Detection of Emerging Stress in Trees Using Hyperspectral Indices as Classification Features
Abstract
1. Introduction
2. Materials and Methods
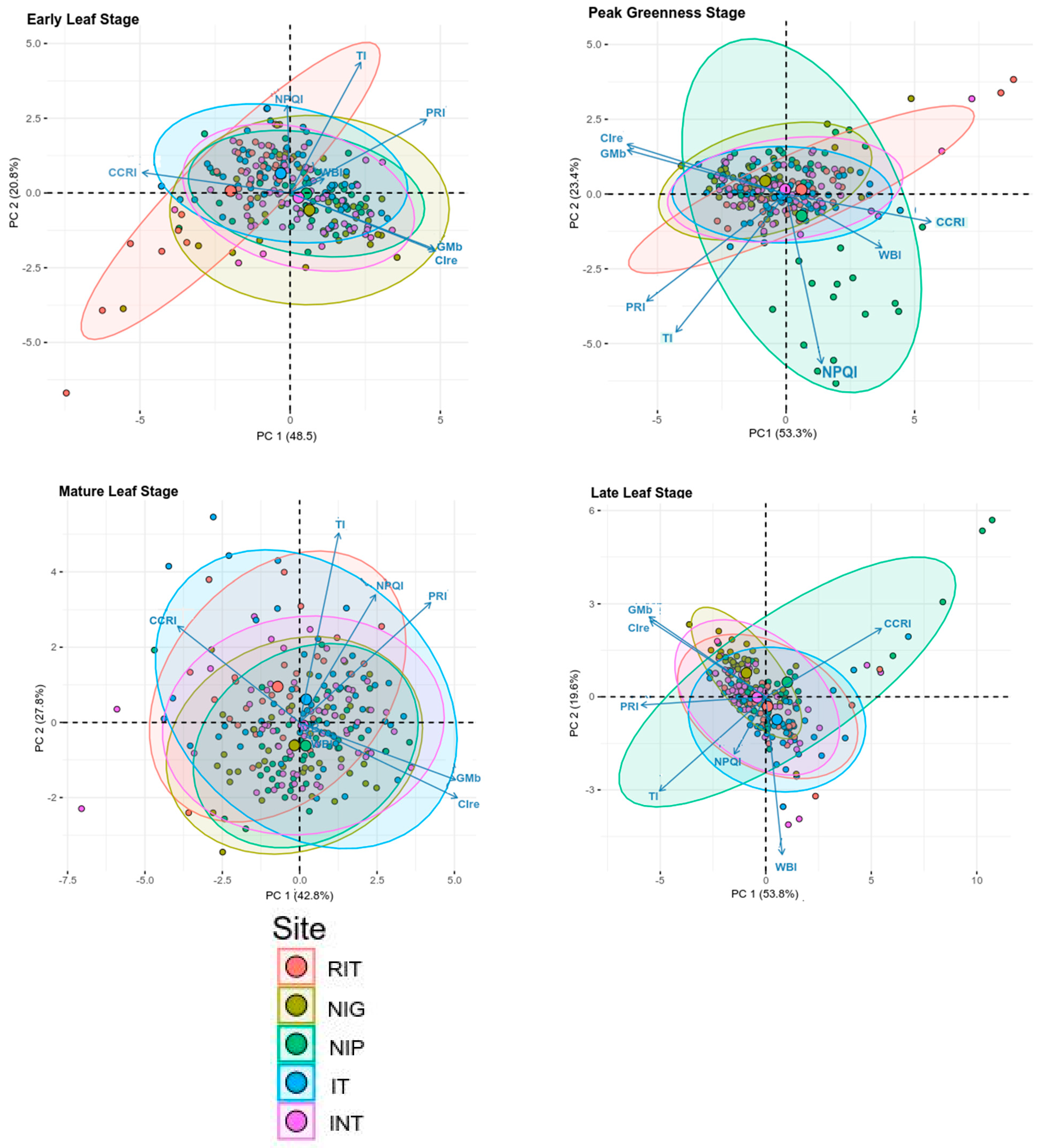
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lassalle, G.; Fabre, S.; Credoz, A.; Hédacq, R.; Bertoni, G.; Dubucq, D.; Elger, A. Application of PROSPECT for Estimating Total Petroleum Hydrocarbons in Contaminated Soils from Leaf Optical Properties. J. Hazard. Mater. 2019, 377, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Pontius, J.; Martin, M.; Plourde, L.; Hallett, R. Ash Decline Assessment in Emerald Ash Borer-Infested Regions: A Test of Tree-Level, Hyperspectral Technologies. Remote Sens. Environ. 2008, 112, 2665–2676. [Google Scholar] [CrossRef]
- Pu, R. Hyperspectral Remote Sensing: Fundamentals and Practices, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2017; ISBN 978-1-315-12060-7. [Google Scholar]
- Shukla, A.; Kot, R. An Overview of Hyperspectral Remote Sensing and Its Applications in Various Disciplines. IRA-JAS 2016, 5, 85. [Google Scholar] [CrossRef]
- Blackburn, G.A. Hyperspectral Remote Sensing of Plant Pigments. J. Exp. Bot. 2006, 58, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wei, C.; Zhang, Y.; Blackburn, G.A.; Wang, X.; Wei, C.; Wang, J. Meta-Analysis of the Detection of Plant Pigment Concentrations Using Hyperspectral Remotely Sensed Data. PLoS ONE 2015, 10, e0137029. [Google Scholar] [CrossRef]
- Curran, P.J. Remote Sensing of Foliar Chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Chutia, D.; Bhattacharyya, D.K.; Sarma, K.K.; Kalita, R.; Sudhakar, S. Hyperspectral Remote Sensing Classifications: A Perspective Survey. Trans. GIS 2016, 20, 463–490. [Google Scholar] [CrossRef]
- Song, G.; Wang, Q. Developing Hyperspectral Indices for Assessing Seasonal Variations in the Ratio of Chlorophyll to Carotenoid in Deciduous Forests. Remote Sens. 2022, 14, 1324. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Q. Informative Bands Used by Efficient Hyperspectral Indices to Predict Leaf Biochemical Contents Are Determined by Their Relative Absorptions. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 616–626. [Google Scholar] [CrossRef]
- Gitelson, A.; Vina, A.; Arkebauer, T.; Rundquist, D.; Keydan, G.; Leavitt, B.; Verma, S. Remote Estimation of Leaf Area Index and Green Leaf Biomass in Maize Canopies. Geophys. Res. Lett. 2003, 30, 1248–1251. [Google Scholar] [CrossRef]
