Abstract
BioWin 6.0 does not accurately predict phosphorus (P) speciation in acidogenic anaerobic digesters under default kinetics characterization and parameterization. The accurate modeling of acid-phase digestion is needed to predict the performance of novel nutrient recovery technologies that act on these digester effluents. The main thrust of this work was to identify and correct the causes of inaccurate P partitioning and precipitation within BioWin models of acid-phase digestion reactors. A BioWin configuration including an organic acid digester was parameterized and recalibrated based on the known traits of acid-phase digestion and then validated against a full-scale digester in a municipal wastewater treatment plant. This digester, with pH 5.14 and 61–74% solubilized P, was predicted by BioWin default parameters to have only 27% soluble P and a net formation of P precipitates. Corrections to the polyphosphate-accumulating organism decay, endogenous product decay, hydrolysis rate, and brushite behavior resulted in 67% solubilization with no precipitate formation. Cabinet configurations showed similar behavior when modified to include an acid-phase digester under default parameters, but predictions were similarly amended by our parameter changes. This improved modeling technique should allow operators to effectively characterize acid digesters for their own treatment trains and allow engineers to predict the performance of novel nutrient recovery technologies acting on acidogenic digest.
1. Introduction
As the deleterious effects of nutrient pollution have gained importance, municipal wastewater treatment plants have been targeted by regulatory agencies as significant point sources for nutrients [1,2,3,4]. In response, treatment plants have explored new technologies for removing nutrients, first from effluent to biosolids, and more recently from treatment to resource recovery. As the circular economy develops, municipal wastewater treatment plants are being increasingly viewed as potential resource recovery facilities to be targeted for fertilizer production [5]. This new wave of experimentation has resulted in different pilot technologies attempting to recycle/recover nutrients.
Anaerobic digestion is a key process in many treatment trains [6]; digesters receive a biomass-rich sludge clarified from other reactors and process it using a microbial community that consumes biomass without the need for oxygen [6,7]. These reactors reduce the pathogen and biomass load while producing a mix of carbon dioxide and methane (i.e., biogas) which provides a potential energy source for the treatment plant [6,7,8]. Digested effluent is typically separated into liquids and recalcitrant sludge [6] with the liquids returned to the headworks for re-processing while the sludge is disposed of through land application, landfilling, or incineration [6].
The anaerobic digester converts biomass into biogas using four steps: hydrolysis, fermentation, acidogenesis, and methanogenesis. Each process is performed by distinct communities of microorganisms [9,10,11]. Hydrolysis, fermentation, and acidogenesis sequentially break down complex organic molecules into volatile fatty acids (VFAs) and soluble inorganic nutrients [9,10]; the primary nutrients released include nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg). Subsequent methanogenesis from metabolizing the VFA substrate raises the pH and can precipitate N, P, and Mg out of solution, most commonly as the ammonium magnesium phosphate mineral struvite (NH4MgPO4·6H2O) [8,12]. Struvite is considered a nuisance precipitate by the wastewater industry because it clogs pipes and reduces the reactor volume, necessitating costly maintenance and repairs; its prevention and removal is an ongoing area of research [8,12,13,14,15,16,17,18,19,20,21,22,23,24]. In addition, the sludge generated from processes such as enhanced biological phosphorus removal (EBPR) contains elevated P loads from the digestion of polyphosphate-accumulating organisms, which can exacerbate struvite precipitation issues [8,12,15,25].
The methanogenesis and acidogenesis stages of digestion have an antagonistic relationship. VFAs from acidogenesis dissociate as weak acids and reduce the solution pH, but methanogenesis can be inhibited by pH conditions under 6.5 [9,11]. Because methanogenesis is typically the rate-limiting step, anaerobic digester operators must ensure sufficient methanogenic substrate without creating inhibitory pH conditions through excess acidogenesis [9,11]. A two-phase system can segregate the acidogenic and methanogenic steps into separate reactors to create more favorable conditions for the adverse microorganisms, leading to a net improvement in solids destruction, loading tolerance, methane production, and/or reactor volume needed [1,6,9,10,11,12]. The first phase cultivates the hydrolysis, fermentation, and acidogenesis communities using a reactor with a short hydraulic retention time (HRT), referred to as an acid-phase digester. Acid-phase digesters contain high concentrations of VFAs and soluble nutrients; the acidic conditions, typically pH 5–6, inhibit the formation of inorganic precipitates and the growth of methanogens [1,7,9,10,12]. The low-pH, high-nutrient effluent is typically fed to a subsequent anaerobic digester which cultivates methanogens using a longer HRT under near-neutral pH. These methanogens convert VFAs into biogas, but the higher pH favors the formation of nuisance precipitates like struvite [4,9,10,12].
The high-nutrient, low-pH effluent of the acid-phase digester is an ideal target for nutrient recovery technologies. Precipitating P from acid digests in a controlled environment prevents their soluble phosphorus from continuing to the methanogenic digesters, where it may precipitate as struvite [12,26,27]. This offers the potential to reduce treatment stoppages for maintenance due to mineral clogging in subsequent digesters, to reduce the total sludge dry weight and P content, to lower biosolids dewatering costs, and to recover a high-strength fertilizer product [12,26,27]. To predict the potential of emerging technologies in an individual treatment train before a full-scale implementation, wastewater engineers often use computer models [6,7,8].
BioWin [28] is widely known in the wastewater treatment industry for its comprehensive biological and chemical modeling suite and its ease of use. The modeling of acid-phase digestion is currently not explicitly supported by BioWin and literature examples are rare. Some authors skip the modeling of the acid-phase reactor [29,30] and others note aberrantly low pH conditions [31,32] associated with the acidogenesis steps. Recent work has confirmed that BioWin does not natively make accurate predictions of acid digester function and developed methods to correct the pH predictions [26,33], allowing for more accurate assessments of model performance with respect to the nutrient content. As of writing, there is no literature validating BioWin’s modeling of nutrient speciation in acid digesters. Without accurate nutrient speciation, models of nutrient recovery or struvite control technologies would be unable to predict critical performance statistics like reagent requirements, nutrient recovery rates, or struvite mitigation. Improved BioWin methods for modeling acid digesters could allow for the efficient and economical evaluation of new nutrient removal/recovery technologies as well as the troubleshooting of pilot-scale projects.
To develop these methods, we used a BioWin configuration composed by the Nine Springs Wastewater Treatment Plant in Madison, WI, USA, to characterize its treatment train. In contrast to the previous work [33], this evaluation is focused entirely on the speciation of P within the acid digest effluent. We used literature on the behavior of acid digesters to identify gaps in the existing model and corrected them by altering kinetic and stoichiometric parameters. Where possible, we minimized the effects that these parameter alterations have on the performance of other reactors and treatment trains. We compared the model predictions of the Nine Springs configuration using default parameters to predictions using our suggested modified parameters; both model predictions were compared against chemical analyses obtained via grab sampling from the Nine Springs plant. To verify the reproducibility, we replicate the methods using cabinet configurations, which come standard with BioWin for demonstration purposes to enable users to verify our modifications for their specific configurations.
The major goal of this study is to determine whether the BioWin modeling suite can accurately reproduce the conditions of an acid-phase digester with respect to P solubilization. An additional goal is to create a configuration and set of model values for which the predicted acid digester’s effluent matches the measured data while being parsimonious about the number of default values altered. This work also seeks to modify the existing model in a way that current users can replicate with available parameter changes without the need for external calculations or additional expertise. This work is intended as groundwork for the future assessment of P removal from acid digester effluent in municipal wastewater treatment plants.
