Global Warming and Fish Diversity Changes in the Po River (Northern Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Water Temperature and Flow Data
2.3. Fish Species Data
2.4. Data Analysis
3. Results
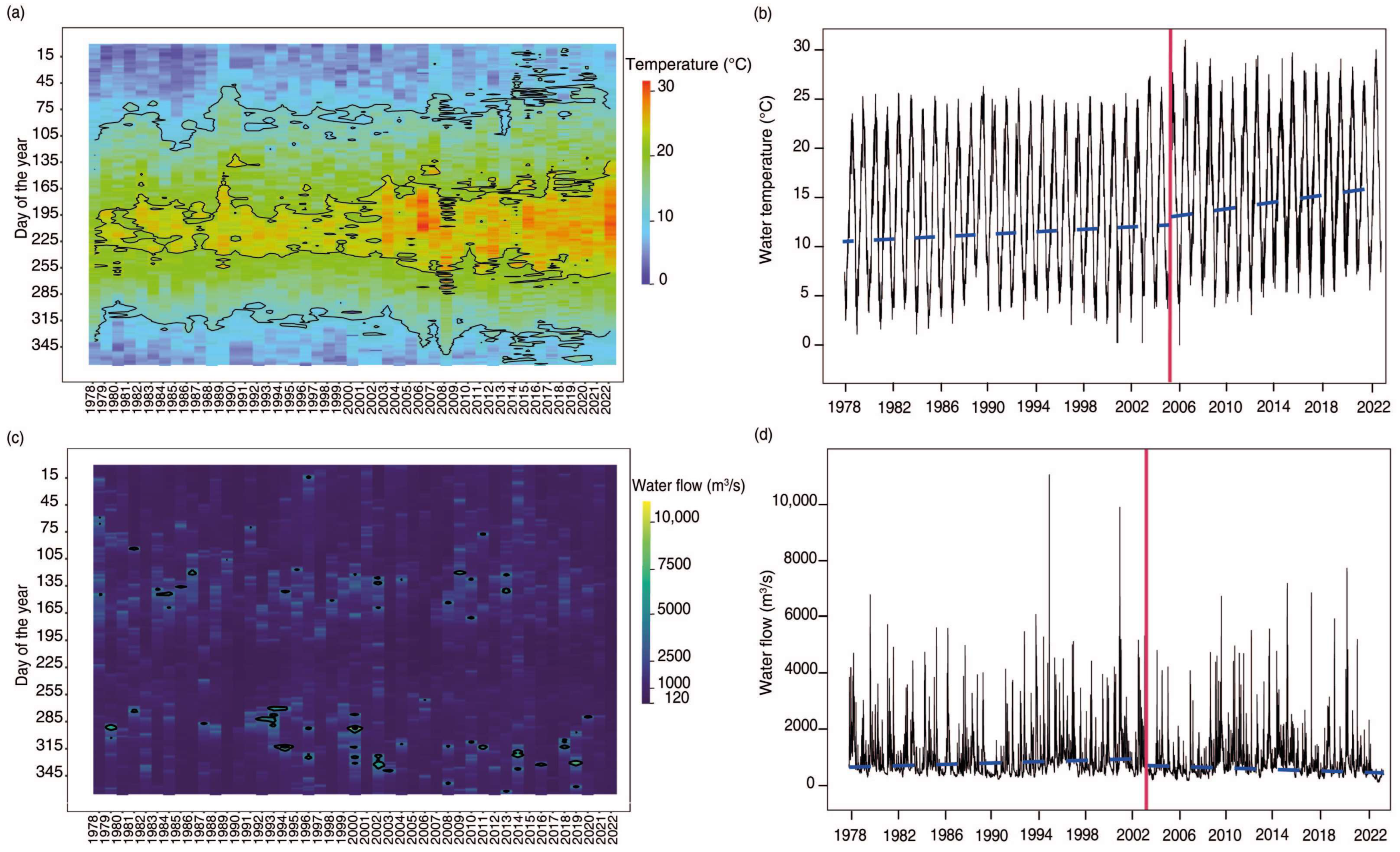
3.1. Water Temperature and Flow Trends
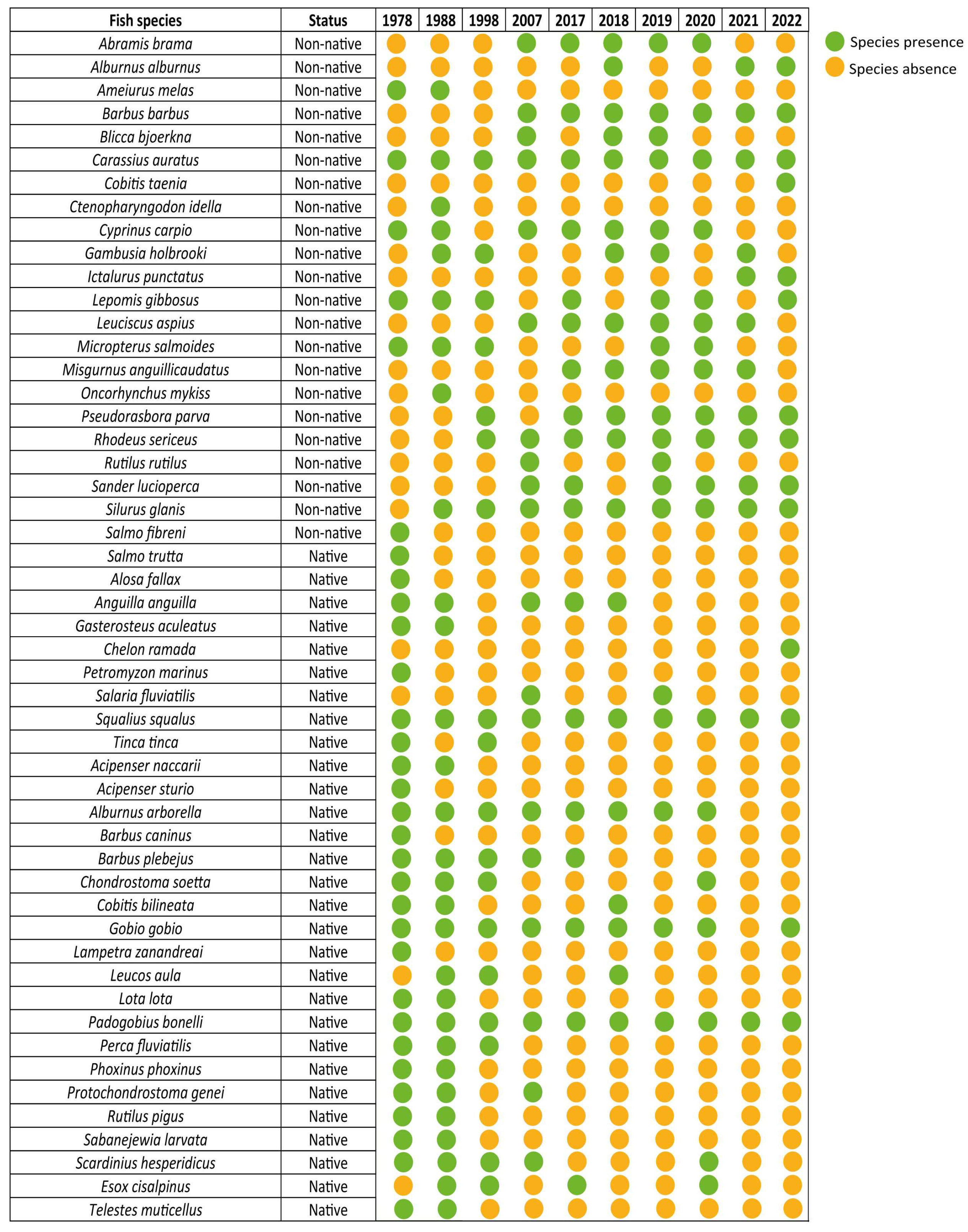
3.2. Fish Community Trends
4. Discussion
4.1. Water Temperature and Flow Trends
4.2. Fish Community Turnover
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, H.; Kemp, D.B.; Tian, L.; Chu, D.; Song, H.; Dai, X. Thresholds of Temperature Change for Mass Extinctions. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- FAO. Fao Strategy on Climate Change (2022–2031); FAO: Rome, Italy, 2022. [Google Scholar]
- Cochrane, K.; De Young, C.; Soto, D.; Bahri, T. Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientifi c Knowledge; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2009; ISBN 9789251063477. [Google Scholar]
- Duffy, K.; Gouhier, T.C.; Ganguly, A.R. Climate-Mediated Shifts in Temperature Fluctuations Promote Extinction Risk. Nat. Clim. Chang. 2022, 12, 1037–1044. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Synthesis Report. A Report of the Intergovernmental Panel on Climate Change. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Dudgeon, D. Multiple Threats Imperil Freshwater Biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R960–R967. [Google Scholar] [CrossRef] [PubMed]
- Paaijmans, K.P.; Heinig, R.L.; Seliga, R.A.; Blanford, J.I.; Blanford, S.; Murdock, C.C.; Thomas, M.B. Temperature Variation Makes Ectotherms More Sensitive to Climate Change. Glob. Chang. Biol. 2013, 19, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, R.; Everett, J.; Blanchard, J.; Sykes, P.; Richardson, A. Climate-Driven Zooplankton Shifts Could Cause Global Declines in Food Quality for Fish. Res. Sq. 2023, 470–477. [Google Scholar] [CrossRef]
- Antão, L.H.; Bates, A.E.; Blowes, S.A.; Waldock, C.; Supp, S.R.; Magurran, A.E.; Dornelas, M.; Schipper, A.M. Temperature-Related Biodiversity Change across Temperate Marine and Terrestrial Systems. Nat. Ecol. Evol. 2020, 4, 927–933. [Google Scholar] [CrossRef]
- Rodgers, E.M. Adding Climate Change to the Mix: Responses of Aquatic Ectotherms to the Combined Effects of Eutrophication and Warming. Biol. Lett. 2021, 17, 20210442. [Google Scholar] [CrossRef]
- Qian, W.; Zhu, Y. Climate Change in China from 1880 to 1998 and Its Impact on the Environmental Condition. Clim. Chang. 2001, 50, 419–444. [Google Scholar] [CrossRef]
