Zinc Accumulation Pattern in Native Cortaderia nitida in High Andes (Ecuador) and Potential for Zinc Phytoremediation in Soil
Abstract
1. Introduction
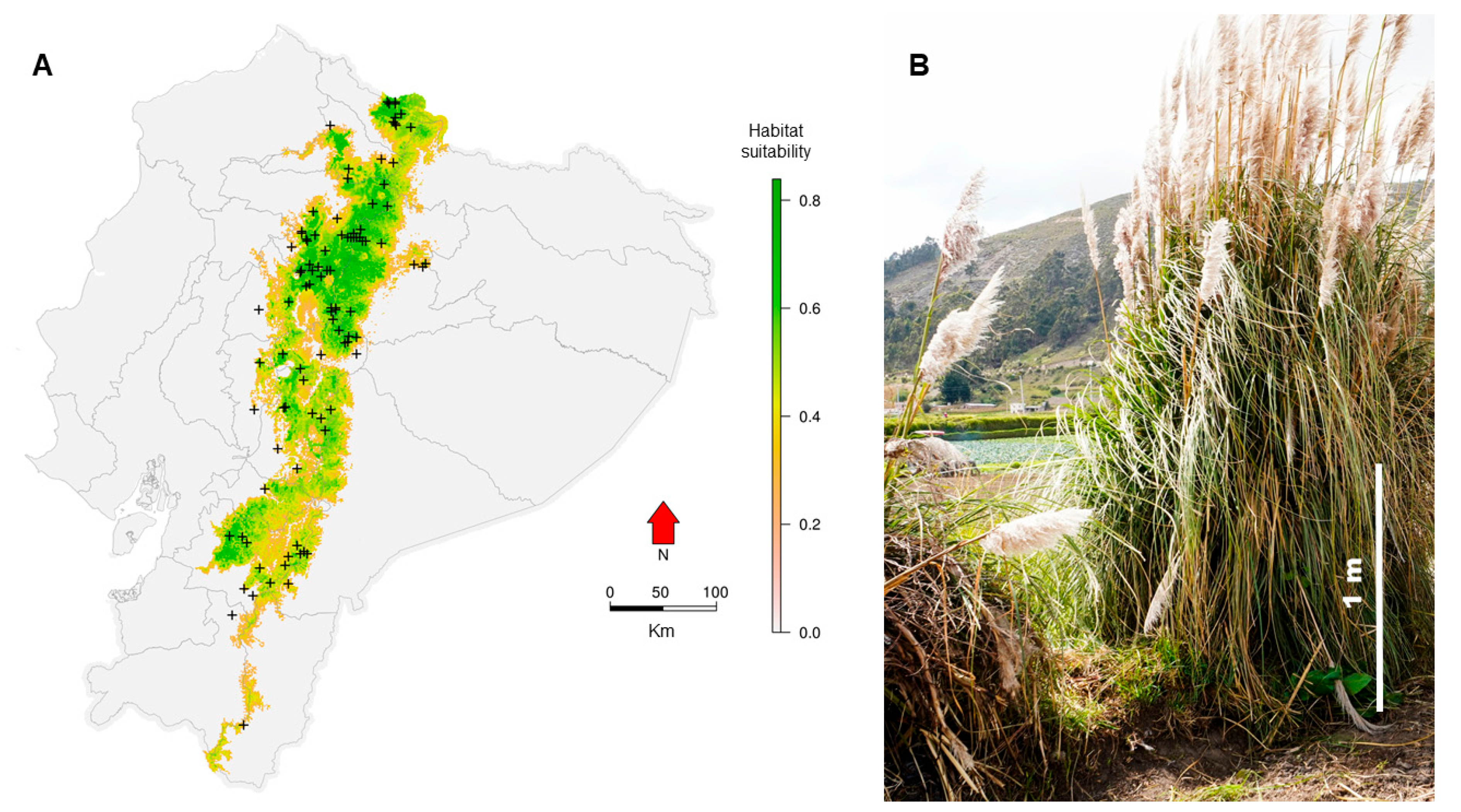
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Sediment and Plant Physico-Chemical Analysis
2.3. Bioconcentration, Bioaccumulation and Translocation Factors
2.4. Statistical Analysis
3. Results
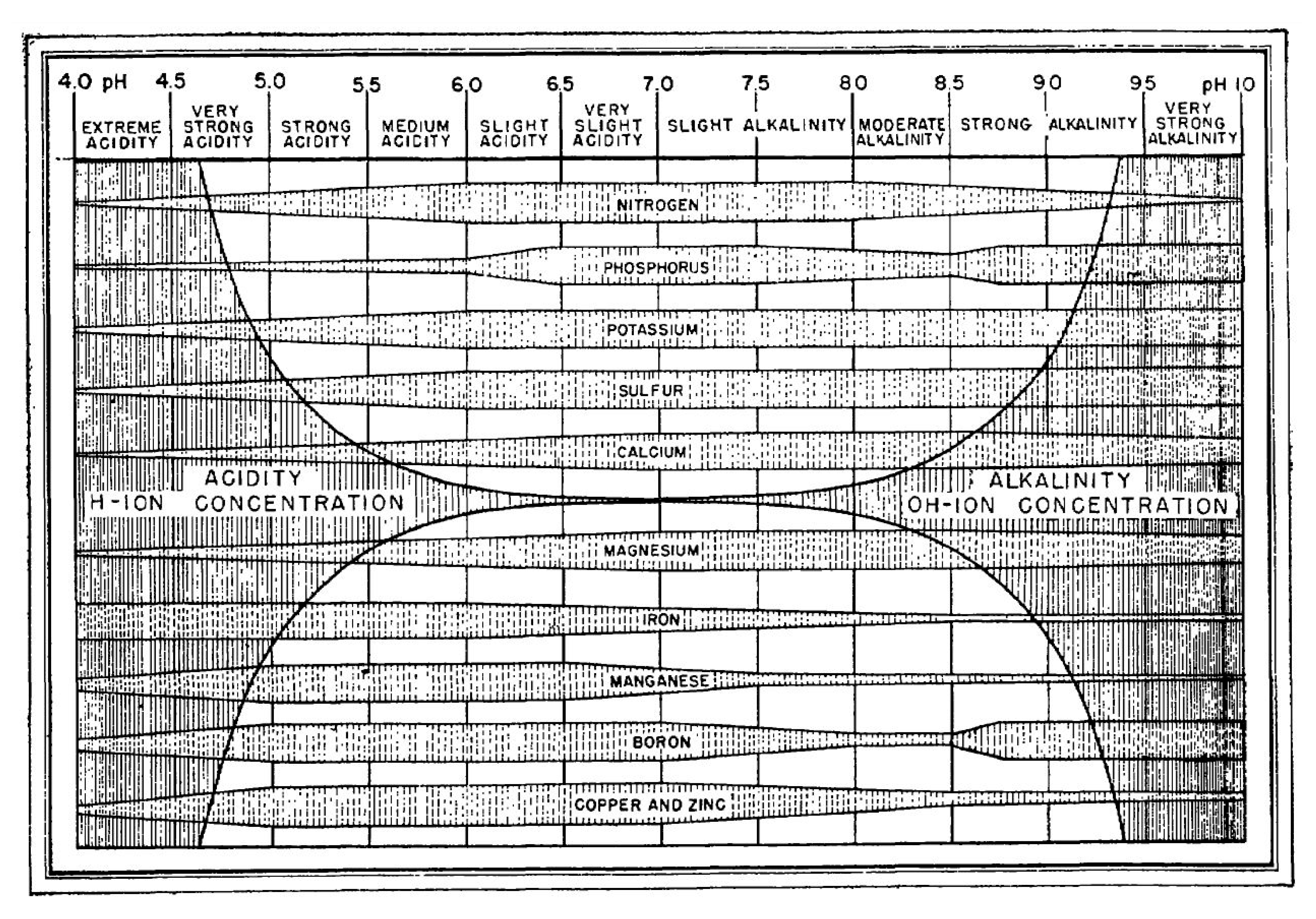
3.1. Physico-Chemical Traits of Bulk Sediments and Rhizosediments
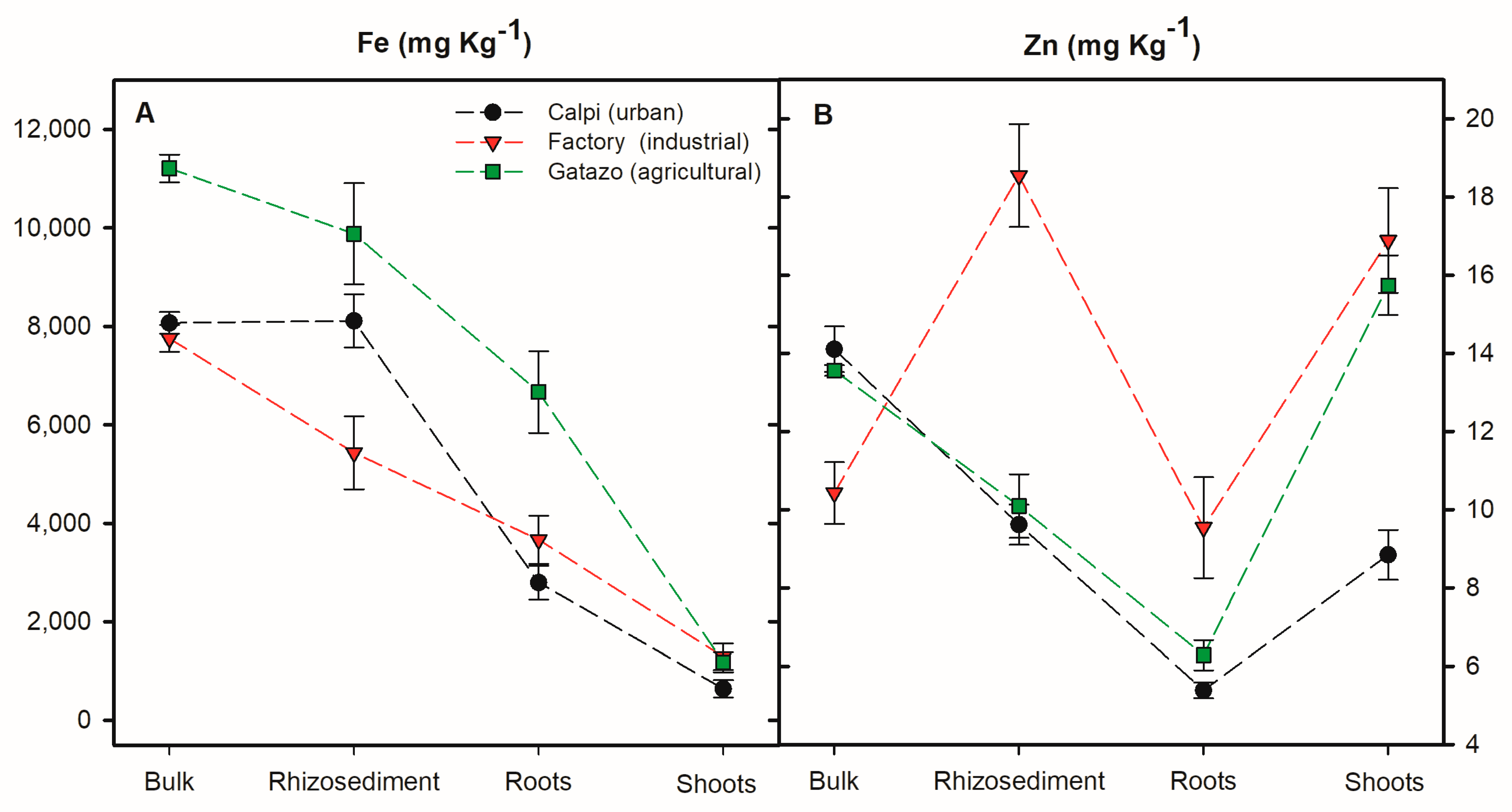
3.2. Metal Content in Sediments and Cortaderia Nitida Tissues
3.3. Zinc Accumulation Capacity of Cortaderia nitida
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pilger, R.K.F. Botanische Jahrbücher Für Systematik. Pflanzengesch. Und Pflanzengeogr. 1906, 37, 375. [Google Scholar]
- Connor, H.E. Catalogue of New World Grasses (Poaceae): III. Subfamilies Panicoideae, Aristidoideae, Arundinoideae and Danthonioideae. Contrib. United States Natl. Herb. 2003, 46, 163–166. [Google Scholar]
- Testoni, D.; Linder, H.P. Synoptic Taxonomy of Cortaderia Stapf (Danthonioideae, Poaceae). PhytoKeys 2017, 76, 39–69. [Google Scholar] [CrossRef]
