Factors Influencing Endangered Marine Species in the Mediterranean Sea: An Analysis Based on IUCN Red List Criteria Using Statistical and Soft Computing Methodologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Analysis
2.2. Univariate Analysis
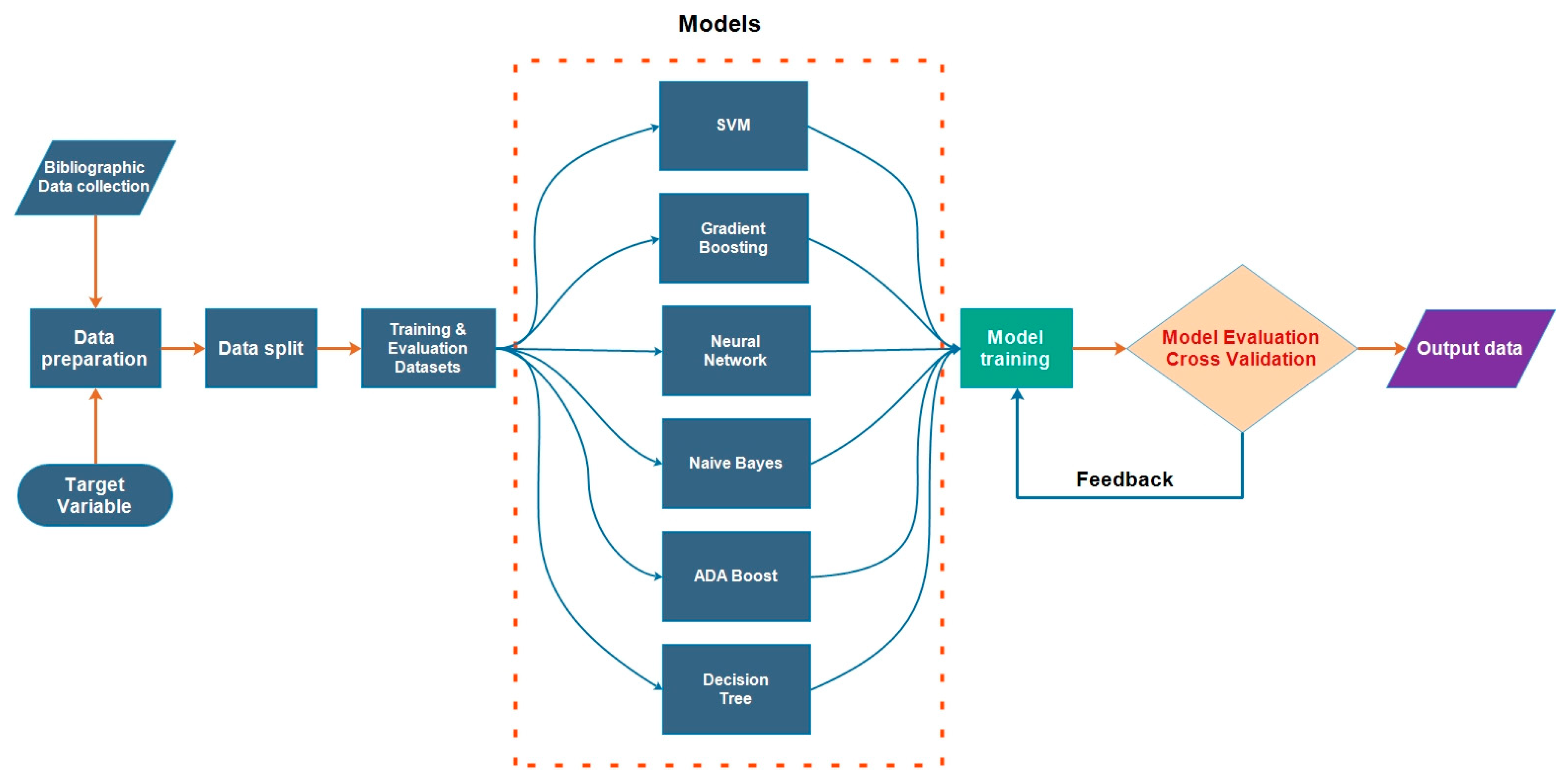
2.3. Machine Learning
2.4. Description of ML Algorithms
2.4.1. The Support Vector Machine Model
2.4.2. Gradient Boosting
2.4.3. Neural Network
2.4.4. Naïve Bayes Classifiers
2.4.5. Adaptive Boosting
2.4.6. Decision Tree
3. Results
3.1. Univariate Statistics
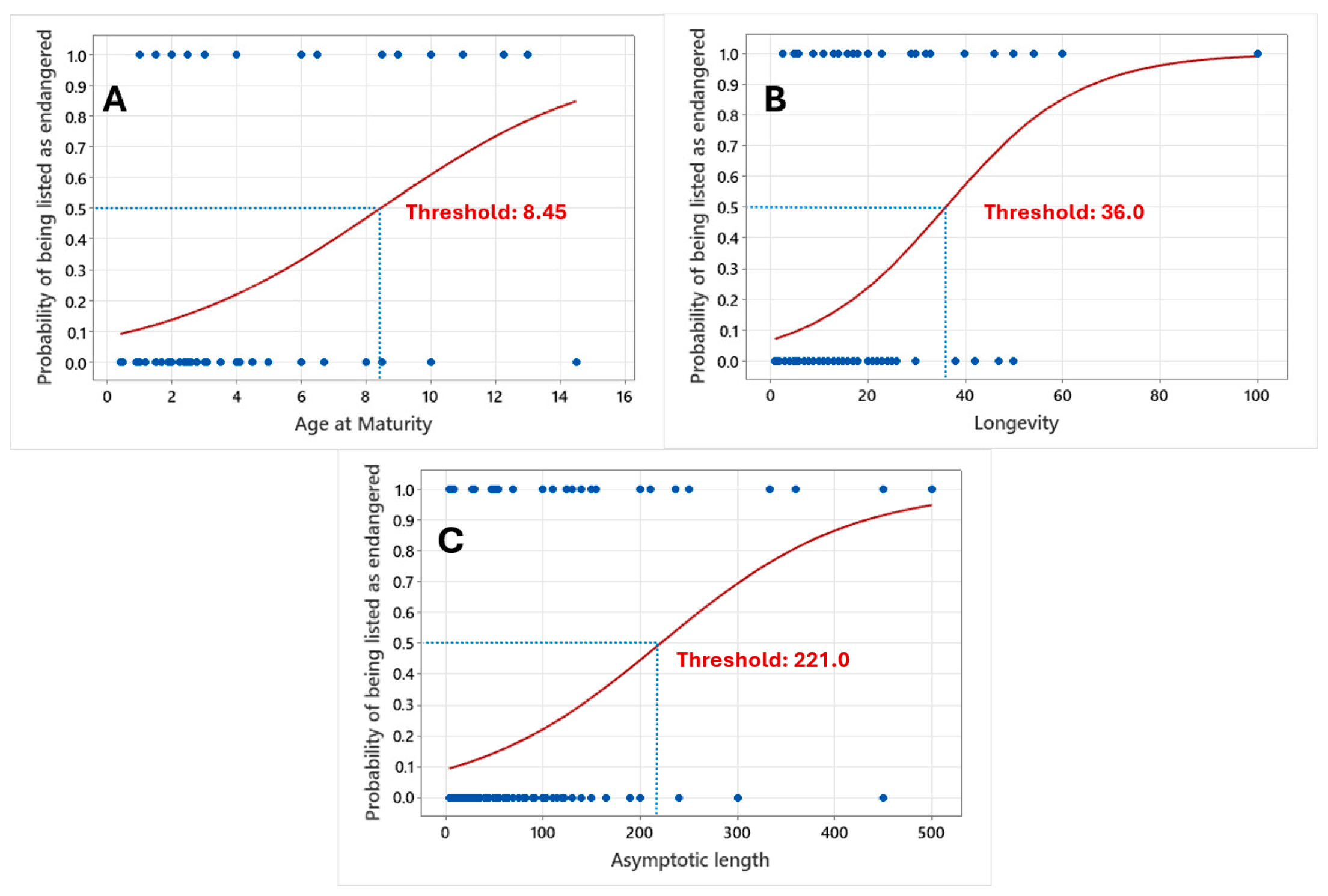
3.2. Machine Learning
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bianchi, C.N.; Morri, C. Marine Biodiversity of the Mediterranean Sea: Situation, Problems and Prospects for Future Research. Mar. Pollut. Bull. 2000, 40, 367–376. [Google Scholar] [CrossRef]
- Cuttelod, A.; García, N.; Malak, D.A.; Temple, H.J.; Katariya, V. The Mediterranean: A biodiversity hotspot under threat. In Wildlife in a Changing World—An Analysis 2008 IUCN Red List Threatened Species; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2009; Volume 89. [Google Scholar]
- Lotze, H.K. Marine biodiversity conservation. Curr. Biol. 2021, 31, R1190–R1195. [Google Scholar] [CrossRef] [PubMed]
- Culotta, E. Exploring biodiversity’s benefits. Science 1996, 273, 1045–1046. [Google Scholar] [CrossRef]
- Bengtsson, J.; Jones, H.; Setälä, H. The value of biodiversity. Trends Ecol. Evol. 1997, 12, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Biodiversity and ecosystem function: The debate deepens. Science 1997, 277, 1260–1261. [Google Scholar] [CrossRef]
- Aarts, B.G.W. Ecological sustainability and biodiversity. Int. J. Sustain. Dev. World Ecol. 1999, 6, 89–102. [Google Scholar] [CrossRef]
- Neeman, N.; Servis, J.A.; Naro-Maciel, E. Conservation issues: Oceanic ecosystems. In Encyclopedia of the Anthropocene; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1–5, pp. 193–201. ISBN 9780128096659. [Google Scholar]
- Asaad, I.; Lundquist, C.J.; Erdmann, M.V.; Costello, M.J. The coral triangle: The most species rich marine region on earth. Encycl. World’s Biomes 2020. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [PubMed]
- IUCN. The IUCN Red List of Threatened Species; Version 2023-1; IUCN: Gland, Switzerland, 2023. [Google Scholar]
- Kontoyiannis, H.; Lykousis, V.; Papadopoulos, V.; Stavrakakis, S.; Anassontzis, E.G.; Belias, A.; Koutsoukos, S.; Resvanis, L.K. Hydrography, Circulation, and Mixing at the Calypso Deep (the Deepest Mediterranean Trough) during 2006-2009. J. Phys. Oceanogr. 2016, 46, 1255–1276. [Google Scholar] [CrossRef]
- Shaltout, M.; Omstedt, A. Recent sea surface temperature trends and future scenarios for the Mediterranean Sea. Oceanologia 2014, 56, 411–443. [Google Scholar] [CrossRef]
- Danovaro, R. Climate change impacts on the biota and on vulnerable habitats of the deep Mediterranean Sea. Rend. Lincei. Sci. Fis. E Nat. 2018, 29, 525–541. [Google Scholar] [CrossRef]
- Azov, Y. Eastern Mediterranean—A marine desert? Mar. Pollut. Bull. 1991, 23, 225–232. [Google Scholar] [CrossRef]