2. Materials and Methods
The primary software used for this study is BioWin 6.2 (EnviroSim Associates, Ltd., Hamilton, ON, Canada), one of the most popular modeling suites for the wastewater treatment industry. BioWin integrates a series of hydraulic, chemical, and biological models to predict changes in the chemical and microbial composition of wastewater as it passes through various reactors. The BioWin software suite is continually updated to meet the needs of the wastewater treatment industry [34], but BioWin has historically not been a tool of choice for modeling P transformations during wastewater treatment [35]. In the past two versions, it has revamped its chemical precipitation models in an attempt to predict formation of P-containing compounds that frequently act as a nuisance; components capturing struvite and brushite formation were incorporated into BioWin version 5 (2016) and version 6 (2019), respectively.
BioWin contains a library of user-adjustable parameters intended to characterize chemical and microbial interactions. BioWin’s activated sludge/anaerobic digestion model (ASDM) is a combination of preexisting anaerobic digestion models (ADMs) and activated sludge models (ASMs) intended to integrate the inputs and outputs of each to cover mechanistic gaps [36] and create a seamless method of modeling whole wastewater treatment plants. The BioWin ASDM alone contains more than fifty state variables and over eighty process expressions. The default values are frequently used as is, but users are able to alter parameters to better represent their own systems when appropriate. BioWin’s recent updates have introduced new chemical models to predict the precipitation and dissolution of the P-containing minerals brushite and struvite.
The primary simulation model used for our evaluation is a proprietary BioWin configuration for the Nine Springs Wastewater Treatment Plant in Madison, WI, operated by the Madison Metropolitan Sewerage District (MMSD), created by on-site engineers to predict the effects of changes to their treatment train (Table 1) [27,37]. This plant utilizes an EBPR treatment train which generates high-P sludge. The plant anaerobically digests a 50:50 mixture of primary sludge and WAS (waste activated sludge) in a series of mesophilic digesters generating biogas and then dewaters the sludge to produce biosolids for land application [37]. The first of these digesters is an acid-phase digester with a HRT of 1.3 days. The plant has incurred significant costs in the form of treatment stoppages, lost reactor volume, and maintenance expenses due to the buildup of nuisance struvite in its anaerobic digesters. Their BioWin configuration was created, in part, to assist with P remediation efforts, but the inability of BioWin to properly model their acidogenic digester limited its usefulness.

Table 1.
Summary statistics for the Nine Springs Wastewater Treatment Plant.
Digester performance data are taken from a Water Environment Federation evaluation of a P recovery technology [12,27], which recorded daily samples for one year from the acid digester effluent at the Nine Springs Wastewater Treatment plant in 2018. Measured pH was generally stable, averaging 5.14 pH (SD 0.20). Percent P solubilization, as reported by Tabanpour et al. [27], was calculated as the concentration of soluble P (determined by ICP-AES on samples passing through a 0.45 µm filter) times the water content of the sludge sample (obtained by drying at 105 °C), divided by the total P of the sludge (determined by dry ashing at 500 °C, dissolution of the ash in acid, and P determination by ICP-AES). Site measurements from the Nine Springs acid digester effluent recorded a consistent P solubilization of 66% ± 5% and other facilities record solubilities in the 65–85% range [12,27]. Soluble P, as a percentage of the total P in the digest, ranged from 60.7 to 74.0% of P in the fall of 2018 [27].
The BioWin configuration supplied by Nine Springs comprehensively covered the major aspects of treatment, including clarification, a plug flow aeration stage, EBPR, anaerobic digestion, and sludge recycle streams, WASSTRIP biosolids processing, and an Ostara Pearl struvite reactor. However, the anaerobic digestion section of the configuration was underdeveloped as it contained a single anaerobic digester unit with an HRT of 35.5 days rather than the 9-tank system of the real plant. The operators of the Nine Springs plant had previously attempted unsuccessfully to incorporate an acid digester process in the configuration to accurately model the behavior of the P recovery technologies that precipitate struvite (Ostara) or brushite (CalPrex). Default Biowin parameters yielded predictions that were not comparable to site measurements, so the operators compensated using static, user-defined streams. A goal of our study is to replace this inflexible solution with a working acid digester model based on literature figures and the acid digest pH and VFA simulation methods of Vineyard et al. [33].
Our modifications began by splitting the existing 35.5-day HRT anaerobic digestion process into two new processes: a 1.3-day HRT anaerobic digester and a subsequent 34-day HRT anaerobic digester. As BioWin does not natively model acidogenic digesters, the methods detailed in Vineyard et al. [33] were used to achieve appropriate pH and VFA concentrations by inhibiting microbial growth within the acid digester using the pH inhibition thresholds of anaerobic heterotrophs and H2-utilizing methanogens. In the initial configuration, BioWin predicted a P solubilization of 27%, too low for a meaningful analysis of new P recovery technologies. We found that BioWin had allocated the effluent P primarily to six compartments: soluble, heterotroph biomass, unreleasable polyphosphate (poly-P) within polyphosphate-accumulating organisms (PAOs), endogenous products, degradable organics, and brushite. This distribution suggests that the disparity between model predictions and site observations cannot be explained by a single variable.
Multiple parameter changes were required to bring model behavior closer to process dynamics and biochemical behavior observed on site and documented in existing literature (Table 2). To minimize the effects of our parameterization on plant-wide performance [38,39], parameter modifications were localized only to the modeled organic acid digester. Universal stoichiometric parameters were avoided as these changes cannot be localized to a single reactor and would affect predictions in the configuration’s entire treatment train; however, the universal brushite solubility product was altered after validation against an external chemical speciation program, Visual MINTEQ v 3.1 [40]. We validate these results using extensive soluble P measurements collected in 2018.

Table 2.
Alteration of parameters to achieve satisfactory predictions of phosphorus solubilization.
Our study considered additional parameters which may be intuitive, but which were found to be unproductive in the pursuit of the major study goals. We tested a range of acid digest HRT values from 0.1 days to 25 days to analyze the effects of HRT on key parameters such as pH, alkalinity, VFA production, and P solubility. We also tested an alternative method of reducing unreleasable poly-P predictions by directly manipulating stoichiometry of the poly-P fractionation. The results of these explorations and their reasons for exclusion from the final parameterization are outlined in the Appendix A. The following changes (Table 2) best influenced the BioWin predictions of P speciation in an acid digester.
Parameter changes and their effects are reported here in sequence for illustrative purposes, but a user can selectively implement any or all of them to better represent their own reactors. The following changes best influenced the BioWin predictions of P speciation in an acid digester:
2.1. Alteration of Local PAO Decay Kinetics
Acid digesters are specialized systems where PAOs are unlikely to survive [9,10,11]. Tabanpour et al. [27] found no evidence of polyphosphates in their samples, suggesting that the acid digest environment either forces PAOs to rapidly eject their ‘unreleasable’ P stores or that the P stores are released during destruction of PAOs. In the simulated acid digester, unreleasable stored poly-Pcontained within the PAOs remains a large reservoir of P. Without solubilizing this component, a good agreement between modeling predictions and the soluble P site measurements is not likely.
In BioWin, PAO decay follows the first-order rate at which PAOs die and are broken down. The default kinetics of the BioWin model do not have a unique decay rate for low pH environments or conditions under which PAOs are likely to be predated upon by other organisms and has no mechanism to replicate these effects. As such, there is a negligible decline in the PAO population over the 1.3-day HRT, which directly leads to unrealistic predictions of their P retention within the digester. Raising the local PAO decay rate allows us to simulate the effects of a hostile, predatory environment on the PAO population. We chose an arbitrary 90% reduction in PAO biomass in the acid digest effluent as our target, which at this HRT, corresponded to an increase in the decay rate from 0.04/d to 7/d.
