event on 6 April 2025. This strong correlation between rainfall intensity and anion concentration suggests enhanced atmospheric washout of reactive nitrogen and sulfur species, as well as general atmospheric deposition. The elevated levels of these anions in rainwater directly affect receiving ecosystems, contributing to possible eutrophication from nitrate and phosphate and acidification from sulfate. The pH values showed a significant negative correlation with nitrate (r2 = −0.83; p < 0.05), sulfate (r2 = −0.97; p < 0.001), and phosphate (r2 = −0.60; p = 0.21) concentrations across the sampling dates (Figure 3). These results indicate that lower pH levels are associated with higher concentrations of these major anions. The strongest correlation was observed between pH and sulfate, suggesting a close link between acidification and sulfate mobilization or retention in the system. As expected, precipitation events with higher sulfate and nitrate concentrations were associated with lower pH values. This reflects the established role of strong acidic anions in controlling precipitation acidity in poorly buffered waters. The trend observed here is consistent with global acid deposition studies. It confirms that these anions strongly influence the ionic composition of rainwater at the study site, with limited evidence of neutralization by base cations. The inverse relationships may reflect acid-driven changes in solubility, adsorption–desorption dynamics, or redox-mediated transformations influencing nutrient availability in the aquatic environment.
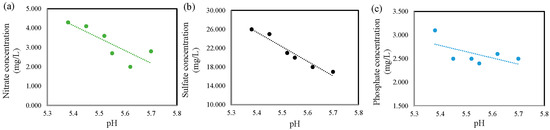
Figure 3.
Graph showing the variation in major anion concentrations as a function of pH in rainwater samples: (a) nitrate, (b) sulfate, and (c) phosphate concentrations (mg/L) exhibit distinct trends across the observed pH range.
Beyond major anions, the study unveiled a chemically diverse array of organic contaminants, encompassing both contemporary-use and legacy pesticides, alongside carcinogenic polycyclic aromatic hydrocarbons (PAHs). Organophosphate insecticides like malathion, chlorpyrifos, and diazinon were consistently detected, indicating ongoing volatilization from agricultural or urban applications, followed by wet deposition. Similarly, widely used herbicides such as atrazine, MCPA, and, notably, exceptionally high levels of glyphosate, were prevalent, reflecting their extensive global application and, particularly for glyphosate, its high solubility and resistance to degradation in the environment. The persistence of banned organochlorine pesticides (OCPs) like heptachlor epoxide, dieldrin, and lindane at sub- to low-ng/L levels further underscores their environmental persistence and propensity for long-range atmospheric transport and re-emission from existing environmental reservoirs. The comprehensive detection of PAHs, including carcinogenic types, with concentrations peaking during rainfall, reinforces the significance of wet deposition in the atmospheric cycling of these toxic compounds, originating from various combustion sources.
The non-targeted screening component of this study provided a more holistic view of the “broad atmospheric burden of anthropogenic contaminants.” This analysis identified numerous organic micropollutants not typically monitored, including a range of plasticizers (phthalate esters), organophosphate flame retardants, industrial chemicals, and metabolites from consumer products. Phthalate esters suggest atmospheric dispersal from industrial sources, waste treatment, or consumer product volatilization, while flame retardants reflect their atmospheric redistribution from treated materials. Furthermore, detecting alkylphenolic surfactants and their degradation products, the antimicrobial agent triclosan, bisphenol A (BPA), and even nicotine, highlights a continuous influx of emerging contaminants from both household and industrial sources. Comparable studies have also reported the co-occurrence of major ions and organic pollutants in rainwater, reflecting both natural inputs and anthropogenic influences, including work from central India, Chengdu, Southwest China, and regional datasets in North America [40,41,42] (Table S5). This study demonstrates that rainwater serves as a significant conduit for various contaminants, ranging from essential nutrients at ecologically impactful levels to highly toxic and persistent organic pollutants. The direct correlation between precipitation events and contaminant concentrations highlights wet deposition as a critical process for transferring these substances from the atmosphere to aquatic and terrestrial ecosystems. The study’s comprehensive approach, particularly the non-targeted analysis, underscores the pervasive nature of anthropogenic pollution in the atmosphere and the continuous introduction of both known and emerging contaminants into our environment, necessitating ongoing monitoring and a more holistic understanding of their ecological and human health implications.