- Danovaro, R.; Canals, M.; Gambi, C.; Heussner, S.; Lampadariou, N.; Vanreusel, A. Exploring benthic biodiversity patterns and hotspots on European margin slopes. Oceanography 2009, 22, 16–25. [Google Scholar] [CrossRef]
- IUCN. Guidelines for Using the IUCN Red List Categories and Criteria; Version 15; IUCN Standards and Petitions Committee: Gland, Switzerland, 2022. [Google Scholar]
- Baum, J.K.; Myers, R.A.; Kehler, D.G.; Worm, B.; Harley, S.J.; Doherty, P.A. Collapse and conservation of shark populations in the Northwest Atlantic. Science 2003, 299, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.A.; Worm, B. Extinction, survival or recovery of large predatory fishes. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 13–20. [Google Scholar] [CrossRef]
- Jennings, S.; Kaiser, M.J. The effects of fishing on marine ecosystems. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 1998; Volume 34, pp. 201–352. ISBN 0065-2881. [Google Scholar]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F., Jr. Fishing down marine food webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef]
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, J.E.; Folke, C.; Halpern, B.S.; Jackson, J.B.C.; Lotze, H.K.; Micheli, F.; Palumbi, S.R. Impacts of biodiversity loss on ocean ecosystem services. Science 2006, 314, 787–790. [Google Scholar] [CrossRef]
- MacKenzie, B.R.; Mosegaard, H.; Rosenberg, A.A. Impending collapse of bluefin tuna in the northeast Atlantic and Mediterranean. Conserv. Lett. 2009, 2, 26–35. [Google Scholar] [CrossRef]
- Lleonart, J.; Maynou, F. Fish stock assessments in the Mediterranean: State of the art. Sci. Mar. 2003, 67, 37–49. [Google Scholar] [CrossRef]
- Papaconstantinou, C.; Farrugio, H. Fisheries in the Mediterranean. Mediterr. Mar. Sci. 2000, 1, 5–18. [Google Scholar] [CrossRef]
- Bas, C.; Maynou, F.; Sarda, F.; Lleonart, J. Variacions Demogràfiques a Les Poblacions D’espècies Demersals Explotades; Institut d’Estudis Catalans: Barcelona, Spain, 2003. [Google Scholar]
- Bombace, G.; Grati, F. Che succede alle risorse di pesca del Mediterraneo. In Notiziario Della SocietaItaliana di Biologia Marina; S.I.B.M.: Livorno, Italy, 2007; Volume 51, pp. 29–38. [Google Scholar]
- Hilborn, R.; Branch, T.A.; Ernst, B.; Magnusson, A.; Minte-Vera, C.V.; Scheuerell, M.D.; Valero, J.L. State of the world’s fisheries. Annu. Rev. Environ. Resour. 2003, 28, 359–399. [Google Scholar] [CrossRef]
- Lee, M.; Safina, C. The effects of overfishing on marine biodiversity. Curr. J. Mar. Educ. 1995, 13, 5–9. [Google Scholar]
- Bell, S.; Hampshire, K.; Topalidou, S. The political culture of poaching: A case study from northern Greece. Biodivers. Conserv. 2007, 16, 399–418. [Google Scholar] [CrossRef]
- Chefaoui, R.M.; Duarte, C.M.; Serrão, E.A. Dramatic loss of seagrass habitat under projected climate change in the Mediterranean Sea. Glob. Chang. Biol. 2018, 24, 4919–4928. [Google Scholar] [CrossRef] [PubMed]
- D’Amen, M.; Azzurro, E. Lessepsian fish invasion in Mediterranean marine protected areas: A risk assessment under climate change scenarios. ICES J. Mar. Sci. 2020, 77, 388–397. [Google Scholar] [CrossRef]
- Klausmeyer, K.R.; Shaw, M.R. Climate change, habitat loss, protected areas and the climate adaptation potential of species in mediterranean ecosystems worldwide. PLoS ONE 2009, 4, e6392. [Google Scholar] [CrossRef] [PubMed]