2.2. Altering the Dissolution Kinetics of Brushite
After implementation of the aforementioned step (Section 2.1), stored polyphosphates are reduced and soluble P becomes the largest P compartment, followed by the biomass of ordinary heterotrophs, endogenous products, and precipitated brushite. The predicted 41.2% soluble P (as a fraction of total P) is still considerably less than the 69% average solubility recorded in the Nine Springs Acid Digester. The prediction of brushite within the system is incongruous with expectations. Tabanpour et al. [27] observed no brushite particulates in the acid digest effluent and they would be unlikely to survive in the acidic, mesophilic environment.
Rate of brushite dissolution is dependent on multiple variables including temperature, agitation, and surface area, but BioWin predicts dissolution using a single static rate. As the BioWin manual specifically warns against setting these rates too high due to computational expenses, it is possible that the default dissolution rate was chosen for user experience rather than devoting large amounts of computing power to a single process. This is a known weakness of precipitation and dissolution modeling [41]. Given that brushite precipitates primarily form as small particles on small nucleating surfaces within the wastewater solution, we assume that the brushite will have a high surface area for dissolution and will rapidly dissolve in the acidic environment. A range of higher brushite dissolution rates, in order-of-magnitude increments, were tested to observe the effects of dissolving brushite particulates to the point of near-instantaneous equilibrium of brushite with its ionic products. The kinetic constant for brushite dissolution in BioWin was finally set to 1 × 109 L/(mol·d) to reduce recalcitrant brushite without a noticeable increase in computation needs.
2.3. Modification of the Brushite Solubility Product
Even at high dissolution rates that simulate near-instantaneous equilibrium, BioWin predicted that 33.9 kg brushite P per day would exit the acidic environment of the acid digester. Given that the P solubility (as a fraction of total P) after prior modifications was still significantly underpredicted compared to measurements, the next logical step was to test whether brushite saturation has been correctly simulated in BioWin.
When the pH and solution concentrations of the BioWin-predicted acid digester effluent were input into Visual MINTEQ, there was a good agreement between the two chemical models in terms of charge balance (0.82% difference in the sum of cations and anions). However, Visual MINTEQ reported that the effluent solution was undersaturated with respect to brushite (Figure 1) while BioWin still predicted brushite in the effluent. The ion activity product for brushite calculated by Visual MINTEQ for the acid digester effluent is about one-third less than BioWin’s default Ksp,brushite value. The Ksp,brushite value used by Visual Minteq is nearly identical to that internally set in BioWin (Figure 2), and both BioWin and Visual MINTEQ use the Davies Equation activity model. We hypothesize the reason that BioWin predictions do not agree with Visual MINTEQ is a failure within BioWin to consider ion pairs appropriate for brushite under these conditions [12], leading to an erroneous prediction of saturation.
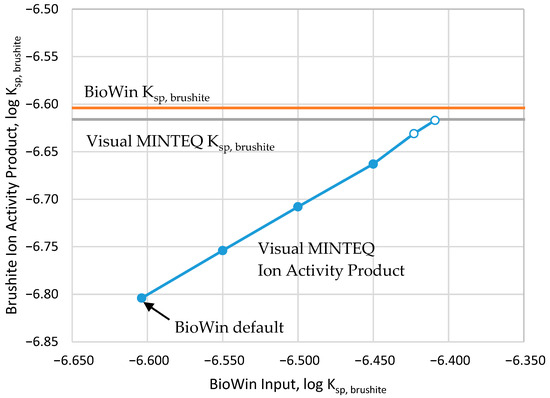
Figure 1.
Relationship between value of Ksp,brushite input into BioWin and the resulting ion activity product of the effluent as predicted by Visual MINTEQ. Open circles represent BioWin predictions requiring brushite addition to ensure brushite saturation.
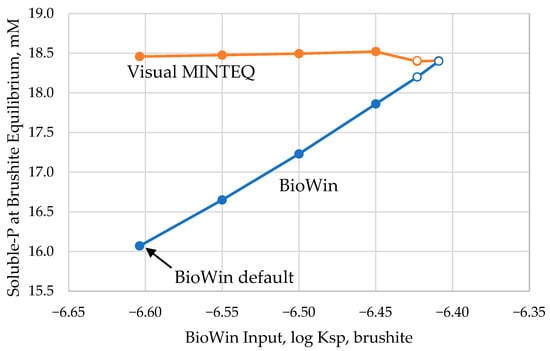
Figure 2.
The relationship between value of Ksp,brushite input into BioWin and the resulting predicted soluble P concentration in the acid digester effluent compared to the Visual MINTEQ predictions of soluble P at brushite equilibrium. Open circles represent BioWin predictions requiring brushite addition to ensure brushite saturation.
Chemical speciation calculations by Visual MINTEQ predicted that free Ca2+ in the acid digest effluent was only 69% of total soluble Ca, with VFA ion pairs alone neutralizing 21% of total soluble Ca. Visual MINTEQ predicted that free PO43− was 93% of the total soluble P, with the primary ion pairs of P species predicted to be with Ca and Mg. Absent consideration of the presence of ion pairs in internal BioWin calculations, particularly those of VFAs, BioWin would overestimate the concentration of free Ca and P and, consequently, overestimate the amount of brushite to be precipitated to relieve supersaturation.
In the absence of an open programming interface within BioWin to correct the chemical modeling of brushite, the ad hoc solution is to alter the Ksp,brushite default value used in BioWin to accommodate the expected ion pair formation, essentially creating an apparent or conditional solubility product. We tested a range of brushite solubility products in BioWin and input the resultant effluent concentrations into Visual MINTEQ to determine a Ksp,brushite value that indicated the same degree of undersaturation as predicted by Visual MINTEQ.
2.4. Increasing the Endogenous Product Decay Rate
The endogenous products in BioWin are the inert fractions of biomass left over by the microbial processes. Their decay rate is a first order equation that converts their mass to a bioavailable substrate. In BioWin, this parameter is conditionally set to 0 under anaerobic conditions, but a literature review by Menniti et al. [42] reported evidence that endogenous products are degradable under anaerobic conditions and that thermophilic conditions can enhance this degradation. To liberate P from the endogenous products, we increase the local decay rate within the reactor from 0 to 1/d, the maximum allowed by BioWin for this parameter.
2.5. Increasing the Local Hydrolysis Rate
After the previous adjustment step (Section 2.4), degradable organics in the effluent is the largest reservoir of P remaining after heterotroph biomass. As an organic acid digester is a mesophilic system with a high rate of microbial activity, it is specifically engineered to have much higher hydrolysis rates than standard anaerobic digesters [9,10,11,43]. To more closely represent literature values [10,43], the local hydrolysis rate is increased from 2.1/d to 3/d and the anaerobic hydrolysis factor increased from 0.5 to 1. The acid-representative hydrolysis rate releases P in the organics that would otherwise be erroneously partitioned to the biosolids.
