Abstract
As an alternative wood source for biochar and a cost-effective renewable energy source, sustainable biomass production based on fast-growing willows irrigated with treated wastewater has been explored. Salix alba L. and Salix viminalis L. were selected for assessment of their potentially high woody biomass productivity and phytoremediation efficiency when irrigated with greywater treated by floating treatment wetlands. Both Salix species produced significantly (p < 0.05) high woody biomass in the second harvest, with a significantly higher fresh woody biomass weight with higher water content (53%) for S. viminalis compared to S. alba. The dry biomass weight of S. alba was greater than of S. viminalis at the first harvest. The element accumulations in substrates changed significantly after irrigation, with greywater compared to the raw substrate following this order: Mg > Fe > Al > Cr > Mn > Cd > Cu > B. Element concentrations accumulated in twigs of S. alba following this order: Ca > Mg > Na > Mn > Zn > Fe > Al > Cd > Cu > Cr > Ni > B, but for S. viminalis the order was Ca > Mg > Mn > Zn > Na > Fe > Al > Cd > Cu > Ni > Cr > B. The accumulations of Al, B, Ca, Fe, Mg, Mn, and Ni were significantly greater in S. alba leaves compared to their twigs, which showed significantly high accumulations of Na and Zn. The accumulations of Al, B, Ca, Fe, Mg, Mn, and Na were significantly greater in S. viminalis leaves compared to their twigs.
1. Introduction
Governments have the responsibility to address adverse anthropogenic activities against the environment [1]. Environmental degradation could be mitigated by following the international standards and guidance for safe daily practices associated with industrial manufacturing, agriculture, as well as disposal of municipal and industrial waste. Furthermore, some sustainable solutions offer a road map for moving towards the restoration of ecosystems [2]. Tree plantations and agroforestry systems such as those cultivated with willow have gained interest for their public health, economic, and environmental benefits occurring over a range of spatial and temporal scales [3]. Willows are traditionally used for public health gains, such as in folk medicine, and as an essential source in phytochemistry, pharmacology, and other medicinal uses [4]. In terms of economic benefits, they are used as biofuel (renewable energy), for the production of timber, and in the furniture industry. Willows are also utilized in horticulture and architecture [3]. More recently, willows have been applied for substantial environment enhancement thought climate change mitigation and adaptation measures [5], soil erosion control, nutrient recycling, and soil fertility improvement. They also provide genetic resources for crops, enhance habitat, increase biodiversity, produce oxygen [6], and enhance carbon sequestration [6]. Willows are also used for flood mitigation as well as soil and water phytoremediation [2]. Willows are of the genus Salix spp. from the traditional family of Salicaceae, which include about 56 genera and 1220 species [7]. Willows comprise around 330–500 species and more than 200 hybrid species of deciduous trees and shrubs that grow in temperate, sub-tropic, and tropic regions [4,8].
In the last decade, communities have increasingly selected sustainable biofuels such as bioethanol and biogas instead of fossil-based fuel [9]. Willows can grow under variable climates and on challenging soils unsuitable for edible crops, which makes them economically attractive [10]. The sequestration of carbon supports global greenhouse gas mitigation [5]. According to the Food and Agriculture Organization (FAO) [10], tree plantations are vital in sequestering both organic and inorganic carbon [6]. Moreover, phytoremediation by willows offers a sustainable technique to clean-up contaminated water, groundwater, soil, sediment, and sludge [2]. Climate change associated with elevated carbon emissions commonly leads to water scarcity and the deterioration of freshwater quality [11]. This has led specialists to investigate an alternative and sustainable source for agricultural irrigation water [12], amounting to about 75% of the total world water demand [13].
Sewage can act as a fertilizer containing nutrients, which are necessary for plant growth [14,15]. Greywater commonly comprises a major proportion (50–80%) of domestic wastewater [16]. It predominantly originates from household washing activities. Therefore, greywater has good public acceptance in terms of reuse because of the absence of fecal waste and, thereby, low content of pathogens [17]. However, it is recommended that greywater should be treated, for example, by wetlands with or without the presence of special substrates such as ochre and wood chips to remove specific pollutants before reuse to meet environmental and public health criteria [15,18]. Floating Treatment Wetlands (FTWs) have been applied in many countries around the world for the purification of different types of wastewaters [19]. The pollutant removal mechanisms and the structure of FTWs are like those of free-water surface constructed wetlands [20], but the floating macrophytes grow in a hydroponic manner on buoyant structures such as mats, and the root network suspends into the water column where the pollutants are trapped, filtered, and degraded biologically and biochemically [21]. Microorganisms associated with rhizomes and roots are responsible for the degradation of organic matter to inorganic nutrients to be absorbed by plants [22].
Within this context, the targets of wastewater treatment and biomass production would be simultaneously achieved when recycling wastewater for the irrigation of willows within a closed-loop concept [23]. Sas et al. [24] have investigated the impact of recycled wastewater on willow biomass production. Willows can be used in the phytoremediation processes of both wastewater and soil [25]. However, some irrigation water may infiltrate into adjacent soil, surface water, and groundwater [26]. Therefore, Gregersen and Brix [27] have developed a constructed wetland system vegetated with willows (S. viminalis) for zero discharge of nutrients. This technology is known as an evapotranspiration willow system to purify domestic wastewater, evaporate water, and recycle nutrients into willow biomass. Vysloužilová et al. [28] have considered a pot-scale study for seven Salix species. Clones were planted at three different pollutant levels of soil to assess the cadmium and zinc accumulation and phytoextraction potential of the willow biomass.
Almost all the referenced published scientific research studies have recommended the further investigation of irrigation wastewater effects on willow biomass production, chemical element accumulation, pollutant loading, and nutrient recovery [23,25,29]. Therefore, this study addresses this apparent need by considering two species of Salix for investigation; namely, white willow (S. alba) and common osier (S. viminalis). The willows were irrigated with synthetic greywater treated by floating treatment wetlands to address the following objectives: (a) to assess the developing biomass growth of both species; (b) to evaluate the accumulation of elements in willow-planted substrate; (c) to compare the accumulation of elements in the biomass of both species; and (d) to study the impact of cement–ochre pellets within the treatment system.
2. Materials and Methods
2.1. Willow, Substrate, and Material Selection
Two species of willows (Salix spp.) were selected for irrigation with treated synthetic greywater, white willow (S. alba) and common osier (S. viminalis), which were grown under the same real environmental conditions. Both species were purchased from Yorkshire Willow Online Shop as cuttings with lengths of 20–25 cm, as well as diameters of 3–5 mm for S. alba and 6–8 mm for S. viminalis (Figure 1a). The synthetic greywater (SGW) effluents were recycled for irrigation, with two replicates (labelled as a and b) for each willow species.
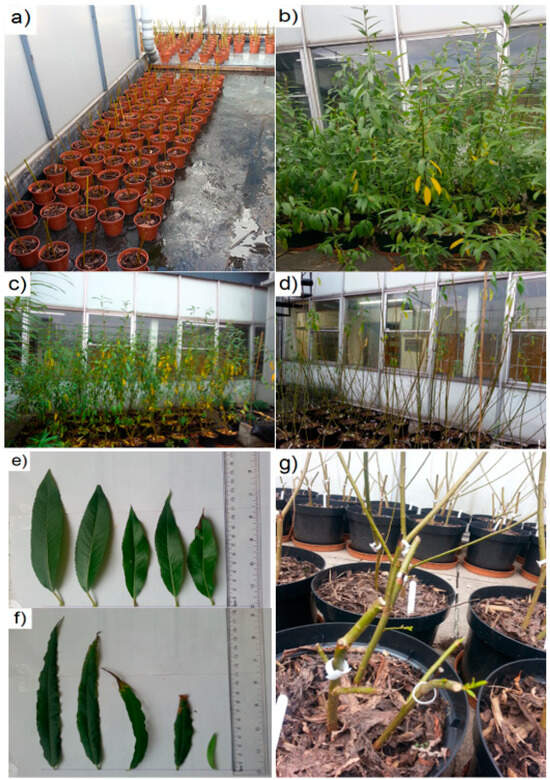
Figure 1.