- Chatzimentor, A.; Doxa, A.; Katsanevakis, S.; Mazaris, A.D. Are Mediterranean marine threatened species at high risk by climate change? Glob. Chang. Biol. 2023, 29, 1809–1821. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Bax, N.; Williamson, A.; Aguero, M.; Gonzalez, E.; Geeves, W. Marine invasive alien species: A threat to global biodiversity. Mar. Policy 2003, 27, 313–323. [Google Scholar] [CrossRef]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ. 2008, 6, 485–492. [Google Scholar] [CrossRef]
- Hulme, P.E.; Pyšek, P.; Nentwig, W.; Vilà, M. Will threat of biological invasions unite the European Union? Science 2009, 324, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Kumschick, S.; Gaertner, M.; Vilà, M.; Essl, F.; Jeschke, J.M.; Pyšek, P.; Ricciardi, A.; Bacher, S.; Blackburn, T.M.; Dick, J.T.A. Ecological impacts of alien species: Quantification, scope, caveats, and recommendations. Bioscience 2015, 65, 55–63. [Google Scholar] [CrossRef]
- Zenetos, A.; Galanidi, M. Mediterranean non indigenous species at the start of the 2020s: Recent changes. Mar. Biodivers. Rec. 2020, 13, 10. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; Garcia, E.L.; Stern, N.; Tsiamis, K.; Galanidi, M. Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterr. Mar. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Kondylatos, G.; Theocharis, A.; Mandalakis, M.; Avgoustinaki, M.; Karagyaurova, T.; Koulocheri, Z.; Vardali, S.; Klaoudatos, D. The Devil Firefish Pterois miles (Bennett, 1828): Life History Traits of a Potential Fishing Resource in Rhodes (Eastern Mediterranean). Hydrobiology 2024, 3, 31–50. [Google Scholar] [CrossRef]
- Kondylatos, G.; Vagenas, G.; Kalaentzis, K.; Mavrouleas, D.; Conides, A.; Karachle, P.K.; Corsini-Foka, M.; Klaoudatos, D. Exploring the Structure of Static Net Fisheries in a Highly Invaded Region: The Case of Rhodes Island (Eastern Mediterranean). Sustainability 2023, 15, 14976. [Google Scholar] [CrossRef]
- Galil, B.; Marchini, A.; Occhipinti-Ambrogi, A.; Ojaveer, H. The enlargement of the Suez Canal—Erythraean introductions and management challenges. Manag. Biol. Invasions 2017, 8, 141–152. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of invasive alien marine species on ecosystem services and biodiversity: A pan-European review. Aquat Invasions 9 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Tempera, F.; Teixeira, H. Mapping the impact of alien species on marine ecosystems: The Mediterranean Sea case study. Divers. Distrib. 2016, 22, 694–707. [Google Scholar] [CrossRef]
- Galil, B.S.; Boero, F.; Campbell, M.L.; Carlton, J.T.; Cook, E.; Fraschetti, S.; Gollasch, S.; Hewitt, C.L.; Jelmert, A.; Macpherson, E. ‘Double trouble’: The expansion of the Suez Canal and marine bioinvasions in the Mediterranean Sea. Biol. Invasions 2015, 17, 973–976. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the Siskiyou mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Levin, L.A.; Etter, R.J.; Rex, M.A.; Gooday, A.J.; Smith, C.R.; Pineda, J.; Stuart, C.T.; Hessler, R.R.; Pawson, D. Environmental influences on regional deep-sea species diversity. Annu. Rev. Ecol. Syst. 2001, 32, 51–93. [Google Scholar] [CrossRef]