- Daufresne, M.; Lengfellner, K.; Sommer, U. Global Warming Benefits the Small in Aquatic Ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12788–12793. [Google Scholar] [CrossRef]
- Buisson, L.; Thuiller, W.; Lek, S.; Lim, P.; Grenouillet, G. Climate Change Hastens the Turnover of Stream Fish Assemblages. Glob. Chang. Biol. 2008, 14, 2232–2248. [Google Scholar] [CrossRef]
- Moore, J.W.; Olden, J.D. Response Diversity, Nonnative Species, and Disassembly Rules Buffer Freshwater Ecosystem Processes from Anthropogenic Change. Glob. Chang. Biol. 2017, 23, 1871–1880. [Google Scholar] [CrossRef]
- Salis, R.K.; Brennan, G.L.; Hansson, L.A. Successful Invasions to Freshwater Systems Double with Climate Warming. Limnol. Oceanogr. 2023, 68, 953–962. [Google Scholar] [CrossRef]
- Kirk, M.A.; Maitland, B.M.; Hickerson, B.T.; Walters, A.W.; Rahel, F.J. Climatic Drivers and Ecological Impacts of a Rapid Range Expansion by Non-Native Smallmouth Bass. Biol. Invasions 2022, 24, 1311–1326. [Google Scholar] [CrossRef]
- Dornelas, M.; Gotelli, N.J.; McGill, B.; Shimadzu, H.; Moyes, F.; Sievers, C.; Magurran, A.E. Assemblage Time Series Reveal Biodiversity Change but Not Systematic Loss. Science (1979) 2014, 344, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Barceló, C.; Ciannelli, L.; Olsen, E.M.; Johannessen, T.; Knutsen, H. Eight Decades of Sampling Reveal a Contemporary Novel Fish Assemblage in Coastal Nursery Habitats. Glob. Chang. Biol. 2016, 22, 1155–1167. [Google Scholar] [CrossRef]
- Tuel, A.; Eltahir, E.A.B. Why Is the Mediterranean a Climate Change Hot Spot? J. Clim. 2020, 33, 5829–5843. [Google Scholar] [CrossRef]
- Straffelini, E.; Tarolli, P. Climate Change-Induced Aridity Is Affecting Agriculture in Northeast Italy. Agric. Syst. 2023, 208, 103647. [Google Scholar] [CrossRef]
- Appiotti, F.; Krželj, M.; Russo, A.; Ferretti, M.; Bastianini, M.; Marincioni, F. A Multidisciplinary Study on the Effects of Climate Change in the Northern Adriatic Sea and the Marche Region (Central Italy). Reg. Environ. Chang. 2014, 14, 2007–2024. [Google Scholar] [CrossRef]
- Fioravanti, G.; Piervitali, E.; Desiato, F. Recent Changes of Temperature Extremes over Italy: An Index-Based Analysis. Theor. Appl. Climatol. 2016, 123, 473–486. [Google Scholar] [CrossRef]
- Formetta, G.; Tootle, G.; Therrell, M. Regional Reconstruction of Po River Basin (Italy) Streamflow. Hydrology 2022, 9, 163. [Google Scholar] [CrossRef]
- Montanari, A.; Nguyen, H.; Rubinetti, S.; Ceola, S.; Galelli, S.; Rubino, A.; Zanchettin, D. Why the 2022 Po River Drought Is the Worst in the Past Two Centuries. Sci. Adv. 2023, 9, eadg8304. [Google Scholar] [CrossRef] [PubMed]