Photos (taken by Suhail N. Abed) of the Salix spp. experimental planting irrigated with greywater effluents of floating treatment wetland systems: (a) Salix spp. cutting cultivations; (b) S. alba growth; (c) S. viminalis growth; (d) leaves fell during the autumn season; (e) S. alba leaves; (f) S. viminalis leaves; and (g) Salix spp. after biomass harvesting.
Compost substrate and bark of the “Verve Brand” were purchased from a local B&Q plc warehouse in Salford, Greater Manchester, UK. Multipurpose peat-based compost substrate (product code: 03717644) was selected as a planting media, while small, chipped bark (product code: 5397007188110) of mixed wood was applied on the top surface of the compost substrate to maintain moisture and insulate the substrate within the pots.
The analysis of dry raw compost substrate before planting was about 89% organic matter, 368 mg/kg total phosphorus, 999 mg/kg total nitrogen, 2776 mg/kg potassium, and 26.59 mg/kg zinc. In terms of physical properties, the compost substrate had a low bulk density and a high organic content proportion, providing a substrate with a high total porosity, stable substrate structure, good hydraulic conductivity, as well as a high water-retention time. A good compost water-holding potential and water-retention capacity are linked to high substrate porosity [30].
Willow planting was carried out in two stages. The initial phase commenced on 25 February 2015, involving the cultivation of willow cuttings in compost substrate within small plastic pots (60 mm diameter) for a duration ranging from three to six weeks (Figure 1a). Subsequently, the plants were exposed directly to natural weather conditions on the top of a flat, open roof. The irrigation regime utilizing treated SGW was initiated on 1 April 2015.
Three healthy willow cuttings were each transplanted into a single large plastic pot (300 mm diameter) with a 10 L volume. These pots, sourced from Scot plants Direct–Hedgehogs Nursery Ltd. (Crompton Road, Glenrothes, Scotland, UK), were filled with multipurpose compost substrate topped with small, chipped bark from mixed wood to enhance moisture and insulate the substrate (Figure 1b–d). The willow growth of both species was monitored and compared to each other until the autumn season. Leaves of S. alba and S. viminalis were randomly collected for element analyses (Figure 1e,f). Furthermore, biomass was harvested to assess fresh and dry weights as well as for chemical analyses of element accumulations (Figure 1g).
2.2. Greywater and Floating Wetland Systems
The SGW was formulated in the laboratory using analytical-grade chemicals purchased from Fisher Scientific Co., Ltd., Bishop Meadow Road, Loughborough, UK. Supplementary material Table S1 shows two different chemical recipes that mimic low and high concentrations of synthetic greywater labelled as LC–SGW and HC–SGW (Table 1), respectively. Stock solutions of both greywaters were kept within a refrigerator at 5 °C. For experimental purposes, the stock solution was diluted with tap water at a ratio of 1 to 10 [31].

Table 1.
Overview of the experimental set-up of floating wetland systems designed for irrigation of S. alba and S. viminalis with treated synthetic greywater.
Bare-rooted Phragmites australis (Cav.) Trin. ex Steud. (Common reed) was selected as the macrophyte for the experimental floating treatment wetland systems [32]. Furthermore, mine acid drainage sludge (ochre) was provided by the Deerplay Coal Mine authority, North Rochdale, UK, to create cement–ochre pellets after mixing with ordinary Portland cement at specific proportional ratios. The purpose of creating cement–ochre pellets was to mitigate the soluble mineral concentration, reduce the risk of losing ochre at high flows, and improve the treatment performance [33]. The treatment system used was of mesocosm scale and consisted of 72 buckets of 14 L each. The buckets were filled with only 10 L of SGW to prevent flooding during heavy precipitation events. All the systems were exposed to similar weather conditions while located on the flat roof of the Newton Building, The University of Salford, Salford, UK.
The experimental set-up aimed to assess the influence of four design and operational parameters: (a) two greywater pollution strengths (LC–SGW and HC–SGW); (b) two hydraulic retention times (HRTs: 2 days and 7 days); (c) presence or absence of P. australis; and (d) presence or absence of cement–ochre pellets (Table 1).
A total of 72 mesocosms comprised of treatment systems T1 to T16 with four replicates and controls C1 to C4 with two replicates. The distribution of these systems was as follows: (a) 2-day HRT (T1–T8, C1, and C2) and 7-day HRT (T9–T16, C3, and C4); (b) greywater pollution strength HC–SGW (T1–T4 and T9–T12) and LC–SGW (T5–T8 and T13–T16); (c) presence of P. australis (T1, T2, T5, T6, T9, T10, T13, T14, C1, and C3); and (d) presence of cement–ochre pellets (T2, T4, T6, T8, T10, T12, T14, and T16). A combination of P. australis and cement–ochre pellets was tested for the systems T2, T6, T10, and T13, while mesocosms of only SGW were associated withT3, T7, T11, and T15 as shown in Table 1. Upon completion of the specified hydraulic retention times (HRTs), SGW effluents were replaced by freshly created SGW influents. Subsequently, the effluents from the treatment systems were recycled for the irrigation of willow plants following the experimental design (Figure 2).
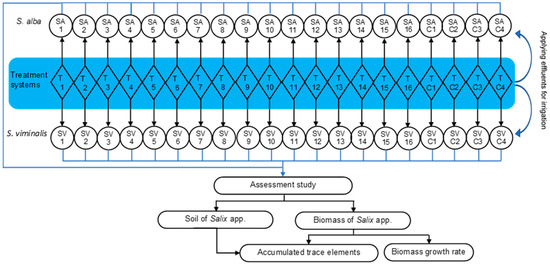
Figure 2.
Schematic drawing of the experimental design and the flow directions within the system.
2.3. Water Quality, Substrate, and Willow Biomass Analysis
Water quality tests for SGW were launched on 1 September 2014 and ended on 1 November 2016. Moreover, the authors monitored the development of biofilms attached to the roots and rhizomes of P. australis as well as to the vessel interior walls between September and October 2014 only.
Water testing was performed according to the American standard methods for the examination of water and wastewater [34]. All sampling kits were cleaned and washed using non-ionic detergents, then rinsed with tap water, soaked overnight within a 10% nitric acid solution, and later rinsed again with deionized water just before use.
A spectrophotometer DR 2800 (Hach Lange) was utilized to assess total suspended solids (TSSs), color, orthophosphate–phosphorus (PO4−P), nitrate–nitrogen (NO3−N), ammonia–nitrogen (NH4−N), and chemical oxygen demand (COD). A mono-metric measurement device (OxiTop IS 12–6 System) was used to calculate the five-day biochemical oxygen demand (BOD5). A digital electrochemistry HQ30d Flexi Meter (Hach Lange) was used for determining the dissolved oxygen (DO). The conductivity meter (METTLER TOLEDO FIVE GOTM) was applied for electric conductivity (EC) measurements. A turbidity meter of type TurbiCheck (Lovibond Water Testing) was operated for the determination of turbidity. A SensION+ benchtop multi-parameter meter (Hach Lange) was used to measure hydrogen ions (pH) and the redox potential (Eh). Following the “SW–846: TEST Method 6010D” of the USEPA [35], the metal concentrations in water samples were obtained by using inductively coupled plasma–optical emission spectrometry (ICP–OES) analysis with a Varian 720–ES provided by Agilent Technologies UK Ltd. Samples for mineral water analysis were prepared according to the USEPA [36] in triplicate water samples of 10 mL each, which were acidified and filtered through a 0.45 μm cellulose filter paper before analysis.
According to Method 200.7 of the USEPA [36], compost substrate and willow tissues were analyzed for accumulated elements using ICP–OES. Substrate samples were taken as 20 samples (10 from each replicate pot) by a substrate sampler kit, reaching to a depth of up to 20 cm from the top surface [37], while willow tissues (leaves and twigs) comprised 48 randomly selected samples (24 from each replicate) from each planting set (separately for both species).
