Tungsten Bioaccessibility and Environmental Availability in Tungsten-Spiked Agricultural Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Characterization and Spiking
2.2. Tungsten Environmental Availability and Bioaccessibility Evaluation
2.3. Tungsten Analysis
2.4. Quality Assurance and Quality Control
2.5. Statistical Analysis
3. Results and Discussion
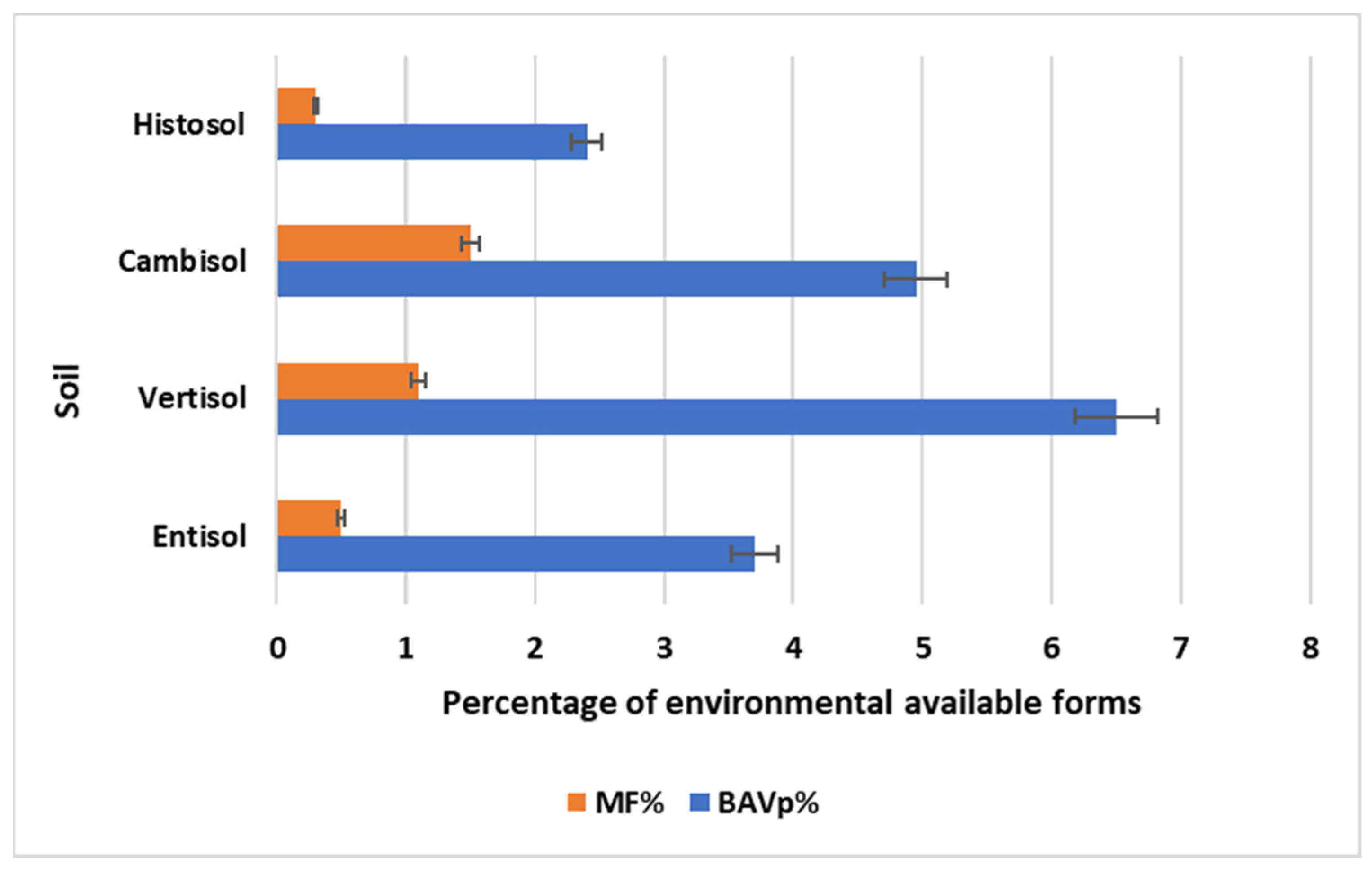
3.1. Environmental Availability
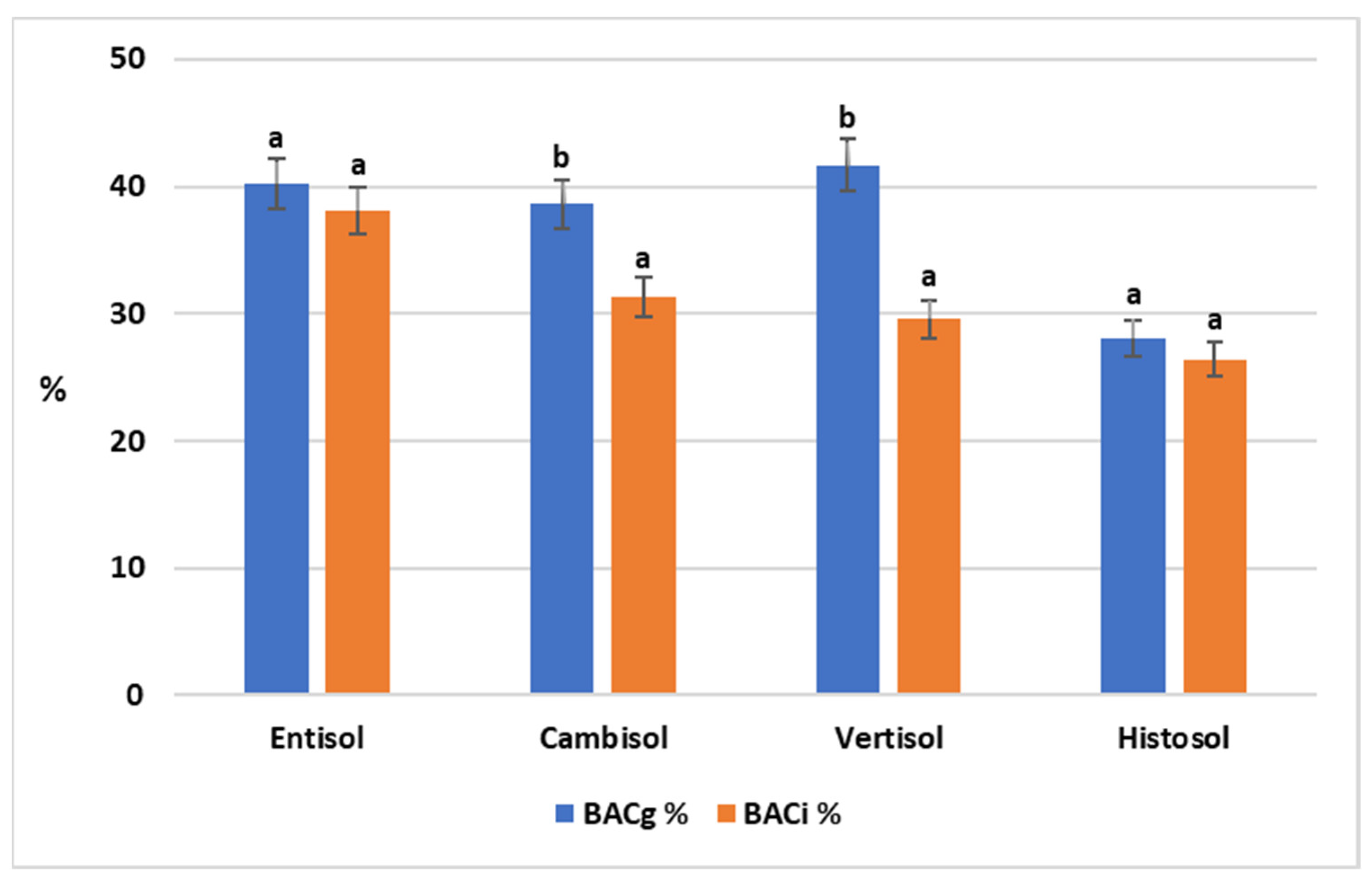
3.2. Bioaccessibility
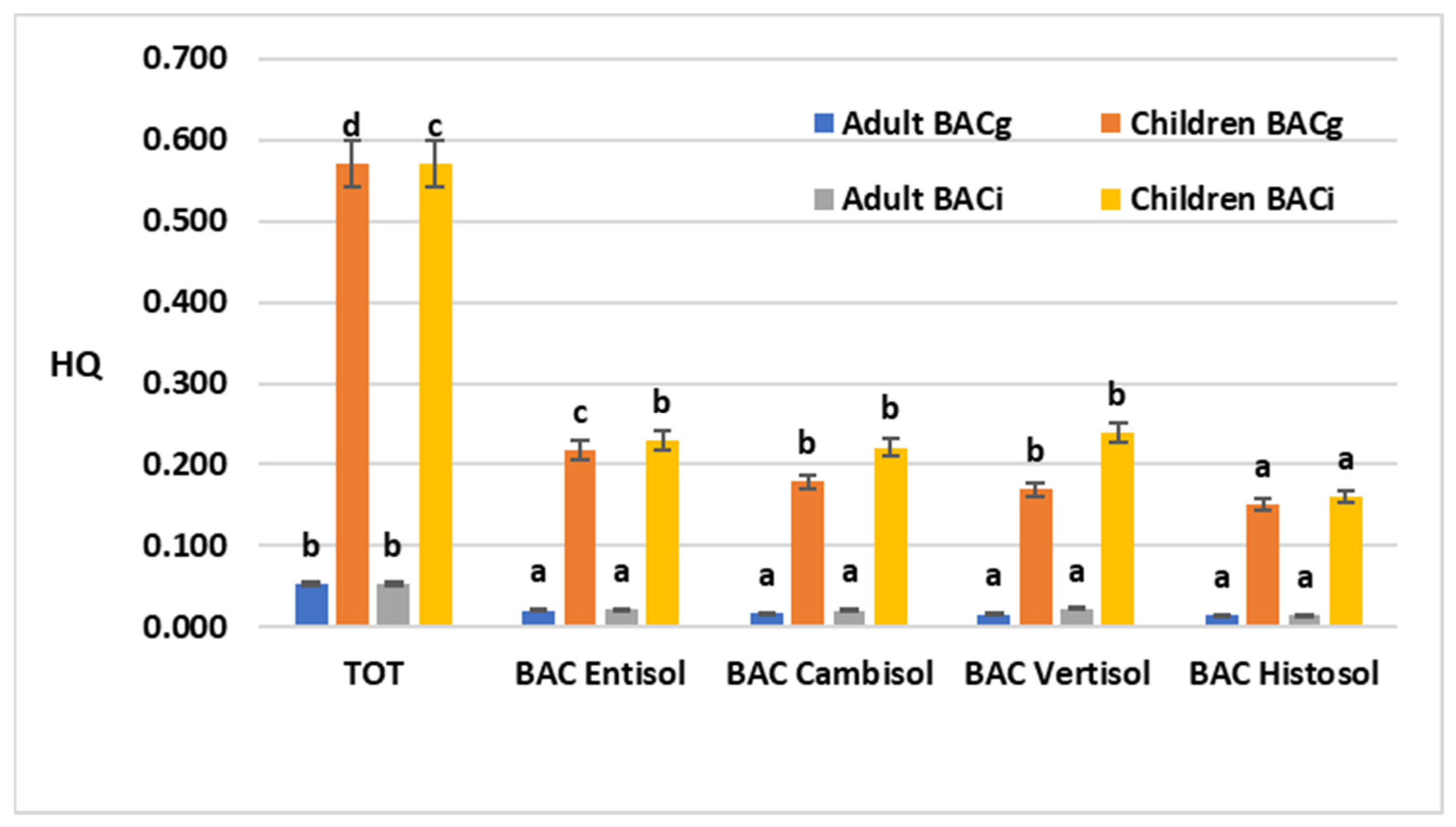
3.3. Environmental Availability and Bioaccessibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graedel, T.E.; Harper, E.M.; Nassar, N.T.; Nuss, P.; Reck, B.K. Criticality of metals and metalloids. Proc. Natl. Acad. Sci. USA 2015, 112, 4257–4262. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Pedron, F. The Dynamics of Tungsten in Soil: An Overview. Environments 2021, 8, 66. [Google Scholar] [CrossRef]
