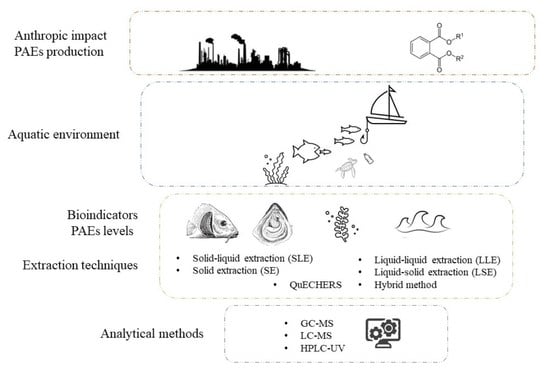
Environmental Aspect Concerning Phthalates Contamination: Analytical Approaches and Assessment of Biomonitoring in the Aquatic Environment
Abstract
1. Introduction
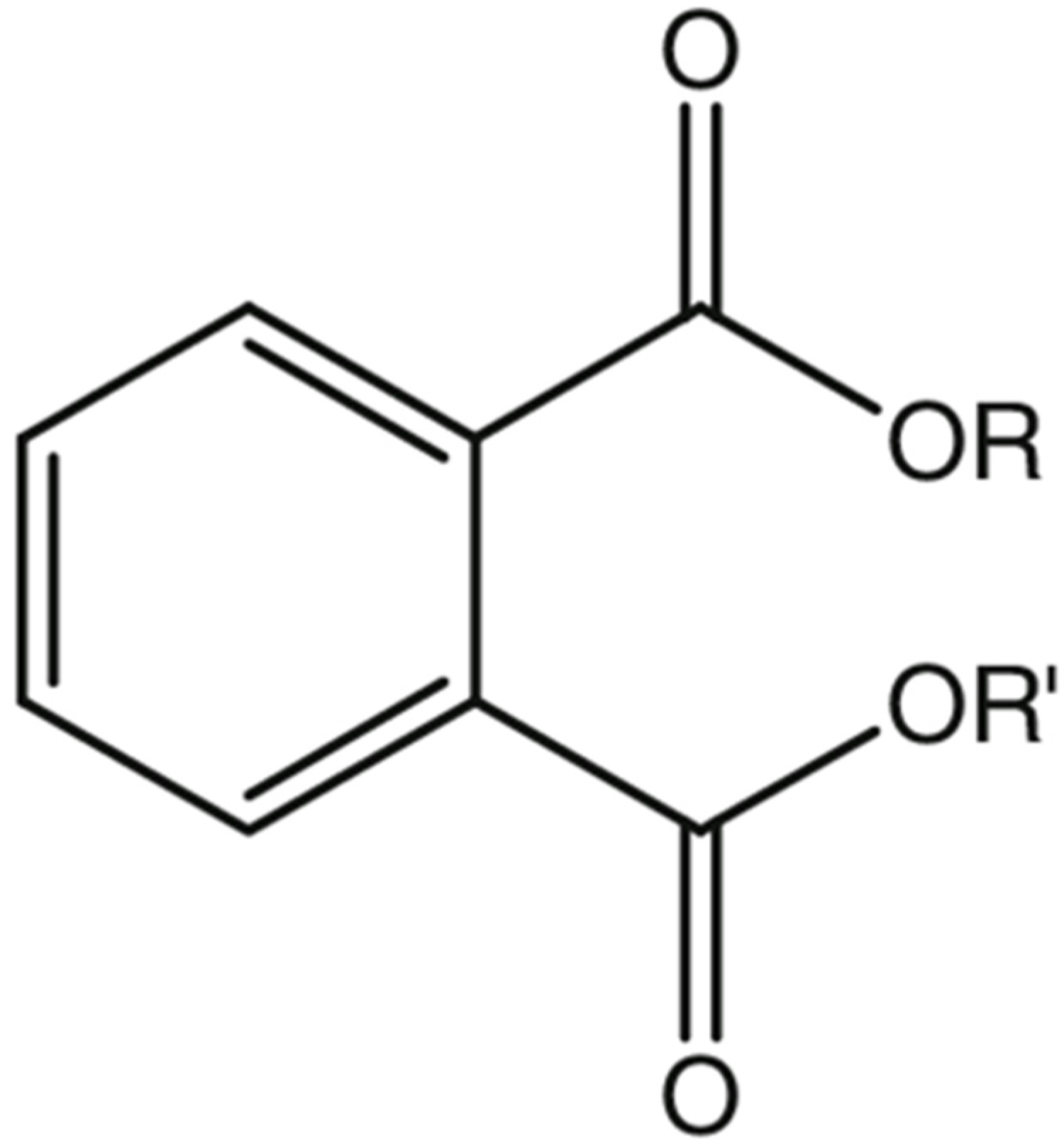
2. Physical, Chemical, and Environmental Properties of Phthalates
| PAE Congeners | Acronym | Molecular Formula | CAS | R1 | R2 | Log Kow |
|---|---|---|---|---|---|---|
| dimethyl phthalate | DMP | C10H10O4 | 131-11-3 | CH3 | CH3 | 1.60 |
| diethyl phthalate | DEP | C12H14O4 | 84-66-2 | CH2CH3 | CH2CH3 | 2.47 |
| diisobutyl phthalate | DiBP | C16H22O4 | 84-69-5 | CH2CH(CH3)2 | CH2CH(CH3)2 | 4.11 |
| dibutyl phthalate | DnBP | C16H22O4 | 84-74-2 | CH2CH2CH2CH3 | CH2CH2CH2CH3 | 4.50 |
| dimethylglycol phthalate | DMEP | C14H18O6 | 117-82-8 | CH2CH2OCH3 | CH2CH2OCH3 | 1.11 * |
| benzyl butyl phthalate | BBzP | C19H20O4 | 85-68-7 | CH2C6H5 | CH2C6H5 | 4.73 |
| dicyclohexyl phthalate | DCHP | C20H26O4 | 84-61-7 | CH(CH2)5 | CH(CH2)5 | 5.6 |
| di-n-pentyl phthalate | DnPP | C18H26O4 | 131-18-0 | CH2(CH2)3CH3 | CH2(CH2)3CH3 | 5.62 |
| bis (2-n-butoxyethyl) phthalate | DBEP | C20H30O6 | 117-83-9 | CH2CH2O(CH2)3CH3 | CH2CH2O(CH2)3CH3 | 4.06 * |
| diphenyl phthalate | DPhP | C24H38O4 | 84-62-8 | C6H5 | C6H5 | n.a. |
| di(2-ethylhexyl) phthalate | DEHP | C20H14O4 | 117-81-7 | CH(CH2)5(CH3)2 | CH(CH2)5(CH3)2 | 7.60 |
| di-n-octyl phthalate | DnOP | C24H38O4 | 117-84-0 | (CH2)7CH3 | (CH2)7CH3 | 8.10 |
| diisononyl phthalate | DiNP | C26H42O4 | 28553-12-0 | C9H19 | C9H19 | 8.8 |
| dinonyl phthalate | DnNP | C26H42O4 | 84-76-4 | C9H19 | C9H19 | 9.52 * |
3. Extraction and Analytical Methods
3.1. Extraction Techniques
3.2. Analytical Method
4. Bioindicators and Levels of PAEs in the Aquatic Environment
| Organisms | Species | Location in Wild | Samples | DMP | DEP | DiBP | DnBP | DMEP | DnPP | BBzP | DBEP | DCHP | DPHP | DEHP | DnOP | DiNP | DnNP | Sum of PAEs (unit) | Ref. Year |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actinopterygii | Mullus barbatus | Tyrrhenian Sea | gills | 649.0 | 245.0 | 305.0 | 284.0 | 1061.0 | 1491.0 | 2544.0 (ng/g d.w.) | [80] | ||||||||
| Actinopterygii | Mullus barbatus | Tyrrhenian Sea | muscles | 191.0 | 97.0 | 101.0 | 776.0 | 103.0 | 144.0 | 1268.0 (ng/g d.w.) | [80] | ||||||||
| Actinopterygii | Acanthopagrus Schlegelii | Xiangshan Bay (East China Sea) | muscles | 61.1 | 9.5 | 397.8 | 364.3 | 11.9 | 35.3 | 16.3 | 6.6 | 253.0 | 4.4 | 1160.0 (ng/g d.w.) | [81] | ||||
| Actinopterygii | Arius maculatus | Yangtze River Delta area (East China Sea) | muscles | 0.9 | 4.5 | n.d. | 643.0 | n.d. | 648.4 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Boleophthalmus pectinirostris | Yangtze River Delta area (East China Sea) | muscles | 1.4 | 0.1 | 21.8 | 133.0 | n.d. | 156.4 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Centropristis striata | Xiangshan Bay (East China Sea) | muscles | 11.3 | 7.2 | 1938.0 | 659.0 | 8.6 | 30.5 | 4.2 | 94.9 | 1.8 | 2168.0 | 3.2 | 4926.7 (ng/g d.w.) | [81] | |||
| Actinopterygii | Chelidonichthys spinosus | Yangtze River Delta area (East China Sea) | muscles | 0.8 | 3.7 | n.d. | 46.1 | n.d. | 50.7 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Clupea pallasii | Yangtze River Delta area (East China Sea) | muscles | n.d. | 1.8 | 2.4 | 119.0 | n.d. | 123.2 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Diplodus annularis | Tyrrhenian Sea | gills | 441.0 | 144.0 | 267.0 | 666.0 | 175.0 | 229.0 | 1693.0 (ng/g d.w.) | [80] | ||||||||
| Actinopterygii | Diplodus annularis | Tyrrhenian Sea | muscles | 231.0 | 96.0 | 108.0 | 187.0 | 186.0 | 200.0 | 808.0 (ng/g d.w.) | [80] | ||||||||
| Actinopterygii | Diplodus vulgaris Oblada melanura Serranus cabrilla Serranus scriba | Cabrera MPA (Balearic Sea) | muscles | 170.0 | 720.0 | 880.0 | 1770.0 (ng/g w.w.) | [69] | |||||||||||
| Actinopterygii | Ditrema temmincki Bleeker | Xiangshan Bay (East China Sea) | muscles | 12.3 | 4.4 | 530.0 | 199.0 | 45.8 | 732.0 | 1523.5 (ng/g d.w.) | [81] | ||||||||
| Actinopterygii | Epinephelus akaara | Xiangshan Bay (East China Sea) | muscles | 7.3 | 7.3 | 854.5 | 351.0 | 9.1 | 11.3 | 42.2 | 1.3 | 337.0 | 1620.9 (ng/g d.w.) | [81] | |||||
| Actinopterygii | Epinephelus goreensis | Xiangshan Bay (East China Sea) | muscles | 18.8 | 12.9 | 541.0 | 369.5 | 7.6 | 246.0 | 1.4 | 1197.2 (ng/g d.w.) | [81] | |||||||
| Actinopterygii | Eucyclogobiusnewberryi | Yangtze River Delta area (East China Sea) | muscles | 1.1 | n.d. | n.d. | 3.9 | n.d. | 5.0 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Gadus morhua | local fishmonger (Tarragona, Spain) | muscles | 11.4 | n.d. | n.d. | 12.7 (ng/g w.w.) | [83] | |||||||||||
| Actinopterygii | Jordanella floridae | Xiangshan Bay (East China Sea) | muscles | 19.6 | 9.9 | 1661.0 | 323.0 | 74.2 | 347.0 | 6.3 | 2440.9 (ng/g d.w.) | [81] | |||||||
| Actinopterygii | Konosirus Punctatus | Xiangshan Bay (East China Sea) | muscles | 145.0 | 13.0 | 35.7 | 672.0 | 9.6 | 53.0 | 201.0 | 51.8 | 1181.1 (ng/g d.w.) | [81] | ||||||
| Actinopterygii | Larimichthys crocea | Xiangshan Bay (East China Sea) | muscles | 159.3 | 10.4 | 665.6 | 1025.5 | 9.2 | 43.1 | 2.4 | 540.6 | 2.2 | 2458.2 (ng/g d.w.) | [81] | |||||
| Actinopterygii | Larimichthys polyactis | Yangtze River Delta area (East China Sea) | muscles | 0.5 | 0.4 | n.d. | 68.8 | n.d. | 69.7 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Lateolabrax Japonicus | Xiangshan Bay (East China Sea) | muscles | 25.0 | 17.4 | 493.7 | 262.7 | 15.0 | 17.2 | 2.7 | 685.7 | 2.3 | 8.2 | 1529.7 (ng/g d.w.) | [81] | ||||