The present study is limited by its short temporal span (three months) and single-site sampling, which constrains its ability to represent local-scale variability in atmospheric deposition. While the analytical approach provides a detailed baseline of ionic and organic compounds in precipitation, interpretation of air pollution environments at micro- or mesoscales requires integration with meteorological and transport processes. Development of rainwater chemistry as a diagnostic tool will therefore necessitate a standardized protocol that combines: (i) longer-term and multi-site sampling to capture spatial and seasonal variability; (ii) parallel atmospheric monitoring (aerosol and gas-phase measurements) to link wet deposition with ambient concentrations; and (iii) trajectory and cloud-dynamics modeling to distinguish between local and transboundary contributions. Such integration would enhance the representativeness of rainwater sampling and allow its use as a more robust diagnostic indicator of atmospheric contamination.
4. Conclusions
This study provides a comprehensive evaluation of rainwater chemistry in a semiurban environment, revealing that precipitation is a significant pathway for transferring a broad range of anthropogenic pollutants from the atmosphere to the surface. Despite the absence of major industrial emitters, rainwater in Athens, Georgia, contained elevated levels of nitrate, sulfate, and phosphate, along with a chemically diverse mixture of organic contaminants. These included contemporary-use and legacy pesticides, polycyclic aromatic hydrocarbons, plasticizers, flame retardants, surfactant degradation products, industrial additives, and other emerging compounds. Within the limits of this dataset, the consistent detection of these substances, particularly during high-intensity rainfall events, suggests the role of wet deposition as an important atmospheric cleansing process and points to the complex chemical burden of the urban atmosphere, while recognizing that confirmation of these patterns would require a larger and longer-term dataset. The integration of targeted and non-targeted analyses allowed for the detection of both commonly monitored and rarely tracked pollutants, offering a more complete picture of atmospheric contamination. The findings demonstrate that semiurban atmospheres can serve as reservoirs of both legacy and modern pollutants, which are readily mobilized and deposited through rainfall. This work emphasizes the value of rainwater chemistry as a complementary approach for environmental monitoring, with potential applications in source attribution when combined with atmospheric modeling. Continued investigation into the temporal dynamics, source pathways, and ecological impacts of these pollutants is essential for understanding the role of rainwater chemical profiling as a diagnostic tool in interpreting the local air pollution activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments12100351/s1, Table S1: Physicochemical characteristics of rainwater samples collected during precipitation events (February–April 2025); Table S2: GC/MS conditions for targeted screening in Scan and SIM mode for pesticides; Table S3: GC/MS conditions for targeted screening in Scan and SIM mode for polycyclic aromatic hydrocarbons; Table S4: Concentration variations and recovery efficiency of organic compounds in rainwater (with tap water as reference); Table S5: Comparative insights into rainwater chemistry and pesticide concentrations [40,41,42,43,44,45,46,47].
Author Contributions
S.D. and A.D. conceived the study idea. G.S. and S.D. prepared the original draft. G.S. and G.B. collected the rainwater samples, while G.S. and A.B. performed sample extraction and analysis. G.B. and S.D. conducted the GC–MS instrumental analysis. A.D., A.B. and S.D. carried out data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by the U.S. Department of Energy, Office of Environmental Management, under Award No. DE-EM0005228 to the University of Georgia Research Foundation. Undergraduate assistantships for Grace Stamm and Arka Bhattacharjee were supported by the National Science Foundation under Award No. EAR-2502654.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liang, L.; Wang, Z.; Li, J. The effect of urbanization on environmental pollution in rapidly developing urban agglomerations. J. Clean. Prod. 2019, 237, 117649. [Google Scholar] [CrossRef]
- Lopez-Aparicio, S.; Grythe, H.; Drabicki, A.; Chwastek, K.; Toboła, K.; Górska-Niemas, L.; Kierpiec, U.; Markelj, M.; Strużewska, J.; Kud, B.; et al. Environmental sustainability of urban expansion: Implications for transport emissions, air pollution, and city growth. Environ. Int. 2025, 196, 109310. [Google Scholar] [CrossRef]
- Pongpiachan, S.; Tipmanee, D.; Khumsup, C.; Hirunyatrakul, P.; Hashmi, M.Z.; Poshyachinda, S. Size-segregated analysis of PAHs in Urban air: Source apportionment and health risk assessment in an Urban canal-adjacent environment. PLoS ONE 2025, 20, e0320405. [Google Scholar]
- Hwang, H.M.; Fiala, M.J.; Wade, T.L.; Park, D. Review of pollutants in urban road dust: Part II. Organic contaminants from vehicles and road management. Int. J. Urban Sci. 2019, 23, 445–463. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, R. Urban areas and air pollution: Causes, concerns, and mitigation. In Geospatial Analytics for Environmental Pollution Modeling: Analysis, Control and Management; Springer Nature: Cham, Switzerland, 2023; pp. 163–185. [Google Scholar]
- Ott, L.M. Ozone Air Pollution and Stage-of-Change Status for Alternative Transportation Usage Among College Students. Doctoral Dissertation, Texas Woman’s University, Denton, TX, USA, 2023. Available online: https://www.proquest.com/docview/305235366 (accessed on 24 September 2025).
