Morphological Alterations and Oxidative Stress Induction in Danio rerio Liver After Short-Term Exposure to the Strobilurin Fungicide Dimoxystrobin
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Maintenance
2.2. Test Substance
2.3. Experimental Design
2.4. Histological and Ultrastructural Methods
2.5. Histopathological and Morphometrical Analyses
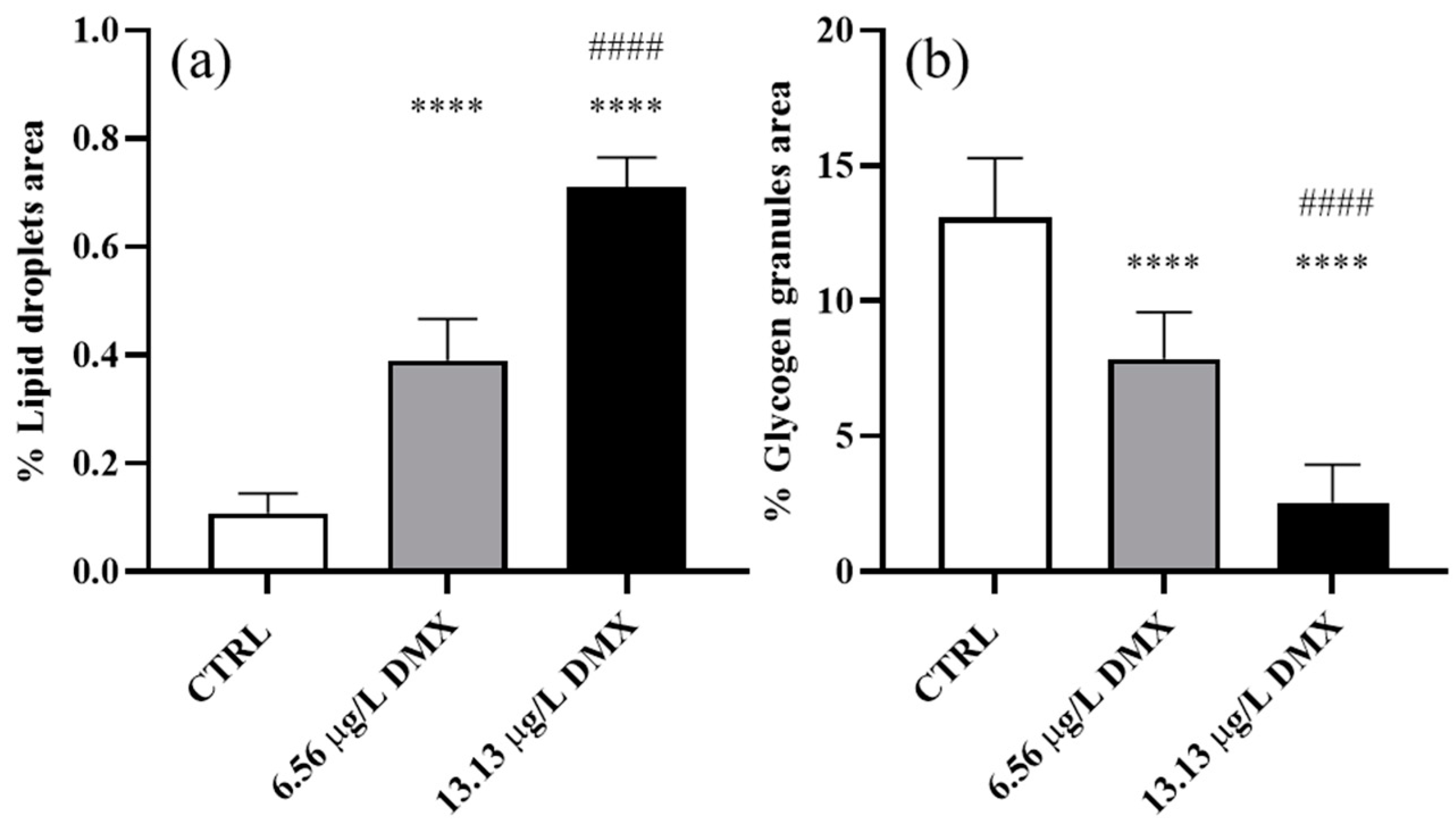
2.6. Lipid Droplet and Glycogen Granule Content
2.7. RNA Isolation and qRT-PCR
2.8. Statistical Analyses
3. Results
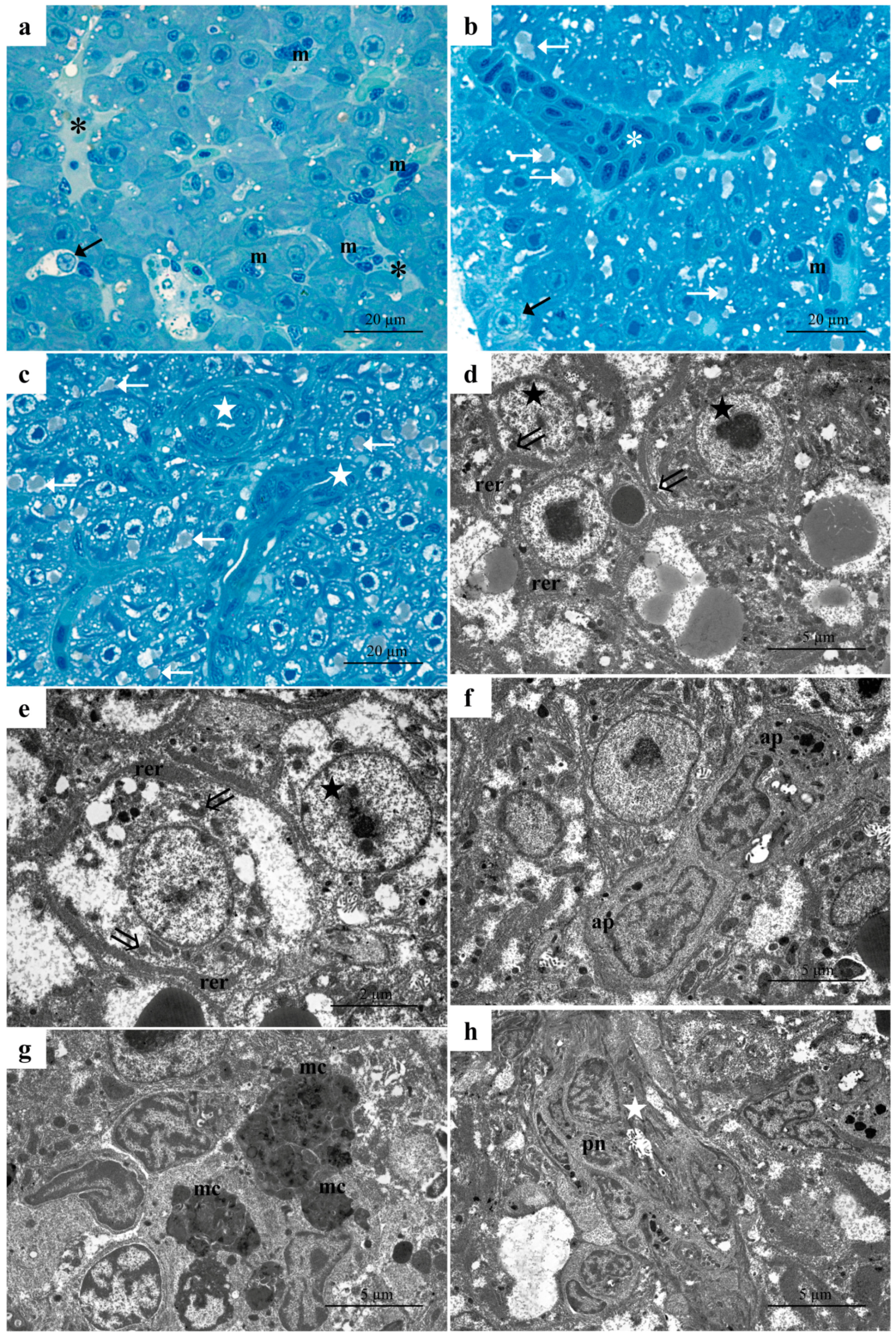
3.1. Histology and Ultrastructure
3.1.1. Control Group
3.1.2. Low-Concentration Group (6.56 µg/L)
3.1.3. High-Concentration Group (13.13 µg/L)
3.2. Organ Index
3.3. Morphometric Analyses
3.4. Real-Time PCR (qRT-PCR)
4. Discussion
4.1. Morphology and Ultrastructure
4.2. Morphometric Evaluation
4.3. Oxidative Stress
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Herrero, M.; Thornton, P.K.; Power, B.; Bogard, J.R.; Remans, R.; Fritz, S.; Gerber, J.S.; Nelson, G.; See, L.; Waha, K.; et al. Farming and the geography of nutrient production for human use: A transdisciplinary analysis. Lancet Planet. Health. 2017, 1, e33–e42. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; Angle, S.; Rabbinge, R. Unlocking the multiple public good services from balanced fertilizers. Food Secur. 2018, 10, 273–285. [Google Scholar] [CrossRef]