| Actinopterygii | Lophius litulon | Yangtze River Delta area (East China Sea) | muscles | 0.6 | 1.3 | 4.9 | 161.2 | n.d. | 168.1 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Merluccius merluccius | local fishmonger (Tarragona, Spain) | muscles | 9.0 | 1.3 | n.d. | 10.3 (ng/g w.w.) | [83] | |||||||||||
| Actinopterygii | Mugil cephalus | Xiangshan Bay (East China Sea) | muscles | 119.4 | 10.0 | 698.5 | 858.5 | 13.6 | 16.4 | 27.8 | 276.0 | 3.1 | 2023.4 (ng/g d.w.) | [81] | |||||
| Actinopterygii | Mugil cephalus | Yangtze River Delta area (East China Sea) | muscles | 0.8 | 3.6 | 3.2 | 587.6 | n.d. | 595.2 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Mugil cephalus | Tyrrhenian Sea | gills | 298.0 | 212.0 | 407.0 | 647.0 | 157.0 | 134.0 | 1721.0 (ng/g d.w.) | [80] | ||||||||
| Actinopterygii | Mugil cephalus | Tyrrhenian Sea | muscles | 182.0 | 86.0 | 132.0 | 316.0 | 59.0 | 116.0 | 775.0 (ng/g d.w.) | [80] | ||||||||
| Actinopterygii | Muraenesox cinereus | Yangtze River Delta area (East China Sea) | muscles | 0.1 | 0.3 | n.d. | n.d. | n.d. | 0.4 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Nibea Albiflora | Xiangshan Bay (East China Sea) | muscles | 79.6 | 23.5 | 556.1 | 643.4 | 15.2 | 26.1 | 34.1 | 1.1 | 219.3 | 3.3 | 1601.6 (ng/g d.w.) | [81] | ||||
| Actinopterygii | Pagrus Major | Xiangshan Bay (East China Sea) | muscles | 74.0 | 11.4 | 288.5 | 655.5 | 23.1 | 13.9 | 1270.5 | 2336.8 (ng/g d.w.) | [81] | |||||||
| Actinopterygii | Pampusargenteus | Yangtze River Delta area (East China Sea) | muscles | 0.9 | 3.0 | n.d. | 1941.0 | 1.5 | 1946.4 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Platycephalus indicus | Yangtze River Delta area (East China Sea) | muscles | 0.3 | 5.9 | 10.5 | 250.0 | n.d. | 266.7 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Salmo salar | local fishmonger (Tarragona, Spain) | muscles | 61.0 | n.d. | n.d. | 61.0 (ng/g w.w.) | [83] | |||||||||||
| Actinopterygii | Sarda orientalis | Yangtze River Delta area (East China Sea) | muscles | 0.9 | 3.3 | 43.6 | 281.1 | n.d. | 328.8 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Sardina pilchardus | local fishmonger (Tarragona, Spain) | muscles | 102.1 | n.d. | n.d. | 323.8 (ng/g w.w.) | [83] | |||||||||||
| Actinopterygii | Sciaemops ocellatus | Xiangshan Bay (East China Sea) | muscles | 32.5 | 3.8 | 1822.0 | 131.0 | 11.0 | 9.1 | 23.8 | 52.6 | 2069.0 | 4154.7 (ng/g d.w.) | [81] | |||||
| Actinopterygii | Scomber japonicus | Yangtze River Delta area (East China Sea) | muscles | 1.0 | 13.6 | 2.5 | 108.5 | n.d. | 125.6 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Scomber vincialis | local fishmonger (Tarragona. Spain) | muscles | 2.9 | n.d. | n.d. | 2.9 (ng/g w.w.) | [83] | |||||||||||
| Actinopterygii | Scomberomorus niphonius | Yangtze River Delta area (East China Sea) | muscles | 1.3 | 0.1 | n.d. | 51.6 | n.d. | 53.0 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Solea solea | local fishmonger (Tarragona, Spain) | muscles | 15.0 | 1.0 | 8.3 | 27.5 (ng/g w.w.) | [83] | |||||||||||
| Actinopterygii | Sphyraenus | Xiangshan Bay (East China Sea) | muscles | 10.9 | 36.9 | 1076.0 | 128.0 | 60.3 | 3.9 | 392.0 | 26.5 | 1734.5 (ng/g d.w.) | [81] | ||||||
| Actinopterygii | Thunnus thynnus | Yangtze River Delta area (East China Sea) | muscles | 0.1 | 2.5 | 2.6 | 7.4 | n.d. | 12.6 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Thunnus thynnus | local fishmonger (Tarragona, Spain) | muscles | 19.4 | n.d. | n.d. | 19.4 (ng/g w.w.) | [83] | |||||||||||
| Actinopterygii | Trachinotus ovatus | Xiangshan Bay (East China Sea) | muscles | 13.9 | 7.4 | 1791.0 | 102.0 | 28.6 | 1.2 | 1148.0 | 10.0 | 3102.1 (ng/g d.w.) | [81] | ||||||
| Actinopterygii | Trachurus japonicus | Yangtze River Delta area (East China Sea) | muscles | 0.7 | 2.5 | n.d. | 137.7 | n.d. | 140.8 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Trichiurus lepturus | Yangtze River Delta area (East China Sea) | muscles | 0.01 | 1.3 | n.d. | 126.0 | n.d. | 127.3 (ng/g w.w.) | [82] | |||||||||
| Actinopterygii | Zeus faber | Yangtze River Delta area (East China Sea) | muscles | 0.5 | 4.2 | n.d. | n.d. | 28.1 | 32.9 (ng/g w.w.) | [82] | |||||||||
| Ascidiacea | Herdmania momus | Mikhmoret beach (Mediterranean Sea) | whole body | 5064.0 | 9095.0 | 14,159.0 (ng/g d.w.) | [84] | ||||||||||||
| Ascidiacea | Herdmania momus | Eilat marina (Red Sea) | whole body | 3757.0 | 5556.0 | 9313.0 (ng/g d.w.) | [84] | ||||||||||||
| Ascidiacea | Microcosmus exasperatus | Palmahim national park (Mediterranean Sea) | whole body | 1643.0 | 4988.0 | 6631.0 (ng/g d.w.) | [84] | ||||||||||||
| Ascidiacea | Microcosmus exasperatus | Bat-Yam beach (Mediterranean Sea) | whole body | 2224.0 | 4851.0 | 7075.0 (ng/g d.w.) | [84] | ||||||||||||
| Bivalvia | Crassostrea virginica | Florida coast (United States) | soft tissues | 1.3 | 1.9 | 3.0 | 3.2 | 70.4 | 0.2 | 79.8 (ng/g w.w.) | [85] | ||||||||
| Bivalvia | Mussels | Estuary of Bilbao (Spain) | soft tissues | 132.1 | 391.1 | 1673.8 | 592.2 | 8355.6 | 37.3 | 11,182.1 (ng/g d.w.) | [86] | ||||||||
| Bivalvia | Mytilus galloprovincialis | local fishmonger (Tarragona, Spain) | soft tissues | 67.3 | 6.6 | n.d. | 73.9 (ng/g w.w.) | [83] | |||||||||||