- Soana, E.; Gervasio, M.P.; Granata, T.; Colombo, D.; Castaldelli, G. Climate Change Impacts on Eutrophication in the Po River (Italy): Temperature-mediated reduction in nitrogen export but no effect on phosphorus. J. Environ. Sci. 2024, 143, 148–163. [Google Scholar] [CrossRef]
- Gervasio, M.P.; Soana, E.; Granata, T.; Colombo, D. An Unexpected Negative Feedback between Climate Change and Eutrophication: Higher Temperatures Increase Denitrification and Buffer Nitrogen Loads in the Po River (Northern Italy). Environ. Res. Lett. 2022, 17, 084031. [Google Scholar] [CrossRef]
- Fenoglio, S.; Bo, T.; Cucco, M.; Mercalli, L.; Malacarne, G. Effects of Global Climate Change on Freshwater Biota: A Review with Special Emphasis on the Italian Situation. Ital. J. Zool. 2010, 77, 374–383. [Google Scholar] [CrossRef]
- Toreti, A.; Masante, D.; Acosta Navarro, J.; Bavera, D.; Cammalleri, C.; De Felice, M.; de Jager, A.; Di Ciollo, C.; Hrast Essenfelder, A.; Maetens, W.; et al. Drought in Europe July 2022; Publications Office of the European Union: Luxembourg, 2022; Volume 22, ISBN 978-92-76-54953. [Google Scholar] [CrossRef]
- Bonaldo, D.; Bellafiore, D.; Ferrarin, C.; Ferretti, R.; Ricchi, A.; Sangelantoni, L.; Vitelletti, M.L. The Summer 2022 Drought: A Taste of Future Climate for the Po Valley (Italy)? Reg. Environ. Chang. 2023, 23, 1–6. [Google Scholar] [CrossRef]
- Colombano, D.D.; Carlson, S.M.; Hobbs, J.A.; Ruhi, A. Four Decades of Climatic Fluctuations and Fish Recruitment Stability across a Marine-Freshwater Gradient. Glob. Chang. Biol. 2022, 28, 5104–5120. [Google Scholar] [CrossRef]
- Loerke, E.; Pohle, I.; Wilkinson, M.E.; Rivington, M.; Wardell-Johnson, D.; Geris, J. Long-Term Daily Stream Temperature Record for Scotland Reveals Spatio-Temporal Patterns in Warming of Rivers in the Past and Further Warming in the Future. Sci. Total Environ. 2023, 890, 164194. [Google Scholar] [CrossRef]
- Viaroli, P.; Soana, E.; Pecora, S.; Laini, A.; Naldi, M.; Fano, E.A.; Nizzoli, D. Space and Time Variations of Watershed N and P Budgets and Their Relationships with Reactive N and P Loadings in a Heavily Impacted River Basin (Po River, Northern Italy). Sci. Total Environ. 2018, 639, 1574–1587. [Google Scholar] [CrossRef]
- Niedrist, G.H.; Hilpold, A.; Kranebitter, P. Unveiling the Rise of Non-Native Fishes in Eastern Alpine Mountain Rivers: Population Trends and Implications. J. Fish. Biol. 2023, 103, 1085–1094. [Google Scholar] [CrossRef]
- Gandolfi, G.; Le Moli, F. A Preliminary Report on Fish Distribution in the Po River. Bolletino Di Zool. 1977, 44, 149–154. [Google Scholar] [CrossRef][Green Version]