- Danovaro, R.; Gambi, C.; Dell’Anno, A.; Corinaldesi, C.; Fraschetti, S.; Vanreusel, A.; Vincx, M.; Gooday, A.J. Exponential decline of deep-sea ecosystem functioning linked to benthic biodiversity loss. Curr. Biol. 2008, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S. The measurement of marine species diversity, with an application to the benthic fauna of the Norwegian continental shelf. J. Exp. Mar. Bio. Ecol. 2000, 250, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Spanou, D.S.; Petroudi, P.; Dimou, E.; Kokkinos, K.; Klaoudatos, D. Walleye (Sander vitreus, Mitchill 1818) age and sex classification using innovative supervised and unsupervised machine learning and soft computing methodologies. Fish. Res. 2024, 275, 107031. [Google Scholar] [CrossRef]
- Kriegl, M.; Elías Ilosvay, X.E.; von Dorrien, C.; Oesterwind, D. Marine protected areas: At the crossroads of nature conservation and fisheries management. Front. Mar. Sci. 2021, 8, 676264. [Google Scholar] [CrossRef]
- Simard, F. Marine Protected Areas and Climate Change: Adaptation and Mitigation Synergies, Opportunities and Challenges; IUCN: Gland, Switzerland, 2016; ISBN 2831718198. [Google Scholar]
- Zhu, J.-J.; Yang, M.; Ren, Z.J. Machine Learning in Environmental Research: Common Pitfalls and Best Practices. Environ. Sci. Technol. 2023, 57, 17671–17689. [Google Scholar] [CrossRef]
- Boukabara, S.-A.; Krasnopolsky, V.; Stewart, J.Q.; Maddy, E.S.; Shahroudi, N.; Hoffman, R.N. Leveraging Modern Artificial Intelligence for Remote Sensing and NWP: Benefits and Challenges. Bull. Am. Meteorol. Soc. 2019, 100, ES473–ES491. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, S.; Liu, J.; Wang, H.; Zhu, J.; Li, D.; Zhao, R. Application of machine learning in intelligent fish aquaculture: A review. Aquaculture 2021, 540, 736724. [Google Scholar] [CrossRef]
- Bonino, G.; Galimberti, G.; Masina, S.; Mcadam, R.; Clementi, E. Machine learning methods to predict sea surface temperature and marine heatwave occurrence: A case study of the Mediterranean Sea. Ocean Sci. 2024, 20, 417–432. [Google Scholar] [CrossRef]
- Bourillon, B.; Feunteun, E.; Acou, A.; Trancart, T.; Teichert, N.; Belpaire, C.; Dufour, S.; Bustamante, P.; Aarestrup, K.; Walker, A. Anthropogenic contaminants shape the fitness of the endangered European eel: A machine learning approach. Fishes 2022, 7, 274. [Google Scholar] [CrossRef]
- Davidson, A.D.; Boyer, A.G.; Kim, H.; Pompa-Mansilla, S.; Hamilton, M.J.; Costa, D.P.; Ceballos, G.; Brown, J.H. Drivers and hotspots of extinction risk in marine mammals. Proc. Natl. Acad. Sci. USA 2012, 109, 3395–3400. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT press: Cambridge, MA, USA, 2016. [Google Scholar]
- Froese, R.; Pauly, D. EDITORS FishBase. In World Wide Web Electronic Publication; ScienceOpen: Lexington, MA, USA, 2024. [Google Scholar]
- Ahyong, S.; Boyko, C.B.; Bailly, N.; Bernot, J.; Bieler, R.; Brandão, S.N.; Daly, M.; De Grave, S.; Gofas, S.; Hernandez, F.; et al. World Register of Marine Species (WoRMS) 2024. Available online: https://www.marinespecies.org/ (accessed on 20 May 2024).