- Blackburn, G.A. Spectral Indices for Estimating Photosynthetic Pigment Concentrations: A Test Using Senescent Tree Leaves. Int. J. Rem. Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, W.; Kong, W.; Ye, H.; Dong, Y.; Casa, R. Assessment of Leaf Carotenoids Content with a New Carotenoid Index: Development and Validation on Experimental and Model Data. Int. J. Appl. Earth Obs. Geoinf. 2017, 57, 24–35. [Google Scholar] [CrossRef]
- Gamon, J.; Serrano, L.; Surfus, J.S. The Photochemical Reflectance Index: An Optical Indicator of Photosynthetic Radiation Use Efficiency across Species, Functional Types, and Nutrient Levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Penuelas, J.; Baret, F.; Filella, I. Semi-Empirical Indices to Assess Carotenoids/Chlorophyll a Ratio from Leaf Spectral Reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Datt, B. Remote Sensing of Water Content in Eucalyptus Leaves. Aust. J. Bot. 1999, 47, 909. [Google Scholar] [CrossRef]
- Penuelas, J.; Fillela, I.; Biel, C.; Serrano, L.; Save, R. The Reflectance at the 950–970 Nm Region as an Indicator of Plant Water Status. Int. J. Rem. Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Moley, L.M.; Goodin, D.G.; Winslow, W.P. Leaf-Level Spectroscopy for Analysis of Invasive Pest Impact on Trees in a Stressed Environment: An Example Using Emerald Ash Borer (Agrilus Planipennis Fairmaire) in Ash Trees (Fraxinus spp.), Kansas, USA. Environments 2022, 9, 42. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P.J. Retrieval of Foliar Information about Plan Pigment Systems from High Resolution Spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef]
- Roberts, D.; Roth, K.; Perroy, R. Hyperspectral Vegetation Indices. In Hyperspectral Remote Sensing of Vegetation; CRC Press: Boca Raton, FL, USA, 2011; pp. 309–328. ISBN 978-1-4398-4537-0. [Google Scholar]
- Hu, B.; Li, J.; Wang, J.; Hall, B. The Early Detection of the Emerald Ash Borer (EAB) Using Advanced Geospacial Technologies. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2014, 40, 213. [Google Scholar] [CrossRef]
- Zhang, K.; Hu, B.; Robinson, J. Early Detection of Emerald Ash Borer Infestation Using Multisourced Data: A Case Study in the Town of Oakville, Ontario, Canada. J. Appl. Remote Sens. 2014, 8, 083602. [Google Scholar] [CrossRef]
- Pontius, J.; Hanavan, R.P.; Hallett, R.A.; Cook, B.D.; Corp, L.A. High Spatial Resolution Spectral Unmixing for Mapping Ash Species across a Complex Urban Environment. Rem. Sens. Environ. 2017, 199, 360–369. [Google Scholar] [CrossRef]
- Carter, G.A.; Knapp, A.K. Leaf Optical Properties in Higher Plants: Linking Spectral Characteristics to Stress and Chlorophyll Concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, W.; Zhang, J.; Kong, W.; Casa, R.; Huang, Y. A Novel Combined Spectral Index for Estimating the Ratio of Carotenoid to Chlorophyll Content to Monitor Crop Physiological and Phenological Status. Int. J. Appl. Earth Obs. Geoinf. 2019, 76, 128–142. [Google Scholar] [CrossRef]