- Mao, S.; Liu, Z.; Zeng, J.; Wu, Q.; Ge, X. Unveiling the urban rainfall chemistry in summer frequent-rainy area: Variation and source identification of air pollutants based on two rainy seasons’ observation. J. Environ. Manag. 2025, 391, 126439. [Google Scholar] [CrossRef]
- Safi, Z.; Miyittah, M.; Offei, B.K.; Amenorpe, G. A systematic review of wet and dry deposition of reactive nitrogen, sulfur, and heavy metals: Ecosystem contamination and food chain disruption in Ghana. Environ. Sci. Atmos. 2025, 5, 756–784. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G.; Zhang, S.; Xiao, X.; Li, Y.; Gao, X.; Wang, D.; Qu, R. Rainwater chemical evolution driven by extreme rainfall in megacity: Implication for the urban air pollution source identification. J. Clean. Prod. 2022, 372, 133732. [Google Scholar] [CrossRef]
- Pacyna, J.M.; Pacyna, E.G. An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environ. Rev. 2001, 9, 269–298. [Google Scholar] [CrossRef]
- Mouli, P.C.; Mohan, S.V.; Reddy, S.J. Rainwater chemistry at a regional representative urban site: Influence of terrestrial sources on ionic composition. Atmos. Environ. 2005, 39, 999–1008. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G.; Zhang, S.; Qu, R. Nitrate dynamics and source identification of rainwater in Beijing during rainy season: Insight from dual isotopes and Bayesian model. Sci. Total Environ. 2023, 856, 159234. [Google Scholar] [CrossRef]
- Yang, F.; Tan, J.; Shi, Z.B.; Cai, Y.; He, K.; Ma, Y.; Duan, F.; Okuda, T.; Tanaka, S.; Chen, G. Five-year record of atmospheric precipitation chemistry in urban Beijing, China. Atmos. Chem. Phys. 2012, 12, 2025–2035. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, X.; Huang, C.; Yang, Y.; Yang, H.; Zhang, J.; Huang, T. High atmospheric wet nitrogen deposition and major sources in two cities of Yangtze River Delta: Combustion-related NH3 and non-fossil fuel NOx. Sci. Total Environ. 2022, 806, 150502. [Google Scholar] [CrossRef]
- He, J.; Balasubramanian, R. Semi-volatile organic compounds (SVOCs) in ambient air and rainwater in a tropical environment: Concentrations and temporal and seasonal trends. Chemosphere 2010, 78, 742–751. [Google Scholar] [CrossRef]
- Rianawati, E.L. Occurrence and Distribution of PAHs in Rainwater and Urban Runoff. Master Dissertation, National University of Singapore, Singapore, 2007. [Google Scholar]
- Fuchte, H. Pollution in Urban Environments: Levels and Profiles of Traffic and Other Organic Contaminants in Street Run-Off and Atmospheric Particles. Doctoral Dissertation, RWTH Aachen University, Aachen, Germany, 2022. [Google Scholar]
- Kilic, S.; Kilic, M. Determination of organic pollutants and pollution sources in sequentially collected rainwater samples in Isparta Province. Environ. Monit. Assess. 2025, 197, 463. [Google Scholar] [CrossRef]