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 4 July 2024).
- Şahin, M.; Aybek, E. Jamovi: An Easy to Use Statistical Software for the Social Scientists. Int. J. Assess. Tools Educ. 2019, 6, 670–692. [Google Scholar] [CrossRef]
- Hampton, R.E.; Havel, J.E. Introductory Biological Statistics; Waveland Press: Long Grove, IL, USA, 2006; ISBN 1577663802. [Google Scholar]
- Sall, J.; Stephens, M.L.; Lehman, A.; Loring, S. JMP Start Statistics: A Guide to Statistics and Data Analysis Using JMP; Sas Institute: Cary, NC, USA, 2017; ISBN 1629608785. [Google Scholar]
- Demsar, J.; Curk, T.; Erjavec, A.; Gorup, C.; Hocevar, T.; Milutinovic, M.; Mozina, M.; Polajnar, M.; Toplak, M.; Staric, A.; et al. Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Jakkula, V. Tutorial on support vector machine (svm). Sch. EECS Washingt. State Univ. 2006, 37, 3. [Google Scholar]
- Trafalis, T. Primal-dual optimization methods in neural networks and support vector machines training. ACAI99 1999, 5, 459–478. [Google Scholar]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Natekin, A.; Knoll, A. Gradient boosting machines, a tutorial. Front. Neurorobot. 2013, 7, 21. [Google Scholar] [CrossRef]
- Nielsen, M.A. Neural Networks and Deep Learning; Determination Press: San Francisco, CA, USA, 2015; Volume 25. [Google Scholar]
- Zhang, H. The optimality of naive Bayes. AAAI 2004, 1, 562–567. [Google Scholar]
- Murphy, K.P. Machine Learning: A Probabilistic Perspective; MIT press: Cambridge, MA, USA, 2012; ISBN 0262304325. [Google Scholar]
- Schapire, R.E. A brief introduction to boosting. In Proceedings of the International Joint Conference on Artificial Intelligence, Stockholm, Sweden, 31 July–6 August 1999; Volume 99, pp. 1401–1406. [Google Scholar]
- Freund, Y.; Schapire, R.E. Experiments with a new boosting algorithm. In Proceedings of the International Conference on Machine Learning, Bari, Italy, 3–6 July 1996; Volume 96, pp. 148–156. [Google Scholar]
- Quinlan, J.R. Induction of decision trees. Mach. Learn. 1986, 1, 81–106. [Google Scholar] [CrossRef]
- Safavian, S.R.; Landgrebe, D. A survey of decision tree classifier methodology. IEEE Trans. Syst. Man. Cybern. 1991, 21, 660–674. [Google Scholar] [CrossRef]
- Song, Y.-Y.; Ying, L.U. Decision tree methods: Applications for classification and prediction. Shanghai Arch. Psychiatry 2015, 27, 130. [Google Scholar] [PubMed]
- Dulvy, N.K.; Simpfendorfer, C.A.; Davidson, L.N.K.; Fordham, S.V.; Bräutigam, A.; Sant, G.; Welch, D.J. Challenges and priorities in shark and ray conservation. Curr. Biol. 2017, 27, R565–R572. [Google Scholar] [CrossRef] [PubMed]