2.6. Validation and Model Testing
Tabanpour et al. [27] sampled eight mass balances over thirty days in the fall of 2018, recording duplicate samples for independent analyses at a local laboratory and the Colorado School of Mines (CSM). The local laboratory reported P solubilities from 60 to 74% while CSM reported from 50 to 85%. Though the averages across all eight mass balances were very similar (66.1% and 64.6%, respectively), the two labs disagreed by an average of ten percentage points on the characterization of any specific sampling date. Using a new influent file comprising 14 days of characteristic variability for the Nine Springs plant from the summer of 2016, we ran two dynamic simulations to record the changes in P solubility predicted by BioWin. The first used default parameters for the acid digester while the second used our modified parameters.
To test for applicability to other datasets, we also replicated these modifications in two new configurations. Because of their ease of access to BioWin users, we selected two preexisting whole-plant configurations in the BioWin cabinet: (i) EBPR with an anaerobic digester, and (ii) N Removal unit with an anaerobic digester. These configurations represent possible EBPR and non-EBPR feeds to acid digesters from realistic treatment trains. Primary modification to the configurations involved pairing a 1.3-day HRT acid digester to the anaerobic digester with appropriate overall HRT adjustment, modifying acid digester performance in accordance with Vineyard et al. [33], and then implementing the above five phosphorus-based parameter changes. We compared the projected acid digester effluent with and without the phosphorus-targeted parameter changes.
3. Results
Five parameter changes were needed to reallocate P to the soluble compartment within a BioWin modeled acid digester for its predictions to match site measurements. For illustrative purposes, these parameters were changed and recorded here one at a time to observe the effects on P speciation within the modeled acid digester effluent. An implementation of these methods can apply them in any order or all at once, depending on the configuration.
3.1. Alteration of the PAO Decay Kinetics
The local decay kinetics for the PAOs under anaerobic conditions were raised from 0.04/d to 7/d to simulate the effects of environments hostile to these organisms and achieve a 90% reduction target. The unreleasable poly-P fraction is reduced in proportion with the biomass, which is then distributed to the soluble P and to the degradable organics fractions (Figure 3). The reduced PAO population in the recycle stream does not significantly affect P reduction in the main treatment train and the effluent P remains within 1% of its value with altered PAO decay rates.
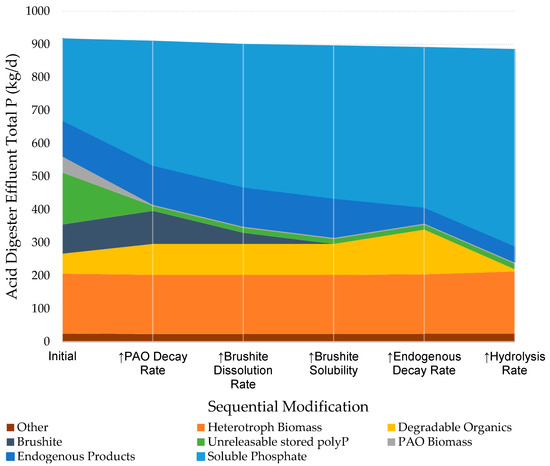
Figure 3.
Effects of parameter alteration methods on P speciation in Nine Springs acid digest effluent. Digester set to maintain a pH of ~5.14 across all steps.
3.2. Altering the Dissolution Kinetics of Brushite
In the default BioWin mineral precipitation module, brushite is modeled to precipitate 100 times faster than it dissolves (106 L/mol/d vs. 104 L/mol/d). This parameterization results in the predictions of brushite, once formed in the activated sludge, not dissolving while in the acid digester, though there are no supporting observations at Nine Springs or elsewhere [27]. Progressively higher brushite dissolution rates predict higher soluble P and lower brushite but ultimately yield diminishing returns, seemingly approaching equilibrium in the modeled acid digester effluent (Figure 4). It may be observed in Figure 3 that the soluble P increases are almost identical to the decrease in the mass of the brushite, suggesting that nearly all the dissolved brushite remains as soluble P rather than being reintegrated into another P compartment. An increase in the dissolution rate from the default to 109 L/mol/d achieves an increase in P solubility from 41% to 48%. As secondary effects, an increased brushite dissolution rate is observed to only slightly increase pH, VFA production, and methane generation.
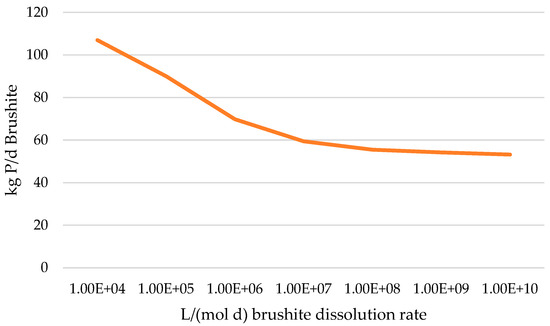
Figure 4.
Response of effluent brushite content to increasing dissolution rates shows diminishing returns.
As BioWin does not isolate this precipitation constant to a single reactor, the modified higher brushite dissolution rate applies to every reactor in the treatment train. The performance of other reactors needs to be monitored for unintended side effects when manipulating global variables. Modifying the brushite dissolution showed no significant changes in the brushite content of other streams and the influent brushite to the acid digester compartment was unchanged, suggesting the unintended effects were minimal. We suggest a 109 L/mol/d dissolution rate to simulate instantaneous dissolution while avoiding the computational needs of higher rates.
3.3. Modification of the Brushite Solubility Product
The BioWin predictions of pH and dissolved ions in the acid digester effluent (NH4-N, Ca, Mg, P, acetate, propionate, total dissolved CO2, sulfate, sulfide, “other cations” (represented as Na+), and “other anions” (represented as Cl−)) were input into the stand-alone program Visual MINTEQ v 3.1, a chemical equilibrium model built upon the USEPA’s MINTEQA2 v 4.06 software using the NIST Standard Reference Data 46 as its database. We tested the effects of Ksp,brushite in BioWin on the modeled acid digester and compared the resulting effluent’s brushite ion activity products as calculated by Visual Minteq. A Ksp,brushite in BioWin of 3.415 × 10−7 (Figure 5) was found to force the BioWin simulations of the brushite equilibrium to match those of Visual Minteq.
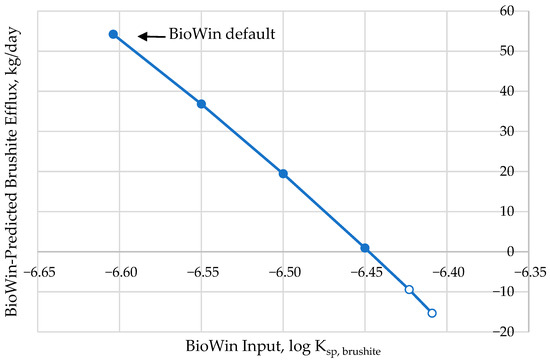
Figure 5.
Comparison of saturation indices calculated in Visual Minteq for the BioWin acid digester effluent conditions produced by a range of log Ksp,brushite Open circles represent BioWin predictions requiring brushite addition to ensure brushite saturation.
Using the updated conditional Ksp,brushite of 3.415 × 10−7, the brushite persistence and/or formation in the acid digester as predicted by BioWin decreases from 33.9 kg P/d to 0 kg P/d, the soluble P concentration in the acid digester effluent increases from 431 kg P/d to 461 kg P/d, with the acid digester effluent now undersaturated with respect to brushite (Figure 3). With this modification to BioWin, the soluble P is 51.4% of the total P in the acid digester effluent of the Nine Springs model (Figure 3), consistent with some other reactors measured in the 45–49% range [27], but still short of the 69% goal based on the measured performance of the modeled reactor.