- Lehnert, L.W.; Meyer, H.; Obermeier, W.A.; Silva, B.; Regeling, B.; Bendix, J. Hyperspectral Data Analysis in R: The Hsdar Package. J. Stat. Softw. 2019, 89. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Spectral Reflectance Changes Associated with Autumn Senescence of Aesculus Hippocastanum L.and Acer Platanoides L. Leaves. Spectral Features and Relation to Chlorophyll Estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A Reappraisal of the Use of DMSO for the Extraction and Determination of Chlorophylls a and b in Lichens and Higher Plants. Env. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote Estimation of Canopy Chlorophyll Content in Crops. Geophys. Res. Lett. 2005, 32, L08403. [Google Scholar] [CrossRef]
- Song, F.; Guo, Z.; Mei, D. Feature Selection Using Principal Component Analysis. In Proceedings of the 2010 International Conference on System Science, Engineering Design and Manufacturing Informatization, Yichang, China, 12 November 2010; Volume 1, pp. 27–30. [Google Scholar]
- Shendryk, Y.; Rossiter-Rachor, N.A.; Setterfield, S.A.; Levick, S.R. Levick Leveraging High-Resolution Satellite Imagery and Gradient Boosting for Invasive Weed Mapping. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 4443–4450. [Google Scholar] [CrossRef]
- De’ath, G.; Fabricius, K.E. Classification and Decision Trees: A Powerful yet Simple Technique for Ecological Data Analysis. Ecology 2000, 81, 3178–3192. [Google Scholar] [CrossRef]
- Funkenberg, T.; Binh, T.T.; Moder, F.; Dech, S. The Ha Tien Plain—Wetland Monitoring Using Remote-Sensing Techniques. Int. J. Remote Sens. 2014, 35, 2893–2909. [Google Scholar] [CrossRef]
- Kavzoglu, T.; Colkesen, I. A Kernel Functions Analysis for Support Vector Machines for Land Cover Classification. Int. J. Appl. Earth Obs. Geoinf. 2009, 11, 352–359. [Google Scholar] [CrossRef]
- Tahir, N.M.; Hussain, A.; Samad, S.A.; Ishak, K.A.; Halim, R.A. Feature Selection for Classification Using Decision Tree. In Proceedings of the 2006 4th Student Conference on Research and Development, Shah Alam, Malaysia, 27 June 2006; pp. 99–102. [Google Scholar]
- Sun, L.; Xu, J.; Yin, Y. Principal Component-Based Feature Selection for Tumor Classification. Bio-Med. Mater. Eng. 2015, 26, S2011–S2017. [Google Scholar] [CrossRef]
- Greenwell, B.; Boehmke, B.; Cunningham, J. Gbm: Generalized Boosted Regression Models 2020. R Package Version 2020, 2.
- Kuhn, M. Caret: Classification and Regression Training 2020. R Package Version 2021, 3.