| Bivalvia | Ruditapes philippinarum | Yangtze River Delta area (East China Sea) | soft tissues | 0.7 | 1.0 | 3.8 | 270.5 | 0.8 | 276.8 (ng/g w.w.) | [82] | |||||||||
| Bivalvia | Sinonovacula constrzcta | Yangtze River Delta area (East China Sea) | soft tissues | 0.9 | 0.02 | 1.5 | 99.2 | n.d. | 101.7 (ng/g w.w.) | [82] | |||||||||
| Bivalvia | Arca noae | Cabrera MPA (Balearic Sea) | soft tissues | 540.0 | 780.0 | 2580.0 | 3900.0 (ng/g w.w.) | [69] | |||||||||||
| Cephalopoda | Loligo vulgaris | local fishmonger (Tarragona, Spain) | soft tissues | n.d. | n.d. | 13.8 | 14.8 (ng/g w.w.) | [83] | |||||||||||
| Crustacea | Aristeus antennatus | local fishmonger (Tarragona, Spain) | soft tissues | 36.9 | n.d. | 10.9 | 49.4 (ng/g w.w.) | [83] | |||||||||||
| Crustacea | Penaeus chinensis | Yangtze River Delta area (East China Sea) | soft tissues | 0.4 | 1.7 | 11.2 | 93.0 | n.d. | 106.2 (ng/g w.w.) | [82] | |||||||||
| Crustacea | Solenocera crassicornis | Yangtze River Delta area (East China Sea) | soft tissues | 0.8 | 0.8 | 5.3 | 82.8 | 0.1 | 89.8 (ng/g w.w.) | [82] | |||||||||
| Crustacea | Talitrus saltator Parhyale plumicornis Parhyale aquilina, Speziorchestia stephenseni, Orchestia montagui | Stagnone di Marsala—Sicily (Mediterranean Sea) | whole body | 108.0 | 97.0 | 23.0 | 46.0 | 292.0 (ng/g w.w.) | [87] | ||||||||||
| Gastropoda | Bullacta exarata | Yangtze River Delta area (East China Sea) | soft tissues | 0.7 | n.d. | 9.6 | 179.0 | n.d. | 189.2 (ng/g w.w.) | [82] | |||||||||
| Holothuroidea | Holothuria forskali, Holothuria poli, Holothuria tubulosa | Cabrera MPA (Balearic Sea) | muscles | 490.0 | 1240.0 | 1480.0 | 3210.0 (ng/g w.w.) | [69] | |||||||||||
| Mammalia | Balaenoptera physalus | Iceland (North Atlantic Ocean) | muscles | 8.0 | 303.0 | 303.0 | 10.0 | 624.0 (ng/g d.w.) * | [88] | ||||||||||
| Mammalia | Globicephala macrorhynchus | Macaronesian Region (Eastern North Atlantic) | muscles | 969.0 | 335.1 | 1304.1 (ng/g w.w.) | [89] | ||||||||||||
| Mammalia | Grampus griseus | Macaronesian Region (Eastern North Atlantic) | muscles | 84.7 | 557.8 | 380.8 | 1023.3 (ng/g w.w.) | [89] | |||||||||||
| Mammalia | Kogia breviceps | Macaronesian Region (Eastern North Atlantic) | muscles | 664.0 | 102.0 | 766.0 (ng/g w.w.) | [89] | ||||||||||||
| Mammalia | Kogia spp. | Atlantic coast of North Carolina and Florida | blubber | 200.0 | 200.0 (ng/g d.w.) * | [90] | |||||||||||||
| Mammalia | Lagenodelphis hosei | Macaronesian Region (Eastern North Atlantic) | muscles | 97.7 | 552.0 | 329.7 | 979.3 (ng/g w.w.) | [89] | |||||||||||
| Mammalia | Lagenorhynchus albirostris | Atlantic coast of North Carolina and Florida | blubber | 13,800.0 | 13,800.0 (ng/g d.w.) * | [90] | |||||||||||||
| Mammalia | Peponocephala electra | Atlantic coast of North Carolina and Florida | blubber | 500.0 | 500 (ng/g d.w.) * | [90] | |||||||||||||
| Mammalia | Stenella spp. | Atlantic coast of North Carolina and Florida | blubber | 70.0 | 70.0 (ng/g d.w.) * | [90] | |||||||||||||
| Mammalia | Stenella coeruleoalba | Macaronesian Region (Eastern North Atlantic) | muscles | 86.2 | 698.7 | 513.9 | 1298.8 (ng/g w.w.) | [89] | |||||||||||
| Mammalia | Tursiops truncatus | Atlantic coast of North Carolina and Florida | blubber | 4800.0 | 4800.0 (ng/g d.w.) * | [90] | |||||||||||||
| Mammalia | Tursiops truncatus | Macaronesian Region (Eastern North Atlantic) | muscles | 413.0 | 783.0 | 1196.0 (ng/g w.w.) | [89] | ||||||||||||
| Reptilia | Caretta caretta | Sicily, Campania, Sardinia (Mediterranean Sea) | blood | 1.2 | 6.8 | 12.1 | 16.2 | 24.9 | 7.4 | 68.4 (ng/mL w.w.) | [91] | ||||||||
| Reptilia | Caretta caretta | Sicily (Mediterranean Sea) | gonads | n.d. | n.d. | 4520.8 | 173.7 | 325.5 | 104.2 | 5124.2 (ng/g w.w.) | [78] | ||||||||
| Reptilia | Caretta caretta | Sicily (Mediterranean Sea) | liver | n.d. | n.d. | 4046.9 | 2018.8 | 361.3 | 540.6 | 6967.5 (ng/g w.w.) | [78] | ||||||||
| Reptilia | Caretta caretta | Sicily (Mediterranean Sea) | blubber | n.d. | n.d. | 2411.0 | 360.0 | 4295.6 | 5481.2 | 12,547.8 (ng/g w.w.) | [78] | ||||||||
| Reptilia | Dermochelys coriacea | Sicily (Mediterranean Sea) | gonads | n.d. | 5718.0 | 12,166.7 | 12,532.6 | 5572.9 | n.d. | 35,990.2 (ng/g w.w.) | [78] | ||||||||
| Reptilia | Dermochelys coriacea | Sicily (Mediterranean Sea) | liver | n.d. | 3937.0 | 6055.6 | 16,014.5 | 1226.9 | n.d. | 27,233.9 (ng/g w.w.) | [78] | ||||||||
| Reptilia | Dermochelys coriacea | Sicily (Mediterranean Sea) | muscles | n.d. | n.d. | 2000.0 | n.d. | n.d. | n.d. | 2000.0 (ng/g w.w.) | [78] | ||||||||
| zooplankton | size > 1000 µm | Marseille (Mediterranean Sea) | whole body | 140.9 | 18.6 | 110.4 | 377.1 | 81.1 | 5586.7 | 262.9 | 6577.7 (ng/g d.w.) | [92] | |||||||
| zooplankton | size: 150–500 µm | Marseille (Mediterranean Sea) | whole body | 52.0 | 33.7 | 73.9 | 183.6 | 63.2 | 6659.2 | 469.6 | 7535.4 (ng/g d.w.) | [92] | |||||||
| zooplankton | size: 500–1000 µm | Marseille (Mediterranean Sea) | whole body | 63.9 | 20.2 | 46.1 | 130.2 | 71.5 | 2981.9 | 173.9 | 3487.7 (ng/g d.w.) | [92] |
5. Environmental Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef]