The new conditional Ksp,brushite determined for the acid digester effluent is applicable only for situations with high concentrations of VFAs and phosphate. For programming reasons, this change must be system-wide in BioWin even though elevated VFAs and phosphate concentrations are typical only of the acid digester. The developers of BioWin could add appropriate ion pairs in later versions and obviate the need for this conditional Ksp.
3.4. Increasing the Endogenous Product Decay Rate
After releasing P from PAO stores and biomass, endogenous products remain one of the largest remaining reservoirs of P. Within the modeled acid digester, the endogenous product decay rate was raised from 0 to 1/d, the maximum allowed. This adjustment compensated for the default state of the endogenous products being labeled as indigestible in an anaerobic digester, which may not apply to acid digesters. This modification liberated 60% of the endogenous product P passing through the digester, repartitioning it into degradable organics and soluble P. The total P solubilization increased from 51.4% to 54.2% (Figure 3).
3.5. Increasing the Hydrolysis Rate
By doubling the local hydrolysis rate, the acid digester effluent was predicted to contain lower quantities of degradable organics and higher quantities of VFAs to correspond with the measurements reported in Tabanpour et al. [27]. Nearly tripling the effective hydrolysis rate resulted in a 95% reduction in the degradable organics in the effluent (Figure 3). The degradable organics were the second largest reservoir of P remaining in the effluent, the digestion of which increased the P solubilization from 54.2% to 67.2% (Figure 3), matching the site observations.
4. Discussion
4.1. Benchmark to Full-Scale Grab Sample Data
The dynamic simulations produced a tight range of P solubilizations (Figure 6). Before modification, BioWin predicted an acid digest of 24.2–26.6% P solubilization, with an average of 25.6%. After modification, BioWin predicted an acid digest of 62.3–63.7% P solubilization, with an average of 63.1%. The modifications create a clear improvement in the predictive power of the software. In both dynamic simulations, the total variability of the predicted solubilization was very small compared to the variability in the grab sample data. There are two possible explanations for this discrepancy: either the methods used to acquire and analyze the grab samples were highly uncertain or BioWin’s perfect mixing model over-homogenizes the system and does not characterize the full effects of the daily fluctuations in a dynamic simulation. Given the large disagreement between the two laboratories analyzing the same samples, we cannot eliminate the possibility of sampling and/or laboratory error. Further reactor sampling could help determine whether the predicted homogeneity is accurate.
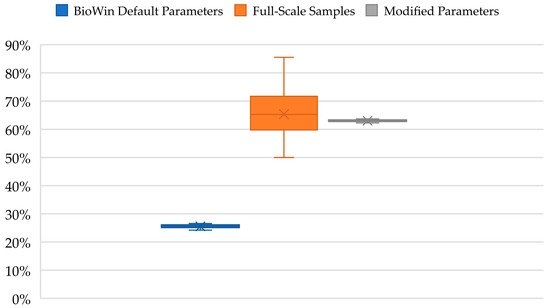
Figure 6.
BioWin predictions of acid digester P solubilization using two weeks of summer 2016 plant influent data compared to laboratory analyses of eight acid digest samples from fall 2018.
4.2. Assessment of Plant-Wide Effects
We assessed the impact on the total anaerobic digester train predictions with parameter modifications tailored specifically for the acid digester. A comparison of the anaerobic digester effluent between the original and re-parameterized BioWin configurations revealed some significant differences (Table 3). Both the VSS and TSS decreased after parameter re-calibration due to the enhanced digestion of the endogenous products and PAOs. The phosphorus distribution between the different compartments was also altered. The greatest percentage difference was in the soluble P compartment attributable to the increased destruction of PAOs liberating P from the unreleasable poly-P compartment. In terms of the total mass, the precipitation of struvite was greatly increased due to the extra soluble P driving the equilibrium towards precipitation.

Table 3.
Predicted final effluent content of the complete Nine Springs anaerobic digester train.
Although all the brushite entering the acid digester was solubilized in the modified (i.e., re-parameterized) reactor, re-calibration had a small effect on the amount of brushite precipitated in the subsequent anaerobic digestion process. Given that the P distribution parameters were altered, their cumulative effect on the P fate can be determined by comparing the P composition in the effluent from the overall configuration before and after the modifications (Table 4). The predicted total P in the plant effluent increased after parameter modification, with changes driven by a shift away from biosolids and towards struvite formation and the treatment plant effluent; the alteration of the brushite solubility and the release of P from endogenous products and PAO biomass, before recirculating to the Ostara reactor, is likely responsible for this change. Modifications to the subsequent anaerobic digester processes to offset these changes may be possible, but it is beyond the scope of this work. Users replicating our methods should be aware of the possible need for rebalancing their treatment train configurations.

Table 4.
Predicted distribution of P across all configuration effluents.
4.3. Application to Other Treatment Plant Models
Both BioWin cabinet configurations were modified by incorporating an acid digester process using the pH correction methods of Vineyard et al. [33]. With default parameters, the acid digesters from both treatment trains projected the P solubility below what is observed in extant acid digesters [27]. After modification using this study’s five parameter changes to enhance the P solubilization, both treatment trains increased their soluble P content to be within the observed range of values from acid digesters as recorded by Tabanpour et al. [27] (Table 5). Therefore, a simple ‘cut and paste’ of new settings into existing configurations could improve the accuracy of the modeled acid digesters. In both cabinet models, it is likely that further calibration using the above methods could bring the predictions of the P distribution closer to the measured/observed values.

Table 5.
Responses of an EBPR Train Digester and an N Removal Train Digester to addition of an acid digester, with acid digester effluent properties before and after the generic changes above.
4.4. Limitations and Future Work
Modeling multiple interactions in a complex treatment train typically encountered in WWTPs is difficult and the assumptions made in the derivation of these estimates may not be valid in certain configurations and circumstances. Our methods sought to increase the accuracy in simulations while making as few changes to the base configuration as possible, but there may be additional complexities that our methods do not capture, which may become significant in alternative treatment trains. As the intended purpose of this work is to better estimate the performance of novel technologies using the acid digester effluent as a starting feed material, future analysts and modelers should be cautious when extending these methods to other applications.
Our work calibrated the acid digester simulation primarily for P solubilization as the groundwork for the evaluation of P recovery by brushite precipitation. This work paves the way for more accurately predicting the performance of nutrient recovery technologies that target soluble P in the acid digester effluent. In the default BioWin settings, such an attempt would predict unrealistically high reagent requirements and unrealistically low recovery rates, leading to inflated estimates of capital and operation costs and low estimates of performance. The consideration of acetate and propionate ion pairs with Ca will be taken up with the programmers at EnviroSim for correction and improvement within future versions of BioWin.
Our re-parameterization process was unable to produce estimates in line with verification data without enacting some plant-wide parameter changes. These new parameter values can still affect the performance of other reactors by altering stream qualities, as recorded in the change in the sludge composition and total plant P distribution despite no changes in the characteristics of the anaerobic digester effluent. A study attempting to predict the plant-wide performance for practical purposes will benefit from accounting for these altered behaviors.
5. Conclusions
The BioWin wastewater treatment plant modeling was unable to match the measured performance of anaerobic acid digesters when using default parameters. It was clear from the data collected from a full-scale facility that a new approach to predicting the P solubility in the model would be required to evaluate the impact of incorporating new nutrient recovery technologies. Through a systematic five-parameter process altering the PAO decay rate, brushite dissolution rate, brushite solubility, endogenous product decay rate, and hydrolysis rate, to align with literature values and account for missing biochemical mechanisms, the BioWin predictions for the soluble P fraction increased from 27% to 69%, matching the site observations. Our novel calibration of the acid digester in BioWin overcomes the limitations of the modeling suite, thus enabling the more rigorous evaluation of novel nutrient treatment/recovery processes.
