Chronic Heat Exposure Modulates Innate and Adaptive Immune Responses in Firefighters
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Blood Sample Collection and Processing
2.3. RNA Isolation
2.4. Gene Expression Analysis
2.5. Plasma HSP70 Protein Analysis (ELISA)
2.6. Statistical Analysis
3. Results
3.1. Demographic Profiles and Exposure Grouping of Study Subjects
3.2. Blood Immune Cell Landscape
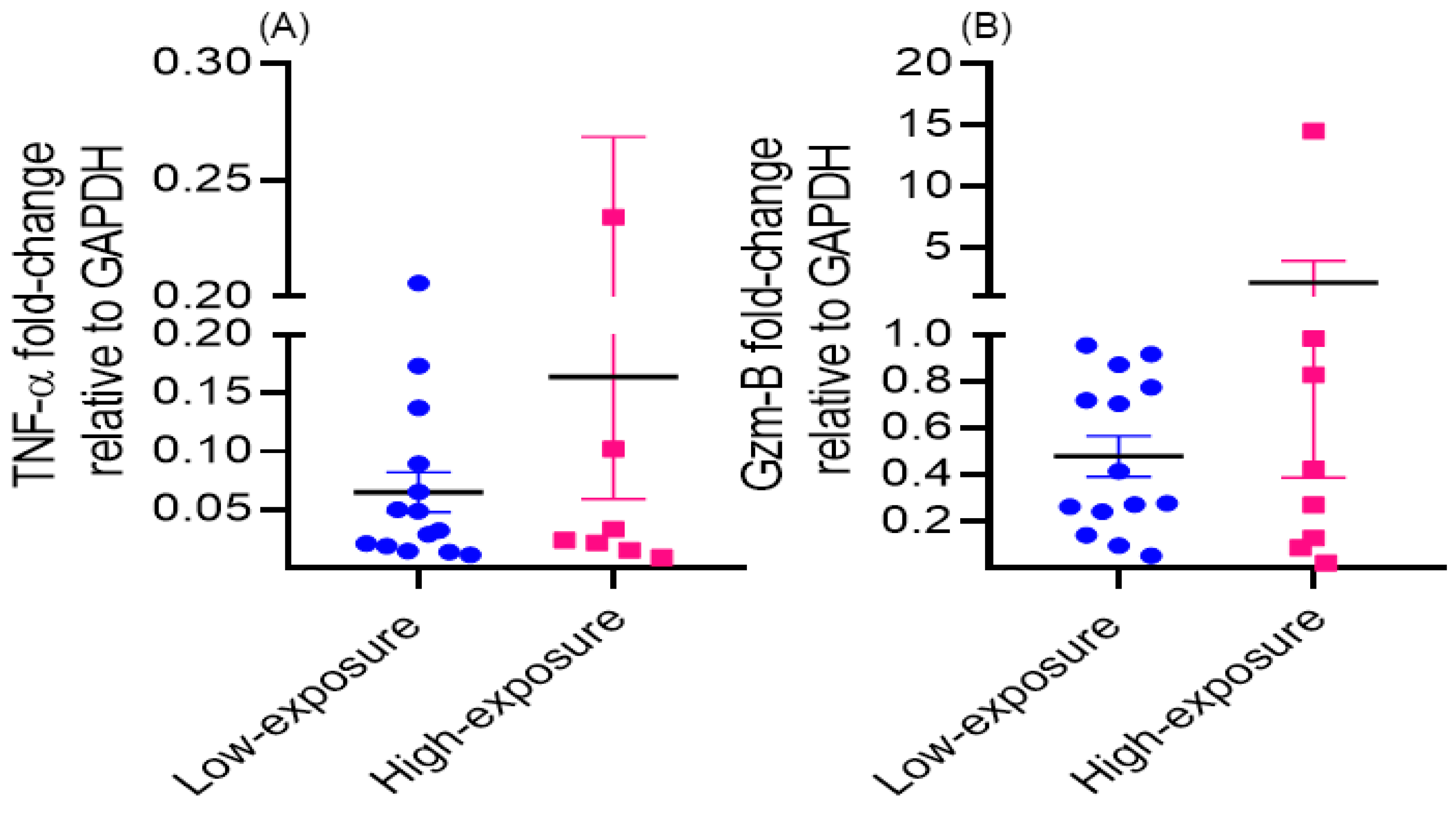
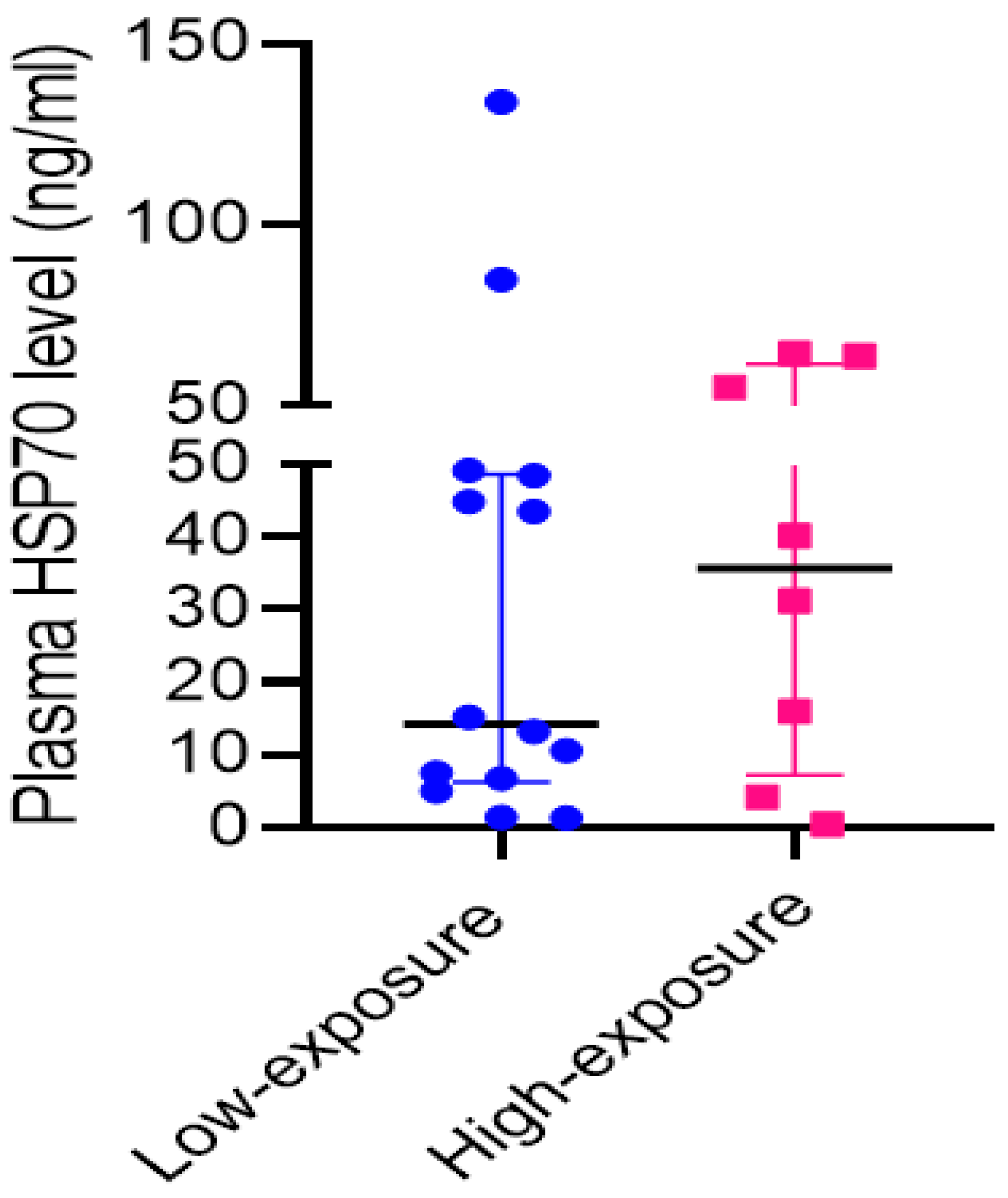
3.3. Dysregulation of Immune Functions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kales, S.N.; Soteriades, E.S.; Christophi, C.A.; Christiani, D.C. Emergency Duties and Deaths from Heart Disease among Firefighters in the United States. N. Engl. J. Med. 2007, 356, 1207–1215. [Google Scholar] [CrossRef]
- Fent, K.W.; Evans, D.E.; Babik, K.; Striley, C.; Bertke, S.; Kerber, S.; Smith, D.; Horn, G.P. Airborne Contaminants during Controlled Residential Fires. J. Occup. Environ. Hyg. 2018, 15, 399–412. [Google Scholar] [CrossRef]
- Abrard, S.; Bertrand, M.; De Valence, T.; Schaupp, T. Physiological, Cognitive and Neuromuscular Effects of Heat Exposure on Firefighters after a Live Training Scenario. Int. J. Occup. Saf. Ergon. 2018, 27, 185–193. [Google Scholar] [CrossRef]