- Bolan, S.; Wijesekara, H.; Ireshika, A.; Zhang, T.; Pu, M.; Petruzzelli, G.; Pedron, F.; Hou, D.; Wang, L.; Zhou, S.; et al. Tungsten contamination, behavior and remediation in complex environmental settings. Environ. Int. 2023, 181, 108276. [Google Scholar] [CrossRef]
- Dodson, J.R.; Hunt, A.J.; Parker, H.L.; Yang, Y.; Clark, J.H. Elemental sustainability: Towards the total recovery of scarce metals. Chem. Eng. Process. Process Intensif. 2012, 51, 69–78. [Google Scholar] [CrossRef]
- Kanianska, R.; Drimal, M.; Varga, J.; Komárek, M.; Ahado, S.K.; Šťastná, M.; Kizeková, M.; Jančová, L. Critically raw materials as potential emerging environmental contaminants, their distribution patterns, risks and behaviour in floodplain soils contaminated by heavy metals. Sci. Rep. 2023, 13, 9597. [Google Scholar] [CrossRef] [PubMed]
- Van der Voet, G.B.; Todorov, T.I.; Centeno, J.A.; Jonas, W.; Ives, J.; Mullick, F.G. Metals and Health: A Clinical Toxicological Perspective on Tungsten and Review of the Literature. Mil. Med. 2007, 172, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Seiler, R.L.; Stollenwerk, K.G.; Garbarino, J.R. Factors controlling tungsten concentrations in ground water, Carson Desert, Nevada. Appl. Geochem. 2005, 20, 423–441. [Google Scholar] [CrossRef]
- Steinberg, K.K.; Relling, M.V.; Gallagher, M.L.; Greene, C.N.; Rubin, C.S.; French, D.; Holmes, A.K.; Carroll, W.L.; Koontz, D.A.; Sampson, E.J.; et al. Genetic studies of a cluster of acute lymphoblastic leukemia cases in Churchill County, Nevada. Environ. Health Perspect. 2007, 115, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, P.R.; Toepfer, P.; Schumacher, E.; Rhodes, K.; Ridenour, G.; Witten, M.L. Morphological and chemical characteristics of airborne tungsten particles of Fallon, Nevada. Microsc. Microanal. 2007, 13, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, C.; Kelly, A.D.R.; Petruccelli, L.A.; Lemaire, M.; Mann, K.K. Exposure to tungsten induces DNA damage and apoptosis in developing B lymphocytes. Leukemia 2011, 25, 1900. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Emerging Contaminant—Tungsten; National Service Center for Environmental Publications: Washington, DC, USA, 2008; pp. 1–4.