- Domènech, R.; Vilà, M. Cortaderia Selloana Seed Germination under Different Ecological Conditions. Acta Oecologica 2008, 33, 93–96. [Google Scholar] [CrossRef]
- Anza, M.; Epelde, L.; Artetxe, U.; Becerril, J.M.; Garbisu, C. Control of Cortaderia Selloana with a Glyphosate-Based Herbicide Led to a Short-Term Stimulation of Soil Fungal Communities. Environ. Monit. Assess. 2016, 188, 631. [Google Scholar] [CrossRef] [PubMed]
- Mgidi, T.N.; Le Maitre, D.C.; Schonegevel, L.; Nel, J.L.; Rouget, M.; Richardson, D.M. Alien Plant Invasions-Incorporating Emerging Invaders in Regional Prioritization: A Pragmatic Approach for Southern Africa. J. Environ. Manag. 2007, 84, 173–187. [Google Scholar] [CrossRef]
- Roldão Almeida, M.; Marchante, E.; Marchante, H. Public Perceptions about the Invasive Pampas Grass, Cortaderia Selloana: A Case Study of Environmentally Conscious Citizens in Southern Europe. Biol. Invasions 2023, 25, 2043–2056. [Google Scholar] [CrossRef]
- Parsons, W.T.; Cuthbertson, E.G. Noxious Weeds of Australia, 2nd ed.; CSIRO Publishing: Collingwood, VIC, Australia, 2001. [Google Scholar]