- Hemmatjo, R.; Motamedzade, M.; Aliabadi, M.; Kalatpour, O.; Farhadian, M. The Effect of Various Hot Environments on Physiological Responses and Information Processing Performance Following Firefighting Activities in a Smoke-Diving Room. Saf. Health Work. 2017, 8, 386–392. [Google Scholar] [CrossRef]
- Aljaroudi, A.M.; Bhattacharya, A.; Strauch, A.; Quinn, T.D.; Jon Williams, W. Effect of Cooling on Static Postural Balance While Wearing Firefighter’s Protective Clothing in a Hot Environment. Int. J. Occup. Saf. Ergon. 2023, 29, 1460–1466. [Google Scholar] [CrossRef]
- Daniels, R.D.; Bertke, S.; Dahm, M.M.; Yiin, J.H.; Kubale, T.L.; Hales, T.R.; Baris, D.; Zahm, S.H.; Beaumont, J.J.; Waters, K.M.; et al. Exposure-Response Relationships for Select Cancer and Non-Cancer Health Outcomes in a Cohort of U.S. Firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occup. Environ. Med. 2015, 72, 699–706. [Google Scholar] [CrossRef]
- Daniels, R.D.; Kubale, T.L.; Yiin, J.H.; Dahm, M.M.; Hales, T.R.; Baris, D.; Zahm, S.H.; Beaumont, J.J.; Waters, K.M.; Pinkerton, L.E. Mortality and Cancer Incidence in a Pooled Cohort of US Firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occup. Environ. Med. 2014, 71, 388–397. [Google Scholar] [CrossRef]