- IARC. Agents Classified by the IARC Monographs. 2022. Available online: https://www.epa.gov/sites/default/files/2020-01/documents/guidelines_for_human_exposure_assessment_final2019.pdf (accessed on 30 November 2023).
- Kabata-Pendias, A.; Sztekewhich, B. Trace Elements in Abiotic and Biotic Environments; CRC Press: Boca Raton, FL, USA, 2015; pp. 341–344. [Google Scholar]
- Lin, C.; Li, R.; Cheng, H.; Wang, J.; Shao, X. Tungsten Distribution in Soil and Rice in the Vicinity of the World’s Largest and Longest-Operating Tungsten Mine in China. PLoS ONE 2014, 9, e91981. [Google Scholar] [CrossRef]
- Koutsospyros, A.; Braida, W.J.; Christodoulatos, C.; Dermatas, D.; Strigul, N.S. A review of tungsten: From environmental obscurity to scrutiny. J. Haz. Mater. 2006, 136, 1–19. [Google Scholar] [CrossRef]
- Koutsospyros, A.D.; Strigul, N.; Braida, W.; Christodoulatos, C. Tungsten: Environmental pollution and health effects. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Burlington, UK, 2011; pp. 418–426. [Google Scholar]
- Clausen, J.L.; Korte, N. Environmental fate of tungsten from military use. Sci. Tot. Environ. 2009, 407, 2887–2893. [Google Scholar] [CrossRef]
- Strigul, N. Does speciation matter for tungsten ecotoxicology? Ecotoxicol. Environ. Saf. 2010, 73, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Vero, S.E.; Hettiarachchi, G.M.; Johannesson, K. Tungsten contamination of soils and sediments: Current state of science. Curr. Pollut. Rep. 2017, 3, 55–64. [Google Scholar] [CrossRef]
- National Research Council (NRC). Bioavailability of Contaminants in Soils and Sediments: Processes, Tools, and Applications; The National Academies Press: Washington, DC, USA, 2003. [Google Scholar] [CrossRef]
- Ng, J.C.; Juhasz, A.; Smith, E.; Naidu, R. Assessing the bioavailability and bioaccessibility of metals and metalloids. Environ. Sci. Pollut. Res. 2015, 22, 8802–8825. [Google Scholar] [CrossRef] [PubMed]
- Oomen, A.G.; Hack, A.; Minekus, M.; Zeijdner, E.; Cornelis, C.; Schoeters, G.; Verstraete, W.; Van de Wiele, T.; Wragg, J.; Rompelberg, C.J.M.; et al. Comparison of five in vitro digestion models to study the bioaccessibility of soil contaminants. Environ. Sci. Technol. 2002, 36, 3326–3334. [Google Scholar] [CrossRef] [PubMed]
- Billmann, M.; Hulot, C.; Pauget, B.; Badreddine, R.; Papin, A.; Pelfrêne, A. Oral bioaccessibility of PTEs in soils: A review of data, influencing factors and application in human health risk assessment. Sci. Tot. Environ. 2023, 896, 165263. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yuan, R.; Wang, X.; Yan, Z. Soil tungsten contamination and health risk assessment of an abandoned tungsten mine site. Sci. Total Environ. 2022, 852, 158461. [Google Scholar] [CrossRef] [PubMed]
- Vasques, I.C.F.; Lima, F.R.D.; Oliveira, J.R.; de Morais, E.G.; Pereira, P.; Guilherme, L.R.G.; Marques, J.J. Comparison of bioaccessibility methods in spiked and field Hg contaminated Soils. Chemosphere 2020, 254, 126904. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yu, Y.; Xiang, M.; Cui, P.; Cui, J.; Zhang, F.; Jiang, J.; Xu, R. Changes in molybdenum bioaccessibility in four spiked soils with respect to soil pH and organic matter. J. Environ. Manag. 2023, 334, 117476. [Google Scholar] [CrossRef]
- Xia, Q.; Peng, C.; Lamb, D.; Mallavarapu, M.; Naidu, R.; Ng, J.C. Bioaccessibility of arsenic and cadmium assessed for in vitro bioaccessibility in spiked soils and their interaction during the Unified BARGE Method (UBM) extraction. Chemosphere 2016, 147, 444–450. [Google Scholar] [CrossRef]
