Abstract
Microplastics (MPs), small plastic particles resulting from the degradation of larger plastic items and from primary sources such as textiles, engineered plastic pellets, etc., have become a ubiquitous environmental pollutant. As their prevalence in the natural environment grows, concerns about their potential impacts on human health have escalated. This review discusses current research findings on the presence of MPs in organs such as the liver, blood, heart, placenta, breast milk, sputum, semen, testis, and urine, while also exploring plausible mechanisms of translocation. Furthermore, the review emphasizes the importance of understanding the potential toxicological effects of MPs on various physiological processes within these organs and their broader implications for human health. This review also examines the pathways through which MPs can enter and accumulate in human organs and bodily fluids, shedding light on the intricate routes of exposure and potential health implications. It is worth noting that the invasive medical procedures may permit direct access of MPs to the bloodstream and tissues, serving as a potential contamination source. However, it is evident that a comprehensive understanding of MPs’ invasion into human organs is vital for effective mitigation strategies and the preservation of both human health and the environment.
1. Introduction
Production of plastics has significantly increased since they were originally developed in the 1950s [1]. The environment’s plastics, which frequently come from improperly disposed of consumer goods, slowly deteriorate due to photo and thermooxidative processes and, to a lesser extent by biodegradation. As a result, the material becomes less stable and breaks down into secondary MPs, which are fragments smaller than 5 mm [2]. According to Browne et al. [3], primary MPs are defined as plastic particles of this size that are intentionally produced for use in products (such as toothpaste or cosmetic exfoliants) or by companies (such as air blasting). MPs pollution has been noted as a rising worldwide problem over the past ten years that may have an impact on ecosystems, biodiversity, and human health [4]. They are persistent pollutants that have been identified as a developing global problem [5].
MPs have been found in a wide range of environments, including freshwater [6,7,8], seawater [9,10], sediment [11,12], soil [13,14], street dust [15], air [16,17], and even consumables such as beer, sea salt, and tap water [18]. Due to the significant deposits of MPs, the modern era has been referred to as “a new historical epoch, the Plasticene” [19]. Recent research has shown that MPs regularly enter the human body through ingestion, inhalation, and dermally, posing a serious threat to health [20]. According to Galloway [21], ingestion is the major route for entering MPs in the human body. The predicted intake of MPs is 39,000–52,000 particles per person per year based on food consumption [22]. Particles may enter the digestive tract through contaminated food or through mucociliary clearance following inhalation, which may trigger an inflammatory response, increase permeability, and alter the composition and metabolism of the gut microbes [23]. According to Prata [24], each person inhales 26–130 airborne MPs every day. A male person with light exercise is predicted to inhale 272 MPs each day based on air sampling using a mannequin [25,26]. The size and density of the particles will affect how they deposit on the respiratory system, with less dense and smaller particles penetrating the lungs deeper. Particle translocation may occur after deposition as a result of macrophage clearance, migration to the bloodstream, or lymphatic system. Dermal contact with MPs is considered a less significant route of exposure, although it has been speculated that nanoplastics (NPs <100 nm) could transverse the dermal barrier [27]. Human epithelial cells suffer oxidative stress from exposure to MPs and NPs as well [28]. Although research on the interactions between MPs and other human organs is ongoing, human absorption models of nanomaterials created by diverse industrial production methods can be used to estimate the potential impacts of MPs. They have been demonstrated to infiltrate the food chain [4], and as a result, they have also been found in human stools [29] and human blood of healthy donors [30]. Due to the prevalence of stool contamination after vaginal birth and investigated that human placenta from caesarean deliveries contains MPs (>50 µm).
From these pathways, MPs have recently been reported in unimaginable parts of the body and bodily fluid systems such as liver [31], blood [31,32], heart [32], placenta [33], breast milk [34], sputum [35], semen [36], testis [36], and urine [37]. This review shines a spotlight on the lesser-known and more unsettling aspect of plastic pollution: the infiltration of MPs into the human body. Through various mechanisms, such as ingestion, inhalation, and dermal absorption, these tiny plastic particles have managed to enter our bloodstream, lymphatic system, and organs. The implications of this phenomenon for human health are profound and multifaceted, as MPs may carry toxic additives, adsorb harmful chemicals, and trigger inflammatory responses. The review examines the pathways through which MPs enter the body, exploring the role of contaminated air, water, and food sources. It also investigates the extent to which MPs are capable of translocating within the body, potentially leading to accumulation in critical organs such as the liver, kidneys, and even the heart. Researchers are actively working to understand the long-term consequences of such internal plastic exposure, including the possible links to chronic diseases such as cancer, autoimmune disorders, and neurological conditions [31,32,33,34,35,36,37].
The review then takes a critical turn, examining the growing body of research suggesting potential health implications linked to MPs exposure. Although the full scope of these health effects is not yet fully understood, the review highlights some concerning findings, such as the ability of MPs to inhibit active enzymes and the potential for inflammation and oxidative stress caused by their presence in bodily tissues. It also underscores the need for further studies to elucidate the long-term consequences of this silent invasion.
2. MPs in the Human Body and Bodily Fluids System
Recent studies are now discovering MPs in more different human organ and bodily systems such as the liver, blood, heart, placenta, breast milk, sputum, semen, testis and urine. These studies are summarized in Table 1, presenting the extraction and analytical methods, particle types, shapes and quantities found. Most studies have relied on potassium hydroxide (KOH) for sample digestions while Proteinase K and calcium chloride (CaCl2) was applied in one study [30]. The highest number of particles were reported in the sputum and the least were reported in the semen.

Table 1.
Summary of MPs studies in different human organ and bodily systems.