Author Contributions
Conceptualization, D.V., K.K. and P.B.; methodology, D.V.; validation, D.V.; formal analysis, D.V.; investigation, D.V.; resources, P.B.; data curation, D.V.; writing—original draft preparation, D.V.; writing—review and editing, D.V., K.K., C.D. and P.B.; visualization, D.V. and P.B.; supervision, K.K., C.D. and P.B.; project administration, K.K., C.D. and P.B.; funding acquisition, P.B., K.K. and C.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by INFEWS/T3 Grant (Reference #2017-67003-26055), USDA/NIFA Hatch Grant No. WIS01920 (Accession # 1009816), and USDA/NIFA Hatch Grant No. WIS04092 (Accession # 7003184).
Data Availability Statement
Some MMSD-related data is confidential but to demonstrate the principles of modeling an acid digester in BioWin, we have made available a modified version of a BioWin cabinet model with full supporting documentation at DOI 10.17605/OSF.IO/8DXM7.
Acknowledgments
We are grateful for the assistance of Matt Seib and the Madison Metropolitan Sewerage District.
Conflicts of Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Phillip Barak reports financial support was provided by the US Department of Agriculture, National Institute of Food and Agriculture, Hatch Grant. Phillip Barak reports a relationship with Nutrient Recovery and Upcycling, LLC, that includes board membership and equity or stocks. Nutrient Recovery and Upcycling, LLC, is rights holder to US Patent 8,568,590 (issued 2013), “Phosphate recovery from acid phase digesters”.
Appendix A
Appendix A.1. Alternative Parameters
Appendix A.1.1. Altering Stored Poly-P Distribution
Polyphosphate-accumulating organisms store polyphosphate (poly-P) in their cells, particularly in the EBPR processes, and BioWin considers that some part of that poly-P is not depolymerized in subsequent digestions. In the simulations for the Nine Springs plant, there remains 147 kg/d of P within the unreleasable poly-P compartment, one of the largest remaining destinations for P within the acid digester effluent. The acid conditions of the digester break poly-P bonds and favor its decomposition to an extent other reactors do not, so this high quantity is unexpected. As no direct measurements support the presence of a large fraction of P retained within the PAOs at Nine Springs, we consider that it can function as a source of soluble P with few unintended side effects for the total treatment train performance. This parameter serves as an alternative to altering the PAO decay rate within the acid digester, to be undertaken after the alterations of low pH inhibition points and increasing the brushite dissolution rate.
Altering low poly-P in the PAOs from the default value (94%) to 99% reduces the throughput of the unreleasable poly-P from 147 kg/d to 29 kg/d (Table A1). Of this 118 kg/d difference, 29 kg/d are solubilized and 48 kg/d are redistributed to brushite precipitate despite the use of a higher dissolution rate. Interestingly, the influent P content is reduced by 41 kg/d, 4% of the total P, because the new P distribution reduces the recirculating P fraction and increases the predicted P capture in the biosolids.

Table A1.
Acid digester effluent profile predictions in response to an increased low poly-P yield in PAOs.
Table A1.
Acid digester effluent profile predictions in response to an increased low poly-P yield in PAOs.
| Item | Unit | 0.94 Low Poly-P (Default) | 0.97 Low Poly-P | 0.99 Low Poly-P |
|---|---|---|---|---|
| Total P | kg P/d | 1050 | 1026 | 1009 |
| Soluble P | kg P/d | 326 | 341 | 355 |
| Brushite | kg P/d | 22.7 | 52.41 | 71.08 |
| Unreleasable Stored PolyP | kg P/d | 147 | 78.3 | 29 |
| Calcium | kg/d | 260 | 247 | 237 |
| VSS Destruction | % | 34.06 | 34.04 | 33.97 |
| Dry Gas Production | m3/hr | 16.64 | 16.62 | 16.68 |
| Gas Methane Content | % | 16.76 | 17.62 | 17.97 |
Appendix A.1.2. Impacts of HRT on Acid Digester Outputs
One of the main parameters a reactor operator might use to alter their reactor conditions is the HRT. BioWin operators may also see HRT as a potential tool for solving the discrepancies due to the incorporation of an acid digester. While VFA production is influenced by the HRT, there is no simple way to characterize all the effects of HRT manipulation. Instead, we experimented with a range of HRTs to analyze their effects on key parameters (Table A2). We chose pH, acetate, propionate, alkalinity, total P, soluble P, and VSS as our response variables for analysis. Our configuration is the base configuration after the separation of the acid digester from the anaerobic digester. As the HRT is increased from 0 d, the pH of the modeled acid digester rapidly decreases to the 4.3–4.5 range, below the measured pH values. At the 1.3–1.8 day HRT range, the predictions of the acid digester effluent soluble P content match the range of observed values, but at this HRT, the predicted pH is still lower than the measured values.

Table A2.
Effects of HRT on key digester effluent parameters.
Table A2.
Effects of HRT on key digester effluent parameters.
| HRT | pH | Acetate | Propionate | Alkalinity | Total P | Soluble P | VSS |
|---|---|---|---|---|---|---|---|
| Days | kg COD/d | kg COD/d | k/d | kg P/d | kg P/d | kg/d | |
| 0.1 | 5.78 | 556 | 1336 | 16.03 | 1004 | 124 | 39,601 |
| 0.125 | 5.46 | 669 | 1659 | 16.35 | 1004 | 141 | 39,302 |
| 0.15 | 5.25 | 779 | 1986 | 16.4 | 1004 | 152 | 39,003 |
| 0.2 | 5.00 | 998 | 2640 | 16.4 | 1004 | 173 | 38,409 |
| 0.25 | 4.86 | 1216 | 3291 | 16.32 | 1004 | 191 | 37,816 |
| 0.3 | 4.77 | 1433 | 3939 | 16.2 | 1004 | 208 | 37,226 |
| 0.4 | 4.65 | 1865 | 5223 | 15.85 | 1004 | 240 | 36,054 |
| 0.5 | 4.57 | 2293 | 6491 | 15.41 | 1004 | 268 | 34,896 |
| 0.6 | 4.52 | 2713 | 7737 | 14.9 | 1004 | 294.5 | 33,755 |
| 0.65 | 4.5 | 2921 | 8352 | 14.65 | 1004 | 307 | 33,192 |
| 0.7 | 4.48 | 3126 | 8958 | 14.39 | 1004 | 319 | 32,635 |
| 0.7125 | 4.47 | 3177 | 9108 | 14.33 | 1004 | 322 | 32,497 |
| 0.725 | 4.47 | 3228 | 9258 | 14.26 | 1004 | 325 | 32,359 |