- Paquotte, P.; Lem, A. Seafood Markets and Trade: A Global Perspective and an Overview of EU Mediterranean Countries; CIHEAM Montpellier: Montpellier, France, 2008. [Google Scholar]
- Claro, R. Características generales de la ictiofauna. Ecol. Los Peces Mar. Cuba 1994, 55–71. [Google Scholar]
- Knijn, R.J.; Boon, T.W.; Heessen, H.J.L.; Hislop, J.R.G. Atlas of North Sea fishes-Based on bottom-trawl survey data for the years 1985–1987. In ICES Cooperative Research Reports; Monday Morning Books: Ashland, OH, USA, 1993; ISBN 8774825321. [Google Scholar]
- Williams, J.T.; Bogorodsky, S.V. Entomacrodus solus, a new species of blenny (Perciformes, Blenniidae) from the Red Sea. Zootaxa 2010, 2475, 64–68. [Google Scholar] [CrossRef]
- Powles, H. Assessing Risk of Extinction of Marine Fishes in Canada—The COSEWIC Experience. Fisheries 2011, 36, 231–246. [Google Scholar] [CrossRef]
- Reynolds, J.D.; Dulvy, N.K.; Goodwin, N.B.; Hutchings, J.A. Biology of extinction risk in marine fishes. Proc. R. Soc. B Biol. Sci. 2005, 272, 2337–2344. [Google Scholar] [CrossRef]
- Hutchings, J.A.; Myers, R.A.; García, V.B.; Lucifora, L.O.; Kuparinen, A. Life-history correlates of extinction risk and recovery potential. Ecol. Appl. 2012, 22, 1061–1067. [Google Scholar] [CrossRef]
- Powles, H. Assessing and protecting endangered marine species. ICES J. Mar. Sci. 2000, 57, 669–676. [Google Scholar] [CrossRef]
- Kvamsdal, S.; Hopland, A.O.; Li, Y.; Selle, S. Expert opinions on threats and impacts in the marine environment. Mar. Policy 2023, 147, 105382. [Google Scholar] [CrossRef]
- Gray, J.S. Marine biodiversity: Patterns, threats and conservation needs. Biodivers. Conserv. 1997, 6, 153–175. [Google Scholar] [CrossRef]
- Mazaris, A.D.; Kallimanis, A.; Gissi, E.; Pipitone, C.; Danovaro, R.; Claudet, J.; Rilov, G.; Badalamenti, F.; Stelzenmüller, V.; Thiault, L. Threats to marine biodiversity in European protected areas. Sci. Total Environ. 2019, 677, 418–426. [Google Scholar] [CrossRef]
- Forster, J.; Lake, I.R.; Watkinson, A.R.; Gill, J.A. Marine biodiversity in the Caribbean UK overseas territories: Perceived threats and constraints to environmental management. Mar. Policy 2011, 35, 647–657. [Google Scholar] [CrossRef]
- Luiz, O.J.; Woods, R.M.; Madin, E.M.P.; Madin, J.S. Predicting IUCN Extinction Risk Categories for the World’s Data Deficient Groupers (Teleostei: Epinephelidae). Conserv. Lett. 2016, 9, 342–350. [Google Scholar] [CrossRef]
- Smith, K.G.; Darwall, W.R.T. The Status and Distribution of Freshwater Fish Endemic to the Mediterranean Basin; World Conservation Union (IUCN): Gland, Switzerland, 2006; ISBN 9782831709161. [Google Scholar]