- Landis, J.; Koch, G. An Application of Hierarchical Kappa-Type Statistics in the Assessment of Majority Agreement among Multiple Observers. Biometrics 1977, 33, 363–374. [Google Scholar] [CrossRef]
- Foody, G.M. Thematic Map Comparison. Photogramm. Eng. Remote Sens. 2004, 70, 627–633. [Google Scholar] [CrossRef]
- Haack, R.A.; Jendak, E.; Houping, L.; Marchant, K.R.; Petrice, T.R.; Poland, T.M.; Ye, H. The Emerald Ash Borer: A New Exotic Pest in North America. Newsl. Mich. Entomol. Soc. 2002, 47, 1–5. [Google Scholar]
- Volkovitsh, M.G.; Orlova-Bienkowskaja, M.J.; Kovalev, A.V.; Bieńkowski, A.O. An Illustrated Guide to Distinguish Emerald Ash Borer (Agrilus planipennis) from Its Congeners in Europe. For. Int. J. For. Res. 2019, 93, 316–325. [Google Scholar] [CrossRef]
- Herms, D.A.; McCullough, D.G. Emerald Ash Borer Invasion of North America: History, Biology, Ecology, Impacts, and Management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Poland, T.M.; McCullough, D.G. Emerald Ash Borer: Invasion of the Urban Forest and the Threat to North America’s Ash Resource. J. For. 2006, 104, 118–124. [Google Scholar] [CrossRef]
- Schlarbaum, S.E.; Hebard, F.; Spaine, P.C.; Kamalay, J.C. Three American Tragedies: Chestnut Blight, Butternut Canker, and Dutch Elm Disease. In Proceedings of the Exotic Pests of Eastern Forests Conference Proceedings; U.S. Forest Service and Tennessee Exotic Pest Plant Council: Nashville, TN, USA, 1998; pp. 45–54. [Google Scholar]
- Webber, J.F. Experimental Studies on Factors Influencing the Transmission of Dutch Elm Disease. For. Syst. 2004, 13, 197–205. [Google Scholar]
- Potter, C.; Harwood, T.; Knight, J.; Tomlinson, I. Learning from History, Predicting the Future: The UK Dutch Elm Disease Outbreak in Relation to Contemporary Tree Disease Threats. Phil. Trans. R. Soc. B 2011, 366, 1966–1974. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between Leaf Chlorophyll Content and Spectral Reflectance and Algorithms for Non-Destructive Chlorophyll Assessment in Higher Plant Leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Horler, D.N.H.; Dockray, M.; Barber, J. The Red Edge of Plant Leaf Reflectance. Int. J. Remote Sens. 1983, 4, 273–288. [Google Scholar] [CrossRef]
- Reflectance at the Red Edge as a Sensitive Indicator of the Damage of Trees and Its Correlation to the State of the Photosynthetic System. Nasr, H.N. (Ed.) In Proceedings of the Image Understanding for Aerospace Applications; SPIE: Munich, Germany, 1991; p. 131. [Google Scholar]
- Zarco-Tejada, P.J.; González-Dugo, V.; Williams, L.E.; Suárez, L.; Berni, J.A.J.; Goldhamer, D.; Fereres, E. A PRI-Based Water Stress Index Combining Structural and Chlorophyll Effects: Assessment Using Diurnal Narrow-Band Airborne Imagery and the CWSI Thermal Index. Remote Sens. Environ. 2013, 138, 38–50. [Google Scholar] [CrossRef]

| Site Type | Sample Size | Collection Dates ¹ | Notes ² | ||||
|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2017 | 2018 | 2019 | ||