Following the USEPA’s Method 3050B [38], both substrate and willow tissue samples were prepared for mineral analysis through overnight drying in an oven at 105 °C. This step was essential to facilitate enzymatic reactions and ensure the stabilization of sample weights [39]. Subsequently, oven-dried and well-grinded samples were acidified by 10 mL of aqua regia mixture containing one part nitric acid (HNO3) with three parts of hydrochloric acid (HCl). This acidification process occurred within a high-pressure-resistance Teflon tube and was followed by digestion using a CEM Mars Xpress microwave. Afterwards, the samples were analyzed by using ICP–OES for the following element concentrations (mg/kg): aluminum (Al), boron (B), calcium (Ca), cadmium (Cd), chromium (Cr), copper (Cu), iron (Fe), potassium (K), magnesium (Mg), manganese (Mn), sodium (Na), nickel (Ni), and zinc (Zn). Three standard calibration solutions were regularly run between the samples to address instrumental drifts. Moreover, blank samples were analyzed at the beginning of each test to identify potential contamination linked to reagents or equipment during the test procedure. The periodical testing of blank samples ensured that the values remained within the detection limits.
2.4. Data Assessment and Statistical Analysis
The collected data underwent a thorough examination of significant differences with a confidence level of 95% using the IBM Statistical Package for the Social Sciences software program version 23. To assess data distribution normality, the Shapiro–Wilk test was applied. The parametric T-test was used for normally distributed independent samples. In cases where the data did not follow a normal distribution, the non-parametric Mann–Whitney U test was performed. These statistical methods were chosen to ensure a comprehensive analysis of the data, considering both normal and non-normal distribution scenarios.
3. Results and Discussion
3.1. Greywater Effluent Quality
Greywater effluent was recycled for the irrigation of two species of S. alba and S. viminalis, as described in Section 2, following the experimental design set-up in Table 1. Table 2 shows the overall effluent water quality for several parameters including the physiochemical and the chemical element concentrations. Notably, the majority of treatment effluents exhibited pH values exceeding 6.5. It was observed that the effluent pH values of treatment systems incorporating the ochre pellets surpassed the recommended pH limit of 8.5 for wastewater intended for agricultural irrigation [14]. However, research suggests allowing a pH of up to 9.5 for wastewater to be recycled for irrigation [40]. This indicates that while the effluent pH values from systems containing ochre pellets may exceed conventional limits, they are within the broader acceptable range established by the relevant research standards.

Table 2.
Willow irrigation water quality: synthetic greywater (SGW) effluents from floating treatment wetland (FTW) systems.
The concentrations of total suspended solids (TSSs) in effluents were found to comply with the recommended values, falling within the range of 100–350 mg/L [15]. The observed TSS concentrations of all the treated LC–SGW were lower than 100 mg/L. Elevated TSS values in this case can be attributed to substrate composition distortion, reduction in substrate porosity, and substrate clogging [30]. Furthermore, electric conductivity (EC) serves as an indicator of water salinity, and it is advisable for EC to remain below the maximum limit of 3000 μS/cm as stated by both the FAO [14] and the WHO [15], since high salinity and EC in agricultural soil and water negatively effect soil structure, water and air exchange in the soil, as well as crop biomass productivity [25].
The five-day biochemical oxygen demand (BOD5) of the treated greywater was much lower than the stated limits, ranging between 110 and 400 mg/L [15]. In fact, greywater is usually characterized by a low content of organic matter compared to black wastewater [16]. As the substrate becomes increasingly clogged, the availability of oxygen within the root zone diminishes, creating conditions conducive to anaerobic microbial activity. Under anaerobic conditions, denitrification processes are more likely to occur, leading to the conversion of nitrate into nitrogen gases such as nitrous oxide and nitrogen. This denitrification process can result in the loss of nitrogen from the root zone, impacting nutrient availability for plants and potentially influencing overall ecosystem dynamics [41].
The greywater effluents of all the treatment systems showed cadmium (Cd) concentrations usually higher than the stated thresholds of 0.01 to 0.05 mg/L [14,42]. The corresponding values were 0.02–0.09 mg/L for LC–SGW and 4.10–7.69 mg/L for HC–SGW (Table 2). Elevated Cd concentrations in irrigation water could lead to accumulations in willow tissue over time [15], as they are more efficient than other plants in the storage of Cd [28,43,44].
In addition, greywater effluents of the systems treating LC–SGW had low concentrations of chromium (Cr), potassium (K), magnesium (Mg), and sodium (Na) compared to the respective threshold limits for irrigation water: 0.1–1.0 mg/L, 0.0–2.0 mg/L, 0.0–5.0 mg/L, and 0.0–40.0 mg/L, respectively [14,42]. In contrast, the corresponding concentrations of the HC–SGW effluents were higher (Table 2). The test results show that the concentrations of copper (Cu), manganese (Mn), nickel (Ni), and zinc (Zn) for all the greywater effluents were lower than the values recommended by the FAO [14] and the USEPA [42]: 0.2–5.0 mg/L, 0.2–10.0 mg/L, 0.2–2.0 mg/L, and 5.0–10.0 mg/L, respectively. Also, the PO4−P concentration within wastewater to be used for irrigation is often limited between 2 mg/L [14] and 5 mg/L [42].
Certain greywater chemicals, such as micronutrients (Al, Ca, Fe, Mg, and Zn) and macronutrients (N and P), may serve as alternatives to industrial fertilizer [3]. However, some greywaters exhibit high concentrations of total phosphorus and total suspended solids, which could limit their water reuse potential. Furthermore, the structure of organic-based compost substrate might be negatively affected by irrigation water with high concentrations of Ca, Mg, and Na, which subsequently increase the sodium adsorption ratio [12].
3.2. Weather Conditions and Willow Growth
The experimental work for both willow species was undertaken in authentic environmental conditions. In general, the weather in Greater Manchester is generally characterized by mostly cloudy, rainy, windy, and cold conditions in the winter, while it is partly cloudy and has moderate in temperature during summer. Therefore, the minimum, maximum, and average temperatures, as well as the relative humidity, were determined to assess the effect of weather conditions on willow growth.
The data were measured in situ and compared to those obtained from the UK weather service, the Met Office (https://www.metoffice.gov.uk/). In March 2015, the temperature was around 3–10 °C, with a noticeable increase observed between May and September 2015. During the summer season, the highest temperature (24 °C) was recorded in June 2015, while the lowest (16 °C) was in July 2015. The relative humidity measurements were around 48.3% and 79.3%, with an approximate average of 65 ± 7.0% (Figure 3).
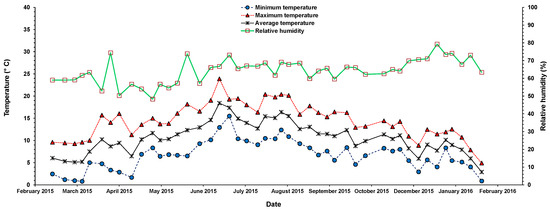
Figure 3.
Recorded temperature and relative humidity during the willow growth period.
Since, Salix spp. is renowned as a fast-growing and easy-to-propagate plant, it has gained importance in short-rotation coppice plantations as a sustainable resource of renewable bioenergy [9]. In the present investigation, the growth rates and biomass productivity of S. alba and S. viminalis were measured on two occasions: November 2015 and November 2016. According to the experimental set-up design, the variable parameters were Salix spp. species and the characteristics of greywater effluent, which depend on the treatment system design (Table 1). In 2015, both species exhibited approximately equal rates of growth after planting, especially at the juvenile stage in April and May. Afterwards, S. alba showed significant growth in terms of the number of leaves compared to S. viminalis. Subsequently, with growth almost all leaves were dropped for both Salix species between September and October. By the end of autumn 2015, biomass productivity was assessed by measuring the average length and average diameter during harvest when the plants were almost dormant to determine the total fresh and dry weights of twigs for both Salix species. Salix spp. had high foliage and also produced a high woody biomass. Biomass production is often correlated with leaf water relations and photosynthesis; however, inconsistent findings have been reported about the relationships between leaves and productivity [45].