- Romoleroux, K.; Cárate-Tandalla, D.; Erler, R.; Navarrete, H. Plantas Vasculares de Los Bosques de Polylepis En Los Páramos de Oyacachi 2019.0 Version. Available online: https://bioweb.bio/floraweb/polylepis/FichaEspecie/Cortaderia%20nitida (accessed on 6 July 2024).
- Flores de la Torre, J.A.; Mitchell, K.; Ramos Gómez, M.S.; Guerrero Barrera, A.L.; Yamamoto Flores, L.; Avelar González, F.J. Effect of Plant Growth on Pb and Zn Geoaccumulation in 300-Year-Old Mine Tailings of Zacatecas, México. Environ. Earth Sci. 2018, 77, 386. [Google Scholar] [CrossRef]
- Flores-Torres, G.; Solis-Hernández, A.P.; Vela-Correa, G.; Rodríguez-Tovar, A.V.; Cano-Flores, O.; Castellanos-Moguel, J.; Pérez, N.O.; Chimal-Hernández, A.; Moreno-Espíndola, I.P.; Salas-Luévano, M.Á.; et al. Pioneer Plant Species and Fungal Root Endophytes in Metal-Polluted Tailings Deposited near Human Populations and Agricultural Areas in Northern Mexico. Environ. Sci. Pollut. Res. 2021, 28, 55072–55088. [Google Scholar] [CrossRef]
- Bech, J.; Roca, N.; Tume, P.; Ramos-Miras, J.; Gil, C.; Boluda, R. Screening for New Accumulator Plants in Potential Hazards Elements Polluted Soil Surrounding Peruvian Mine Tailings. CATENA 2016, 136, 66–73. [Google Scholar] [CrossRef]