- Korhonen, J.J.; Soininen, J.; Hillebrand, H. A Quantitative Analysis of Temporal Turnover in Aquatic Species Assemblages across Ecosystems. Ecology 2010, 91, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.; Miqueleiz, I.; Darwall, W.; Sayer, C.; Dulvy, N.K.; Carpenter, K.E.; Polidoro, B.; Dewhurst-Richman, N.; Pollock, C.; Hilton-Taylor, C.; et al. Monitoring Extinction Risk and Threats of the World’s Fishes Based on the Sampled Red List Index. Rev. Fish. Biol. Fish. 2022, 32, 975–991. [Google Scholar] [CrossRef]
- Po River Water Authority. Monitoraggio Dell’ittiofauna e Redazione Della Carta Ittica Del Fiume Po; Qualità Dell’ittiofauna e Del Macrobenthos Del Fiume Po: Parma, Italy, 2008. [Google Scholar]
- Amministrazione Provinciale di Pavia. Carta Ittica Di Pavia; Amministrazione Provinciale di Pavia: Pavia, Italy, 1988. [Google Scholar]
- Carletti, M. La Fauna Ittica Dell’Emilia-Romagna Nell’ambito Del Progetto BioItaly; Dipartimento Biologia Animale Università Degli Studi di Modena e Reggio Emilia: Modena, Italy, 1999; p. 104. [Google Scholar]
- Graia srl. Environmental Monitoring Program for AIPO (Agenzia Interegionale per Il Fiume PO); Graia srl: Parma, Italy, 2022. [Google Scholar]
- Graia srl. Action D.6—Monitoring the Efficacy of Action C.7. Final Report. Rapporto Tecnico Consegnato Alla Commissione Europea Nell’ambito Del Progetto LIFE15NAT/IT/000989 “LifeTicinoBiosource”; Varese; Graia srl: Parma, Italy, 2020; p. 87. [Google Scholar]
- APAT (Agenzia per la Protezione Dell’ambiente e per i Servizi Tecnici). Protocollo Di Campionamento E Analisi Della Fauna Ittica Dei Sistemi Lotici; A. Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Università “Tor Vergata”; ICRAM: Rome, Italy, 2007; p. 31. [Google Scholar]
- IUCN, Lista Rossa IUCN dei Vertebrati Italiani; Comitato Italiano IUCN e Ministero dell’Ambiente e della Sicurezza Energetica, Roma, 2022. p. 57. Available online: https://www.iucn.it/liste-rosse-italiane.php (accessed on 1 January 2023).
- Nesbø, C.L.; Fossheim, T.; Vøllestad, L.A.; Jakobsen, K.S. Genetic Divergence and Phylogeographic Relationships among European Perch (Perca Fluviatilis) Populations Reflect Glacial Refugia and Postglacial Colonization. Mol. Ecol. 1999, 8, 1387–1404. [Google Scholar] [CrossRef] [PubMed]
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. (Eds.) Eschmeyer’s Catalog of Fishes: Genera, Species, References; California Academy of Sciences: San Francisco, CA, USA, 2018; Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 7 October 2024).