| 0.75 | 4.46 | 3323 | 9556 | 14.13 | 1004 | 331 | 32,084 |
| 0.8 | 4.45 | 3528 | 10,144 | 13.87 | 1004 | 342 | 31,541 |
| 0.9 | 4.42 | 3915 | 11,280 | 13.37 | 1004 | 363 | 30,482 |
| 1 | 4.41 | 4280 | 12,345 | 12.9 | 1005 | 380 | 29,467 |
| 1.2 | 4.38 | 4925 | 14,196 | 12.13 | 1005 | 403 | 27,618 |
| 1.3 | 4.37 | 5209 | 15,002 | 11.76 | 1005 | 413 | 26,821 |
| 1.5 | 4.36 | 5725 | 16,444 | 10.8 | 1005 | 430 | 25,497 |
| 1.8 | 4.34 | 6295 | 17,959 | 9.43 | 1005 | 448 | 24,218 |
| 2.1 | 4.33 | 6640 | 18,731 | 9.05 | 1005 | 461 | 23,460 |
| 2.5 | 4.34 | 6962 | 19,284 | 8.95 | 1005 | 474 | 22,815 |
| 3 | 4.34 | 7287 | 19,647 | 8.86 | 1005 | 487 | 22,263 |
| 3.5 | 4.34 | 7592 | 19,807 | 8.60 | 1005 | 497 | 21,845 |
| 4 | 4.34 | 7904 | 19,819 | 8.11 | 1005 | 507 | 21,503 |
| 4.5 | 4.34 | 8230 | 19,716 | 7.45 | 1005 | 515 | 21,212 |
| 5 | 4.35 | 8566 | 19,530 | 6.71 | 1005 | 522 | 20,957 |
| 5.5 | 4.35 | 8901 | 19,295 | 5.96 | 1005 | 529 | 20,729 |
| 6 | 4.35 | 9228 | 19,038 | 5.25 | 1005 | 535 | 20,524 |
| 6.5 | 4.35 | 9540 | 18,776 | 4.62 | 1005 | 541 | 20,336 |
| 7 | 4.35 | 9835 | 18,521 | 4.06 | 1005 | 546 | 20,163 |
| 10 | 4.35 | 11,236 | 17,286 | 1.91 | 1006 | 571 | 19,355 |
| 15 | 4.36 | 12,646 | 16,124 | 0.46 | 1006 | 598 | 18,498 |
| 20 | 4.36 | 13,491 | 15,945 | −0.17 | 1006 | 616 | 17,946 |
| 25 | 4.36 | 14,060 | 15,104 | −0.52 | 1006 | 629 | 17,557 |
Using the 1.3-day HRT of the verification system as a baseline, we can track how the key parameters of the effluent are affected by alterations to the acid digester HRT (Figure A2). The predicted pH, VSS, and alkalinity all respond negatively to an increasing HRT; the pH shows a rapid decline due to the logarithmic scaling of the metric, while the alkalinity increases briefly before the onset of the negative trend. The acetate, propionate, and soluble P concentrations all respond positively to an increase in the HRT.
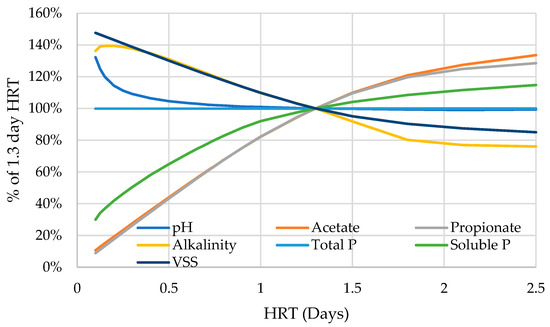
Figure A1.
Behavior of various indicators of acid digester function as HRT is adjusted in comparison to the 1.3-day default. Range of 0-2.5 days.
As the HRT is further increased beyond 2.5 days, the reactor in the base configuration shows an interesting behavior. Increasing the HRT by small steps and then allowing the model to iteratively converge to a new steady state will continue to yield acid digester conditions far beyond the HRT range under which those conditions would be expected (Figure A2).
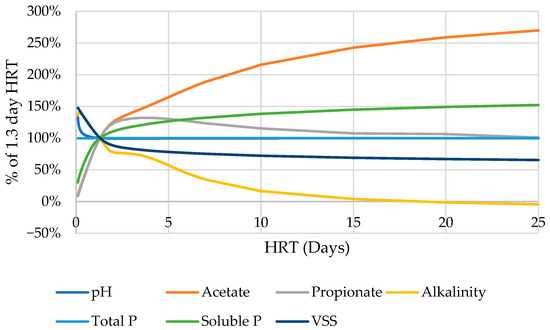
Figure A2.
Behavior of various indicators of default acid digester function as HRT is adjusted in comparison to the 1.3 day default. Range of 0–25 days.
Performing the same HRT analysis on the finalized version of the system, with all the parameter changes made, shows similar trends (Figure A3). The primary differences are that the pH remains much more stable over HRT changes and that the alkalinity shows an inverse trend from the original, increasing with higher HRT. This is likely due to the increased significance of brushite and calcium in the solution.
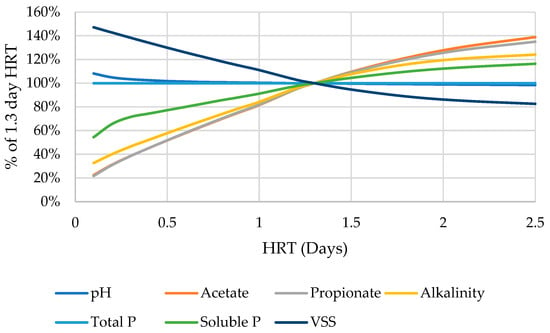
Figure A3.
Behavior of various indicators of modified digester function as HRT is adjusted in comparison to the 1.3 day default. Range of 0–2.5 days.
References
- Jeyanayagam, S.; Khunjar, W.; Mehta, C. Accelerating the Implementation of Extractive Nutrient Recovery as an Integral Component of Sustainable Nutrient Management. Water e-J. 2016, 1, 1–8. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Case Studies on Implementing Low-Cost Modifications to Improve Nutrient Reduction at Wastewater Treatment Plants; United States Environmental Protection Agency: Washington, DC, USA, 2015; Volume 2. [Google Scholar]
- Egle, L.; Rechberger, H.; Krampe, J.; Zessner, M. Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci. Total Environ. 2016, 571, 522–542. [Google Scholar] [CrossRef]
- Clark, D.L.; Hunt, G.; Kasch, M.S.; Lemonds, P.J.; Moen, G.M.; Neethling, J.B. WERF Nutrient Management Report; Montana Department of Environmental Quality: Helena, MT, USA, 2010. [Google Scholar]
- Rahman, S.M.; Eckelman, M.J.; Onnis-Hayden, A.; Gu, A.Z. Life-Cycle Assessment of Advanced Nutrient Removal Technologies for Wastewater Treatment. Environ. Sci. Technol. 2016, 50, 3020–3030. [Google Scholar] [CrossRef]
- Vesilind, P.A. Wastewater Treatment Plant Design; Water Environment Federation: Alexandria, VA, USA, 2003. [Google Scholar]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications; McGraw-Hill: Singapore, 2001. [Google Scholar]
- Water Environment Federation. Introduction to Water Resource Recovery Facility Design, 2nd ed.; McGraw-Hill Professional: New York, NY, USA, 2014. [Google Scholar]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigün, O. Two-Phase Anaerobic Digestion Processes: A Review. J. Chem. Technol. Biotechnol. 1002, 77, 743–755. [Google Scholar] [CrossRef]
- Jain, S.; Jain, S.; Wolf, I.T.; Lee, J.; Tong, Y.W. A comprehensive review on operating parameters and different pretreatment methodologies for anaerobic digestion of municipal solid waste. Renew. Sustain. Energy Rev. 2015, 52, 142–154. [Google Scholar] [CrossRef]
- Barak, P.; Davidson, C.; Minks, A. Chemical composition of organic acid digest from a municipal wastewater treatment plant and chemical modeling of nuisance struvite formation and phosphorus recovery as brushite. PLOS Water 2023, 2, e0000120. [Google Scholar] [CrossRef]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef] [PubMed]
- Elliot, T.J. Benefits of Ferric Chloride Addition on Struvite and Sulfide Control; University of Wisconsin-Madison: Madison, WI, USA, 2002. [Google Scholar]
- Fattah, K.P.; Sabrina, N.; Mavinic, D.S.; Koch, F.A. Reducing operating costs for struvite formation with a carbon dioxide stripper. Water Sci. Technol. 2008, 58, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Lobanov, S.; Lo, V.K. An overview of technologies to recover phosphorus as struvite from wastewater: Advantages and shortcomings. Environ. Sci. Pollut. Res. 2019, 26, 19063–19077. [Google Scholar] [CrossRef]