- Freyhof, J.; Chebanov, M.; Pourkazemi, M. Acipenser nudiventris. In IUCN Red List Threatened Species 2022 (Errata Version Publish 2023); IUCN: Gland, Switzerland, 2022. [Google Scholar]
- Bronzi, P.; Congiu, L.; Freyhof, J. Acipenser naccarii. In IUCN Red List Threatened Species 2022; IUCN: Gland, Switzerland, 2022. [Google Scholar]
- Mugue, N.; Friedrich, T.; Chebanov, M.; Ruban, G. Acipenser stellatus. In IUCN Red List Threatened Species 2022; IUCN: Gland, Switzerland, 2022. [Google Scholar]
- Gessner, J.; Williot, P.; Rochard, E.; Freyhof, J.; Kottelat, M. Acipenser sturio. In IUCN Red List Threatened Species 2022 (Errata Version Publish 2023); IUCN: Gland, Switzerland, 2022. [Google Scholar]
- Gessner, J.; Freyhof, J.; Kottelat, M. Acipenser gueldenstaedtii. In IUCN Red List Threatened Species 2022; IUCN: Gland, Switzerland, 2022. [Google Scholar]
- Food and Agriculture Organization. The State of World Fisheries and Aquaculture 2022. In Towards Blue Transformation; Food and Agriculture Organization: Rome, Italy, 2022. [Google Scholar]
- Hall, M.A.; Alverson, D.L.; Metuzals, K.I. By-catch: Problems and solutions. Mar. Pollut. Bull. 2000, 41, 204–219. [Google Scholar] [CrossRef]
- Kelleher, K. Discards in the World’s Marine Fisheries: An Update; Food and Agriculture Organization: Rome, Italy, 2005; Volume 470, ISBN 9251052891. [Google Scholar]
- Bellido, J.M.; Santos, M.B.; Pennino, M.G.; Valeiras, X.; Pierce, G.J. Fishery discards and bycatch: Solutions for an ecosystem approach to fisheries management? Hydrobiologia 2011, 670, 317–333. [Google Scholar] [CrossRef]
- Squires, D.; Garcia, S. The least-cost biodiversity impact mitigation hierarchy with a focus on marine fisheries and bycatch issues. Conserv. Biol. 2018, 32, 989–997. [Google Scholar] [CrossRef] [PubMed]
- IUCN. IUCN Red List Categories and Criteria: Version 3.1; IUCN: Gland, Switzerland, 2001; ISBN 2831706335. [Google Scholar]
- Otero, J.; Hidalgo, M. Life-history traits and environment shape small pelagic fish demography and responses to fishing and climate across European Atlantic seas. ICES J. Mar. Sci. 2023, 80, 1447–1461. [Google Scholar] [CrossRef]
- Gladju, J.; Kamalam, B.S.; Kanagaraj, A. Applications of data mining and machine learning framework in aquaculture and fisheries: A review. Smart Agric. Technol. 2022, 2, 100061. [Google Scholar] [CrossRef]


| Factor | Units | Classification |
|---|---|---|
| Longevity | years | Numerical factor |
| Asymptotic length | cm | Numerical factor |
| Maximum recorded depth | m | Numerical factor |
| Age at maturity | years | Numerical factor |
| Area of range | km2 | Numerical factor |
| Overfishing | 0 = not vulnerable 1 = vulnerable | Categorical factor |