The measurements revealed that the average twig length per pot for all S. alba was 158 ± 22.7 cm (mean ± standard deviation), and the average twig diameter was 9 ± 0.9 mm. The highest average twig length and diameter observed for S. alba (SA11) were 197 cm and 11 mm, respectively, while the lowest average height was 122 cm for SA/C4, and the lowest average diameter was 8 mm for SA/C1. Regarding the growth of S. viminalis, the average measured twig length per pot was 129 ± 19.3 cm, which was statistically significantly (p < 0.05) smaller than the average twig length of S. alba. (Figure 4a). The measurements for the Salix spp. species grown from cuttings during the first year can be used to predict the growth rates for subsequent seasons in terms of numbers of stems or twigs, their dimeters, long-term biomass production, and final yields. However, the goodness of prediction may vary from species to species [46,47].
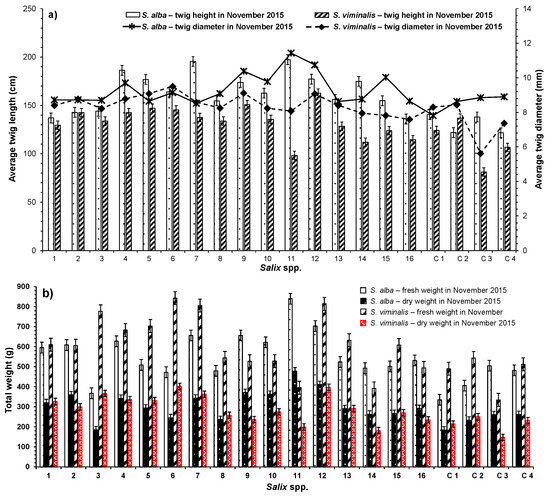
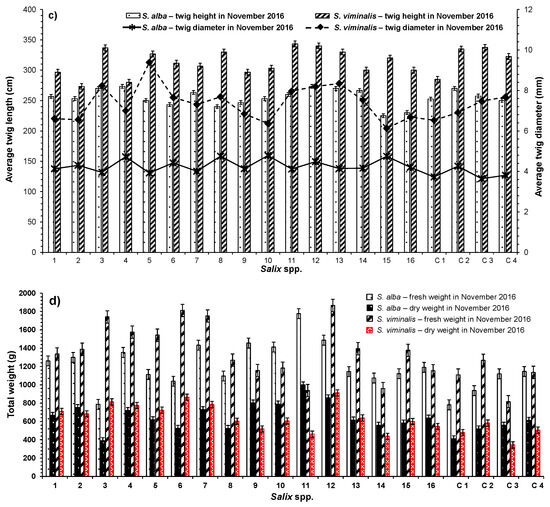
Figure 4.
Comparison of harvested biomass between S. alba and S. viminalis in terms of (a) average twig lengths and average diameters in 2015; (b) total fresh and dry weights in 2015; (c) average twig lengths and average diameters in 2016; and (d) total fresh and dry weights in 2016. Sample number: 6.
The average fresh and dry weights of the harvested biomass per each pot in 2015 for S. alba were 546 ± 119.7 g and 300 ± 74.3 g, respectively, with an average water content of about 45 ± 2.8% (ranging from 41 to 51%). The overall fresh and dry biomass weights produced by all S. alba which were harvested in 2015 were 10.9 kg and 6.0 kg, respectively. The average total fresh and dry weights of the harvested biomass per pot planted with S. viminalis were 592 ± 144.9 g and 281 ± 71.6 g, respectively, with average water content of about 53 ± 2.7% (ranging between 46% and 56%). The overall fresh and dry biomass weights produced by all harvested S. viminalis were 11.8 kg and 5.6 kg, respectively, (Figure 4b).
For the harvested biomass in 2016, the measurements show that the average twig length and diameter per pot of S. alba were 255 ± 13.8 cm and 4 ± 0.3 mm, correspondingly (Figure 4c). The average twig diameters in 2016 recorded a significant reduction compared to their measurements in 2015 (Figure 4a,c).
From the measurements of S. viminalis grown in 2016, the statistical assessment presents significant average twig lengths per pot (314 ± 21.3 cm) and corresponding average twig diameters (7 ± 0.8 mm) in comparison to measurements of (a) comparable S. alba in 2016 (excluding SV2 and SV4) (Figure 4c) and (b) corresponding S. viminalis in 2015 (Figure 4a,c). The above findings agree with previous studies on S. schwerinii, which have reported that willow diameters decreased by 11% after the first year of applying wastewater for irrigation and then increased to 90% after the second year [46].
Regarding biomass production in 2016, the average harvested fresh and dry weights per pot of S. alba were 1201 ± 241.9 g (ranged between 781 and 1777 g) and 643 ± 149.9 g (ranged between 389 and 999 g) in this order, with average water content proportions of 47 ± 2.7% (ranged between 42 and 52%). The overall weights of fresh and dry biomass produced by all S. alba were around 24 kg and 13 kg, respectively, which were significantly greater than the biomass weights obtained in 2015 (Figure 4b,d).
In terms of the harvested S. viminalis biomass in 2016, the average fresh and dry weights per pot were 1338 ± 300.3 g (ranging between 816 and 1866 g) and 629 ± 152.4 g (344–911 g), respectively, with an average water content of 53 ± 2.7% (ranging between 47 and 58%), as shown in Figure 4d. The overall weights of fresh and dry biomass produced by all S. viminalis were around 27 kg and 13 kg, correspondingly, which were significantly greater than the biomass weights obtained in 2015 (Figure 4b,d). As indicated in the literature for a field case study [46], applying wastewater for the irrigation of S. schwerinii improved the biomass productivity by 69% during the first season of growth and between 432% and 446% during successive seasons. The likelihood of real willow plantations achieving the environmental, economic, and industrial objectives depend on biomass production, which is aimed at covering the expenditures for land, irrigation water, materials, labor, as well as operational and maintenance costs [3]. The environmental and economic benefits of cultivating high-biomass-yielding trees for short-rotation forestry should be considered in practice [45]. Growth monitoring and biomass production assessment of both species of Salix spp. grown in pots can be used to consistently predict long–-term biomass production on a field scale [47].
3.3. Element Accumulations in Substrate Used to Grow Willows
Both species of Salix were irrigated with two types of greywater, each varying in contamination strength and treatment experimental set-up designs of floating wetland systems (Table 1 and Table S1). Therefore, an investigation into the compost substrate constituents with a specific focus on element concentrations was conducted. The objective was to assess and compare the phytoremediation efficiencies of S. alba and S. viminalis under the influence of different greywater compositions and experimental conditions.
The chemical analysis of the irrigated substrates of both Salix spp. revealed significant changes (p < 0.05) in element content compared to the raw substrate. The detected accumulated element concentrations indicated the following order: Mg > Fe > Al > Cr > Mn > Cd > Cu > B, as shown in Table 3. These results align with previous observations indicating Fe > Al [47]. Element accumulations and take-ups by Salix spp. can affect substrate pH and organic content [44]. The transport of elements from substrate to plant tissues may contribute to a decrease in the pH value [48]. Moreover, the wide variety of chemical reactions and the high cation-exchange capacity of organic-based compost substrate can lead to significant fluctuations in the concentrations of element accumulations within a substrate [49]. The mobility and solubility of Al at different pH levels could be limited to organic compost and agricultural substrates with high clay proportions. However, the authors have not found any reported toxicity cases linked to human health or the environment due to the accumulation of Al in substrates and plant tissues. For high concentrations of Ca ions in a substrate, the mobility of Al is constrained within plants due to a negative correlation between Al ion exchange and substrate pH [30,50]. Furthermore, traces of B were detected in a few substrate samples (Table 3 and Figure 5b).

Table 3.