- Helsel, D.R.; Hirsch, R.M.; Ryberg, K.R.; Archfield, S.A.; Gilroy, E.J. Statistical Methods in Water Resources Techniques and Methods 4—A3. In USGS Techniques and Methods; US Geological Survey: Reston, VA, USA, 2020; Volume 458. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smitt, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models; Analytical Methods for Social Research; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Hurvich, C.M.; Tsai, C. A Corrected Akaike Information Criterion for Vector Autoregressive Model Selection. J. Time Ser. Anal. 1993, 14, 271–279. [Google Scholar] [CrossRef]
- Snipes, M.; Taylor, D.C. Model Selection and Akaike Information Criteria: An Example from Wine Ratings and Prices. Wine Econ. Policy 2014, 3, 3–9. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2023; Available online: http://www.rstudio.com/ (accessed on 1 January 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Pohlert, T. Trend: Non-Parametric Trend Tests and Change-Point Detection, R Package Version 1.1.6; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Mazerolle, M. Model Selection and Multimodel Inference Based on (Q)AIC(c) Version 2.2-2. 2019, pp. 1–212. Date. Available online: https://cran.r-project.org/web/packages/AICcmodavg/AICcmodavg.pdf (accessed on 1 January 2023).
- Liu, S.; Xie, Z.; Liu, B.; Wang, Y.; Gao, J.; Zeng, Y.; Xie, J.; Xie, Z.; Jia, B.; Qin, P.; et al. Global River Water Warming Due to Climate Change and Anthropogenic Heat Emission. Glob. Planet. Chang. 2020, 193, 103289. [Google Scholar] [CrossRef]
- Kaushal, S.S.; Likens, G.E.; Jaworski, N.A.; Pace, M.L.; Sides, A.M.; Seekell, D.; Belt, K.T.; Secor, D.H.; Wingate, R.L. Rising Stream and River Temperatures in the United States. Front. Ecol. Environ. 2010, 8, 461–466. [Google Scholar] [CrossRef]
- Soto, B. Climate-Induced Changes in River Water Temperature in North Iberian Peninsula. Theor. Appl. Climatol. 2018, 133, 101–112. [Google Scholar] [CrossRef]
- Gizińska, J.; Sojka, M. How Climate Change Affects River and Lake Water Temperature in Central-West Poland—A Case Study of the Warta River Catchment. Atmosphere 2023, 14, 330. [Google Scholar] [CrossRef]
- Webb, B.W.; Nobilis, F. Long-Term Changes in River Temperature and the Influence of Climatic and Hydrological Factors. Hydrol. Sci. J. 2007, 52, 74–85. [Google Scholar] [CrossRef]
- Niedrist, G.H. Substantial Warming of Central European Mountain Rivers under Climate Change. Reg. Environ. Chang. 2023, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Michel, A.; Brauchli, T.; Lehning, M.; Schaefli, B.; Huwald, H. Stream Temperature and Discharge Evolution in Switzerland over the Last 50 Years: Annual and Seasonal Behaviour. Hydrol. Earth Syst. Sci. 2020, 24, 115–142. [Google Scholar] [CrossRef]
- Hardenbicker, P.; Viergutz, C.; Becker, A.; Kirchesch, V.; Nilson, E.; Fischer, H. Water Temperature Increases in the River Rhine in Response to Climate Change. Reg. Environ. Chang. 2017, 17, 299–308. [Google Scholar] [CrossRef]
- Vezzoli, R.; Mercogliano, P.; Pecora, S.; Zollo, A.L.; Cacciamani, C. Hydrological Simulation of Po River (North Italy) Discharge under Climate Change Scenarios Using the RCM COSMO-CLM. Sci. Total Environ. 2015, 521–522, 346–358. [Google Scholar] [CrossRef]
- Thackeray, S.J.; Henrys, P.A.; Hemming, D.; Bell, J.R.; Botham, M.S.; Burthe, S.; Helaouet, P.; Johns, D.G.; Jones, I.D.; Leech, D.I.; et al. Phenological Sensitivity to Climate across Taxa and Trophic Levels. Nature 2016, 535, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Bonacina, L.; Fasano, F.; Mezzanotte, V.; Fornaroli, R. Effects of Water Temperature on Freshwater Macroinvertebrates: A Systematic Review. Biol. Rev. 2023, 98, 191–221. [Google Scholar] [CrossRef]
- Van Vliet, M.T.H.; Franssen, W.H.P.; Yearsley, J.R.; Ludwig, F.; Haddeland, I.; Lettenmaier, D.P.; Kabat, P. Global River Discharge and Water Temperature under Climate Change. Glob. Environ. Chang. 2013, 23, 450–464. [Google Scholar] [CrossRef]
- Arnell, N.W.; Gosling, S.N. The Impacts of Climate Change on River Flow Regimes at the Global Scale. J. Hydrol. 2013, 486, 351–364. [Google Scholar] [CrossRef]