- Bouchama, A.; al-Sedairy, S.; Siddiqui, S.; Shail, E.; Rezeig, M. Elevated Pyrogenic Cytokines in Heatstroke. Chest 1993, 104, 1498–1502. [Google Scholar] [CrossRef]
- Lefferts, W.K.; Heffernan, K.S.; Hultquist, E.M.; Fehling, P.C.; Smith, D.L. Vascular and Central Hemodynamic Changes Following Exercise-Induced Heat Stress. Vasc. Med. 2015, 20, 222–229. [Google Scholar] [CrossRef]
- Miller-Archie, S.A.; Izmirly, P.M.; Berman, J.R.; Brite, J.; Walker, D.J.; Dasilva, R.C.; Petrsoric, L.J.; Cone, J.E. Systemic Autoimmune Disease Among Adults Exposed to the September 11, 2001 Terrorist Attack. Arthritis Rheumatol. 2020, 72, 849–859. [Google Scholar] [CrossRef]
- Lee, C.T.; Ventura, I.B.; Phillips, E.K.; Leahy, A.; Jablonski, R.; Montner, S.; Chung, J.H.; Vij, R.; Adegunsoye, A.; Strek, M.E. Interstitial Lung Disease in Firefighters: An Emerging Occupational Hazard. Front. Med. 2022, 9, 864658. [Google Scholar] [CrossRef]
- International Association of Firefighters (IAFF), Disease and Illness, Infectious Disease. Available online: https://www.iaff.org/Infectious-Disease/ (accessed on 2 April 2024).