| By-catch | 0 = not vulnerable 1 = vulnerable | Categorical factor |
| Pollution | 0 = not vulnerable 1 = vulnerable | Categorical factor |
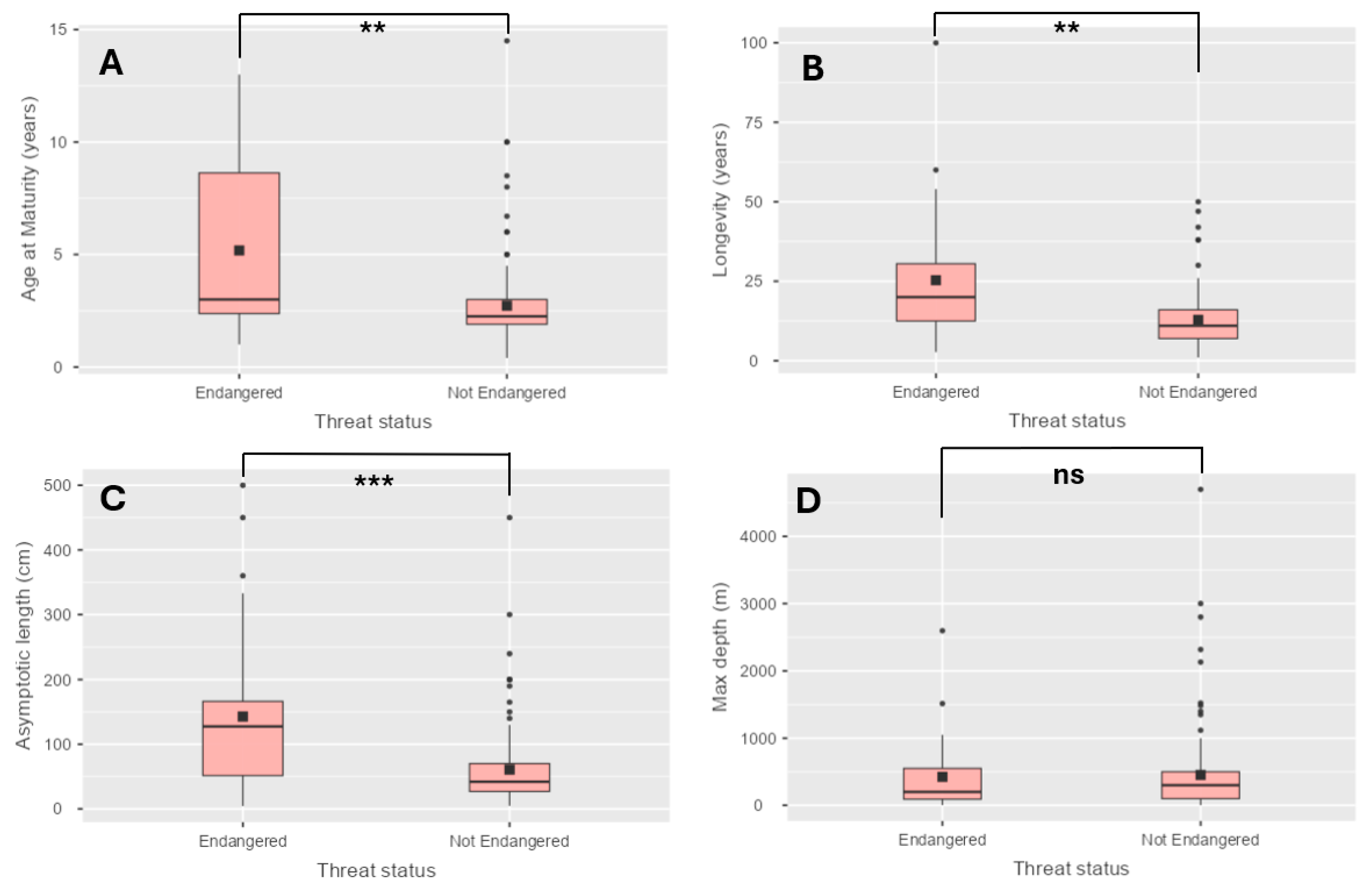
| Threat Category | N | Mean | SE | Median | SD | Min. | Max. | |
|---|---|---|---|---|---|---|---|---|
| Age at Maturity (years) | Endangered | 32 | 5.18 | 3.0 | 3.97 | 3.90 | 1.0 | 13.0 |
| Not Endangered | 123 | 2.72 | 2.3 | 1.97 | 1.97 | 0.4 | 14.5 | |
| Longevity (years) | Endangered | 32 | 25.32 | 20.0 | 20.31 | 20.31 | 2.7 | 100.0 |
| Not Endangered | 123 | 12.85 | 11.0 | 8.59 | 8.59 | 1.0 | 50.0 | |
| Max depth (m) | Endangered | 32 | 424.41 | 200.0 | 563.32 | 563.32 | 3.0 | 2600 |
| Not Endangered | 123 | 452.12 | 300.0 | 643.48 | 643.48 | 2.0 | 4700 | |
| Asymptotic length (cm) | Endangered | 32 | 142.83 | 127.5 | 124.4 | 124.80 | 4.3 | 500 |
| Not Endangered | 123 | 60.69 | 42.0 | 60.74 | 60.74 | 4.7 | 450 |
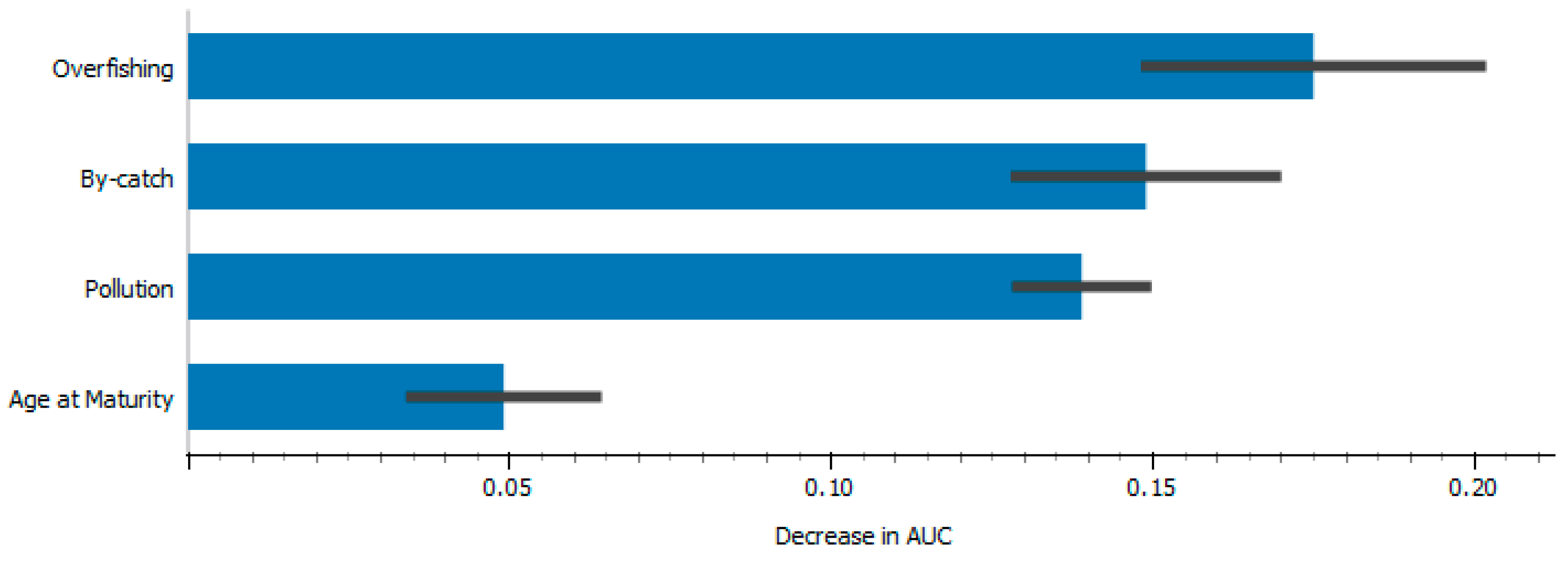
| Source | Logworth | p-Value | |
|---|---|---|---|
| Overfishing | 7.721 |  | 0.00000 |
| Pollution | 4.416 |  | 0.00004 |
| By-catch | 3.697 |  | 0.00020 |
| Age at Maturity | 1.633 |  | 0.02328 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klaoudatos, D.; Karagyaurova, T.; Pitropakis, T.G.I.; Mari, A.; Patas, D.R.; Vidiadaki, M.; Kokkinos, K. Factors Influencing Endangered Marine Species in the Mediterranean Sea: An Analysis Based on IUCN Red List Criteria Using Statistical and Soft Computing Methodologies. Environments 2024, 11, 151. https://doi.org/10.3390/environments11070151
Klaoudatos D, Karagyaurova T, Pitropakis TGI, Mari A, Patas DR, Vidiadaki M, Kokkinos K. Factors Influencing Endangered Marine Species in the Mediterranean Sea: An Analysis Based on IUCN Red List Criteria Using Statistical and Soft Computing Methodologies. Environments. 2024; 11(7):151. https://doi.org/10.3390/environments11070151
Chicago/Turabian StyleKlaoudatos, Dimitris, Teodora Karagyaurova, Theodoros G. I. Pitropakis, Aikaterini Mari, Dimitris R. Patas, Maria Vidiadaki, and Konstantinos Kokkinos. 2024. "Factors Influencing Endangered Marine Species in the Mediterranean Sea: An Analysis Based on IUCN Red List Criteria Using Statistical and Soft Computing Methodologies" Environments 11, no. 7: 151. https://doi.org/10.3390/environments11070151
APA StyleKlaoudatos, D., Karagyaurova, T., Pitropakis, T. G. I., Mari, A., Patas, D. R., Vidiadaki, M., & Kokkinos, K. (2024). Factors Influencing Endangered Marine Species in the Mediterranean Sea: An Analysis Based on IUCN Red List Criteria Using Statistical and Soft Computing Methodologies. Environments, 11(7), 151. https://doi.org/10.3390/environments11070151