- Tumu, K.; Vorst, K.; Curtzwiler, G. Endocrine Modulating Chemicals in Food Packaging: A Review of Phthalates and Bisphenols. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1337–1359. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-Based Plasticizers and Biopolymer Films: A Review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Liu, X.; Shi, J.; Bo, T.; Li, H.; Crittenden, J.C. Occurrence and Risk Assessment of Selected Phthalates in Drinking Water from Waterworks in China. Environ. Sci. Pollut. Res. 2015, 22, 10690–10698. [Google Scholar] [CrossRef]
- Hlisníková, H.; Petrovičová, I.; Kolena, B.; Šidlovská, M.; Sirotkin, A. Effects and Mechanisms of Phthalates’ Action on Reproductive Processes and Reproductive Health: A Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 6811. [Google Scholar] [CrossRef]
- Staples, C.A.; Adams, W.J.; Parkerton, T.F.; Gorsuch, J.W.; Biddinger, G.R.; Reinert, K.H. Aquatic Toxicity of Eighteen Phthalate Esters. Environ. Toxicol. Chem. 1997, 16, 875–891. [Google Scholar] [CrossRef]
- Dutta, S.; Haggerty, D.K.; Rappolee, D.A.; Ruden, D.M. Phthalate Exposure and Long-Term Epigenomic Consequences: A Review. Front. Genet. 2020, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, P.; Fossati, S.; Warembourg, C.; Casas, M.; Clemente, D.B.P.; Garcia-Esteban, R.; Nawrot, T.S.; Vrijheid, M. Prenatal Exposure to Phthalates and Phenols and Preclinical Vascular Health during Early Adolescence. Int. J. Hyg. Environ. Health 2022, 240, 113909. [Google Scholar] [CrossRef]
- Grindler, N.M.; Vanderlinden, L.; Karthikraj, R.; Kannan, K.; Teal, S.; Polotsky, A.J.; Powell, T.L.; Yang, I.V.; Jansson, T. Exposure to Phthalate, an Endocrine Disrupting Chemical, Alters the First Trimester Placental Methylome and Transcriptome in Women. Sci. Rep. 2018, 8, 6086. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Li, J.; Xu, S.; Wan, Y.; Li, Y.; Jiang, Y.; Zhao, H.; Zhou, Y.; Liao, J.; Liu, H.; et al. Prenatal Exposure to Phthalates and Neurocognitive Development in Children at Two Years of Age. Environ. Int. 2019, 131, 105023. [Google Scholar] [CrossRef]
- Rowdhwal, S.S.S.; Chen, J. Toxic Effects of Di-2-Ethylhexyl Phthalate: An Overview. Biomed. Res. Int. 2018, 2018, 1750368. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, H.; Pao, P.-C.; Lee, A.; Wang, J.; Suen Chan, Y.; Manno, F.A.M., III; Wan Chan, S.; Han Cheng, S.; Chen, X. Exposure to Phthalates Impaired Neurodevelopment through Estrogenic Effects and Induced DNA Damage in Neurons. Aquat. Toxicol. 2020, 222, 105469. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, Y.; Yang, Z.; Cai, Z.; Mizuno, T.; Tsuno, H.; Zhu, W.; Zhang, X. Toxicity of Seven Phthalate Esters to Embryonic Development of the Abalone Haliotis Diversicolor Supertexta. Ecotoxicology 2009, 18, 293–303. [Google Scholar] [CrossRef]
- Ghanem, S.F. Effect of Endocrine Disrupting Chemicals Exposure on Reproduction and Endocrine Functions Using the Zebrafish Model. Egypt J. Aquat. Biol. Fish 2021, 25, 951–981. [Google Scholar] [CrossRef]
- Rajvanshi, J.; Sogani, M.; Kumar, A.; Arora, S.; Syed, Z.; Sonu, K.; Gupta, N.S.; Kalra, A. Perceiving Biobased Plastics as an Alternative and Innovative Solution to Combat Plastic Pollution for a Circular Economy. Sci. Total Environ. 2023, 874, 162441. [Google Scholar] [CrossRef]
- Da Costa, J.M.; Kato, L.S.; Galvan, D.; Lelis, C.A.; Saraiva, T.; Conte-Junior, C.A. Occurrence of Phthalates in Different Food Matrices: A Systematic Review of the Main Sources of Contamination and Potential Risks. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2043–2080. [Google Scholar] [CrossRef]
- Neves, R.A.F.; Miralha, A.; Guimarães, T.B.; Sorrentino, R.; Marques Calderari, M.R.C.; Santos, L.N. Phthalates Contamination in the Coastal and Marine Sediments of Rio de Janeiro, Brazil. Mar. Pollut. Bull. 2023, 190, 114819. [Google Scholar] [CrossRef]
- Carney Almroth, B.; Slunge, D. Circular Economy Could Expose Children to Hazardous Phthalates and Chlorinated Paraffins via Old Toys and Childcare Articles. J. Hazard Mater. 2022, 7, 100107. [Google Scholar] [CrossRef]
- Orecchio, S.; Indelicato, R.; Barreca, S. Determination of Selected Phthalates by Gas Chromatography–Mass Spectrometry in Personal Perfumes. J. Toxicol. Environ. Health Part A 2015, 78, 1008–1018. [Google Scholar] [CrossRef]
- Barreca, S.; Indelicato, R.; Orecchio, S.; Pace, A. Photodegradation of Selected Phthalates on Mural Painting Surfaces under UV Light Irradiation. Microchem. J. 2014, 114, 192–196. [Google Scholar] [CrossRef]
- Amin, M.M.; Parastar, S.; Ebrahimpour, K.; Shoshtari-Yeganeh, B.; Hashemi, M.; Mansourian, M.; Kelishadi, R. Association of Urinary Phthalate Metabolites Concentrations with Body Mass Index and Waist Circumference. Environ. Sci. Pollut. Res. 2018, 25, 11143–11151. [Google Scholar] [CrossRef]
- Savoca, D.; Lo Coco, R.; Melfi, R.; Pace, A. Uptake and Photoinduced Degradation of Phthalic Acid Esters (PAEs) in Ulva lactuca Highlight Its Potential Application in Environmental Bioremediation. Environ. Sci. Pollut. Res. 2022, 29, 90887–90897. [Google Scholar] [CrossRef]
- Marturano, V.; Cerruti, P.; Ambrogi, V. Polymer Additives. Phys. Sci. Rev. 2017, 2, 20160130. [Google Scholar] [CrossRef]
- Sridharan, S.; Kumar, M.; Saha, M.; Kirkham, M.B.; Singh, L.; Bolan, N.S. The Polymers and Their Additives in Particulate Plastics: What Makes Them Hazardous to the Fauna? Sci. Total Environ. 2022, 824, 153828. [Google Scholar] [CrossRef]
- Gao, D.-W.; Wen, Z.-D. Phthalate Esters in the Environment: A Critical Review of Their Occurrence, Biodegradation, and Removal during Wastewater Treatment Processes. Sci. Total Environ. 2016, 541, 986–1001. [Google Scholar] [CrossRef]