- Rameshwaran, P.; Bell, V.A.; Davies, H.N.; Kay, A.L. How Might Climate Change Affect River Flows across West Africa? Clim. Chang. 2021, 169, 1–27. [Google Scholar] [CrossRef]
- Zanchettin, D.; Traverso, P.; Tomasino, M. Po River Discharges: A Preliminary Analysis of a 200-Year Time Series. Clim. Chang. 2008, 89, 411–433. [Google Scholar] [CrossRef]
- Marchina, C.; Natali, C.; Bianchini, G. The Po River Water Isotopes during the Drought Condition of the Year 2017. Water 2019, 11, 150. [Google Scholar] [CrossRef]
- Montanari, A. Hydrology of the Po River: Looking for Changing Patterns in River Discharge. Hydrol. Earth Syst. Sci. 2012, 16, 3739–3747. [Google Scholar] [CrossRef]
- Black, E.; Blackburn, M.; Harrison, G.; Hoskins, B.; Methven, J. Factors Contributing to the Summer 2003 European Heatwave. Weather 2004, 59, 217–223. [Google Scholar] [CrossRef]
- Bonino, G.; Masina, S.; Galimberti, G.; Moretti, M. Southern Europe and Western Asia Marine Heat Waves (SEWA-MHWs): A Dataset Based on Macro Events. Earth Syst. Sci. Data 2023, 15, 1269–1285. [Google Scholar] [CrossRef]
- Poff, N.L.; Brinson, M.M.; Day, J.W. Aquatic Ecosystems & Global Climate Change: Potential Impacts on Inland Freshwater and Coastal Wetland Ecosystems in the United States. In Pew Center for Global Change; Pew Charitable Trust: Philadelphia, PA, USA, 2002. [Google Scholar]
- Morales-Marín, L.A.; Rokaya, P.; Sanyal, P.R.; Sereda, J.; Lindenschmidt, K.E. Changes in Streamflow and Water Temperature Affect Fish Habitat in the Athabasca River Basin in the Context of Climate Change. Ecol. Modell. 2019, 407, 108718. [Google Scholar] [CrossRef]
- Poff, N.L.; Zimmerman, J.K.H. Ecological Responses to Altered Flow Regimes: A Literature Review to Inform the Science and Management of Environmental Flows. Freshw. Biol. 2009, 55, 194–205. [Google Scholar] [CrossRef]
- Warren, D.R.; Ernst, A.G.; Baldigo, B.P. Influence of Spring Floods on Year-Class Strength of Fall- and Spring-Spawning Salmonids in Catskill Mountain Streams. Trans. Am. Fish. Soc. 2009, 138, 200–210. [Google Scholar] [CrossRef]
- Tedesco, P.A.; Oberdorff, T.; Cornu, J.F.; Beauchard, O.; Brosse, S.; Dürr, H.H.; Grenouillet, G.; Leprieur, F.; Tisseuil, C.; Zaiss, R.; et al. A Scenario for Impacts of Water Availability Loss Due to Climate Change on Riverine Fish Extinction Rates. J. Appl. Ecol. 2013, 50, 1105–1115. [Google Scholar] [CrossRef]
- Gonçalves-Silva, M.; Manna, L.R.; Rodrigues-Filho, C.A.S.; Teixeira, F.K.; Rezende, C.F. Effect of Drying Dynamics on the Functional Structure of a Fish Assemblage from an Intermittent River Network. Front. Environ. Sci. 2022, 10, 903974. [Google Scholar] [CrossRef]
- Surian, N.; Rinaldi, M. Morphological Response to River Engineering and Management in Alluvial Channels in Italy. Geomorphology 2003, 50, 307–326. [Google Scholar] [CrossRef]
- Bolpagni, R.; Laini, A.; Mutti, T.; Viaroli, P.; Bartoli, M. Connectivity and Habitat Typology Drive CO2 and CH4 Fluxes across Land–Water Interfaces in Lowland Rivers. Ecohydrology 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Gavioli, A.; Milardi, M.; Soininen, J.; Soana, E.; Lanzoni, M.; Castaldelli, G. How Does Invasion Degree Shape Alpha and Beta Diversity of Freshwater Fish at a Regional Scale? Ecol. Evol. 2022, 12, e9493. [Google Scholar] [CrossRef] [PubMed]
- Castaldelli, G.; Pluchinotta, A.; Milardi, M.; Lanzoni, M.; Giari, L.; Rossi, R.; Fano, E.A. Introduction of Exotic Fish Species and Decline of Native Species in the Lower Po Basin, North-Eastern Italy. Aquat. Conserv. 2013, 23, 405–417. [Google Scholar] [CrossRef]
- Rossi, R.; Trisolini, R.; Rizzo, M.G.; Dezfuli, B.S.; Franzoi, P.; Grandi, G. Biologia Ed Ecologia Di Una Specie Alloctona, Il Siluro (Silurus glanis L.) (Osteichthyes, Siluridae), Nella Parte Terminale Del Fiume Po. Atti Della Soc. Ital. Di Sci. Nat. E Del Mus. Civ. Di Stor. Nat. Di Milano 1991, 132, 69–87. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. Available online: www.fishbase.org (accessed on 1 January 2023).