- Macirella, R.; Madeo, G.; Sesti, S.; Tripepi, M.; Bernabò, I.; Godbert, N.; La Russa, D.; Brunelli, E. Exposure and post-exposure effects of chlorpyrifos on Carassius auratus gills: An ultrastructural and morphofunctional investigation. Chemosphere 2020, 251, 126434. [Google Scholar] [CrossRef] [PubMed]
- Nagesh, P.; Edelenbosch, O.Y.; Dekker, S.C.; de Boer, H.J.; Mitter, H.; van Vuuren, D.P. Extending shared socio-economic pathways for pesticide use in Europe: Pest-Agri-SSPs. J. Environ. Manag. 2023, 342, 118078. [Google Scholar] [CrossRef] [PubMed]
- EUROSTAT. Agri-Environmental Indicator-Consumption of Pesticides. 2024. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agri-environmental_indicaPandeytor_-_consumption_of_pesticides (accessed on 16 September 2024).
- Pandey, G.; Rathore, H. Toxicity of Strobilurins fungicides: A comprehensive review. J. Chem. Health Risks 2023, 13, 207–218. [Google Scholar] [CrossRef]
- Weaver, C.R.; Brockman, M.; Mundahl, N.D.; Arnold, W.A.; Blumentritt, D.; Varela, W.L.; Franz, J.L. Detection of strobilurin fungicides in trout streams within an agricultural watershed. Hydrology 2024, 11, 13. [Google Scholar] [CrossRef]
- Li, H.; Cao, F.; Zhao, F.; Yang, Y.; Teng, M.; Wang, C.; Qiu, L. Developmental toxicity, oxidative stress and immunotoxicity induced by three strobilurins (pyraclostrobin, trifloxystrobin and picoxystrobin) in zebrafish embryos. Chemosphere 2018, 207, 781–790. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Wang, Y.; Qin, Y.; Yan, B.; Martyniuk, C.J. A comprehensive review of strobilurin fungicide toxicity in aquatic species: Emphasis on mode of action from the zebrafish model. Environ. Pollut. 2021, 275, 116671. [Google Scholar] [CrossRef]