- Gambino, I.; Bagordo, F.; Grassi, T.; Panico, A.; De Donno, A. Occurrence of Microplastics in Tap and Bottled Water: Current Knowledge. Int. J. Environ. Res. Public Health 2022, 19, 5283. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, Fate, Behavior and Ecotoxicological State of Phthalates in Different Environmental Matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhou, W.; Chen, L.; Wu, L.; Christie, P.; Liu, W. Toxicity of Phthalate Esters to Lettuce (Lactuca sativa) and the Soil Microbial Community under Different Soil Conditions. PLoS ONE 2018, 13, e0208111. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Q.; Gao, R.; Hou, H.; Tan, W.; He, X.; Zhang, H.; Yu, M.; Ma, L.; Xi, B.; et al. Contamination of Phthalate Esters (PAEs) in Typical Wastewater-Irrigated Agricultural Soils in Hebei, North China. PLoS ONE 2015, 10, e0137998. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Zhang, H.-H.; Zhang, J.; Wang, Q.-W.; Yang, G.-P. Occurrence, Distribution, and Ecological Risks of Phthalate Esters in the Seawater and Sediment of Changjiang River Estuary and Its Adjacent Area. Sci. Total Environ. 2018, 619–620, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Savoca, D.; Pace, A. Bioaccumulation, Biodistribution, Toxicology and Biomonitoring of Organofluorine Compounds in Aquatic Organisms. Int. J. Mol. Sci. 2021, 22, 6276. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 Update. Nucleic. Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef] [PubMed]
- Amritha, P.S.; Vinod, V.; Harathi, P.B. A Critical Review on Extraction and Analytical Methods of Phthalates in Water and Beverages. J. Chromatogr. A 2022, 1675, 463175. [Google Scholar] [CrossRef]
- Russo, M.V.; Avino, P.; Perugini, L.; Notardonato, I. Extraction and GC-MS Analysis of Phthalate Esters in Food Matrices: A Review. RSC Adv. 2015, 5, 37023–37043. [Google Scholar] [CrossRef]
- Prokůpková, G.; Holadová, K.; Poustka, J.; Hajšlová, J. Development of a Solid-Phase Microextraction Method for the Determination of Phthalic Acid Esters in Water. Anal. Chim. Acta 2002, 457, 211–223. [Google Scholar] [CrossRef]
- Del Carlo, M.; Pepe, A.; Sacchetti, G.; Compagnone, D.; Mastrocola, D.; Cichelli, A. Determination of Phthalate Esters in Wine Using Solid-Phase Extraction and Gas Chromatography–Mass Spectrometry. Food Chem. 2008, 111, 771–777. [Google Scholar] [CrossRef]
- Shen, H. Simultaneous Screening and Determination Eight Phthalates in Plastic Products for Food Use by Sonication-Assisted Extraction/GC–MS Methods. Talanta 2005, 66, 734–739. [Google Scholar] [CrossRef]
- He, M.; Yang, C.; Geng, R.; Zhao, X.; Hong, L.; Piao, X.; Chen, T.; Quinto, M.; Li, D. Monitoring of Phthalates in Foodstuffs Using Gas Purge Microsyringe Extraction Coupled with GC–MS. Anal. Chim. Acta 2015, 879, 63–68. [Google Scholar] [CrossRef]
- Cai, Q.-Y.; Mo, C.-H.; Wu, Q.-T.; Zeng, Q.-Y.; Katsoyiannis, A. Quantitative Determination of Organic Priority Pollutants in the Composts of Sewage Sludge with Rice Straw by Gas Chromatography Coupled with Mass Spectrometry. J. Chromatogr. A 2007, 1143, 207–214. [Google Scholar] [CrossRef]
- González-Sálamo, J.; Socas-Rodríguez, B.; Hernández-Borges, J. Analytical Methods for the Determination of Phthalates in Food. Curr. Opin. Food Sci. 2018, 22, 122–136. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; De La Guardia, M.; Namieśnik, J. Modern Trends in Solid Phase Extraction: New Sorbent Media. TrAC Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Loos, R.; Wollgast, J.; Castro-Jiménez, J.; Mariani, G.; Huber, T.; Locoro, G.; Hanke, G.; Umlauf, G.; Bidoglio, G.; Hohenblum, P.; et al. Laboratory Intercomparison Study for the Analysis of Nonylphenol and Octylphenol in River Water. TrAC Trends Anal. Chem. 2008, 27, 89–95. [Google Scholar] [CrossRef]
- Luo, Y.-B.; Yu, Q.-W.; Yuan, B.-F.; Feng, Y.-Q. Fast Microextraction of Phthalate Acid Esters from Beverage, Environmental Water and Perfume Samples by Magnetic Multi-Walled Carbon Nanotubes. Talanta 2012, 90, 123–131. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, M.; Yan, H.; Yang, G. Dispersive Solid-Phase Extraction Based on Magnetic Dummy Molecularly Imprinted Microspheres for Selective Screening of Phthalates in Plastic Bottled Beverages. J. Agric. Food Chem. 2014, 62, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Armada, D.; Celeiro, M.; Dagnac, T.; Llompart, M. Green Methodology Based on Active Air Sampling Followed by Solid Phase Microextraction and Gas Chromatography-Tandem Mass Spectrometry Analysis to Determine Hazardous Substances in Different Environments Related to Tire Rubber. J. Chromatogr. A 2022, 1668, 462911. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, N.; Sasamoto, K.; David, F.; Sandra, P. Recent Developments of Stir Bar Sorptive Extraction for Food Applications: Extension to Polar Solutes. J. Agric. Food Chem. 2018, 66, 7249–7255. [Google Scholar] [CrossRef]
- Cai, Y.; Cai, Y.; Shi, Y.; Liu, J.; Mou, S.; Lu, Y. A Liquid–Liquid Extraction Technique for Phthalate Esters with Water-Soluble Organic Solvents by Adding Inorganic Salts. Microchim. Acta 2007, 157, 73–79. [Google Scholar] [CrossRef]
- Sørensen, L.K. Determination of Phthalates in Milk and Milk Products by Liquid Chromatography/Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 1135–1143. [Google Scholar] [CrossRef]