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The Impact of Heat Stress on the Immune System in Dairy Cattle: A Review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef]
- Cartwright, S.L.; McKechnie, M.; Schmied, J.; Livernois, A.M.; Mallard, B.A. Effect of In-Vitro Heat Stress Challenge on the Function of Blood Mononuclear Cells from Dairy Cattle Ranked as High, Average and Low Immune Responders. BMC Vet. Res. 2021, 17, 233. [Google Scholar] [CrossRef]
- Yamamoto, S.; Katagiri, K.; Ando, M. The Effect of High Temperature on Pulmonary Antibacterial Defense in Mice. Jpn. J. Biometeorol. 1999, 36, 145–151. [Google Scholar]
- Jin, Y.; Hu, Y.; Han, D.; Wang, M. Chronic Heat Stress Weakened the Innate Immunity and Increased the Virulence of Highly Pathogenic Avian Influenza Virus H5N1 in Mice. J. Biomed. Biotechnol. 2011, 2011, 367846. [Google Scholar] [CrossRef]
- Al-Otaibi, S.T. Hematological Parameters in a Population of Male Bakers Exposed to High Heat Work Environment. PLoS ONE 2022, 17, e0274782. [Google Scholar] [CrossRef]
- Marrero, M.G.; Dado-Senn, B.; Field, S.L.; Yang, G.; Driver, J.P.; Laporta, J. Chronic Heat Stress Delays Immune System Development and Alters Serotonin Signaling in Pre-Weaned Dairy Calves. PLoS ONE 2021, 16, e0252474. [Google Scholar] [CrossRef]
- O’Connell, P.J.; Wang, X.; Leon-Ponte, M.; Griffiths, C.; Pingle, S.C.; Ahern, G.P. A Novel Form of Immune Signaling Revealed by Transmission of the Inflammatory Mediator Serotonin between Dendritic Cells and T Cells. Blood 2006, 107, 1010–1017. [Google Scholar] [CrossRef]
- Presbitero, A.; Melnikov, V.R.; Krzhizhanovskaya, V.V.; Sloot, P.M.A. A Unifying Model to Estimate the Effect of Heat Stress in the Human Innate Immunity during Physical Activities. Sci. Rep. 2021, 11, 16688. [Google Scholar] [CrossRef]
- Glaser, J.; Lemery, J.; Rajagopalan, B.; Diaz, H.F.; García-Trabanino, R.; Taduri, G.; Madero, M.; Amarasinghe, M.; Abraham, G.; Anutrakulchai, S.; et al. Climate Change and the Emergent Epidemic of CKD from Heat Stress in Rural Communities: The Case for Heat Stress Nephropathy. Clin. J. Am. Soc. Nephrol. 2016, 11, 1472–1483. [Google Scholar] [CrossRef]
- Soneja, S.; Jiang, C.; Fisher, J.; Upperman, C.R.; Mitchell, C.; Sapkota, A. Exposure to Extreme Heat and Precipitation Events Associated with Increased Risk of Hospitalization for Asthma in Maryland, U.S.A. Environ. Health 2016, 15, 57. [Google Scholar] [CrossRef]
- Norloei, S.; Jafari, M.J.; Omidi, L.; Khodakarim, S.; Bashash, D.; Abdollahi, M.B.; Jafari, M. The Effects of Heat Stress on a Number of Hematological Parameters and Levels of Thyroid Hormones in Foundry Workers. Int. J. Occup. Saf. Ergon. 2017, 23, 481–490. [Google Scholar] [CrossRef]