- Pomal, N.C.; Bhatt, K.D.; Kundariya, D.S.; Desai, R.A.; Bhatt, V.; Kongor, A. Calix[4]pyrrole-Grafted Gold Nanoparticles as a Turn-On Fluorescence Sensor for Noxious Fungicide Dimoxystrobin and Their Anti-Cancer Activity against the KB-3-1 Cell Line. ChemistrySelect 2023, 8, e202204252. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Liu, Y.; Lan, Y.; Zhu, J.; Cai, Y.; Guo, F.; Li, F.; Zhang, Y.; Zhang, T.; et al. Evidence of strobilurin fungicides and their metabolites in Dongjiang River ecosystem, southern China: Bioaccumulation and ecological risks. Sci. Total Environ. 2024, 908, 168427. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Peer review of the pesticide risk assessment of the active substance dimoxystrobin. EFSA J. 2023, 21, e08329. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Pesticide Properties DataBase (PPDB). Dimoxystrobin (Ref: BAS 505F). 2024. Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/246.htm (accessed on 20 September 2024).
- CLH Report. Proposal for Harmonised Classification and Labelling Based on Regulation (EC) No 1272/2008 (CLP Regulation), Annex VI, Part 2 Substance Name: Dimoxystrobin. 2019. Available online: https://echa.europa.eu/documents/10162/71711b06-bc63-d700-6553-dd633d93ea8b (accessed on 25 August 2024).