- Bergström, S.; Barri, T.; Norberg, J.; Jönsson, J.Å.; Mathiasson, L. Extracting Syringe for Extraction of Phthalate Esters in Aqueous Environmental Samples. Anal. Chim. Acta 2007, 594, 240–247. [Google Scholar] [CrossRef]
- Rezaee, M.; Yamini, Y.; Faraji, M. Evolution of Dispersive Liquid–Liquid Microextraction Method. J. Chromatogr. A 2010, 1217, 2342–2357. [Google Scholar] [CrossRef]
- Net, S.; Delmont, A.; Sempéré, R.; Paluselli, A.; Ouddane, B. Reliable Quantification of Phthalates in Environmental Matrices (Air, Water, Sludge, Sediment and Soil): A Review. Sci. Total Environ. 2015, 515–516, 162–180. [Google Scholar] [CrossRef]
- Hidalgo-Serrano, M.; Borrull, F.; Marcé, R.M.; Pocurull, E. Simple Method for Determining Phthalate Diesters and Their Metabolites in Seafood Species Using QuEChERS Extraction and Liquid Chromatography-High Resolution Mass Spectrometry. Food Chem. 2021, 336, 127722. [Google Scholar] [CrossRef]
- Verma, J.; Jha, R.R.; Gupta, N.; Singh Thakur, R.; Ansari, N.G.; Patel, D.K. QuEChERS Based Analysis of Multiple Pesticides and Phthalates in Packaged Food Products. Microchem. J. 2021, 171, 106882. [Google Scholar] [CrossRef]
- Yadav, S.; Rai, S.; Srivastava, A.K.; Panchal, S.; Patel, D.K.; Sharma, V.P.; Jain, S.; Srivastava, L.P. Determination of Pesticide and Phthalate Residues in Tea by QuEChERS Method and Their Fate in Processing. Environ. Sci. Pollut. Res. 2017, 24, 3074–3083. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H. High-Performance Liquid Chromatography (HPLC): A review. Ann Adv Chem. 2022, 6, 010–020. [Google Scholar] [CrossRef]
- Salazar-Beltrán, D.; Hinojosa-Reyes, L.; Ruiz-Ruiz, E.; Hernández-Ramírez, A.; Guzmán-Mar, J.L. Phthalates in Beverages and Plastic Bottles: Sample Preparation and Determination. Food Anal. Methods 2018, 11, 48–61. [Google Scholar] [CrossRef]
- Gómez-Ramos, M.M.; Ucles, S.; Ferrer, C.; Fernández-Alba, A.R.; Hernando, M.D. Exploration of Environmental Contaminants in Honeybees Using GC-TOF-MS and GC-Orbitrap-MS. Sci. Total Environ. 2019, 647, 232–244. [Google Scholar] [CrossRef]
- Huysman, S.; Van Meulebroek, L.; Janssens, O.; Vanryckeghem, F.; Van Langenhove, H.; Demeestere, K.; Vanhaecke, L. Targeted Quantification and Untargeted Screening of Alkylphenols, Bisphenol A and Phthalates in Aquatic Matrices Using Ultra-High-Performance Liquid Chromatography Coupled to Hybrid Q-Orbitrap Mass Spectrometry. Anal. Chim. Acta 2019, 1049, 141–151. [Google Scholar] [CrossRef]
- Yarsan, E.; Yipe, M. The Important Terms of Marine Pollution “Biomarkers and Biomonitoring, Bioaccumulation, Bioconcentration, Biomagnification”. J. Mol. Biomark Diagn. 2013, s1, 003. [Google Scholar] [CrossRef]
- Zaghloul, A.; Saber, M.; Gadow, S.; Awad, F. Biological Indicators for Pollution Detection in Terrestrial and Aquatic Ecosystems. Bull. Natl. Res. Cent. 2020, 44, 127. [Google Scholar] [CrossRef]
- Parmar, T.K.; Rawtani, D.; Agrawal, Y.K. Bioindicators: The Natural Indicator of Environmental Pollution. Front. Life Sci. 2016, 9, 110–118. [Google Scholar] [CrossRef]
- Savoca, D.; Melfi, R.; Palumbo Piccionello, A.; Barreca, S.; Buscemi, S.; Arizza, V.; Arculeo, M.; Pace, A. Presence and Biodistribution of Perfluorooctanoic Acid (PFOA) in Paracentrotus lividus Highlight Its Potential Application for Environmental Biomonitoring. Sci. Rep. 2021, 11, 18763. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, J.; Fu, J.; Shi, J.; Jiang, G. Biomonitoring: An Appealing Tool for Assessment of Metal Pollution in the Aquatic Ecosystem. Anal. Chim. Acta 2008, 606, 135–150. [Google Scholar] [CrossRef]
- Bossart, G.D. Marine Mammals as Sentinel Species for Oceans and Human Health. Vet. Pathol. 2011, 48, 676–690. [Google Scholar] [CrossRef]
- Streit, B. Bioaccumulation Processes in Ecosystems. Experientia 1992, 48, 955–970. [Google Scholar] [CrossRef]
- Cao, Y.; Li, J.; Wu, R.; Lin, H.; Lao, J.-Y.; Ruan, Y.; Zhang, K.; Wu, J.; Leung, K.M.Y.; Lam, P.K.S. Phthalate Esters in Seawater and Sediment of the Northern South China Sea: Occurrence, Distribution, and Ecological Risks. Sci. Total Environ. 2022, 811, 151412. [Google Scholar] [CrossRef]
- He, M.-J.; Lu, J.-F.; Wang, J.; Wei, S.-Q.; Hageman, K.J. Phthalate Esters in Biota, Air and Water in an Agricultural Area of Western China, with Emphasis on Bioaccumulation and Human Exposure. Sci. Total Environ. 2020, 698, 134264. [Google Scholar] [CrossRef]
- Rios-Fuster, B.; Alomar, C.; Paniagua González, G.; Garcinuño Martínez, R.M.; Soliz Rojas, D.L.; Fernández Hernando, P.; Deudero, S. Assessing Microplastic Ingestion and Occurrence of Bisphenols and Phthalates in Bivalves, Fish and Holothurians from a Mediterranean Marine Protected Area. Environ. Res. 2022, 214, 114034. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Coppola, D.; Baini, M.; Giannetti, M.; Guerranti, C.; Marsili, L.; Panti, C.; de Sabata, E.; Clò, S. Large Filter Feeding Marine Organisms as Indicators of Microplastic in the Pelagic Environment: The Case Studies of the Mediterranean Basking Shark (Cetorhinus maximus) and Fin Whale (Balaenoptera physalus). Mar. Environ. Res. 2014, 100, 17–24. [Google Scholar] [CrossRef]
- Shumaila Naz, S.N.; Muhammad Nadir, M.N.; Pirzada Jamal Ahmed Siddiqui, P.J.A.S.; Amir Ahmed, A.A.; Muhammad Noman Syed, M.N.S.; Munawwer Rasheed, M.R. Lipophilic Constituents of the Blubber from Blue Whale, Balaenoptera musculus, Washed Ashore at Pakistan Coast. JCS Pak. 2022, 44, 393. [Google Scholar] [CrossRef]