| Infested, Not Treated (INT) | 19 | 27 | 16 | Early: 29 April–6 May Mature: 25–28 June Peak: 26 August–8 September Late: 17–25 October | Early: 9–13 May Mature: 1–9 July Peak: 1–7 September Late: 29–30 September | Early: 13–19 May Mature: 18–25 June Peak: 30 July–6 August Late: 15–18 September | Residential site, Johnson County |
| Infested, Treated (IT) | 18 | 17 | 17 | Recreation ctr. site, Johnson County | |||
| Recently Infested, Treated (RIT) | 9 | 9 | 9 | Park site, Johnson County | |||
| Not Infested, Good Condition (NIG) | 15 | 15 | 15 | Campus site, Riley County | |||
| Not Infested, Poorer Condition (NIP) | 16 | 15 | 15 | Campus site, Riley County | |||
| Index | Definition 1 | Descriptive Purpose of Index 2 | Reference |
|---|---|---|---|
| Water Band Index (WBI) | R970/R900 | Water stress, general organism stress | [17] |
| Gitelson–Merzlyak B Index (GMb) | R750/R700 | Chlorophyll estimation, leaf senescence | [27] |
| Normalized Phaeophytization Index (NPQI) | R435−R415/R435+R415 | Chlorophyll breakdown and general environmental stress | [28] |
| Combined Carotenoid/Chlorophyll Ratio Index (CCRI) | ((R720−R521)/R521)/((R750− R705)/R705) | Combination of a carotenoid index with a red-edge chlorophyll index | [13] |
| Photochemical Reflectance Index (PRI) | (R531−R570)(R531+R570) | General organism stress | [14] |
| Red-Edge Chlorophyll Index (CIre) | (R750−R705)/R705 | Chlorophyll content | [29] |
| Test Index (TI) | PRI/CIre | Composite of PRI and CIre | This Study |
| Early Leaf | Mature Leaf | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Index | PC1 | PC2 | PC3 | PC4 | Index | PC1 | PC2 | PC3 | PC4 |
| PRI | 0.46 | 0.38 | −0.08 | 0.09 | PRI | 0.44 | 0.41 | 0.05 | 0.29 |
| CIRE | 0.49 | −0.30 | 0.15 | −0.15 | CIRE | 0.53 | −0.26 | 0.07 | −0.11 |
| CCRI | −0.49 | 0.11 | 0.01 | −0.16 | CCRI | −0.41 | 0.33 | 0.06 | −0.40 |
| WBI | 0.11 | 0.07 | −0.81 | −0.55 | WBI | 0.04 | −0.06 | −0.97 | 0.12 |
| NPQI | −0.01 | 0.45 | 0.52 | −0.69 | NPQI | 0.25 | 0.44 | −0.19 | −0.72 |
| GMB | 0.48 | −0.29 | 0.17 | −0.16 | GMB | 0.52 | −0.20 | 0.09 | −0.20 |
| TI | 0.24 | 0.68 | −0.08 | 0.36 | TI | 0.13 | 0.65 | 0.04 | 0.41 |
| Peak Greenness | Late Leaf | ||||||||
| Index | PC1 | PC2 | PC3 | PC4 | Index | PC1 | PC2 | PC3 | PC4 |
| PRI | −0.41 | −0.41 | −0.07 | 0.18 | PRI | −0.48 | −0.04 | −0.09 | −0.02 |
| CIRE | −0.47 | 0.19 | 0.26 | −0.30 | CIRE | −0.44 | 0.33 | −0.10 | 0.38 |
| CCRI | 0.43 | −0.11 | −0.37 | −0.28 | CCRI | 0.44 | 0.29 | −0.29 | 0.21 |
| WBI | 0.28 | −0.21 | 0.84 | 0.25 | WBI | 0.06 | −0.68 | −0.05 | 0.73 |
| NPQI | 0.11 | −0.65 | 0.12 | −0.66 | NPQI | −0.12 | −0.25 | −0.90 | −0.28 |
| GMB | −0.47 | 0.17 | 0.15 | −0.38 | GMB | −0.45 | 0.34 | −0.11 | 0.32 |
| TI | −0.33 | −0.53 | −0.21 | 0.40 | TI | −0.41 | −0.41 | 0.26 | −0.32 |
| All Collections | |||||||||
| Index | PC1 | PC2 | PC3 | PC4 | |||||
| PRI | −0.48 | 0.02 | 0.21 | 0.01 | |||||
| CIRE | −0.44 | −0.40 | −0.12 | −0.30 | |||||
| CCRI | 0.45 | −0.41 | 0.17 | −0.14 | |||||
| WBI | 0.14 | 0.38 | −0.37 | −0.82 | |||||
| NPQI | −0.05 | 0.23 | 0.87 | −0.35 | |||||
| GMB | −0.45 | −0.41 | −0.06 | −0.25 | |||||