- Das, S.; Jean, J.S.; Kar, S. Bioaccessibility and health risk assessment of arsenic in arsenic-enriched soils, Central India. Ecotoxicol. Environ. Saf. 2013, 92, 252–257. [Google Scholar] [CrossRef]
- Wilson, B.; Pyatt, F.B. Bioavailability of Tungsten and Associated Metals in Calcareous Soils in the Vicinity of an Ancient Metalliferous Mine in the Corbières Area, Southwestern France. J. Toxicol. Environ. Health Part A 2009, 72, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Li, Y.; Wan, D.; Sun, C.; Sun, J. Tungsten distribution and vertical migration in soils near a typical abandoned tungsten smelter. J. Haz. Mater. 2022, 429, 128292. [Google Scholar] [CrossRef] [PubMed]
- Oburger, E.; Vergara Cid, C.; Preiner, J.; Hu, J.; Hann, S.; Wanek, W.; Richter, A. pH-Dependent Bioavailability, Speciation, and Phytotoxicity of Tungsten (W) in Soil Aspect Growth and Molybdoenzyme Activity of Nodulated Soybeans. Environ. Sci. Technol. 2018, 52, 6146–6156. [Google Scholar] [CrossRef] [PubMed]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. GeoJournal 1969, 2, 109–118. [Google Scholar]
- Loska, K.; Wiechuła, D.; Korus, I. Metal contamination of farming soils affected by industry. Environ. Int. 2004, 30, 159–165. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhang, Q.C.; Kang, S.G.; Li, M.Y.; Zhang, M.Y.; Xu, W.M.; Xiang, P.; Ma, L.Q. Heavy metal(loid)s in agricultural soil from main grain production regions of China: Bioaccessibility and health risks to humans. Sci. Tot. Environ. 2023, 858, 159819. [Google Scholar] [CrossRef]
- Barbieri, M. The importance of enrichment factor (EF) and geoaccumulation index (Igeo) to evaluate the soil contamination. J. Geol. Geophys. 2016, 5, 237–240. [Google Scholar] [CrossRef]
- Zhang, J.W.; Tian, B.; Luo, J.J.; Wu, F.; Zhang, C.; Liu, Z.T.; Wang, X.N. Effect factors and model prediction of soil heavy metal bioaccessibility. Environ. Sci. 2022, 43, 3811–3824. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis Part 3—Chemical Methods; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996. [Google Scholar]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Li, R.; Wang, W.; Wang, S.; Lin, C.; Wang, X.; Meng, L.; Yuan, X. Comparison of two sequential extraction procedures for tungsten fractionation in the tungsten mining soils. RSC Adv. 2019, 9, 35456. [Google Scholar] [CrossRef]
- BARGE—INERIS UBM procedure for the measurement of inorganic contaminant bioaccessibility from solid matrices. Food Chem. 2011, 277, 1–10.
- Duggan, M.J.; Inskip, M.J.; Rundle, S.A.; Moorcroft, J.S. Lead in playground dust and on the hands of schoolchildren. Sci. Total Environ. 1985, 44, 65–79. [Google Scholar] [CrossRef]
- Rodriguez, R.R.; Basta, N.T.; Casteel, S.W.; Pace, L.W. An in-vitro gastrointestinal method to estimate bioavailability arsenic in contaminated soils and media. Environ. Sci. Technol. 1999, 33, 642–649. [Google Scholar] [CrossRef]
- Griggs, J.L.; Thomas, D.J.; Fry, R.; Bradham, K.D. Improving the predictive value of bioaccessibility assays and their use to provide mechanistic insights into bioavailability for toxic metals/metalloids—A research prospectus. J. Toxicol. Environ. Health B 2021, 24, 307–324. [Google Scholar] [CrossRef]
- ISO 17924; Soil Quality. Assessment of Human Exposure From Ingestion of Soil and Soil Material—Procedure for the Estimation of the Human Bioaccessibility/Bioavailability of Metals in Soil). ISO: Geneva, Switzerland, 2018.