- Caracappa, S.; Persichetti, M.F.; Gentile, A.; Caracappa, G.; Currò, V.; Freggi, D.; Arculeo, M. New Records of Leatherback Sea Turtle, Dermochelys coriacea (Vandelli, 1761) (Testudines: Dermochelyidae) in the Strait of Sicily. Cah. Biol. Mar. 2017, 58, 353–357. [Google Scholar] [CrossRef]
- Alduina, R.; Gambino, D.; Presentato, A.; Gentile, A.; Sucato, A.; Savoca, D.; Filippello, S.; Visconti, G.; Caracappa, G.; Vicari, D.; et al. Is Caretta caretta a Carrier of Antibiotic Resistance in the Mediterranean Sea? Antibiotics 2020, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Gambino, D.; Savoca, D.; Sucato, A.; Gargano, V.; Gentile, A.; Pantano, L.; Vicari, D.; Alduina, R. Occurrence of Antibiotic Resistance in the Mediterranean Sea. Antibiotics 2022, 11, 332. [Google Scholar] [CrossRef]
- Savoca, D.; Arculeo, M.; Arizza, V.; Pace, A.; Melfi, R.; Caracappa, S.; Caracappa, G.; Vullo, C.; Cambera, I.; Visconti, G.; et al. Impact of Heavy Metals in Eggs and Tissues of C. caretta along the Sicilian Coast (Mediterranean Sea). Environments 2022, 9, 88. [Google Scholar] [CrossRef]
- Sucato, A.; Vecchioni, L.; Savoca, D.; Presentato, A.; Arculeo, M.; Alduina, R. A Comparative Analysis of Aquatic and Polyethylene-Associated Antibiotic-Resistant Microbiota in the Mediterranean Sea. Biology 2021, 10, 200. [Google Scholar] [CrossRef]
- Matiddi, M.; Hochsheid, S.; Camedda, A.; Baini, M.; Cocumelli, C.; Serena, F.; Tomassetti, P.; Travaglini, A.; Marra, S.; Campani, T.; et al. Loggerhead Sea Turtles (Caretta caretta): A Target Species for Monitoring Litter Ingested by Marine Organisms in the Mediterranean Sea. Environ. Pollut. 2017, 230, 199–209. [Google Scholar] [CrossRef]
- Savoca, D.; Arculeo, M.; Barreca, S.; Buscemi, S.; Caracappa, S.; Gentile, A.; Persichetti, M.F.; Pace, A. Chasing Phthalates in Tissues of Marine Turtles from the Mediterranean Sea. Mar. Pollut. Bull. 2018, 127, 165–169. [Google Scholar] [CrossRef]
- Savoca, D.; Arculeo, M.; Vecchioni, L.; Cambera, I.; Visconti, G.; Melfi, R.; Arizza, V.; Palumbo Piccionello, A.; Buscemi, S.; Pace, A. Can Phthalates Move into the Eggs of the Loggerhead Sea Turtle Caretta caretta? The Case of the Nests on the Linosa Island in the Mediterranean Sea. Mar. Pollut. Bull. 2021, 168, 112395. [Google Scholar] [CrossRef]
- Squillante, J.; Scivicco, M.; Ariano, A.; Nolasco, A.; Esposito, F.; Cacciola, N.A.; Severino, L.; Cirillo, T. Occurrence of Phthalate Esters and Preliminary Data on Microplastics in Fish from the Tyrrhenian Sea (Italy) and Impact on Human Health. Environ. Pollut. 2023, 316, 120664. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Wang, L.-Y.; Gu, Y.-Y.; Sun, A.-L.; You, J.-J.; Shi, X.-Z.; Chen, J. Probing the Contamination Characteristics, Mobility, and Risk Assessments of Typical Plastic Additive–Phthalate Esters from a Typical Coastal Aquaculture Area, China. J. Hazard Mater. 2021, 416, 125931. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gu, Y.; Huang, W.; Yin, D. Phthalate Monoesters as Markers of Phthalate Contamination in Wild Marine Organisms. Environ. Pollut. 2016, 218, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Castro, Ó.; Borrull, S.; Borrull, F.; Pocurull, E. High Production Volume Chemicals in the Most Consumed Seafood Species in Tarragona Area (Spain): Occurrence, Exposure, and Risk Assessment. Food Chem. Toxicol. 2023, 173, 113625. [Google Scholar] [CrossRef] [PubMed]
- Vered, G.; Kaplan, A.; Avisar, D.; Shenkar, N. Using Solitary Ascidians to Assess Microplastic and Phthalate Plasticizers Pollution among Marine Biota: A Case Study of the Eastern Mediterranean and Red Sea. Mar. Pollut. Bull. 2019, 138, 618–625. [Google Scholar] [CrossRef]
- Lemos, L.; Gantiva, L.; Kaylor, C.; Sanchez, A.; Quinete, N. American Oysters as Bioindicators of Emerging Organic Contaminants in Florida, United States. Sci. Total Environ. 2022, 835, 155316. [Google Scholar] [CrossRef]
- Bartolomé, L.; Etxebarria, N.; Martínez-Arkarazo, I.; Raposo, J.C.; Usobiaga, A.; Zuloaga, O.; Raingeard, D.; Cajaraville, M.P. Distribution of Organic Microcontaminants, Butyltins, and Metals in Mussels From the Estuary of Bilbao. Arch. Environ. Contam Toxicol. 2010, 59, 244–254. [Google Scholar] [CrossRef]
- Lo Brutto, S.; Iaciofano, D.; Lo Turco, V.; Potortì, A.G.; Rando, R.; Arizza, V.; Di Stefano, V. First Assessment of Plasticizers in Marine Coastal Litter-Feeder Fauna in the Mediterranean Sea. Toxics 2021, 9, 31. [Google Scholar] [CrossRef]
- Garcia-Garin, O.; Sahyoun, W.; Net, S.; Vighi, M.; Aguilar, A.; Ouddane, B.; Víkingsson, G.A.; Chosson, V.; Borrell, A. Intrapopulation and Temporal Differences of Phthalate Concentrations in North Atlantic Fin Whales (Balaenoptera physalus). Chemosphere 2022, 300, 134453. [Google Scholar] [CrossRef]
- Montoto-Martínez, T.; De la Fuente, J.; Puig-Lozano, R.; Marques, N.; Arbelo, M.; Hernández-Brito, J.J.; Fernández, A.; Gelado-Caballero, M.D. Microplastics, Bisphenols, Phthalates and Pesticides in Odontocete Species in the Macaronesian Region (Eastern North Atlantic). Mar. Pollut. Bull. 2021, 173, 113105. [Google Scholar] [CrossRef]
- Page-Karjian, A.; Lo, C.F.; Ritchie, B.; Harms, C.A.; Rotstein, D.S.; Han, S.; Hassan, S.M.; Lehner, A.F.; Buchweitz, J.P.; Thayer, V.G.; et al. Anthropogenic Contaminants and Histopathological Findings in Stranded Cetaceans in the Southeastern United States, 2012–2018. Front. Mar. Sci. 2020, 7, 630. [Google Scholar] [CrossRef]