- Asif, M.R.; Ye, B.; Ye, C. Acid sulfate soils: Formation, identification, environmental impacts, and sustainable remediation practices. Environ. Monit. Assess. 2025, 197, 484. [Google Scholar] [CrossRef]
- Liggio, J.; Makar, P.; Li, S.M.; Hayden, K.; Darlington, A.; Moussa, S.; Wren, S.; Staebler, R.; Wentzell, J.; Wheeler, M.; et al. Organic carbon dry deposition outpaces atmospheric processing with unaccounted implications for air quality and freshwater ecosystems. Sci. Adv. 2025, 11, eadr0259. [Google Scholar] [CrossRef]
- Duttagupta, S.; Mukherjee, A.; Bhattacharya, A.; Bhattacharya, J. Wide exposure of persistent organic pollutants (PoPs) in natural waters and sediments of the densely populated Western Bengal basin, India. Sci. Total Environ. 2020, 717, 137187. [Google Scholar] [CrossRef]
- Stamm, G.; Basapuram, G.; Duttagupta, S.; Dutta, A. Anion-mediated pathways in organophosphate degradation in the Oconee River watershed in Georgia. Emerg. Contam. 2025, 11, 100542. [Google Scholar] [CrossRef]
- Basapuram, G.; Duttagupta, S.; Dutta, A. Detection and screening of organic contaminants in A riverine system of Georgia using non-targeted analysis. Environments 2024, 11, 89. [Google Scholar] [CrossRef]
- Duttagupta, S.; Basapuram, G.; Cottrell, W.; Dutta, A. Landuse and land cover shape organic contaminants distribution in the Oconee River watershed in Georgia. npj Emerg. Contam. 2025, 1, 3. [Google Scholar] [CrossRef]
- Babayemi, J.O.; Ogundiran, M.B.; Osibanjo, O. Overview of environmental hazards and health effects of pollution in developing countries: A case study of Nigeria. Environ. Qual. Manag. 2016, 26, 51–71. [Google Scholar] [CrossRef]
- Pillay, K.; Duttagupta, S.; Basapuram, G.; Dutta, A. Draft genome sequence of Rossellomorea marisflavi DL-A, a malathion-degrading bacterium. Microbiol. Resour. Announc. 2025, 14, e00220-25. [Google Scholar] [CrossRef]
- Kolde, R.; Kolde, M.R. Package ‘pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- National Oceanic and Atmospheric Administration (NOAA). Weather and Climate Resources. Available online: https://www.noaa.gov/tools-and-resources/weather-and-climate-resources (accessed on 30 July 2025).
- Wu, Z.; Lin, T.; Hu, L.; Guo, T.; Guo, Z. Atmospheric legacy organochlorine pesticides and their recent exchange dynamics in the Northwest Pacific Ocean. Sci. Total Environ. 2020, 1, 138408. [Google Scholar] [CrossRef]
- Cabrerizo, A.; Muir, D.C.; De Silva, A.O.; Wang, X.; Lamoureux, S.F.; Lafrenière, M.J. Legacy and emerging persistent organic pollutants (POPs) in terrestrial compartments in the high Arctic: Sorption and secondary sources. Environ. Sci. Technol. 2018, 52, 14187–14197. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency (ECHA). Understanding REACH. Registering, Evaluating, Authorising and Restricting Chemicals under REACH. Available online: https://echa.europa.eu/regulations/reach/understanding-reach (accessed on 30 July 2025).