- Martínez-Manchego, L.; Sarmiento-Sarmiento, G.; Bocardo-Delgado, E. Native Plant Species with Potential for Phytoremediation of High-Andean Soils Contaminated by Residues from Mining Activity. Bioagro 2021, 33, 161–170. [Google Scholar] [CrossRef]
- Sepúlveda, B.; Rojos, S.; Silva, W.; Sepúlveda, B.; Tume, P.; Pavez, O. Uptake of Cu, Hg, and As in Wild Vegetation, Associated to Surface Water in the Copiapó Valley, before the 2015 Alluvium. Environ. Geochem. Health 2022, 45, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Rabêlo, F.H.S.; Vangronsveld, J.; Baker, A.J.M.; van der Ent, A.; Alleoni, L.R.F. Are Grasses Really Useful for the Phytoremediation of Potentially Toxic Trace Elements? A Review. Front. Plant Sci. 2021, 12, 778275. [Google Scholar] [CrossRef] [PubMed]
- Mesa, J.; Rodríguez-Llorente, I.D.; Pajuelo, E.; Piedras, J.M.B.; Caviedes, M.A.; Redondo-Gómez, S.; Mateos-Naranjo, E. Moving Closer towards Restoration of Contaminated Estuaries: Bioaugmentation with Autochthonous Rhizobacteria Improves Metal Rhizoaccumulation in Native Spartina Maritima. J. Hazard. Mater. 2015, 300, 263–271. [Google Scholar] [CrossRef]