- Denys, S.; Caboche, J.; Tack, K.; Rychen, G.; Wragg, J.; Cave, M.R.; Jondreville, C.; Cyril, F. In Vivo Validation of the Unified BARGE Method to Assess the Bioaccessibility of Arsenic, Antimony, Cadmium, and Lead in Soils. Environ. Sci. Technol. 2012, 46, 6252–6260. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Exposure Factors Handbook; National Technical Information Service: Springfield, VA, USA; Washington, DC, USA, 2011; (EPA/600/R-09/052F).
- USEPA. Regional Screening Levels (RSLs). 2020. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls (accessed on 30 September 2023).
- Ning, Z.P.; Liu, E.G.; Yao, D.J.; Xiao, T.F.; Ma, L.; Liu, Y.Z.; Li, H.; Liu, C.S. Contamination, oral bioaccessibility and human health risk assessment of thallium and other metal(loid)s in farmland soils around a historic Tl Hg mining area. Sci. Total Environ. 2021, 758, 143577. [Google Scholar] [CrossRef] [PubMed]
- Dermatas, D.; Braida, W.; Christodoulatos, C.; Strigul, N.; Panikov, N.; Los, M.; Larson, S. Solubility, sorption, and soil respiration effects of tungsten and tungsten alloys. Environ. Forensics 2004, 5, 5–13. [Google Scholar] [CrossRef]
- Bednar, A.J.; Jones, W.T.; Chappell, M.A.; Johnson, D.R.; Ringelberg, D.B. A modified acid digestion procedure for extraction of tungsten from soil. Talanta 2010, 80, 1257–1263. [Google Scholar] [CrossRef]
- Sallman, B.; Rakshit, S.; Lefèvre, G. Influence of phosphate on tungstate sorption on hematite: A macroscopic and spectroscopic evaluation of the mechanism. Chemosphere 2018, 213, 596–601. [Google Scholar] [CrossRef]
- Salbu, B.; Krekling, T.; Oughton, D.H. Characterisation of radioactive particles in the environment. Analyst 1998, 123, 843–849. [Google Scholar] [CrossRef]
- Beeston, M.P.; van Elteren, J.T.; Šlejkovec, Z.; Jan, H. Glass Migration of arsenic from old tailings ponds—A case study on the King Edward Mine, Cornwall, UK. Environ. Res. 2008, 108, 28–34. [Google Scholar] [CrossRef]
- Kalyvas, G.; Gasparatos, D.; Massas, I. A critical assessment on arsenic partitioning in mine-affected soils by using two sequential extraction protocols. Arch. Agron. Soil. Sci. 2018, 64, 1549–1563. [Google Scholar] [CrossRef]
- Oburger, E.; Vergar Cid, C.; Schwertberger, D.; Roschitz, C.; Wenzel, W.W. Response of tungsten (W) solubility and chemical fractionation to changes in soil pH and soil aging. Sci. Total Environ. 2020, 731, 139224. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Srivastava, P.K. Bioavailability of arsenic in agricultural soils under the influence of different soil properties. SN Appl. Sci. 2020, 2, 153. [Google Scholar] [CrossRef]
- Semhi, K.; Boutin, R.; Nallusamy, S.; Al Busaidi, W.; Al Hamdi, A.; Al Dhafri, K.; Al Busaidi, A. Impact of a Variable Tungsten Pollution on the Elemental Uptake of Two Plant Species. Water Air Soil. Pollut. 2018, 229, 294. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Pedron, F. Influence of Increasing Tungsten Concentrations and Soil Characteristics on Plant Uptake: Greenhouse Experiments with Zea mays. Appl. Sci. 2019, 9, 3998. [Google Scholar] [CrossRef]
- EPA 505-F-17-004; N-Nitroso-dimethylamine (NDMA). USEPA. Office of Land and Emergency Management: Washington, DC, USA, 2017; p. 5106.