- Matthies, M.; Solomon, K.; Vighi, M.; Gilman, A.; Tarazona, J.V. The origin and evolution of assessment criteria for persistent, bioaccumulative and toxic (PBT) chemicals and persistent organic pollutants (POPs). Environ. Sci. Process. Impacts 2016, 18, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.R. Assessment of Contamination Associated with Tobacco Product Waste Within the Kendall-Frost Reserve. Masters Dissertation, San Diego State University, San Diego, CA, USA, 2022. [Google Scholar]
- Waaijers, S.L.; Kong, D.; Hendriks, H.S.; de Wit, C.A.; Cousins, I.T.; Westerink, R.H.; Leonards, P.E.; Kraak, M.H.; Admiraal, W.; de Voogt, P.; et al. Persistence, bioaccumulation, and toxicity of halogen-free flame retardants. Rev. Environ. Contam. Toxicol. 2012, 222, 1–71. [Google Scholar]
- Bamal, D.; Duhan, A.; Pal, A.; Beniwal, R.K.; Kumawat, P.; Dhanda, S.; Goyat, A.; Hooda, V.S.; Yadav, R. Herbicide risks to non-target species and the environment: A review. Environ. Chem. Lett. 2024, 22, 2977–3032. [Google Scholar] [CrossRef]
- Masoner, J.R.; Kolpin, D.W.; Cozzarelli, I.M.; Barber, L.B.; Burden, D.S.; Foreman, W.T.; Forshay, K.J.; Furlong, E.T.; Groves, J.F.; Hladik, M.L.; et al. Urban stormwater: An overlooked pathway of extensive mixed contaminants to surface and groundwaters in the United States. Environ. Sci. Technol. 2019, 53, 10070–10081. [Google Scholar] [CrossRef]
- Yang, Y.; Vance, M.; Tou, F.; Tiwari, A.; Liu, M.; Hochella, M.F. Nanoparticles in road dust from impervious urban surfaces: Distribution, identification, and environmental implications. Environ. Sci. Nano 2016, 3, 534–544. [Google Scholar] [CrossRef]
- Alkan, N.; Alkan, A.; Salih, B.; Yilmaz, C.; Üçüncü, O. Environmental distributions of phthalates in sediments affected by municipal wastewater in the South-eastern Black Sea. Chemosphere 2025, 377, 144364. [Google Scholar] [CrossRef]
- Qin, R.X.; Fu, C.Z.; Zhuo, L.; Zhang, S.Y.; Cai, F.S.; Wu, K.Y.; Yan, X.; Luo, W.K.; Li, M.; Shi, Y.G.; et al. Estimating wastewater emissions and environmental levels of typical organic contaminants based on regionalized modelling. Environ. Res. 2025, 270, 120965. [Google Scholar] [CrossRef]
- Salve, P.R.; Maurya, A.; Wate, S.R.; Devotta, S. Chemical composition of major ions in rainwater. Bull. Environ. Contam. Toxicol. 2008, 80, 242–246. [Google Scholar] [CrossRef]
- Cottrell, B.A.; Gonsior, M.; Isabelle, L.M.; Luo, W.; Perraud, V.; McIntire, T.M.; Pankow, J.F.; Schmitt-Kopplin, P.; Cooper, W.J.; Simpson, A.J. A regional study of the seasonal variation in the molecular composition of rainwater. Atmos. Environ. 2013, 77, 588–597. [Google Scholar] [CrossRef]
- Wang, H.; Han, G. Chemical composition of rainwater and anthropogenic influences in Chengdu, Southwest China. Atmos. Res. 2011, 99, 190–196. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, J.; Liu, C.; Jing, R.; Lu, Q. Spatial and Temporal Variations in Rainwater Chemistry in a Rapid Urbanization Area of Shenzhen, China. Atmosphere 2024, 15, 1536. [Google Scholar] [CrossRef]
- Keresztesi, Á.; Nita, I.A.; Boga, R.; Birsan, M.V.; Bodor, Z.; Szép, R. Spatial and long-term analysis of rainwater chemistry over the conterminous United States. Environ. Res. 2020, 188, 109872. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.A.; Santos, V.S.; Vizioli, B.C.; Ferreira, B.S.; Montagner, C.C. Pesticides in rainwater: A two-year occurrence study in an unexplored environmental compartment in regions with different land use in the State of São Paulo–Brazil. Chemosphere 2025, 372, 144093. [Google Scholar] [CrossRef]
- Zamora, C.; Kratzer, C.R.; Majewski, M.S.; Knifong, D.L. Diazinon and chlorpyrifos loads in precipitation and urban and agricultural storm runoff during January and February 2001 in the San Joaquin River Basin, California. Water-Resour. Investig. Rep. 2003, 3, 4091. [Google Scholar]
- World Health Organization. Nitrate and Nitrite in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2017; Available online: https://www.who.int/docs/default-source/wash-documents/wash-chemicals/nitrate-nitrite-background-document.pdf (accessed on 4 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).