- Paredes-Páliz, K.I.; Mateos-Naranjo, E.; Doukkali, B.; Caviedes, M.A.; Redondo-Gómez, S.; Rodríguez-Llorente, I.D.; Pajuelo, E. Modulation of Spartina densiflora Plant Growth and Metal Accumulation upon Selective Inoculation Treatments: A Comparison of Gram Negative and Gram Positive Rhizobacteria. Mar. Pollut. Bull. 2017, 125, 77–85. [Google Scholar] [CrossRef]
- Veloz-Mayorga, N.C.; Carbonel, C.A.A. Evaluation of the Water Quality of the Microcuenca of the Chibunga-Ecuador River in Seasonal Variations, Period 2013–2017. 2018. Available online: https://alicia.concytec.gob.pe/vufind/Record/REVUNMSM_98734652b0f25abca82b5a5d87efc778 (accessed on 10 December 2023).
- Romero-Granja, C.; Wollni, M. Opportunistic Behaviour and Trust: Experimental Results from Broccoli Farmers in Ecuador. J. Agric. Econ. 2019, 70, 62–80. [Google Scholar] [CrossRef]
- Iglesias-Quintana, J.X.; Puerta-Martínez, Y.; Cangas-Oña, L.X.; Álvarez-Enríquez, G.F. State of the Enviromental Legal Situation and Co-Responsibility with the Ecosystem of the Chibunga River Communities—Riobamba, Ecuador. Dilemas Contemp. Educ. Política Y Valores 2019, 78, 1–15. Available online: https://dilemascontemporaneoseducacionpoliticayvalores.com/index.php/dilemas/article/view/1430/1725 (accessed on 10 December 2023).