Element concentrations (mg/kg) accumulated in substrate planted with (a) S. alba and (b) S. viminalis.
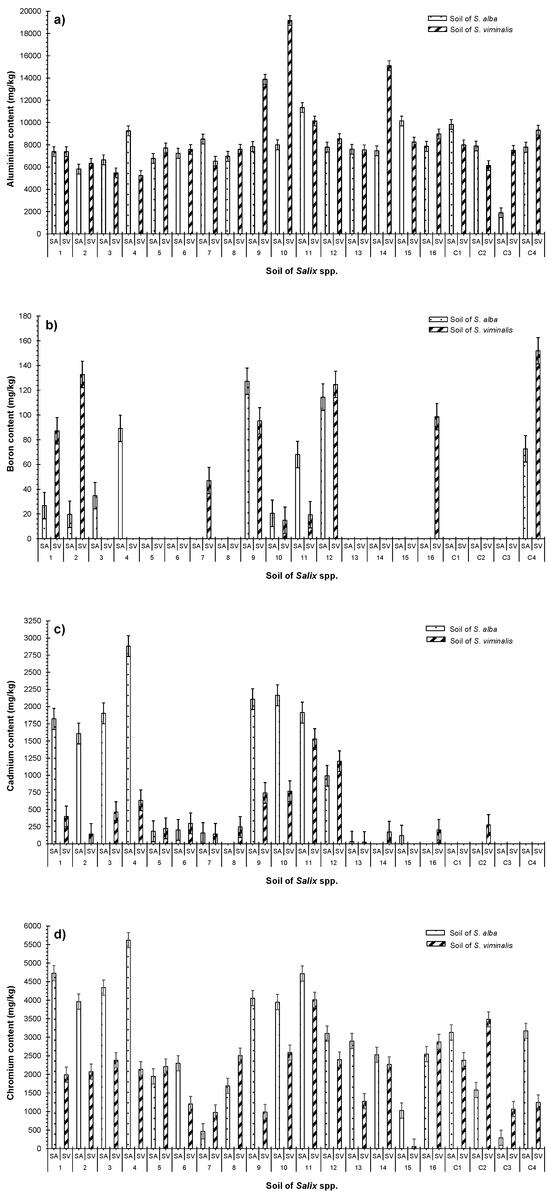
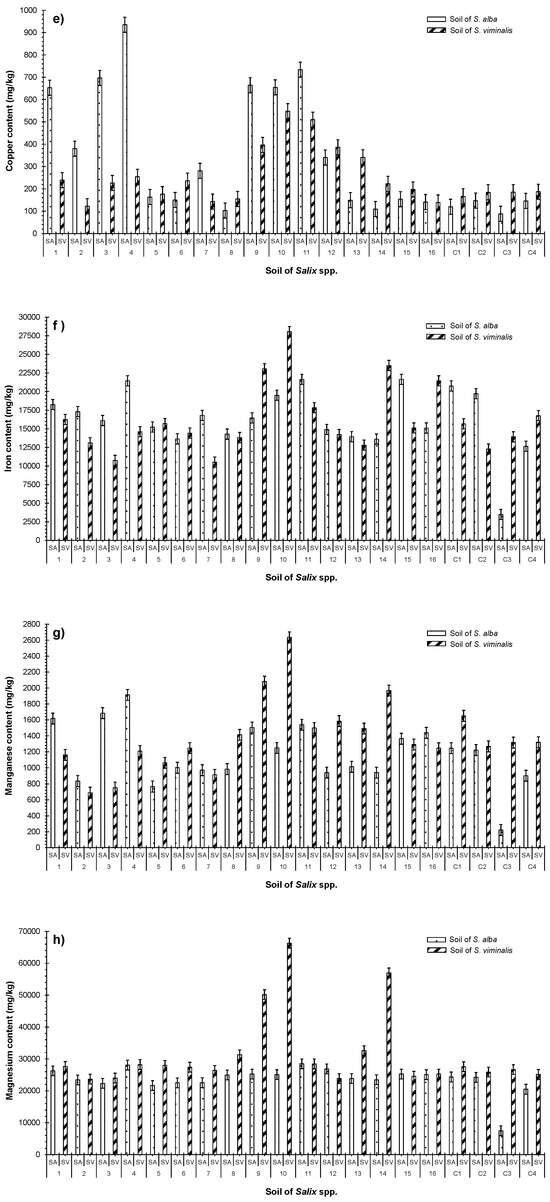
Figure 5.
Comparison between the concentrations of accumulated elements in substrates linked to S. alba and S. viminalis in terms of (a) aluminum (Al); (b) boron (B); (c) cadmium (Cd); (d) chromium (Cr); (e) copper (Cu); (f) iron (Fe); (g) manganese (Mn); and (h) magnesium (Mg). Sample number: 20.
Cd, Cr, and Cu accumulated in the substrates of S. alba irrigated with HC–SGW. The accumulations were significantly (p < 0.05) higher compared (a) to the elements accumulated in the substrates linked to S. viminalis; and (b) to those accumulated in the substrates irrigated with LC–SGW (Figure 5c–e). The fixation of metals such as Cd in agricultural substrates can occur through various mechanisms. This may include the application of phosphoric fertilizers, recycling contaminated irrigation water, and exposing substrates to contamination via deposition from the air [51]. Finally, the patterns of accumulated Fe and Mn in the substrates of both species of Salix fluctuated and had no clear trend (Figure 5f).
Concentrations of Mn were significantly (p < 0.05) higher in the substrates used for S. alba which were irrigated with HC–SGW effluent of 2-day HRT. Almost all substrates planted with S. viminalis, excluding SV1–SV4, showed significant accumulations of Mn compared to the corresponding substrates linked to S. alba receiving the same effluent quality (Figure 5g). Metal bioavailability is influenced by the oxygen and pH conditions in substrates. These boundary conditions play a crucial role in respiration and photosynthesis processes. The involvement of microorganisms is vital in the oxidation of metals and the formation of metal hydroxides in substrates. Hydroxides are more stable than free metal ions and cations, contributing to the overall metal bioavailability in a system [51,52].
Significantly (p < 0.05) higher concentrations of Mg were detected in substrates planted with S. viminalis irrigated with effluents subjected to a long HRT, which were greater than the concentrations in the corresponding substrates for S. alba but were not significant (p > 0.05) (Table 3, Figure 5h). Low plant growth rates are linked to the high Mg contents within the substrate since they correlated positively with Fe. However, Mg is essential for plant photosynthesis [49,52].
The reuse of wastewater for agricultural irrigation poses a risk of element accumulations and subsequent leaching in acid substrates [41]. To safeguard human health and the environment, it is recommended to monitor substrate pollutants, considering chemical composition, substrate salinity, and contamination mobility [12,49]. Long-term irrigation with treated wastewater can lead to elevated accumulations of elements such as Cd, Cu, Zn, Ni, and Cr in agricultural substrates above regulatory thresholds [51,52]. The root systems of Salix spp. are typically associated with fungus colonies that protect roots and reduce the risk of mineral contamination. Mycorrhizal fungus efficiently stores metals, immobilizing metal ions below ground and decreasing metal translocations from roots to other plant tissues [43,48]. This symbiotic relationship plays a crucial role in mitigating the potential negative impacts of element accumulation in the substrate.
3.4. Element Accumulation in Willow Biomass
Both species of Salix were considered for element accumulations in their biomass. Both twigs (woody biomass) and leaves (foliage biomass) were subjected to chemical analysis to assess phytoremediation efficiency. The accumulated mineral contents in S. alba and S. viminalis were compared, and the results are presented in Table S3.
The statistical analysis of element concentrations accumulated within twigs of S. alba revealed sequences of metal contents following this order: Ca > Mg > Na > Mn > Zn > Fe > Al > Cd > Cu > Cr > Ni > B. For S. viminalis, the order was as follows: Ca > Mg > Mn > Zn > Na > Fe > Al > Cd > Cu > Ni > Cr > B,(Table S3; Figure 6a,b).