- Blasi, M.F.; Avino, P.; Notardonato, I.; Di Fiore, C.; Mattei, D.; Gauger, M.F.W.; Gelippi, M.; Cicala, D.; Hochscheid, S.; Camedda, A.; et al. Phthalate Esters (PAEs) Concentration Pattern Reflects Dietary Habitats (Δ13C) in Blood of Mediterranean Loggerhead Turtles (Caretta caretta). Ecotoxicol. Environ. Saf. 2022, 239, 113619. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.; Castro-Jiménez, J.; Oursel, B.; Sempéré, R. Phthalates and Organophosphate Esters in Surface Water, Sediments and Zooplankton of the NW Mediterranean Sea: Exploring Links with Microplastic Abundance and Accumulation in the Marine Food Web. Environ. Pollut. 2021, 272, 115970. [Google Scholar] [CrossRef]
- Shero, M.R.; Pearson, L.E.; Costa, D.P.; Burns, J.M. Improving the Precision of Our Ecosystem Calipers: A Modified Morphometric Technique for Estimating Marine Mammal Mass and Body Composition. PLoS ONE 2014, 9, e91233. [Google Scholar] [CrossRef]
- Toruan, R.L.; Coggins, L.X.; Ghadouani, A. Response of Zooplankton Size Structure to Multiple Stressors in Urban Lakes. Water 2021, 13, 2305. [Google Scholar] [CrossRef]
- Sun, C.; Chen, L.; Zhao, S.; Guo, W.; Luo, Y.; Wang, L.; Tang, L.; Li, F.; Zhang, J. Seasonal Distribution and Ecological Risk of Phthalate Esters in Surface Water and Marine Organisms of the Bohai Sea. Mar. Pollut. Bull. 2021, 169, 112449. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Jang, J.-H.; Cho, H.-Y.; Lee, Y.-B. Risk Assessment for Humans Using Physiologically Based Pharmacokinetic Model of Diethyl Phthalate and Its Major Metabolite, Monoethyl Phthalate. Arch. Toxicol. 2020, 94, 2377–2400. [Google Scholar] [CrossRef]
- Corsolini, S.; Ancora, S.; Bianchi, N.; Mariotti, G.; Leonzio, C.; Christiansen, J.S. Organotropism of Persistent Organic Pollutants and Heavy Metals in the Greenland Shark Somniosus microcephalus in NE Greenland. Mar. Pollut. Bull. 2014, 87, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wei, T.; Goldberg, H.; Wang, W.; Cullion, K.; Kohane, D.S. Getting Drugs Across Biological Barriers. Adv. Mater. 2017, 29, 1606596. [Google Scholar] [CrossRef] [PubMed]
- Savoca, D.; Pace, A.; Arizza, V.; Arculeo, M.; Melfi, R. Controlled uptake of PFOA in adult specimens of Paracentrotus lividus and evaluation of gene expression in their gonads and embryos. Environ. Sci. Pollut. Res. 2023, 30, 26094–26106. [Google Scholar] [CrossRef]
- Yan, Y.; Guo, F.; Liu, K.; Ding, R.; Wang, Y. The Effect of Endocrine-Disrupting Chemicals on Placental Development. Front. Endocrinol. 2023, 14, 1059854. [Google Scholar] [CrossRef]
- Das, M.T.; Kumar, S.S.; Ghosh, P.; Shah, G.; Malyan, S.K.; Bajar, S.; Thakur, I.S.; Singh, L. Remediation Strategies for Mitigation of Phthalate Pollution: Challenges and Future Perspectives. J. Hazard Mater. 2021, 409, 124496. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhu, X.; Zhou, S.; Cheng, Z.; Shi, K.; Zhang, C.; Shao, H. Phthalic Acid Esters: Natural Sources and Biological Activities. Toxins 2021, 13, 495. [Google Scholar] [CrossRef] [PubMed]
| Phthalates Extraction in Different Environmental Matrices | Extraction Methods | Type of Extraction Procedures |
|---|---|---|
| Water | Liquid–liquid extraction (LLE) | Separation funnel Ultrasound/vortex to assist LLE |
| Liquid–solid extraction (LSE) | Solid-phase extraction (SPE) Dispersive solid-phase extraction (d-SPE) Solid-phase microextraction (SPME) Stir bar sorptive extraction (SBSE) | |
| Hybrid method | Solid-supported LLE (SLE) Liquid-phase microextraction (LPME) Single-drop microextraction (SDME), Hollow-fibre LPME (HF-LPME) Dispersive liquid–liquid microextraction (DLLME) Microporous membrane LLE (MMLLE) | |
| Sediments and Biota | Solid–liquid extraction (SLE) | Ultrasound/vortex to assist SLE Soxhlet extraction Accelerated solvent extraction (ASE) |
| Solid extraction (SE) | Thermodesorption (TD) | |
| Water, Sediments and Biota | Quick, easy, cheap, effective, rugged, and safe (QuECHERS) | Combination of LLE and d-SPE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savoca, D.; Barreca, S.; Lo Coco, R.; Punginelli, D.; Orecchio, S.; Maccotta, A. Environmental Aspect Concerning Phthalates Contamination: Analytical Approaches and Assessment of Biomonitoring in the Aquatic Environment. Environments 2023, 10, 99. https://doi.org/10.3390/environments10060099
Savoca D, Barreca S, Lo Coco R, Punginelli D, Orecchio S, Maccotta A. Environmental Aspect Concerning Phthalates Contamination: Analytical Approaches and Assessment of Biomonitoring in the Aquatic Environment. Environments. 2023; 10(6):99. https://doi.org/10.3390/environments10060099
Chicago/Turabian StyleSavoca, Dario, Salvatore Barreca, Riccardo Lo Coco, Diletta Punginelli, Santino Orecchio, and Antonella Maccotta. 2023. "Environmental Aspect Concerning Phthalates Contamination: Analytical Approaches and Assessment of Biomonitoring in the Aquatic Environment" Environments 10, no. 6: 99. https://doi.org/10.3390/environments10060099
APA StyleSavoca, D., Barreca, S., Lo Coco, R., Punginelli, D., Orecchio, S., & Maccotta, A. (2023). Environmental Aspect Concerning Phthalates Contamination: Analytical Approaches and Assessment of Biomonitoring in the Aquatic Environment. Environments, 10(6), 99. https://doi.org/10.3390/environments10060099