- Prieto, J.; Gonzales, C.; Roman, A.; Prieto, F. Plant Contamination and Phytotoxicity Due to Heavy Metals from Soil and Water. Trop. Subtrop. Agroecosyst. 2009, 10, 29–44. [Google Scholar]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive Mechanisms of Heavy Metal Toxicity in Plants, Detoxification, and Remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef]
- López-Rosas, H.; Romero, J. Medición Del Potencial Redox Del Suelo y Construcción de Electrodos de Platino. In Breviario Para Describir, Observar y Manejar Humedales; Moreno-Casasola, P., Warner, B., Eds.; RAMSAR, INECOL, CONANP, US Fish and Wildlife Service, US State Department: Xalapa, Mexico, 2009; pp. 131–138. ISBN 9786077579120. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Guidelines for Soil Description, 4th ed.; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2006. [Google Scholar]
- Zagal, E.; Sadzawka, A. Protocolo de Métodos de Análisis Para Suelos y Lodos; Universidad de Concepción y Comisión de Normalización y Acreditación de La Sociedad Chilena de La Ciencia Del Suelo: Chillán, Chile, 2007. [Google Scholar]
- República del Ecuador. Norma de Calidad Ambiental del Recurso Suelo y Criterios de Remediación Para Suelos Contaminados; República del Ecuador: Quito, Ecuador, 2016. [Google Scholar]
- República del Ecuador. Acuerdo Ministerial No. 028 de 2015, Norma de Calidad Ambiental Del Recurso Suelo y Criterios de Remediación Para Suelos Con-Taminados de Ecuador; República del Ecuador: Quito, Ecuador, 2015. [Google Scholar]
- Briceño, J.; Armado, A.; Sequera, Á.; Niño-Ruiz, Z. Estimation of Base and Reference Levels for Iron, Manganese, Nickel and Zinc in Soils of an Area Not Intervened Anthropogenically. Rev. Investig. Talent. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in Plants: Tansley Review. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Preeti, P.; Tripathi, A.K. Effect of Heavy Metals on Morphological and Biochemical Characteristics of Albizia Procera (Roxb.) Benth. Seedlings. Int. J. Environ. Sci. 2011, 1, 1009–1018. [Google Scholar]
- Milner, P.; Barker, A.V. Factors Affecting Zinc Concentrations in Plants Grown in Sludge-Amended Soils. Commun. Soil Sci. Plant Anal. 1989, 20, 1–21. [Google Scholar] [CrossRef]
- Tagwira, F.; Mugwira, L.; Piha, M. Effect of PH, and Phosphorus and Organic Matter Contents on Zinc Availability and Distribution in Two Zimbabwean Soils. Commun. Soil Sci. Plant Anal. 1992, 23, 1485–1500. [Google Scholar] [CrossRef]
- Xian, X.; In Shokohifard, G. Effect of PH on Chemical Forms and Plant Availability of Cadmium, Zinc, and Lead in Polluted Soils. Water Air Soil Pollut. 1989, 45, 265–273. [Google Scholar] [CrossRef]
- Lindsay, W.L. Zinc in Soils and Plant Nutrition. Adv. Agron. 1972, 24, 147–186. [Google Scholar] [CrossRef]
- Truog, E. Soil Reaction Influence on Availability of Plant Nutrients. Soil Sci. Soc. Am. J. 1947, 11, 305–308. [Google Scholar] [CrossRef]
- Hartemink, A.E.; Barrow, N.J. Soil PH—Nutrient Relationships: The Diagram. Plant Soil 2023, 486, 209–215. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised Pourbaix Diagrams for Zinc at 25–300 °C. Corros. Sci. 1997, 39, 107–114. [Google Scholar] [CrossRef]