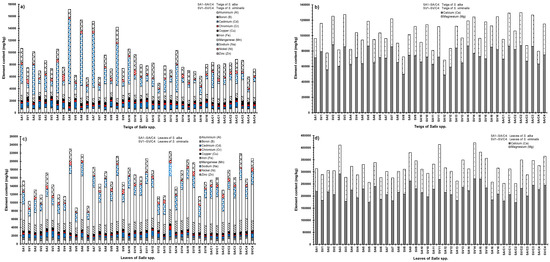
Figure 6.
Comparisons between element contents accumulated in biomass of S. alba and S. viminalis in terms of (a) twigs (aluminum, boron, cadmium, chromium, copper, iron, manganese, sodium, nickel, and zinc); (b) twigs (calcium and magnesium); (c) leaves (aluminum, boron, cadmium, chromium, copper, iron, manganese, sodium, nickel, and zinc); and (d) leaves (calcium and magnesium). Sample number: 48.
Certain elements are classified either as macronutrients (N, K, P, S, Mg, and Ca) or micronutrients (Fe, Mn, B, Zn, Cu, Cl, Mo, and Na). These elements are crucial for promoting healthy plant growth in moderate quantities. However, elevated concentrations of these elements can be toxic to some plants [53]. In the case of Salix spp., the required corresponding proportional macronutrients were 100, 65, 13, 9, 8.5, and 7 parts of weight, respectively, and 0.7, 0.4, 0.2, 0.06, 0.03, 0.007, 0.003, and 0.003 parts for micronutrients in this order. However, the exact nutritional needs for willows under various boundary conditions are not well known as they are a function of the substrate nutrient level, microorganism community, harvest regime, maturity of plants, and irrigation water quality [54]. Furthermore, the greatest total accumulations of elements were observed in twigs for almost all S. alba samples compared to the total accumulations in twigs linked to S. viminalis. (Table 1, Figure 6a,b).
In addition, the statistical analysis elucidated that the concentrations of Al, B, Ca, Cd, Mg, and Ni accumulated and were significantly (p < 0.05) higher in the woody biomass of S. viminalis twigs compared to those from S. alba, which were associated with significantly high accumulations of Cu, Na, and Zn concentrations in their twig biomass. However, Cr and Fe concentrations were not significantly different (p > 0.05) in terms of biomass accumulations for both Salix spp. Furthermore, the concentrations of Mn were significantly higher in terms of accumulation within twigs obtained from S. viminalis which were irrigated with greywater effluents from systems associated with long (7-day) HRT. The Mn concentrations were significantly higher in terms of accumulation within S. alba, which received greywater effluent from systems of short HRT.
The cultivation and subsequent harvesting of Salix spp. provide a mechanism for removing unwanted elements from the land. Moreover, the extraction of metals from plant tissues could be explored for reuse purposes, potentially offering an economical and environmentally sustainable approach in certain case studies [43].
The leaves of both species of Salix were also investigated for element concentrations. The rank orders of element accumulation occurrences were as follows: Ca > Mg > Mn > Na > Fe > Zn > Al > Cd > Ni > B > Cu > Cr for S. alba, and Ca > Mg > Mn > Na > Fe > Zn > Cd > Al > B > Ni > Cu > Cr for S. viminalis (Table S3). The leaves of both species had Ca as the highest accumulated element and Cr as the lowest accumulated one. This is positive but not so important, as the dry weight of leaves is small in comparison to twigs and stems.
The overall accumulations of elements were significantly (p < 0.05) higher in the leaves of almost all S. alba, particularly in those irrigated with greywater effluents of short HRT compared to S. viminalis (Figure 6c,d).
Elements such as Cu, Na, Ni, and Zn within the leaves fluctuated without a clear trend for both of the willow species. However, the leaves of S. alba showed significantly (p < 0.05) higher concentrations of elements linked to effluent treatment parameters, such as Al (for effluents of long HRT), Ca and Mn (for effluents of short HRT), Cd (for effluents of HC–SGW), and Fe (for almost all effluents). Accumulations of B and Mg were higher in the leaves of S. viminalis associated with effluents of long HRT compared to S. alba. In contrast, Cr accumulation showed no significant differences when comparing accumulations in leaves of both species with each other (Figure 6c,d).
Salix spp. demonstrated resilience and survival despite the accumulation of elements in their tissues. The metals absorbed by willows tend to accumulate in the aerial tissues, with the highest accumulations associated with woody tissues. However, there is a considerable variety of metal distributions in newly grown willows, where metals are more evenly distributed between foliage and woody biomass [43].
The findings show that the accumulations of Al, B, Ca, Fe, Mg, Mn, and Ni were significantly (p < 0.05) greater in the leaves compared to the twigs of S. alba. Moreover, woody biomass accumulations compared to those in twigs showed significantly higher concentrations of Na and Zn. However, the highest accumulations of Cd, Cr, and Cu fluctuated between leaves and twigs without a specific trend. The accumulations of Al, B, Ca, Fe, Mg, Mn, and Na were significantly greater in S. viminalis leaves compared to twigs. Cd, Cr, Cu, Ni, and Zn showed variations in their highest accumulations between leaves and twigs (Figure 6).
Elements can be stored in various parts of willow biomass, including in below-ground (roots) and above-ground woody plant parts (stems, twig branches and shoots), and foliage (leaves). It is more common to observe higher accumulations of elements in plant roots and stems than in other willow parts [54]. However, the challenge lies in the enhanced spatial distribution of accumulated metals, particularly through leaf fall in autumn [43].
The substrate to plant transfer factor provides an indication of metal accumulation rates into plant biomass tissues. For certain metals, such as Cd, Cu, and Ni, the transfer factor is higher for edible leafy vegetable crops compared to woody biomass crops [43,48]. Phytoextraction and phytostabilization are the two crucial phytoremediation processes. In phytoextraction, contaminants are adsorbed and extracted from the substrate by plants and accumulate in their tissues. This process allows for the removal of contaminants from both the substrate and plants, either through harvest or dead leaves. The methods of element adsorption and intracellular translocation can vary among different plants. Toxicity tolerance is affected by several parameters including water pH, microorganism populations, as well as plant species and their enzymes [28]. These factors collectively contribute to the effectiveness of phytoremediation in mitigating environmental contamination.
4. Conclusions and Recommendations
This study focused on investigating the woody biomass productivity of S. alba and S. viminalis, as well as the remediation potential of substrates irrigated with synthetic greywater (with two pollutant strengths) treated by floating wetland systems. The irrigation greywater quality complied with international standards and thresholds for the safe reuse of wastewater for irrigation, in particular for low-contamination-strength synthetic greywater. However, high-contamination-strength synthetic greywater comprised some elements with concentrations above the regulatory thresholds. High total suspended solids, salinity, and electric conductivity in agricultural substrates and water had adverse effects on substrate structure, water and air exchange into the substrate, and crop biomass productivity. As a result, the use of high-contamination-strength synthetic greywater in practice should be avoided to ensure optimal agricultural conditions. The growth rates and biomass productivity of both species of Salix spp. were assessed by measuring their twig numbers, lengths, and diameters, as well as their fresh and dry woody biomasses. During the juvenile stage, both species exhibited approximately equal rates of growth after planting. At the first harvest, S. alba produced a high woody biomass weight with twig lengths and diameters greater than those of S. viminalis for. For the second growth season, S. alba had a high number of slim and long twigs compared to the smaller number of long twigs for S. viminalis, which was linked to a significantly high fresh biomass weight with a high average biomass water content compared to S. alba. However, the dry biomass weight of S. alba was higher. Nevertheless, the utilization of both species for biomass production is recommended, taking into consideration their distinct characteristics and potential applications.
The element contents in the compost substrates underwent significant changes after irrigation with greywater effluents. The substrates of both Salix spp. recorded the highest accumulations of Mg, Fe, and Al. Fluctuations in the highest accumulations of Cu, Cd, and Mg in the substrates of S. alba were observed, which were particularly linked to irrigation with high-contamination-strength greywater. Consequently, the dilution of such waters should be considered.