- Hao, X.D.; van Loosdrecht, M.C.M. Model-based evaluation of struvite recovery from an in-line stripper in a BNR process (BCFS). Water Sci. Technol. 2006, 53, 191–198. [Google Scholar] [CrossRef]
- Hao, X.D.; Wang, C.C.; Lan, L.; van Loosdrecht, M.C. Struvite formation, analytical methods and effects of pH and Ca2+. Water Sci. Technol. 2008, 58, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.K.; Boyer, T.H. Life cycle comparison of centralized wastewater treatment and urine source separation with struvite precipitation: Focus on urine nutrient management. Water Res. 2015, 79, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resour. Conserv. Recycl. 2016, 107, 142–156. [Google Scholar] [CrossRef]
- Marti, N.; Bouzas, A.; Seco, A.; Ferrer, J. Struvite precipitation assessment in anaerobic digestion processes. Chem. Eng. J. 2008, 141, 67–74. [Google Scholar] [CrossRef]
- Sharp, R.; Vadiveloo, E.; Fergen, R.; Moncholi, M.; Pitt, P.; Wankmuller, D.; Latimer, R. A theoretical and practical evaluation of struvite control and recovery. Water Environ. Res. 2013, 85, 675–686. [Google Scholar] [CrossRef]
- Song, Y.H.; Qiu, G.L.; Yuan, P.; Cui, X.Y.; Peng, J.F.; Zeng, P.; Duan, L.; Xiang, L.C.; Qian, F. Nutrients removal and recovery from anaerobically digested swine wastewater by struvite crystallization without chemical additions. J. Hazard. Mater. 2011, 190, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Barak, P.; Stafford, A. Struvite: A recovered and recycled phosphorus fertilizer. In Proceedings of the 2006 Wisconsin Fertilizer, Aglime & Pest Management Conference, Madison, WI, USA, 17–19 January 2006; p. 199. [Google Scholar]
- Booker, N.; Priestley, A.; Fraser, I. Struvite formation in wastewater treatment plants: Opportunities for nutrient recovery. Environ. Technol. 1999, 20, 777–782. [Google Scholar] [CrossRef]
- Vineyard, D.L., II. Phosphorus Recovery of Brushite at Wastewater Treatment Plants: BioWin Modeling, Economic and Life Cycle Assessment of CalPrex. Ph.D. Thesis, The University of Wisconsin, Madison, WI, USA, 2022. [Google Scholar]
- Tabanpour, M.; Downing, L.; Kuhnjar, W. Demonstrating the CalPrex System for High Efficiency Phosphorus Recovery; Water Research Foundation: Alexandria, VA, USA, 2021. [Google Scholar]
- BioWin 6 Help Manual; Envirosim Associates Ltd.: Hamilton, ON, USA, 2020.
- Pritty, C. Anaerobic Digestion Modeling of a High Rate Acidification Pre-Treatment Technology. Master’s Thesis, The University of Western Ontario, Ontario, CA, 2019. [Google Scholar]
- Forgacs, G.; Smyth, M.; Law, I.; Arnot, T. BioWin modelling to reduce the Avonmouth digester commissioning programme from 6 months to 6 weeks. In Proceedings of the European Biosolids and Organic Resources Conference, Leeds, UK, 20–21 November 2017. [Google Scholar]
- Yang, W.; Young, S.; Munoz, A.; Palmarin, M.J. Dynamic modeling of a full-scale anaerobic mesophilic digester start-up process for the treatment of primary sludge. J. Environ. Chem. Eng. 2019, 7, 103091. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Wickham, R.; Ohandja, D.-G. Enhanced biogas production and performance assessment of a full-scale anaerobic digester with acid phase digestion. Int. Biodeterior. Biodegrad. 2017, 124, 162–168. [Google Scholar] [CrossRef]
- Vineyard, D.; Karthikeyan, K.G.; Davidson, C.; Barak, P. Modeling an acid-phase digester in BioWin with parameter optimization from site data. J. Water Process Eng. 2023, 54, 103971. [Google Scholar] [CrossRef]
- Envirosim. A Brief History of Activated Sludge Process Models and Simulators. 2000. Available online: https://www.envirosim.com/downloads/Model%20history.pdf (accessed on 7 November 2023).
- Flores-Alsina, X.; Solon, K.; Kazadi Mbamba, C.; Tait, S.; Gernaey, K.V.; Jeppsson, U.; Batstone, D.J. Modelling phosphorus (P), sulfur (S) and iron (Fe) interactions for dynamic simulations of anaerobic digestion processes. Water Res. 2016, 95, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Guo, W.; Batstone, D.; Makinia, J.; Li, Y. Modifications to the anaerobic digestion model no. 1 (ADM1) for enhanced understanding and application of the anaerobic treatment processes—A comprehensive review. Water Res. 2023, 244, 120504. [Google Scholar] [CrossRef] [PubMed]
- AECOM. Chloride Compliance Study: Nine Springs Wastewater Treatment Plant Final Report. Madison Metropolitan Sewerage District. 2015. Available online: https://web.archive.org/web/20160505154642/ http://www.madsewer.org/Portals/0/ProgramInitiatives/ChlorideReduction/MMSD%20Chloride%20Compliance%20Study%20Report%20-%20Final%206-19-15bookmarks.pdf (accessed on 7 November 2023).
- Liwarska-Bizukojc, E.; Biernacki, R. Identification of the most sensitive parameters in the activated sludge model implemented in BioWin software. Bioresour. Technol. 2010, 101, 7278–7285. [Google Scholar] [CrossRef] [PubMed]
- Liwarska-Bizukojc, E.; Olejnik, D.; Biernacki, R.; Ledakowicz, S. Calibration of a complex activated sludge model for the full-scale wastewater treatment plant. Bioprocess Biosyst. Eng. 2011, 34, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J. Visual MINTEQ ver. 3.1. 2013. Available online: https://vminteq.com (accessed on 7 November 2023).
- Wang, R.; Li, Y.; Chen, W.; Zou, J.; Chen, Y. Phosphate release involving PAOs activity during anaerobic fermentation of EBPR sludge and the extension of ADM1. Chem. Eng. J. 2016, 287, 436–447. [Google Scholar] [CrossRef]
- Menniti, A.; Rieger, L.; Boltz, J.P.; Johnson, B.; Daigger, G.; Habermacher, J.; Derlon, N.; Morgenroth, E. Critical review on modeling of endogenous processes and the degradability of endogenous decay products. In Proceedings of the WWTmod, Mont-Sainte-Anne, QC, Canada, 26–28 February 2012. [Google Scholar]
- Eastman, J.A.; Ferguson, J.F. Solubilization of particulate organic carbon during the acid phase of anaerobic digestion. J. Water Pollut. Control Fed. 1981, 53, 352–366. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).