- Wragg, J.; Cave, M.; Basta, N.; Brandon, E.; Casteel, S.; Denys, S.; Gron, C.; Oomen, A.; Reimer, K.; Tack, K.; et al. An inter-laboratory trial of the unified BARGE bioaccessibility method for arsenic, cadmium and lead in soil. Sci. Total. Environ. 2011, 409, 4016–4030. [Google Scholar] [CrossRef] [PubMed]
- Pelfrêne, A.; Douay, F. Assessment of oral and lund bioaccessibility of Cd and Pb from smelter-impacted dust. Environ. Sci. Pollut. Res. Int. 2018, 25, 3718–3730. [Google Scholar] [CrossRef] [PubMed]
- Pelfrêne, A.; Waterlot, C.; Douay, F. In vitro digestion and DGT techniques for estimating cadmium and lead bioavailability in contaminated soils: Influence of gastric juice pH. Sci. Total Environ. 2011, 409, 5076–5085. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, M.; Zhong, W.; Yin, Z.; Jing, C. Speciation, leachability and bioaccessibility of tungsten in tungsten ore processing residue. Chemosphere 2022, 302, 134856. [Google Scholar] [CrossRef]
- Li, S.W.; Li, J.; Li, H.B.; Naidu, R.; Ma, L.Q. Arsenic bioaccessibility in contaminated soils: Coupling in vitro assays with sequential and HNO3 extraction. J. Haz. Mater. 2015, 295, 145–152. [Google Scholar] [CrossRef]
- Mokhtarzadeh, Z.; Keshavarzi, B.; Moore, F.; Marsan, F.A.; Padoan, E. Potentially toxic elements in the Middle East oldest oil refinery zone soils: Source apportionment, speciation, bioaccessibility and human health risk assessment. Environ. Sci. Pollut. Res. 2020, 27, 40573–40591. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Pedron, F. Tungstate adsorption onto Italian soils with different characteristics. Environ. Monit. Assess. 2017, 189, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Bednar, A.J.; Boyd, R.E.; Jones, W.T.; McGrath, C.J.; Johnson, D.R.; Chappell, M.A.; Ringelberg, D.B. Investigations of tungsten mobility in soil using column tests. Chemosphere 2009, 75, 1049–1056. [Google Scholar] [CrossRef]
- Girouard, E.; Zagury, G.J. Arsenic bioaccessibility in CCA-contaminated soils: Influence of soil properties, arsenic fractionation, and particle-size fraction. Sci. Total Environ. 2009, 407, 2576–2585. [Google Scholar] [CrossRef]
- Hiller, E.; Pilková, Z.; Filová, L.; Mihaljevič, M.; Špirová, V.; Jurkovič, Ľ. Metal(loid) concentrations, bioaccessibility and stable lead isotopes in soils and vegetables from urban community gardens. Chemosphere 2022, 305, 135499. [Google Scholar] [CrossRef]
- Chi, H.; Hou, Y.; Li, G.; Zhang, Y.; Coulon, F.; Cai, C. In vitro model insights into the role of human gut microbiota on arsenic bioaccessibility and its speciation in soils. Environ. Pollut. Part A 2020, 263, 114580. [Google Scholar] [CrossRef]
- Sowers, T.D.; Nelson, C.M.; Blackmon, M.D.; Jerden, M.L.; Kirby, A.M.; Diamond, G.L.; Bradham, K.D. Interconnected soil iron and arsenic speciation effects on arsenic bioaccessibility and bioavailability: A scoping review. J. Toxicol. Environ. Health B 2022, 25, 1–22. [Google Scholar] [CrossRef]
- Luo, X.S.; Jing, D.; Bo, X.; Wang, Y.J.; Li, H.B.; Shen, Y. Incorporating bioaccessibility into human health risk assessments of heavy metals in urban park soils. Sci. Total Environ. 2012, 424, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Ouyang, W.Y.; Shu, Y.L.; Tian, Y.Z.; Feng, Y.C.; Zhang, T.; Chen, W. Incorporating bioaccessibility into health risk assessment of heavy metals in particulate matter originated from different sources of atmospheric pollution. Environ. Pollut. 2019, 254, 113113. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Cipullo, S.; Cocerva, T.; Coulon, F.; Dion, G.A.; Ajmone-Marsan, F.; Padoan, E.; Cox, S.F.; Cave, M.R.; Luca, D.A.D. Incorporating oral bioaccessibility into human health risk assessment due to potentially toxic elements in extractive waste and contaminated soils from an abandoned mine site. Chemosphere 2020, 255, 126927. [Google Scholar] [CrossRef] [PubMed]
- Zingaretti, D.; Baciocchi, R. Different approaches for incorporating bioaccessibility of inorganics in human health risk assessment of contaminated soils. Appl. Sci. 2021, 11, 3005. [Google Scholar] [CrossRef]