- Joseph, L.N.; Field, S.A.; Wilcox, C.; Possingham, H.P. Presence-Absence versus Abundance Data for Monitoring Threatened Species. Conserv. Biol. 2006, 20, 1679–1687. [Google Scholar] [CrossRef]
- Bianco, P.G. Mediterranean Endemic Freshwater Fishes of Italy. Biol. Conserv. 1995, 72, 159–170. [Google Scholar] [CrossRef]
- Cavallari, A.; Resta, C.; Sandias, M.; Melotti, P.; Zaccanti, F.; Roncarati, A.; Ferrari, G.; Bigazzi, M.; Tongiorgi, P.; Sala, L.; et al. Elementi Di Base per La Predisposizione Della Carta Ittica Regionale; Emilia-Romagna, R., Ed.; GreenTime: Bologna, Italy, 1992; Volume 1. [Google Scholar]
- Bronzi, P.; Castaldelli, G.; Cataudella, S.; Rossi, R. The Historical and Contemporary Status of the European Sturgeon, Acipenser sturio L., in Italy. In Biology and Conservation of the European Sturgeon Acipenser Sturio L. 1758; Springer: Berlin, Heidelberg, 2011; pp. 227–241. ISBN 9783642206115. [Google Scholar] [CrossRef]
- Bronzi, P.; Vecsei, P.; Arlati, G. Threatened Fishes of the World: Acipenser Naccarii Bonaparte, 1836 (Acipenseridae). Environ. Biol. Fishes 2005, 72, 66. [Google Scholar] [CrossRef]
- Milardi, M.; Aschonitis, V.; Gavioli, A.; Lanzoni, M.; Fano, E.A.; Castaldelli, G. Run to the Hills: Exotic Fish Invasions and Water Quality Degradation Drive Native Fish to Higher Altitudes. Sci. Total Environ. 2018, 624, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Milardi, M.; Gavioli, A.; Soana, E.; Lanzoni, M.; Fano, E.A.; Castaldelli, G. The Role of Species Introduction in Modifying the Functional Diversity of Native Communities. Sci. Total Environ. 2020, 699, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Daufresne, M.; Veslot, J.; Capra, H.; Carrel, G.; Poirel, A.; Olivier, J.M.; Lamouroux, N. Fish Community Dynamics (1985-2010) in Multiple Reaches of a Large River Subjected to Flow Restoration and Other Environmental Changes. Freshw. Biol. 2015, 60, 1176–1191. [Google Scholar] [CrossRef]
- Bianco, P.G. Freshwater Fish Transfers in Italy: History, Local Changes in Fish Fauna and a Prediction on the Future of Native Populations. In Stocking and Introductions Fishes; Cowx, I.G., Ed.; Fishing News Books; Blackwell Science: Oxford, UK, 1998; Volume 456, pp. 165–197. [Google Scholar]
- Stefani, F.; Schiavon, A.; Tirozzi, P.; Gomarasca, S.; Marziali, L. Functional Response of Fish Communities in a Multistressed Freshwater World. Sci. Total Environ. 2020, 740, 139902. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D.B.; Tobler, M.; Winemiller, K.O. From Richer to Poorer: Successful Invasion by Freshwater Fishes Depends on Species Richness of Donor and Recipient Basins. Glob. Chang. Biol. 2016, 22, 2440–2450. [Google Scholar] [CrossRef]
- Makki, T.; Mostafavi, H.; Matkan, A.A.; Valavi, R.; Hughes, R.M.; Shadloo, S.; Aghighi, H.; Abdoli, A.; Teimori, A.; Eagderi, S.; et al. Predicting climate heating impacts on riverine fish species diversity in a biodiversity hotspot region. Sci. Rep. 2023, 13, 14347. [Google Scholar] [CrossRef]
- Manjarrés-Hernández, A.; Guisande, C.; García-Roselló, E.; Heine, J.; Pelayo-Villamil, P.; Pérez-Costas, E.; González-Vilas, L.; González-Dacosta, J.; Duque, S.R.; Granado-Lorencio, C.; et al. Predicting the effects of climate change on future freshwater fish diversity at global scale. Nat. Conserv. 2021, 43, 1–24. [Google Scholar] [CrossRef]


| Abbreviation | Variable | Unit | Min | Max | Mean | d.s. |
|---|---|---|---|---|---|---|
| meanTemp | Annual mean water temperature | °C | 12.73 | 17.61 | 14.89 | 1.41 |
| minTemp | Annual minimum water temperature | °C | 1.00 | 7.49 | 3.68 | 1.68 |
| maxTemp | Annual maximum water temperature | °C | 23.50 | 31.00 | 26.49 | 1.92 |
| Summer_meanTemp | Summer mean water temperature | °C | 20.60 | 25.93 | 22.57 | 1.45 |
| Winter_meanTemp | Winter mean water temperature from | °C | 4.18 | 11.51 | 7.98 | 1.68 |
| Summer_days | Number of days during the summer season with water temperatures exceeding the seasonal mean | Number of days | 6.00 | 82.00 | 48.82 | 20.02 |
| Winter_days | Number of days during the summer season with water temperatures exceeding the seasonal mean | Number of days | 0 | 79 | 39.42 | 19.97 |