- Ahmed, A.I.M.; Macirella, R.; Talarico, F.; Curcio, V.; Trotta, G.; Aiello, D.; Gharbi, N.; Mezzasalma, M.; Brunelli, E. Short-term effects of the strobilurin fungicide dimoxystrobin on zebrafish gills: A morpho-functional study. Chemosphere 2023, 333, 138914. [Google Scholar] [CrossRef]
- Ahmed, A.I.M.; Macirella, R.; Talarico, F.; Muoio, M.F.; Mezzasalma, M.; Tronci, V.; Lal, P.; Gharbi, N.; Brunelli, E. Effect of short-term exposure to the strobilurin fungicide dimoxystrobin: Morphofunctional, behavioural and mitochondrial alterations in Danio rerio embryos and larvae. Ecotoxicol. Environ. Saf. 2024, 279, 116493. [Google Scholar] [CrossRef] [PubMed]
- Macirella, R.; Guardia, A.; Pellegrino, D.; Bernabò, I.; Tronci, V.; Ebbesson, L.O.; Sesti, S.; Tripepi, S.; Brunelli, E. Effects of two sublethal concentrations of mercury chloride on the morphology and metallothionein activity in the liver of zebrafish (Danio rerio). Int. J. Mol. Sci. 2016, 17, 361. [Google Scholar] [CrossRef]
- Popović, N.T.; Čižmek, L.; Babić, S.; Strunjak-Perović, I.; Čož-Rakovac, R. Fish liver damage related to the wastewater treatment plant effluents. Environ. Sci. Pollut. Res. 2023, 30, 48739–48768. [Google Scholar] [CrossRef]
- Araújo, F.G.; Gomes, I.D.; do Nascimento, A.A.; dos Santos, M.A.J.; Sales, A. Histopathological analysis of liver of the catfish Pimelodus maculatus in a tropical eutrophic reservoir from Southeastern Brazil. Acta Sci. Biol. Sci. 2019, 41, 41039. [Google Scholar] [CrossRef]
- Rohani, M.F. Pesticides toxicity in fish: Histopathological and hemato-biochemical aspects—A review. Emerg. Contam. 2023, 9, 100234. [Google Scholar] [CrossRef]
- Levina, I.L.; Fedorova, E.A.; Kuznetsova, L.Y.; Zinchuk, O.A. Dynamics of antioxidant protection and detoxication processes affected by strobilurin fungicides in the liver of cyprinids. Inland Water Biol. 2012, 5, 222–228. [Google Scholar] [CrossRef]
- Valenti, M.T.; Marchetto, G.; Mottes, M.; Dalle Carbonare, L. Zebrafish: A suitable tool for the study of cell signaling in bone. Cells 2020, 9, 1911. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Chen, Q.; Jin, L.; Peng, R. Research progress of zebrafish model in aquatic ecotoxicology. Water 2023, 15, 1735. [Google Scholar] [CrossRef]
- Adhish, M.; Manjubala, I. Effectiveness of zebrafish models in understanding human diseases—A review of models. Heliyon 2023, 9, e14557. [Google Scholar] [CrossRef]
- Cortes, L.G.; Marinov, D.; Sanseverino, I.; Cuenca, A.N.; Niegowska, M.; Rodriguez, E.P.; Lettieri, T. Selection of Substances for the 3rd Watch List Under the Water Framework Directive; EUR 30297 EN; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, G.A.; Herrera-Vázquez, S.E. Zebrafish as Model Organism in Aquatic Ecotoxicology: Current Trends and Future Perspectives. In Zebrafish Research—An Ever-Expanding Experimental Model; Disner, G.D., Ed.; Zebrafish Research; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Van Dyk, J.C.; Marchand, M.J.; Smit, N.J.; Pieterse, G.M. A histology-based fish health assessment of four commercially and ecologically important species from the Okavango Delta panhandle, Botswana. Afr. J. Aquat. Sci. 2009, 34, 273–282. [Google Scholar] [CrossRef]
- Sun, Y.; Heng, J.; Liu, F.; Zhang, S.; Liu, P. Isolation and proteomic study of fish liver lipid droplets. Biophys. Rep. 2023, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Manera, M.; Castaldelli, G.; Fano, E.A.; Giari, L. Perfluorooctanoic acid-induced cellular and subcellular alterations in fish hepatocytes. Environ. Toxicol. Pharmacol. 2021, 81, 103548. [Google Scholar] [CrossRef]