- Bolt, A.M. Tungsten toxicity and carcinogenesis. Adv. Pharmacol. 2023, 96, 119–150. [Google Scholar] [CrossRef] [PubMed]
- Marschner, B.; Welge, P.; Hack, A.; Wittsiepe, J.; Wilhelm, M. Comparison of soil Pb in vitro bioaccessibility and in vivo bioavailability with Pb pools from a sequential soil extraction. Environ. Sci. Technol. 2006, 40, 2812–2818. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.W.; Du, J.J.; Luo, T.; Huang, Y.Y.; Jing, C.Y. Evaluation of chromium bioaccessibility in chromite ore processing residue using in vitro gastrointestinal method. J. Haz. Mater. 2012, 209, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Bradham, K.D.; Diamond, G.L.; Nelson, C.M.; Noerpel, M.; Scheckel, K.G.; Elek, B.; Chaney, R.I.; Ma, Q.; Thomas, D.J. Long-Term in Situ Reduction in Soil Lead Bioavailability Measured in a Mouse Model. Environ. Sci. Technol. 2018, 52, 13908–13913. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Barbafieri, M.; Franchi, E.; Fusini, D.; Vocciante, M.; Pedron, F. Effect of Soil Aging on Cadmium Bioavailability and Bioaccessibility at a Contaminated Site. Environments 2023, 10, 105. [Google Scholar] [CrossRef]

| Soil Classification | Entisol | Cambisol | Vertisol | Histosol |
|---|---|---|---|---|
| Textural class | Sandy loam | Loamy | Loamy | Sandy loam |
| pH | 6.2 (0.02) | 7.3 (0.04) | 8.1 (0.03) | 4.7 (0.03) |
| Organic matter% | 3.1 (0.33) | 1.08 (0.22) | 1.1 (0.27) | 5.32 (0.48) |
| C.E.C (cmol (+) kg−1) | 21.4 (1.4) | 10,6 (0.7) | 16.2 (0.8) | 25.6 (1.1) |
| Clay% | 15.6 (0.4) | 13.3 (0.7) | 23 (1.1) | 10.4 (1.0) |
| Silt% | 26.6 (0.5) | 46.4 (0.9) | 42 (0.7) | 23.6 (0.9) |
| Sand% | 57.8 (0.5) | 40.3 (1.2) | 35 (1.0) | 66.0 (1.4) |
| Total W mg kg−1 | 0.25 (0.4) | 0.36 (0.4) | 0.29 (0.4) | 0.32 (0.4) |
| Fe% | 2.8 (0.17) | 3.1 (0.11) | 2.4 (0.16) | 4.2 (0.15) |
| Fractions | Entisol | Cambisol | Vertisol | Histosol |
|---|---|---|---|---|
| F1 | 0.25 (0.05) | 0.55 (0.04) | 0.75 (0.05) | 0.15 (0.04) |
| F2 | 1.60 (0.4) | 2.42 (0.6) | 2.53 (0.5) | 1.05 (0.1) |
| F3 | 7.51 (0.5) | 6.53 (0.3) | 5.52 (0.3) | 4.51 (0.2) |
| F4 | 16.0 (1.2) | 14.0 (1.1) | 11.5 (1.2) | 8.50 (1.0) |
| F5 | 24.6 (1.8) | 26.5 (1.7) | 29.7 (1.8) | 35.9 (1.9) |
| Soil Properties | Equation | R2 |
|---|---|---|
| pH | BAVp = 1.441 pH + 3.26 | 0.977 |
| OM | BAVp = −1.950 OM + 7.13 | 0.955 |
| Clay | BAVp = 3.962 Clay + 6.46 | 0.552 |
| Fe | BAVp = −0.615 Fe + 4.53 | 0.649 |
| Soil | BACg | BACi |
|---|---|---|
| Entisol | 19.1 (0.21) | 20.1 (0.51) |
| Cambisol | 15.6 (0.11) | 19.3 (0.32) |
| Vertisol | 14.8 (0.09) | 20.8 (0.50) |
| Histosol | 13.2 (0.09) | 14.0 (0.10) |
| Soil Properties | Equation | R2 |
|---|---|---|
| pH | BACg = 0.311 pH + 13.63 | 0.0344 |
| BACi = 1.830 pH + 6.54 | 0.7644 | |
| OM | BACg = −0.303 OM + 16.48 | 0.0612 |
| BACi = −1.317 OM + 22.06 | 0.742 | |
| Clay | BACg = 0.0741 Clay + 14.52 | 0.0261 |
| BACi = 0.444 Clay + 11.65 | 0.603 | |
| Fe | BACg = −1.747 Fe + 21.13 | 0.298 |
| BACi = −3.931 Fe + 30.86 | 0.969 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruzzelli, G.; Pedron, F. Tungsten Bioaccessibility and Environmental Availability in Tungsten-Spiked Agricultural Soils. Environments 2024, 11, 26. https://doi.org/10.3390/environments11020026
Petruzzelli G, Pedron F. Tungsten Bioaccessibility and Environmental Availability in Tungsten-Spiked Agricultural Soils. Environments. 2024; 11(2):26. https://doi.org/10.3390/environments11020026
Chicago/Turabian StylePetruzzelli, Gianniantonio, and Francesca Pedron. 2024. "Tungsten Bioaccessibility and Environmental Availability in Tungsten-Spiked Agricultural Soils" Environments 11, no. 2: 26. https://doi.org/10.3390/environments11020026
APA StylePetruzzelli, G., & Pedron, F. (2024). Tungsten Bioaccessibility and Environmental Availability in Tungsten-Spiked Agricultural Soils. Environments, 11(2), 26. https://doi.org/10.3390/environments11020026