- Uno, S.; Dalton, T.P.; Dragin, N.; Curran, C.P.; Derkenne, S.; Miller, M.L.; Shertzer, H.G.; Gonzalez, F.J.; Nebert, D.W. Oral Benzo[a]Pyrene in Cyp1 Knockout Mouse Lines: CYP1A1 Important in Detoxication, CYP1B1 Metabolism Required for Immune Damage Independent of Total-Body Burden and Clearance Rate. Mol. Pharmacol. 2006, 69, 1103–1114. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Bosi, S.; Bassi, C.; Ferracin, M.; Lanza, G.; Gafà, R.; Magri, E.; Selvatici, R.; Torresani, S.; Marci, R.; et al. Gene Expression Changes in Progression of Cervical Neoplasia Revealed by Microarray Analysis of Cervical Neoplastic Keratinocytes. J. Cell Physiol. 2015, 230, 806–812. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Ju, Z.; Ragab, A.A.; Lundbäck, P.; Long, W.; Valdes-Ferrer, S.I.; He, M.; Pribis, J.P.; Li, J.; et al. MD-2 Is Required for Disulfide HMGB1-Dependent TLR4 Signaling. J. Exp. Med. 2015, 212, 5–14. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Fan, J.; Yu, S.; Chen, S.; Luo, Y.; Prestwich, G.D.; Mascarenhas, M.M.; Garg, H.G.; Quinn, D.A.; et al. Regulation of Lung Injury and Repair by Toll-like Receptors and Hyaluronan. Nat. Med. 2005, 11, 1173–1179. [Google Scholar] [CrossRef]
- Ramirez-Ortiz, Z.G.; Prasad, A.; Griffith, J.W.; Pendergraft, W.F.; Cowley, G.S.; Root, D.E.; Tai, M.; Luster, A.D.; El Khoury, J.; Hacohen, N.; et al. The Receptor TREML4 Amplifies TLR7-Mediated Signaling during Antiviral Responses and Autoimmunity. Nat. Immunol. 2015, 16, 495–504. [Google Scholar] [CrossRef]
- Lu, J.; Li, H.; Yu, D.; Zhao, P.; Liu, Y. Heat Stress Inhibits the Proliferation and Differentiation of Myoblasts and Is Associated with Damage to Mitochondria. Front. Cell Dev. Biol. 2023, 11, 1171506. [Google Scholar] [CrossRef]
- Martinez-Sanchez, M.E.; Huerta, L.; Alvarez-Buylla, E.R.; Villarreal Luján, C. Role of Cytokine Combinations on CD4+ T Cell Differentiation, Partial Polarization, and Plasticity: Continuous Network Modeling Approach. Front. Physiol. 2018, 9, 877. [Google Scholar] [CrossRef]
- Leung, S.; Liu, X.; Fang, L.; Chen, X.; Guo, T.; Zhang, J. The Cytokine Milieu in the Interplay of Pathogenic Th1/Th17 Cells and Regulatory T Cells in Autoimmune Disease. Cell Mol. Immunol. 2010, 7, 182–189. [Google Scholar] [CrossRef]
- Yadav, B.; Prasad, N.; Agrawal, V.; Jain, M.; Agarwal, V. Role of Pathogenic T-helper Cells-17 in Chronic Antibody-mediated Rejection in Renal Allograft Recipients. Indian J. Transplant. 2022, 16, 88–95. [Google Scholar] [CrossRef]
- Boivin, W.A.; Shackleford, M.; Vanden Hoek, A.; Zhao, H.; Hackett, T.L.; Knight, D.A.; Granville, D.J. Granzyme B Cleaves Decorin, Biglycan and Soluble Betaglycan, Releasing Active Transforming Growth Factor-Β1. PLoS ONE 2012, 7, e33163. [Google Scholar] [CrossRef]
- Zeiler, R.J.; Bhattacharya, A.B. Effects of Chronic Heat Stress and Shift Work on Postural Stability in Firefighters: A Pilot Study; AIHce EXP 2023: Phoenix, AZ, USA, 2023; Available online: https://www.coeh.berkeley.edu/21ew1117 (accessed on 13 May 2024).
- Yao, C.; Oh, J.-H.; Lee, D.H.; Bae, J.-S.; Jin, C.L.; Park, C.-H.; Chung, J.H. Toll-like Receptor Family Members in Skin Fibroblasts Are Functional and Have a Higher Expression Compared to Skin Keratinocytes. Int. J. Mol. Med. 2015, 35, 1443–1450. [Google Scholar] [CrossRef]
- Available online: https://www.origene.com/Catalog/Gene-Expression/Qpcr-Primer-Pairs/Hp212370/Tlr-Human-Qpcr-Primer-Pair-Nm (accessed on 1 July 2018).