- Flores-Lopes, F.; Correia, M.A.; da Silva, D.M.L. Histological and ultrastructural analysis of Tilapia rendalli liver as an environmental assessment tool for Cachoeira River, Bahia, Brazil. Int. J. Zool. Investig. 2020, 6, 31–48. [Google Scholar] [CrossRef]
- Rašković, B.; Poleksić, V. Fish histopathology as biomarker in ecotoxicology. Trends Fish. Aquat. Anim. Health 2017, 27, 155–181. [Google Scholar]
- Huang, X.; Li, Y.; Wang, T.; Liu, H.; Shi, J.; Zhang, X. Evaluation of the oxidative stress status in zebrafish (Danio rerio) liver induced by three typical organic UV filters (BP-4, PABA and PBSA). Int. J. Environ. Res. Public Health 2020, 17, 651. [Google Scholar] [CrossRef]
- Rossi, A.S.; Fantón, N.; Michlig, M.P.; Repetti, M.R.; Cazenave, J. Fish inhabiting rice fields: Bioaccumulation, oxidative stress and neurotoxic effects after pesticides application. Ecol. Indic. 2020, 113, 106186. [Google Scholar] [CrossRef]
- Santana, M.S.; de Melo, G.D.; Sandrini-Neto, L.; Di Domenico, M.; Prodocimo, M.M. A meta-analytic review of fish antioxidant defense and biotransformation systems following pesticide exposure. Chemosphere 2022, 291, 132730. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Wu, P.; Huang, L.; Li, H.; Qian, L.; Pang, S.; Qiu, L. Short-term developmental effects and potential mechanisms of azoxystrobin in larval and adult zebrafish (Danio rerio). Aquat. Toxicol. 2018, 198, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Martyniuk, C.J.; Wu, P.; Zhao, F.; Pang, S.; Wang, C.; Qiu, L. Long-term exposure to environmental concentrations of azoxystrobin delays sexual development and alters reproduction in zebrafish (Danio rerio). Environ. Sci. Technol. 2019, 53, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development (OECD). OECD Guidelines for the Testing of Chemicals, Fish Embryo Acute Toxicity (FET) Test. Section 2; OECD Publishing: Paris, France, 2013. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). OECD Guidance Document on Aqueous-Phase Aquatic Toxicity Testing of Difficult Test Chemicals. No. 23, 2nd ed.; OECD Publishing: Paris, France, 2019; Available online: https://one.oecd.org/document/ENV/JM/MONO(2000)6/REV1/en/pdf (accessed on 30 August 2024).
- Wallace, C.K.; Bright, L.A.; Marx, J.O.; Andersen, R.P.; Mullins, M.C.; Carty, A.J. Effectiveness of rapid cooling as a method of euthanasia for young zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 58–63. [Google Scholar] [PubMed]
- Macirella, R.; Curcio, V.; Ahmed, A.I.; Talarico, F.; Sesti, S.; Paravani, E.; Odetti, L.; Mezzasalma, M.; Brunelli, E. Morphological and Functional Alterations in Zebrafish (Danio rerio) Liver after Exposure to Two Ecologically Relevant Concentrations of Lead. Fishes 2023, 8, 342. [Google Scholar] [CrossRef]
- Macirella, R.; Curcio, V.; Ahmed, A.I.M.; Pellegrino, D.; Brunelli, E. Effect of short-term exposure to low concentration of tebuconazole: Morphological, histometric and functional modifications in Danio rerio liver. Eur. Zool. J. 2022, 89, 331–345. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, L.; Luo, Y.; Zhang, C.; Liu, X.; Fang, N.; Wang, X.; Zhao, X.; Jiang, J. Trifloxystrobin induced developmental toxicity by disturbing the ABC transporters, carbohydrate and lipid metabolism in adult zebrafish. Chemosphere 2024, 349, 140747. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, Y.; Wen, C.; Liu, W.; Cao, L.; Feng, X.; Chen, J.; Wang, H.; Tang, Y.; Tian, L.; et al. Effects of environmental contaminants in water resources on nonalcoholic fatty liver disease. Environ. Int. 2021, 154, 106555. [Google Scholar] [CrossRef]