| Meanflow | Annual mean water flow | m3 s−1 | 315 | 1435 | 913 | 255 |
| Minflow | Annual minimum water flow | m3 s−1 | 124 | 564 | 330 | 90 |
| Maxflow | Annual maximum water flow | m3 s−1 | 595 | 11,068 | 4786 | 2016 |
| Summer_meanflow | Summer mean water flow | m3 s−1 | 203 | 1119 | 675 | 218 |
| maxTemp | Annual maximum water temperature from 1978 to 2022 | °C | 23.50 | 31.00 | 26.49 | 1.92 |
| Summer_meanTemp | Summer mean water temperature from 1978 to 2022 | °C | 20.60 | 25.93 | 22.57 | 1.45 |
| Winter_meanTemp | Winter mean water temperature from 1978 to 2022 | °C | 4.18 | 11.51 | 7.98 | 1.68 |
| (a) Mod. | Explanatory Variable | Component Model | K | AIC | AICc | ΔAICc | AICcWt | CumWt | LL |
| M4 | Total richness | summer_days | 4 | 14.06 | 22.06 | 0 | 0.94 | 0.94 | −3.03 |
| M3 | Total richness | summer_days + summer_meanflow | 5 | 14.48 | 29.48 | 7.42 | 0.02 | 0.96 | −2.24 |
| M5 | Total richness | summer_days + meanTemp | 5 | 14.76 | 29.76 | 7.7 | 0.02 | 0.98 | −2.38 |
| M6 | Total richness | summer_days + winter_days | 5 | 14.79 | 29.79 | 7.73 | 0.02 | 1 | −2.4 |
| M2 | Total richness | summer_days + winter_days+ summer_days_year + summer_meanflow | 7 | 14.36 | 70.36 | 48.3 | 0 | 1 | −0.18 |
| M1 | Total richness | meanTemp + summer_days+ winter_days + summer_days_year+ meanFlow | 8 | 12.17 | 156.17 | 134.11 | 0 | 1 | 1.91 |
| (b) | Explanatory Variable | Component Model | K | AIC | AICc | ΔAICc | AICcWt | CumWt | LL |
| N4 | Native richness | summer_days + NNS | 5 | 9.85 | 24.85 | 0 | 0.99 | 0.99 | 0.07 |
| N3 | Native richness | summer_days + summer_meanflow + NNS | 6 | 7.42 | 35.42 | 10.56 | 0.01 | 1 | 2.29 |
| N5 | Native richness | summer_days + meanTemp + NNS | 6 | 8.13 | 36.13 | 11.28 | 0 | 1 | 1.93 |
| N6 | Native richness | summer_days + winter_days + NNS | 6 | 11.04 | 39.04 | 14.18 | 0 | 1 | 0.48 |
| N1 | Native richness | summer_days + winter_days+ summer_days_year + summer_meanflow + NNS | 8 | 2.73 | 146.73 | 121.88 | 0 | 1 | 6.63 |
| N2 | Native richness | meanTemp + summer_days+ winter_days + summer_days_year+ meanFlow + NNS | 8 | 5.09 | 149.09 | 124.24 | 0 | 1 | 5.45 |
| (c) | Explanatory Variable | Component Model | K | AIC | AICc | ΔAICc | AICcWt | CumWt | LL |
| E4 | Non-native richness | summer_days + Nat | 5 | 2.71 | 17.71 | 0 | 0.74 | 0.74 | 3.65 |
| E5 | Non-native richness | summer_days + meanTemp + Nat | 6 | −8.13 | 19.87 | 2.16 | 0.25 | 0.99 | 10.07 |
| E3 | Non-native richness | summer_days + summer_meanflow + Nat | 6 | 0.35 | 28.35 | 10.65 | 0 | 1 | 5.82 |
| E6 | Non-native richness | summer_days + winter_days + Nat | 6 | 1.95 | 29.95 | 12.24 | 0 | 1 | 5.03 |
| E2 | Non-native richness | meanTemp + summer_days+ winter_days + summer_days_year+ meanFlow + Nat | 8 | −6.09 | 137.91 | 120.2 | 0 | 1 | 11.04 |
| E1 | Non-native richness | summer_days + winter_days+ summer_days_year + summer_meanflow + Nat | 8 | −2.82 | 141.18 | 123.48 | 0 | 1 | 9.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavioli, A.; Castaldelli, G.; Trasforini, S.; Puzzi, C.; Gervasio, M.P.; Granata, T.; Colombo, D.; Soana, E. Global Warming and Fish Diversity Changes in the Po River (Northern Italy). Environments 2024, 11, 226. https://doi.org/10.3390/environments11100226
Gavioli A, Castaldelli G, Trasforini S, Puzzi C, Gervasio MP, Granata T, Colombo D, Soana E. Global Warming and Fish Diversity Changes in the Po River (Northern Italy). Environments. 2024; 11(10):226. https://doi.org/10.3390/environments11100226
Chicago/Turabian StyleGavioli, Anna, Giuseppe Castaldelli, Stefania Trasforini, Cesare Puzzi, Maria Pia Gervasio, Tommaso Granata, Daniela Colombo, and Elisa Soana. 2024. "Global Warming and Fish Diversity Changes in the Po River (Northern Italy)" Environments 11, no. 10: 226. https://doi.org/10.3390/environments11100226
APA StyleGavioli, A., Castaldelli, G., Trasforini, S., Puzzi, C., Gervasio, M. P., Granata, T., Colombo, D., & Soana, E. (2024). Global Warming and Fish Diversity Changes in the Po River (Northern Italy). Environments, 11(10), 226. https://doi.org/10.3390/environments11100226