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Żwirowski, S.; Kłosowska, A.; Obuchowski, I.; Nillegoda, N.B.; Piróg, A.; Ziętkiewicz, S.; Bukau, B.; Mogk, A.; Liberek, K. Hsp70 Displaces Small Heat Shock Proteins from Aggregates to Initiate Protein Refolding. EMBO J. 2017, 36, 783–796. [Google Scholar] [CrossRef]
- Xu, J.; Tang, S.; Song, E.; Yin, B.; Bao, E. Inhibition of Heat Shock Protein 70 Intensifies Heat-Stressed Damage and Apoptosis of Chicken Primary Myocardial Cells in Vitro. Mol. Med. Rep. 2017, 15, 2881–2889. [Google Scholar] [CrossRef]
- Shimoni, C.; Goldstein, M.; Ribarski-Chorev, I.; Schauten, I.; Nir, D.; Strauss, C.; Schlesinger, S. Heat Shock Alters Mesenchymal Stem Cell Identity and Induces Premature Senescence. Front. Cell Dev. Biol. 2020, 8, 565970. [Google Scholar] [CrossRef]
- Gharibi, V.; Khanjani, N.; Heidari, H.; Ebrahimi, M.H.; Hosseinabadi, M.B. The Effect of Heat Stress on Hematological Parameters and Oxidative Stress among Bakery Workers. Toxicol. Ind. Health 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Paley, M.J.; Tepas, D.I. Fatigue and the Shiftworker: Firefighters Working on a Rotating Shift Schedule. Hum. Factors 1994, 36, 269–284. [Google Scholar] [CrossRef]
- Justiz Vaillant, A.A.; Zito, P.M. Neutropenia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Starkebaum, G. Chronic Neutropenia Associated with Autoimmune Disease. Semin. Hematol. 2002, 39, 121–127. [Google Scholar] [CrossRef]
- Visser, G.; Rake, J.P.; Fernandes, J.; Labrune, P.; Leonard, J.V.; Moses, S.; Ullrich, K.; Smit, G.P. Neutropenia, Neutrophil Dysfunction, and Inflammatory Bowel Disease in Glycogen Storage Disease Type Ib: Results of the European Study on Glycogen Storage Disease Type I. J. Pediatr. 2000, 137, 187–191. [Google Scholar] [CrossRef]
- Kroemer, G.; Galassi, C.; Zitvogel, L.; Galluzzi, L. Immunogenic Cell Stress and Death. Nat. Immunol. 2022, 23, 487–500. [Google Scholar] [CrossRef]
- Arumugam, T.V.; Okun, E.; Tang, S.-C.; Thundyil, J.; Taylor, S.M.; Woodruff, T.M. Toll-like Receptors in Ischemia-Reperfusion Injury. Shock 2009, 32, 4–16. [Google Scholar] [CrossRef]
- Hammami, M.M.; Bouchama, A.; Shail, E.; Aboul-Enein, H.Y.; Al-Sedairy, S. Lymphocyte Subsets and Adhesion Molecules Expression in Heatstroke and Heat Stress. J. Appl. Physiol. 1998, 84, 1615–1621. [Google Scholar] [CrossRef]
- Glick, A.B.; Wodzinski, A.; Fu, P.; Levine, A.D.; Wald, D.N. Impairment of Regulatory T-Cell Function in Autoimmune Thyroid Disease. Thyroid 2013, 23, 871–878. [Google Scholar] [CrossRef]
- Xu, S.; Cao, X. Interleukin-17 and Its Expanding Biological Functions. Cell Mol. Immunol. 2010, 7, 164–174. [Google Scholar] [CrossRef]
- Kimura, A.; Naka, T.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl Hydrocarbon Receptor Regulates Stat1 Activation and Participates in the Development of Th17 Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 9721–9726. [Google Scholar] [CrossRef]