- Cao, F.; Zhu, L.; Li, H.; Yu, S.; Wang, C.; Qiu, L. Reproductive toxicity of azoxystrobin to adult zebrafish (Danio rerio). Environ. Pollut. 2016, 219, 1109–1121. [Google Scholar] [CrossRef]
- Agamy, E. Histopathological changes in the livers of rabbit fish (Siganus canaliculatus) following exposure to crude oil and dispersed oil. Toxicol. Pathol. 2012, 40, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Zhou, Y.; Song, M.; Dong, K.; Chen, X.; Wang, C.; Bi, S.; Zhu, W. Chronic toxic effects of flutolanil on the liver of zebrafish (Danio rerio). Chem. Res. Toxicol. 2019, 32, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Mishra, A.; Verma, A.; Jain, A.; Khan, A.A.; Dwivedi, S.; Trivedi, S.P. Assessment of oxidative stress, genotoxicity, and histopathological alterations in freshwater food fish Channa punctatus exposed to fungicide, Mancozeb. J. Appl. Biol. 2024, 12, 159–164. [Google Scholar] [CrossRef]
- Shan, Z.; Ju, C. Hepatic macrophages in liver injury. Front. Immunol. 2020, 11, 322. [Google Scholar] [CrossRef]
- Bjørgen, H.; Koppang, E.O. The melano-macrophage: The black leukocyte of fish immunity. Fish Shellfish Immunol. 2024, 148, 109523. [Google Scholar] [CrossRef]
- Oliveira, S.E.; Costa, P.M.; Nascimento, S.B.; Castro, W.V.; de Azambuja Ribeiro, R.I.M.; Santos, H.B.; Thomé, R.G. Atrazine promotes immunomodulation by melanomacrophage centre alterations in spleen and vascular disorders in gills from Oreochromis niloticus. Aquat. Toxicol. 2018, 202, 57–64. [Google Scholar] [CrossRef]
- Hamed, M.; Said, R.E.; Soliman, H.A.; Osman, A.G.; Martyniuk, C.J. Immunotoxicological, histopathological, and ultrastructural effects of waterborne pyrogallol exposure on African catfish (Clarias gariepinus). Chemosphere 2024, 349, 140792. [Google Scholar] [CrossRef]
- Sayed, A.E.D.H.; Abd-Elkareem, M.; Abou Khalil, N.S. Immunotoxic effects of 4-nonylphenol on Clarias gariepinus: Cytopathological changes in hepatic melanomacrophages. Aquat. Toxicol. 2019, 207, 83–90. [Google Scholar] [CrossRef]
- Narra, M.R.; Rajender, K.; Reddy, R.R.; Rao, J.V.; Begum, G. The role of vitamin C as antioxidant in protection of biochemical and haematological stress induced by chlorpyrifos in freshwater fish Clarias batrachus. Chemosphere 2015, 132, 172–178. [Google Scholar] [CrossRef]
- Singh, D.P.; Kumar, A. Changes in liver glycogen contents in fresh water fishes due to cobalt toxicity. J. Exp. Zool. India 2013, 16, 519–521. [Google Scholar]
- Roy George, K.; Malini, N.A.; Rani, G.S. Biochemical changes in liver and muscle of the cichlid, Oreochromis mossambicus (Peters, 1852) exposed to sub-lethal concentration of mercuric chloride. Indian J. Fish. 2012, 59, 147–152. [Google Scholar]
- Banaee, M.; Sagvand, S.; Sureda, A.; Amini, M.; Haghi, B.N.; Sopjani, M.; Faggio, C. Evaluation of single and combined effects of mancozeb and metalaxyl on the transcriptional and biochemical response of zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. 2023, 268, 109597. [Google Scholar] [CrossRef]
- Schwaiger, J.; Fent, K.; Stecher, H.; Ferling, H.; Negele, R.D. Effects of sublethal concentrations of triphenyltinacetate on rainbow trout (Oncorhynchus mykiss). Arch. Environ. Contam. Toxicol. 1996, 30, 327–334. [Google Scholar] [CrossRef]
- Sharma, R.; Jindal, R. Assessment of cypermethrin induced hepatic toxicity in Catla catla: A multiple biomarker approach. Environ. Res. 2020, 184, 109359. [Google Scholar] [CrossRef] [PubMed]