The overall highest accumulations of elements were observed in the twigs of almost all S. alba compared to S. viminalis. Significantly high concentrations of Al, B, Ca, Cd, Mg, and Ni were linked to the woody biomass of S. viminalis twigs compared to S. alba. The highest Ca and Mg accumulations were detected in the twigs of both Salix spp. The twigs of S. alba were highly efficient in Na accumulation compared to S. viminalis, which showed a high ability to accumulate Mn, Zn, and Ni. It follows that these willow species are recommended for bioremediation purposes.
The accumulations of elements in the foliage (leaves) of both species showed the highest concentrations for Ca, and the lowest ones for Cr. The leaves of S. viminalis accumulated Cd and B better than Al and Ni. However, the opposite was the case for the leaves of S. alba. The overall accumulations of elements were significantly high in the leaves of almost all S. alba compared to S. viminalis, particularly for those irrigated with greywater effluents of short HRT. The leaves of S. alba were efficient in the accumulation of Al, B, Ca, Fe, Mg, Mn, and Ni compared to their twigs, which showed significantly high accumulations of Na and Zn. Furthermore, the leaves of S. viminalis were effective in accumulating Al, B, Ca, Fe, Mg, Mn, and Na in comparison to its twigs. The elements Cd, Cr, Cu, Ni, and Zn showed variations in their highest accumulations between leaves and twigs.
A plant’s ability to accumulate and store certain elements in its biomass can contribute to the remediation of contaminated soils. This highlights the potential for phytoremediation strategies involving Salix spp. to not only mitigate environmental contamination but also to recover valuable resources from the harvested biomass. The dynamic metal distributions within the plants’ different tissues suggest an adaptive response of Salix spp. to element uptake and storage, contributing to their overall resilience in the face of varying environmental conditions and contamination levels.
The natural shedding of leaves can lead to the redistribution of accumulated elements in the environment, posing a challenge for managing and controlling the impact of metal accumulation in the broader ecosystem. This aspect underscores the complexity of phytoremediation processes and the need for careful consideration of their long-term effects.
Therefore, regular harvesting of short-rotation coppices during the growing season is recommended to maximize removals of nutrients and metals, promoting the use of willows, for example, for biochar production or as a solid fuel for energy creation.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/environments11030044/s1. Table S1 shows chemical formulas of synthetic greywaters (SGWs) for low (LC) and high (HC) pollutant concentrations. Table S2 indicates physiochemical characteristics of synthetic greywater. Table S3 summarizes detected element concentrations (mg/kg) accumulated in Salix spp. biomass for S. alba leaves, S. alba twigs, S. viminalis leaves, and S. viminalis twigs.
Author Contributions
Conceptualization, S.A.A.A.N.A., S.N.A. and M.S.; methodology, S.N.A. and M.S.; software, S.A.A.A.N.A. and S.N.A.; validation, S.A.A.A.N.A., S.N.A. and M.S.; formal analysis, S.A.A.A.N.A. and S.N.A.; investigation, S.A.A.A.N.A. and S.N.A.; resources, S.A.A.A.N.A. and M.S.; data curation, S.A.A.A.N.A. and S.N.A.; writing—original draft preparation, S.A.A.A.N.A. and S.N.A.; writing—review and editing, M.S.; visualization, S.A.A.A.N.A. and S.N.A.; supervision, M.S.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
The actual experimental work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. However, the staff’s time for data analysis and paper writing was subsequently paid for by the Water JPI grant RainSolutions and the European Union Horizon 2020 grant WATERAGRI (no. 858375).
Data Availability Statement
The data can be made available by the authors on reasonable request.
Acknowledgments
Guillaume Drouin, Pauline Francois, Rubén García, Guillaume Gourdikian, Claire Guillin, Elena Manjon Alfaro, César Moya, Anita Paul, Moe Thazin Aung, Hasan Rezaee, and Carlos Verdú helped with data collection.
Conflicts of Interest
The author Miklas Scholz is employed by Kunststoff-Technik Adams and Nexus by Sweden. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- UNEP. Global Environment Outlook GEO5: Environment for the Future We Want; United Nations Environment Programme (UNEP): Nairobi, Kenya, 2012. [Google Scholar]
- Zalesny, R.S.; Headlee, W.L.; Gopalakrishnan, G.; Bauer, E.O.; Hall, R.B.; Hazel, D.W.; Isebrands, J.G.; Licht, L.A.; Negri, M.C.; Nichols, E.G.; et al. Ecosystem services of poplar at long-term phytoremediation sites in the Midwest and Southeast, United States. Wiley Interdiscip. Rev. Energy Environ. 2019, 8, e349. [Google Scholar] [CrossRef]
- Izac, A.M.N. Economic aspect of soil fertility management and agroforestry practices. In Tree Crop and Soil Fertility: Concept and Research Methods; Scroth, G., Sinclair, F., Eds.; CABI: Wallingford, UK, 2003; p. 464. [Google Scholar]
- Tawfeek, N.; Mahmoud, M.F.; Hamdan, D.I.; Sobeh, M.; Farrag, N.; Wink, M.; El–Shazly, A.M. Phytochemistry, pharmacology and medicinal uses of plants of the genus Salix: An updated review. Front. Pharmacol. 2021, 12, 50–80. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.K.R. Climate change mitigation: A low hanging fruit of agroforestry. In Agroforestry: The Future of Global Land–Use; Nair, P.K.R., Garitty, D., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 31–68. [Google Scholar]
- Nair, P.K.R.; Kumar, B.M.; Nair, V.D. Agroforestry as a strategy for carbon sequestration. J. Plant Nutr. Soil Sci. 2009, 172, 10–23. [Google Scholar] [CrossRef]
- Christenhusz, M.J.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Ostaff, D.P.; Mosseler, A.; Johns, R.C.; Javorek, S.; Klymko, J.; Ascher, J.S. Willows (Salix spp.) as pollen and nectar sources for sustaining fruit and berry pollinating insects. Can. J. Plant Sci. 2015, 95, 505–516. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, D.; Singh, P.M.; Ragauskas, A.J. The new forestry biofuels sector. Biofuels Bioprod. Bioref. 2008, 2, 58–73. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2010; Main report; Forestry Paper 163; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 2010. [Google Scholar]
- UN Water. Coping with Water Scarcity—A Strategic Issue and Priority for System–Wide Action; UN—Water Thematic Initiatives: Geneva, Switzerland, 2006. [Google Scholar]
- Almuktar, S.A.; Abed, S.N.; Scholz, M. Recycling of domestic wastewater treated by vertical–flow wetlands for irrigation of two consecutive Capsicum annuum generations. Ecol. Eng. 2017, 107, 82–98. [Google Scholar] [CrossRef]
- UNESCO. Water for People–Water for Life: A Joint Report by the Twenty-Three United Nations Agencies Concerned with Freshwater; United Nations Educational, Scientific and Cultural Organization (UNESCO): Barcelona, Spain, 2003. [Google Scholar]
- FAO. User Manual for Irrigation with Treated Wastewater; Food and Agriculture Organization (FAO) of the United Nations, Regional Office for Near East: Cairo, Egypt, 2003. [Google Scholar]
- WHO. Guidelines for the Safe Use of Wastewater, Excreta and Greywater: Wastewater Use in Agriculture; World Health Organization (WHO): Geneva, Switzerland, 2006; Volume 2. [Google Scholar]
- Eriksson, E.; Auffarth, K.; Henze, M.; Ledin, A. Characteristics of grey wastewater. Urban Water 2002, 4, 85–104. [Google Scholar] [CrossRef]