- Hu, Y.; Jin, H.; Du, X.; Xiao, C.; Luo, D.; Wang, B.; She, R. Effects of Chronic Heat Stress on Immune Responses of the Foot-and-Mouth Disease DNA Vaccination. DNA Cell Biol. 2007, 26, 619–626. [Google Scholar] [CrossRef]
- Kleinewietfeld, M.; Hafler, D.A. The Plasticity of Human Treg and Th17 Cells and Its Role in Autoimmunity. Semin. Immunol. 2013, 25, 305–312. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, C.; Xu, Y.; Wang, M.; Rao, Q.; Xing, H.; Tian, Z.; Tang, K.; Mi, Y.; Wang, Y.; et al. Hyperfunction of CD4 CD25 Regulatory T Cells in de Novo Acute Myeloid Leukemia. BMC Cancer 2020, 20, 472. [Google Scholar] [CrossRef]
- Wensink, A.C.; Hack, C.E.; Bovenschen, N. Granzymes Regulate Proinflammatory Cytokine Responses. J. Immunol. 2015, 194, 491–497. [Google Scholar] [CrossRef]
- Velotti, F.; Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Granzyme B in Inflammatory Diseases: Apoptosis, Inflammation, Extracellular Matrix Remodeling, Epithelial-to-Mesenchymal Transition and Fibrosis. Front. Immunol. 2020, 11, 587581. [Google Scholar] [CrossRef]
- Yadav, B.; Prasad, N.; Agrawal, V.; Agarwal, V.; Jain, M. Lower Circulating Cytotoxic T-Cell Frequency and Higher Intragraft Granzyme-B Expression Are Associated with Inflammatory Interstitial Fibrosis and Tubular Atrophy in Renal Allograft. Medicina 2023, 59, 1175. [Google Scholar] [CrossRef]
| Firefighter Characteristics | Low-Exposure Group N = 14 (Mean ± SE) | High-Exposure Group N = 8 (Mean ± SE) | p-Value |
|---|---|---|---|
| Age (Years) | 36.42 ± 1.13 | 34.25 ± 1.56 | 0.27 |
| BMI (kg/m2) | 28.96 ± 0.92 | 29.04 ± 0.80 | 0.95 |
| Structure fires/week (number of heavy smoke conditions or structure fires in previous year ÷ number of weeks in the year) | 0.10 ± 0.017 | 0.26 ± 0.094 | 0.051 |
| Number of times total in the last year worked in a structure fire or heavy smoke conditions | 5.50 ± 0.92 | 13.50 ± 4.90 | 0.051 |
| Years of full-time firefighter work | 10.10 ± 1.80 | 8.1 ± 2.4 | 0.52 |
| Additional years worked as a part-time firefighter | 5.64 ± 0.94 | 4.75 ± 1.47 | 0.60 |
| Total Years as firefighter (full-time + part-time) | 15.75 ± 1.33 | 12.87 ± 2.53 | 0.28 |
| Average hours per week worked as a firefighter over the past 6 months | 60.43 ± 2.43 | 41.62 ± 4.47 | 0.001 |
| Average shiftwork hours/year (per week × 52 weeks) | 3141.98 ± 122.55 | 2164.50 ± 232.95 | 0.001 |
| Average shiftwork hours/month (per week × 52 weeks)/12 | 261.83 ± 10.21 | 180.37 ± 19.41 | 0.001 |
| Career shift work (hours/year × years as firefighter) | 49,539.65 ± 4429.48 | 28,236.0 ± 6876.90 | 0.013 |
| On average, number of EMS runs per week over the past 6 months | 14.78 ± 3.10 | 15.87 ± 5.37 | 0.852 |
| CBC Parameters * | Low-Exposure Group N = 14 (Mean ± SE) | High-Exposure Group N = 8 (Mean ± SE) | p-Value | Reference Range ϒ (Normal Value) |
|---|---|---|---|---|
| WBC (k/mcl) | 6.8 ± 0.47 | 6.07 ± 0.89 | 0.29 | 9.00–30.00 |
| RBC(m/mcl) | 5.18 ± 0.08 | 5.07 ± 0.14 | 0.48 | 3.90–5.50 |
| HGB (gm/dcl) | 15.40 ± 0.23 | 14.98 ± 0.23 | 0.25 | 13.5–19.5 |
| HCT (%) | 46.19 ± 0.62 | 44.61 ± 060 | 0.11 | 42.0–60.0 |
| MCV (fL) | 89.18 ± 0.74 | 86.96 ± 0.94 | 0.08 | 98.0–118 |