- Vani, G.; Veeraiah, K.; Kumar, M.V.; Parveen, S.; GRao, D.P. Biochemical changes induced by Cartap hydrochloride (50% SP), carbamate insecticide in freshwater fish Cirrhinus mrigala (Hamilton, 1822). Nat. Environ. Pollut. Technol. 2020, 19, 1821–1828. [Google Scholar] [CrossRef]
- Yadavrao, W.S. Confidor and Bavistin induced effects on total glycogen content in liver and gonads of snakeheaded fish, Channa gachua. Pharm. Innov. 2017, 6, 41–43. [Google Scholar]
- Georgieva, E.; Kovacheva, E.; Yancheva, V.; Velcheva, I.; Hrischev, P.; Atanassova, P.; Tomov, S.; Stoyanova, S. Pesticides induce fatty degeneration in liver of Cyprinus carpio (Linnaeus 1758) after acute exposure. Ecol. Balk. 2023, 15, 77–82. [Google Scholar]
- Stoyanova, S.; Georgieva, E.; Velcheva, I.; Yancheva, V. Histochemical Alterations in Bighead Carp (Hypophthalmichthys nobilis Richardson, 1845) Liver Under Two Pesticides Exposure: A Comparative Study. Ecol. Balk. 2019, 11, 63–71. [Google Scholar]
- Sun, L.; Li, J.; Zuo, Z.; Chen, M.; Wang, C. Chronic exposure to paclobutrazol causes hepatic steatosis in male rockfish Sebastiscus marmoratus and the mechanism involved. Aquat. Toxicol. 2013, 126, 148–153. [Google Scholar] [CrossRef]
- Arellano, J.M.; Storch, V.; Sarasquete, C. Histological changes and copper accumulation in liver and gills of the Senegales sole, Solea senegalensis. Ecotoxicol. Environ. Saf. 1999, 44, 62–72. [Google Scholar] [CrossRef]
- Bonifacio, A.F.; Hued, A.C. Single and joint effects of chronic exposure to chlorpyrifos and glyphosate based pesticides on structural biomarkers in Cnesterodon decemmaculatus. Chemosphere 2019, 236, 124311. [Google Scholar] [CrossRef] [PubMed]
- Bleier, L.; Dröse, S. Superoxide generation by complex III: From mechanistic rationales to functional consequences. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Dröse, S.; Brandt, U. The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J. Biol. Chem. 2008, 283, 21649–21654. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Fang, N.; Zhang, C.; Hu, H.; Li, Y.; Wang, X.; Zhao, X.; Jiang, J. Histology and metabonomics reveal the toxic effects of kresoxim-methyl on adult zebrafish. Chemosphere 2022, 309, 136739. [Google Scholar] [CrossRef]
- Jia, W.; Mao, L.; Zhang, L.; Zhang, Y.; Jiang, H. Effects of two strobilurins (azoxystrobin and picoxystrobin) on embryonic development and enzyme activities in juveniles and adult fish livers of zebrafish (Danio rerio). Chemosphere 2018, 207, 573–580. [Google Scholar] [CrossRef]
- Mao, L.; Jia, W.; Zhang, L.; Zhang, Y.; Zhu, L.; Sial, M.U.; Jiang, H. Embryonic development and oxidative stress effects in the larvae and adult fish livers of zebrafish (Danio rerio) exposed to the strobilurin fungicides, kresoxim-methyl and pyraclostrobin. Sci. Total Environ. 2020, 729, 139031. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, T.; Wang, J.; Zhang, S.; Zhu, L.; Du, Z.; Wang, J. Acute and chronic toxic effects of fluoxastrobin on zebrafish (Danio rerio). Sci. Total Environ. 2018, 610, 769–775. [Google Scholar] [CrossRef]