| TI | −0.39 | 0.54 | −0.12 | 0.21 | |||||
| Reference | ||||||
|---|---|---|---|---|---|---|
| Prediction | NIG | NIP | IT | INT | RIT | |
| NIG | 37 | 3 | 5 | 10 | 1 | |
| NIP | 4 | 32 | 7 | 8 | 4 | |
| IT | 6 | 8 | 43 | 8 | 2 | |
| INT | 12 | 5 | 11 | 42 | 2 | |
| RIT | 4 | 1 | 3 | 9 | 22 | |
| Accuracy | 60.9% | |||||
| Kappa | 0.505 | |||||
| a.Error Matrices, Collection 1 (Early Leaf Stage) | ||||||
| Reference | ||||||
| Prediction | NIG | NIP | IT | INT | RIT | |
| NIG | 12 | 1 | 0 | 0 | 0 | |
| NIP | 2 | 9 | 2 | 2 | 0 | |
| IT | 2 | 1 | 12 | 1 | 1 | |
| INT | 2 | 3 | 0 | 11 | 1 | |
| RIT | 0 | 2 | 0 | 3 | 4 | |
| Accuracy | 67.6% | |||||
| Kappa | 0.598 | |||||
| b. Error Matrices, Collection 2 (Mature Leaf Stage) | ||||||
| Reference | ||||||
| Prediction | NIG | NIP | IT | INT | RIT | |
| NIG | 11 | 0 | 1 | 2 | 0 | |
| NIP | 1 | 12 | 1 | 1 | 0 | |
| IT | 1 | 2 | 12 | 1 | 3 | |
| INT | 0 | 0 | 3 | 11 | 1 | |
| RIT | 1 | 0 | 0 | 1 | 7 | |
| Accuracy | 73.6% | |||||
| Kappa | 0.668 | |||||
| c. Error Matrices, Collection 3 (Peak Greenness Stage) | ||||||
| Reference | ||||||
| Prediction | NIG | NIP | IT | INT | RIT | |
| NIG | 8 | 0 | 2 | 3 | 0 | |
| NIP | 1 | 7 | 0 | 5 | 2 | |
| IT | 1 | 2 | 9 | 4 | 1 | |
| INT | 1 | 1 | 1 | 16 | 0 | |
| RIT | 0 | 1 | 2 | 2 | 4 | |
| Accuracy | 60.3% | |||||
| Kappa | 0.490 | |||||
| d. Error Matrices, Collection 4 (Late Leaf Stage) | ||||||
| Reference | ||||||
| Prediction | NIG | NIP | IT | INT | RIT | |
| NIG | 16 | 1 | 1 | 3 | 0 | |
| NIP | 0 | 10 | 4 | 3 | 0 | |
| IT | 1 | 1 | 21 | 3 | 1 | |
| INT | 2 | 2 | 3 | 24 | 1 | |
| RIT | 1 | 2 | 6 | 2 | 4 | |
| Accuracy | 67% | |||||
| Kappa | 0.573 | |||||
| Combined | Early Leaf Stage | Mature Leaf Stage | Peak Greenness | Late Leaf Stage | |
|---|---|---|---|---|---|
| Combined | - | - | - | - | - |
| Early Leaf Stage | 1.07 (0.142) | - | - | - | - |
| Mature Leaf Stage | 2.05 * (0.020) | 1.18 (0.119) | - | - | - |
| Peak Greenness | 0.084 (0.467) | 1.45 ** (0.074) | 2.79 * (0.003) | - | - |
| Late Leaf Stage | 1.17 (0.121) | 0.21 (0.492) | 1.31 (0.095) | 1.83 * (0.034) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moley, L.M.; Goodin, D.G.; Winslow, W.P., III. Detection of Emerging Stress in Trees Using Hyperspectral Indices as Classification Features. Environments 2024, 11, 85. https://doi.org/10.3390/environments11040085
Moley LM, Goodin DG, Winslow WP III. Detection of Emerging Stress in Trees Using Hyperspectral Indices as Classification Features. Environments. 2024; 11(4):85. https://doi.org/10.3390/environments11040085
Chicago/Turabian StyleMoley, Laura M., Douglas G. Goodin, and William P. Winslow, III. 2024. "Detection of Emerging Stress in Trees Using Hyperspectral Indices as Classification Features" Environments 11, no. 4: 85. https://doi.org/10.3390/environments11040085
APA StyleMoley, L. M., Goodin, D. G., & Winslow, W. P., III. (2024). Detection of Emerging Stress in Trees Using Hyperspectral Indices as Classification Features. Environments, 11(4), 85. https://doi.org/10.3390/environments11040085