- Al–Jayyousi, O.R. Greywater reuse: Towards sustainable water management. Desalination 2003, 156, 181–192. [Google Scholar] [CrossRef]
- Scholz, M.; Lee, B.H. Constructed wetlands: A review. Int. J. Environ. Stud. 2005, 62, 421–447. [Google Scholar] [CrossRef]
- Borne, K.E.; Fassman–Beck, E.A.; Winston, R.J.; Hunt, W.F.; Tanner, C.C. Implementation and maintenance of floating treatment wetlands for urban stormwater management. J. Environ. Eng. 2015, 141, 04015030. [Google Scholar] [CrossRef]
- Headley, T.; Tanner, C. Constructed wetlands with floating emergent macrophytes: An innovative stormwater treatment technology. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2261–2310. [Google Scholar] [CrossRef]
- Rehman, K.; Ijaz, A.; Arslan, M.; Afzal, M. Floating treatment wetlands as biological buoyant filters for wastewater reclamation. Int. J. Phytoremediat. 2019, 10, 1273–1289. [Google Scholar] [CrossRef]
- Shahid, M.J.; Arslan, M.; Ali, S.; Siddique, M.; Afzal, M. Floating wetlands: A sustainable tool for wastewater treatment. Clean Soil Air Water 2018, 46, 1800120. [Google Scholar] [CrossRef]
- Nissim, W.G.; Jerbi, A.; Lafleur, B.; Fluet, R.; Labrecque, M. Willows for the treatment of municipal wastewater: Performance under different irrigation rates. Ecol. Eng. 2015, 81, 395–404. [Google Scholar] [CrossRef]
- Sas, E.; Hennequin, L.M.; Frémont, A.; Jerbi, A.; Legault, N.; Lamontagne, J.; Fagoaga, N.; Sarrazin, M.; Hallett, J.P.; Fennell, P.S.; et al. Biorefinery potential of sustainable municipal wastewater treatment using fast–growing willow. Sci. Total Environ. 2021, 792, 148146. [Google Scholar] [CrossRef]
- Istenič, D.; Božič, G. Short–rotation willows as a wastewater treatment plant: Biomass production and the fate of macronutrients and metals. Forests 2021, 12, 554. [Google Scholar] [CrossRef]
- Jama, A.; Nowak, W. Willow (Salix viminalis L.) in purifying sewage sludge treated soils. Pol. J. Agron. 2012, 9, 3–6. [Google Scholar]
- Gregersen, P.; Brix, H. Zero–discharge of nutrients and water in a willow dominated constructed wetland. Water Sci. Technol. 2001, 44, 407–412. [Google Scholar] [CrossRef]
- Vysloužilová, M.; Tlustoš, P.; Száková, J. Cadmium and zinc phytoextraction potential of seven clones of Salix spp. planted on heavy metal contaminated soils. Plant Soil Environ. 2003, 49, 542–547. [Google Scholar] [CrossRef]
- Brix, H.; Arias, C.A. Use of Willows in Evapotranspirative Systems for Onsite Wastewater Management–Theory and Experiences from Denmark. In Proceedings of the “STREPOW” International Workshop, Andrevlje-Novi Sad, Serbia, 21–22 February 2011; Orlovi’c, S., Ed.; Institute of Lowland Forestry and Environment: Novi Sad, Serbia, 2011; pp. 15–29. [Google Scholar]
- Almuktar, S.A.A.A.N.; Abed, S.N.; Scholz, M.; Uzomah, V.C. Assessment of Capsicum annuum L. grown in controlled and semi–controlled environments irrigated with greywater treated by floating wetland systems. Agronomy 2021, 11, 1817. [Google Scholar] [CrossRef]
- Abed, S.N.; Scholz, M. Chemical simulation of greywater. Environ. Technol. 2016, 37, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- Abed, S.N.; Almuktar, S.A.A.A.N.; Scholz, M. Remediation of synthetic greywater in mesocosm–scale floating treatment wetlands. Ecol. Eng. 2017, 102, 303–319. [Google Scholar] [CrossRef]
- Abed, S.N.; Almuktar, S.A.A.A.N.; Scholz, M. Treatment of contaminated greywater using pelletised mine water sludge. J. Environ. Manag. 2017, 197, 10–23. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association (APHA), American Water Works Association, and Water and Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- USEPA. SW–846: Test Method 6010D: Inductively Coupled Plasma Optical Emission Spectrometry (ICP–OES); Revision 4; United States Environmental Protection Agency (USEPA): Washington, DC, USA, 2014.
- USEPA. Method 200.7: Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma Atomic Emission Spectrometry; Revision 4.4; United States Environmental Protection Agency (USEPA): Washington, DC, USA, 1994.
- Chary, N.S.; Kamala, C.T.; Raj, D.S.S. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf. 2008, 69, 513–524. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils; Revision 2; United States Environmental Protection Agency (USEPA): Washington, DC, USA, 1996.
- Plank, C.O. Plant Analysis Reference Procedures for the Southern Region of the United States; Southern Cooperative Series Bulletin number 368; University of Georgia: Athens, GA, USA, 1992. [Google Scholar]
- Decreto Ministeriale. Regulating Technical Standards for Wastewater Reuse; Decreto Ministeriale: Rome, Italy, 2003; Volume 185. [Google Scholar]
- Almuktar, S.A.A.A.N.; Abed, S.N.; Scholz, M. Contaminations of soil and two Capsicum annuum generations irrigated by reused urban wastewater treated by different reed beds. Int. J. Environ. Res. Public Health 2018, 15, 1776. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Guidelines for Water Reuse; Report (EPA/600/R–12/618); United States Environmental Protection Agency (USEPA): Washington, DC, USA, 2012.
- Kuzovkina, Y.A.; Knee, M.; Quigley, M.F. Cadmium and copper uptake and translocation in five willow (Salix L.) species. Int. J. Phytoremediat. 2004, 6, 269–287. [Google Scholar] [CrossRef]
- Berndes, G.; Fredrikson, F.; Börjesson, P. Cadmium accumulation and Salix–based phytoextraction on arable land in Sweden. Agric. Ecosyst. Environ. 2004, 103, 207–223. [Google Scholar] [CrossRef]
- Aasamaa, K.; Heinsoo, K.; Holm, B. Biomass production, water use and photosynthesis of Salix clones grown in a wastewater purification system. Biomass Bioenergy 2010, 34, 897–905. [Google Scholar] [CrossRef]
- Mohsin, M.; Kaipiainen, E.; Salam, M.M.; Evstishenkov, N.; Nawrot, N.; Villa, A.; Wojciechowska, E.; Kuittinen, S.; Pappinen, A. Biomass Production and Removal of Nitrogen and Phosphorus from Processed Municipal Wastewater by Salix schwerinii: A Field Trial. Water 2021, 13, 2298. [Google Scholar] [CrossRef]
- Bouman, O.T.; Sylliboy, J. Biomass allocation and photosynthetic capacity of willow (Salix spp.) bio–energy varieties. Forstarchiv 2012, 83, 139–143. [Google Scholar]
- Sharma, R.K.; Agrawal, M.; Marshall, F. Heavy metal contamination in vegetables grown in wastewater irrigated areas of Varanasi, India. Bull. Environ. Contam. Toxicol. 2006, 77, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, Y.; Sun, Q.; Zhang, H. Trace elements in soils and selected agricultural plants in the Tongling mining area of China. Int. J. Environ. Res. Public Health 2018, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Taib, S.M.; Din, M.F.M.; Dahalan, F.A.; Kamyab, H. Comprehensive review on phytotechnology: Heavy metals removal by diverse aquatic plants species from wastewater. J. Hazard. Mater. 2016, 318, 587–599. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, R.K.; Agrawal, M.; Marshall, F.M. Risk assessment of heavy metal toxicity through contaminated vegetables from waste water irrigated area of Varanasi, India. Trop. Ecol. 2010, 51, 375–387. [Google Scholar]
- Millaleo, R.; Reyes–Díaz, M.; Ivanov, A.; Mora, M.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Net. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Wickham, J.; Rice, B.; Finnan, J.; McConnon, R. A Review of Past and Current Research on Short Rotation Coppice in Ireland and Abroad; Coford: Dublin, Ireland, 2010. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).