- Crupkin, A.C.; Fulvi, A.B.; Iturburu, F.G.; Medici, S.; Mendieta, J.; Panzeri, A.M.; Menone, M.L. Evaluation of hematological parameters, oxidative stress and DNA damage in the cichlid Australoheros facetus exposed to the fungicide azoxystrobin. Ecotoxicol. Environ. Saf. 2021, 207, 111286. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, J.; Rajeshkumar, S.; Feng, Y.; Liu, Y.; Li, X.; Zhang, B. Hepatopancreas toxicity and immunotoxicity of a fungicide, pyraclostrobin, on common carp. Comp. Biochem. Physiol. Part C Toxicol. 2022, 262, 109445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, J.; Zhang, S.; Zhu, L.; Du, Z.; Wang, J. Acute and subchronic toxicity of pyraclostrobin in zebrafish (Danio rerio). Chemosphere 2017, 188, 510–516. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, C.; Wu, Z.Q.; Gong, Y.X.; Wang, G.X. Toxic effects of three strobilurins (trifloxystrobin, azoxystrobin and kresoxim-methyl) on mRNA expression and antioxidant enzymes in grass carp (Ctenopharyngodon idella) juveniles. Ecotoxicol. Environ. Saf. 2013, 98, 297–302. [Google Scholar] [CrossRef]
- Casalino, E.; Calzaretti, G.; Sblano, C.; Landriscina, C. Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology 2002, 179, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Ghelichpour, M.; Mirghaed, A.T.; Hoseinifar, S.H.; Khalili, M.; Yousefi, M.; Van Doan, H.; Perez-Jimenez, A. Expression of immune, antioxidant and stress related genes in different organs of common carp exposed to indoxacarb. Aquat. Toxicol. 2019, 208, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.S.; Michlig, M.P.; Repetti, M.R.; Cazenave, J. Single and joint toxicity of azoxystrobin and cyproconazole to Prochilodus lineatus: Bioconcentration and biochemical responses. Sci. Total Environ. 2024, 907, 167992. [Google Scholar] [CrossRef]
- Tabassum, H.; Dawood, A.Q.; Sharma, P.; Khan, J.; Raisuddin, S.; Parvez, S. Multi-organ toxicological impact of fungicide propiconazole on biochemical and histological profile of freshwater fish Channa punctata Bloch. Ecol. Indic. 2016, 63, 359–365. [Google Scholar] [CrossRef]
- Ahmad, I.; Pacheco, M.; Santos, M.A. Enzymatic and nonenzymatic antioxidants as an adaptation to phagocyte-induced damage in Anguilla anguilla L. following in situ harbor water exposure. Ecotoxicol. Environ. Saf. 2004, 57, 290–302. [Google Scholar] [CrossRef]
- Isik, I.; Celik, I. Acute effects of methyl parathion and diazinon as inducers for oxidative stress on certain biomarkers in various tissues of rainbowtrout (Oncorhynchus mykiss). Pestic. Biochem. Physiol. 2008, 92, 38–42. [Google Scholar] [CrossRef]



| CTRL | 6.56 µg/L DMX | 13.13 µg/L DMX |
|---|---|---|
| 3.50 ± 1.91 | 31.00 ± 5.03 (a****) | 47.00 ± 1.15 (ab****) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macirella, R.; Ahmed, A.I.M.; Talarico, F.; Gharbi, N.; Mezzasalma, M.; Brunelli, E. Morphological Alterations and Oxidative Stress Induction in Danio rerio Liver After Short-Term Exposure to the Strobilurin Fungicide Dimoxystrobin. Environments 2024, 11, 282. https://doi.org/10.3390/environments11120282
Macirella R, Ahmed AIM, Talarico F, Gharbi N, Mezzasalma M, Brunelli E. Morphological Alterations and Oxidative Stress Induction in Danio rerio Liver After Short-Term Exposure to the Strobilurin Fungicide Dimoxystrobin. Environments. 2024; 11(12):282. https://doi.org/10.3390/environments11120282
Chicago/Turabian StyleMacirella, Rachele, Abdalmoiz I. M. Ahmed, Federica Talarico, Naouel Gharbi, Marcello Mezzasalma, and Elvira Brunelli. 2024. "Morphological Alterations and Oxidative Stress Induction in Danio rerio Liver After Short-Term Exposure to the Strobilurin Fungicide Dimoxystrobin" Environments 11, no. 12: 282. https://doi.org/10.3390/environments11120282
APA StyleMacirella, R., Ahmed, A. I. M., Talarico, F., Gharbi, N., Mezzasalma, M., & Brunelli, E. (2024). Morphological Alterations and Oxidative Stress Induction in Danio rerio Liver After Short-Term Exposure to the Strobilurin Fungicide Dimoxystrobin. Environments, 11(12), 282. https://doi.org/10.3390/environments11120282