| MCH (pg) | 29.73 ± 0.23 | 29.18 ± 0.28 | 0.16 | 31.0–37.0 |
| MCHC (gm/dL) | 33.34 ± 0.25 | 33.56 ± 0.22 | 0.56 | 30.0–36.0 |
| RDWCV (%) | 14.18 ± 1.91 | 12.43 ± 0.14 | 0.50 | NA |
| Platelets(k/mcl) | 264.78 ± 11.56 | 253.25 ± 12.35 | 0.52 | 135–466 |
| MPV (fL) | 10.22 ± 0.22 | 10.43 ± 0.29 | 0.56 | 10.2–11.9 |
| SEGS (%) | 58.73 ± 1.66 | 61.11 ± 3.69 | 0.50 | 67.0–87.0 |
| Lymphocyte (%) | 30.55 ± 1.60 | 28.18 ± 3.04 | 0.45 | 28.0–34.0 |
| Monocyte (%) | 7.8 ± 0.36 | 8.13 ± 0.66 | 0.63 | 0.0–12.0 |
| Eosinophils (%) | 1.88 ± 0.33 | 1.51 ± 0.31 | 0.46 | 0.0–4.0 |
| Basophils (%) | 0.43 ± 0.09 | 0.76 ± 0.08 | 0.025 | 0.0–1.0 |
| Immature granulocyte (%) | 0.25 ± 0.034 | 0.52 ± 0.28 | 0.21 | NA |
| Neutrophils absolute (K/mcl) | 4.05 ± 0.35 | 3.76 ± 0.40 | 0.60 | 6.00–28.00 |
| Lymphocyte absolute (K/mcl) | 3.70 ± 1.67 | 1.67 ± 0.15 | 0.38 | 2.00–11.00 |
| Monocyte absolute (K/mcl) | 0.86 ± 0.32 | 0.52 ± 0.05 | 0.43 | 0.00–2.20 |
| Eosinophils absolute counts (K/mcl) | 0.47 ± 0.25 | 0.08 ± 0.01 | 0.27 | 0.00–1.20 |
| Basophils absolute counts (K/mcl) | 0.06 ± 0.026 | 0.046 ± 0.003 | 0.68 | 0.00–0.10 |
| Immature Granulocyte absolute counts | 0.099 ± 0.033 | 0.033 ± 0.023 | 0.17 | 0.0–0.7 |
| Target Gene/Protein | Fold-Change (FC) Relative to House-Keeping Gene | Fold-Difference | |
|---|---|---|---|
| Low-Exposure Group N = 14 (Mean ± SE) | High-Exposure Group N = 8 (Mean ± SE) | (high-Exposure FC/Low-Exposure FC) | |
| TLR2 | 0.48 ± 0.09 | 0.31 ± 0.12 | −1.51 |
| TLR4 | 0.44 ± 0.09 | 0.20 ± 0.07 | −2.21 |
| TLR7 | 0.088 ± 0.036 | 0.16 ± 0.12 | 1.58 |
| TLR9 | 0.15 ± 0.05 | 0.15 ± 0.10 | −1.03 |
| MYD88 | 0.40 ± 0.10 | 0.27 ± 0.11 | −1.48 |
| T-bet (Th1) | 5.46 ± 2.12 | 5.58 ± 2.65 | −1.19 |
| RORC (Th17) | 54.86 ± 17.21 | 92.78 ± 68.47 | 1.69 |
| FoxP3 (Treg) | (0.03 ± 0.006) × 10−2 | (0.12 ± 0.07) × 10−2 | 3.52 |
| T-bet/FoxP3 (Th1/Treg) | (15.64 ± 4.02) × 103 | (11.9 ± 5.93) × 103 | −1.31 |
| RORC/FoxP3 (Th17/Treg) | (180.6 ± 53.3) × 103 | (102.6 ± 3.5) × 103 | −1.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, B.; Mohammed, A.N.; Graham, B.; Bhattacharya, A.; Yadav, J.S. Chronic Heat Exposure Modulates Innate and Adaptive Immune Responses in Firefighters. Environments 2024, 11, 131. https://doi.org/10.3390/environments11060131
Yadav B, Mohammed AN, Graham B, Bhattacharya A, Yadav JS. Chronic Heat Exposure Modulates Innate and Adaptive Immune Responses in Firefighters. Environments. 2024; 11(6):131. https://doi.org/10.3390/environments11060131
Chicago/Turabian StyleYadav, Brijesh, Afzaal Nadeem Mohammed, Brittney Graham, Amit Bhattacharya, and Jagjit Singh Yadav. 2024. "Chronic Heat Exposure Modulates Innate and Adaptive Immune Responses in Firefighters" Environments 11, no. 6: 131. https://doi.org/10.3390/environments11060131
APA StyleYadav, B., Mohammed, A. N., Graham, B., Bhattacharya, A., & Yadav, J. S. (2024). Chronic Heat Exposure Modulates Innate and Adaptive Immune Responses in Firefighters. Environments, 11(6), 131. https://doi.org/10.3390/environments11060131