- Nouri, J.; Khorasani, N.; Lorestani, B.; Karami, M.; Hassani, A.H.; Yousefi, N. Accumulation of Heavy Metals in Soil and Uptake by Plant Species with Phytoremediation Potential. Environ. Earth Sci. 2009, 59, 315–323. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Andrades-Moreno, L.; Mateos-Naranjo, E.; Parra, R.; Valera-Burgos, J.; Aroca, R. Synergic Effect of Salinity and Zinc Stress on Growth and Photosynthetic Responses of the Cordgrass, Spartina densiflora. J. Exp. Bot. 2011, 62, 5521–5530. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Naranjo, E.; Pérez-romero, J.A.; Redondo-gómez, S.; Mesa-Marín, J.; Manuel, E.; John, A. Salinity Alleviates Zinc Toxicity in the Saltmarsh Zinc-Accumulator Juncus acutus. Ecotoxicol. Environ. Saf. 2018, 163, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Khoshgoftar, A.H.; Shariatmadari, H.; Karimian, N.; Kalbasi, M.; van der Zee, S.E.A.T.M.; Parker, D.R. Salinity and Zinc Application Effects on Phytoavailability of Cadmium and Zinc. Soil Sci. Soc. Am. J. 2004, 68, 1885–1889. [Google Scholar] [CrossRef]
- Baker, A.J.M.; McGrath, S.P.; Reeves, R.D.; Smith, J.A.C. Metal Hyperaccumulator Plants: A Review of the Ecology and Physiology of a Biological Resource for Phytoremediation of Metal-Polluted Soils. In Phytoremediation of Contaminated Soil and Water; Terry, N., Bañuelos, G., Eds.; Lewis Publishers: Boca Ratón, FL, USA, 2000; pp. 85–107. [Google Scholar]
- Abhari, P.S.; Manteghi, F.; Tehrani, Z. Adsorption of Lead Ions by a Green AC/HKUST-1 Nanocomposite. Nanomaterials 2020, 10, 1647. [Google Scholar] [CrossRef]


| Location | Sediment | pH | Conductivity (μS/cm) | Redox Potential (mV) | Texture |
|---|---|---|---|---|---|
| Calpi (urban) | Bulk | 8.32 ± 0.10 a | 89.00 ± 14.57 a | 141.53 ± 4.72 ab | Loamy |
| Rhizosediment | 7.98 ± 0.09 b | 155.33 ± 12.91 c | 149.00 ± 3.28 ab | Silty loam | |
| Factory (industrial) | Bulk | 8.47 ± 0.15 a | 82.00 ± 2.00 a | 141.36 ± 7.23 ab | Loamy |
| Rhizosediment | 8.52 ± 0.08 a | 108.67 ± 16.47 ab | 138.63 ± 1.07 a | Silty loam | |
| Gatazo (agricultural) | Bulk | 7.85 ± 0.02 b | 131.33 ± 6.48 bc | 151.16 ± 0.49 bc | Silty loam |
| Rhizosediment | 7.46 ± 0.03 c | 261.66 ± 11.46 d | 163.33 ± 2.63 c | Silty loam |
| Location | Fe | Zn | ||||
|---|---|---|---|---|---|---|
| BAF | BCF | TF | BAF | BCF | TF | |
| Calpi (urban) | 0.42 * | 0.34 * | 0.23 | 1.48 | 0.56 | 1.64 |
| Factory (industrial) | 0.91 * | 0.67 | 0.35 * | 1.42 | 0.51 | 1.77 |
| Gatazo (agricultural) | 0.79 * | 0.67 | 0.18 | 2.18 * | 0.62 | 2.51 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paredes-Páliz, K.I.; Mendoza, B.; Mesa-Marín, J. Zinc Accumulation Pattern in Native Cortaderia nitida in High Andes (Ecuador) and Potential for Zinc Phytoremediation in Soil. Environments 2024, 11, 205. https://doi.org/10.3390/environments11090205
Paredes-Páliz KI, Mendoza B, Mesa-Marín J. Zinc Accumulation Pattern in Native Cortaderia nitida in High Andes (Ecuador) and Potential for Zinc Phytoremediation in Soil. Environments. 2024; 11(9):205. https://doi.org/10.3390/environments11090205
Chicago/Turabian StyleParedes-Páliz, Karina I., Benito Mendoza, and Jennifer Mesa-Marín. 2024. "Zinc Accumulation Pattern in Native Cortaderia nitida in High Andes (Ecuador) and Potential for Zinc Phytoremediation in Soil" Environments 11, no. 9: 205. https://doi.org/10.3390/environments11090205
APA StyleParedes-Páliz, K. I., Mendoza, B., & Mesa-Marín, J. (2024). Zinc Accumulation Pattern in Native Cortaderia nitida in High Andes (Ecuador) and Potential for Zinc Phytoremediation in Soil. Environments, 11(9), 205. https://doi.org/10.3390/environments11090205