2.1. MPs in Human Liver, Kidney and Spleen
The human liver, kidney, and spleen are crucial organs for overall health. The liver, located in the right abdomen, detoxifies the blood, aids in digestion, regulates blood sugar, and produces clotting proteins. Kidneys, positioned on either side of the spine, filter waste, regulate fluid balance, blood pressure, and activate vitamin D. The spleen, found on the left side behind the stomach, serves immune functions, filters blood, and stores essential blood components, helping fight infections caused by bacteria [39,40,41,42,43,44]. These three organs, the liver, kidneys, and spleen, work in harmony to maintain the body’s internal balance, support immune function, and ensure proper waste elimination. Caring for these organs through a balanced diet, regular exercise, and avoiding harmful substances can help promote overall health and well-being. However, they have been faced with contamination by MPs [31]. In Horvatits et al. [31], tissue samples from six patients with liver cirrhosis and five healthy subjects were compared. 17 samples in all, comprising 11 liver samples, 3 kidney samples, and 3 spleen samples, were examined. The scientists used a well-known methodology to find MPs particles in human tissue that were between 4 and 30 µm in size. The tissue samples were chemically digested as part of this technique, then they were stained with Nile red. The properties and make-up of the MPs were further evaluated using µRaman and fluorescence microscopy.
According to the findings, liver, kidney, and spleen samples from healthy people did not contain any MPs when compared to the limit of detection. In contrast, cirrhosis-affected liver tissues revealed positive MPs quantities that were significantly greater than liver samples from people without liver disease. The research discovered six different kinds of MPs polymers [PS, PVC, PET, PMMA, POM, and PP] in the cirrhotic liver tissues, ranging in size from 4 to 30 µm.
These results highlight the existence of MPs in cirrhotic liver tissues and their much greater quantities as compared to healthy persons. Additionally, the identification of distinct MPs offers important new information on the make-up of MPs in relation to liver cirrhosis. However, in animal studies, research [45] has confirmed the accumulation of PS NPs in the liver and kidneys of mice, leading to noticeable structural and functional changes in these organs. In a related study [46], the inflammatory impact of PS NPs of varying sizes (100 and 50 nm) and PS MPs on transgenic zebrafish larvae was investigated. Smaller NPs were found to provoke higher levels of neutrophil aggregation and macrophage apoptosis in the larvae’s abdomen, correlating with increased hepatic inflammation. Furthermore, NPs were observed to dose- and size-dependently enhance the expression of the liver-specific inflammatory binding protein fabp10a. The 50 nm PS particles at a concentration of 0.1 mg/L increased fabp10a expression in larval livers by 21.90%. The authors propose that these effects are contingent on NP distribution and the generation of reactive oxygen species within the larvae. These findings deepen our comprehension of the possible effects of MPs pollution on liver function and emphasize the value of more studies in this area to fully comprehend the effects of MPs pollution on human health.
2.2. MPs in Human Blood
Blood, a crucial bodily fluid, plays a crucial function in preserving general health. It offers a great matrix for looking at plastic particles through human biomonitoring as a result of its function as a transport route and its capability to take blood samples directly from the body without interaction with plastic materials.
In light of these elements, research was carried out to examine the presence of plastic particles in blood and their possible effects on human health [30]. This study provided the first evidence of MPs in blood. The extent of plastic pollution and its effects on the human body were investigated through the analysis of blood samples.
A double shot Py-GC/MS analytical and sampling method that was precise and sensitive to assessed MPs in blood was developed by Leslie et al. [30]. Quantification of MPs (≤700 nm) or less was performed on whole blood samples from 22 healthy persons. PET was the most commonly encountered polymer, with quantifiable values found in 50% of all tested donors. Following these were PMMA (5%), PE (23%), and PS (36%). The blood samples had maximal concentrations of PET, PS, and PE of 2.4 µg/mL, 4.8 µg/mL, and 7.1 µg/mL, respectively. Based on a limited number of donors, this study offers a unique way to assess the bulk concentration of plastic particles in human blood. The polymeric component of plastic in the blood was found to be present at a mean total measurable concentration of 1.6 µg/mL. Their results demonstrated the presence of different plastic particles in human blood, demonstrating the possibility of bloodstream exposure to MPs. Since there is no information on the long-term health impacts of MPs in human blood, further study is required to better comprehend the dangers. This entails examining the origins and routes by which MPs enters the bloodstream, looking into the distribution and accumulation trends, and determining how they could affect various physiological and cardiovascular systems as well as immunological responses and other aspects of human health. In a recent investigation conducted by Yang et al. [32], a comprehensive analysis of venous blood samples taken before and after cardiac surgery revealed a consistent presence of MPs across all samples, with sizes spanning from 20 to 184 μm. Notably, the most prevalent types of MPs were identified as PA (49%) and PET (22%), collectively constituting over 70% of the total microplastic content. The study also unveiled concerning fluctuations in the composition of MPs before and after surgery. For instance, PET dominated the pre-surgery blood samples, accounting for 67% of the total MPs, while PA took precedence in the post-surgery samples, making up 57%. The researchers identified eight distinct types of MPs in the post-surgery blood samples, in contrast to the six types detected in the pre-surgery blood samples. Remarkably, the prevailing diameter range of MPs shifted between the pre- and post-surgery phases. Before the surgery, MPs primarily fell within the 30 to 50 μm diameter range (67%), while after the surgery, a smaller diameter range prevailed (20 to 30 μm, 51%). These findings have notable implications for both human health and environmental awareness. The consistent presence of MPs in the bloodstream, along with the discernible shifts in MPs’ composition and diameter range following cardiac surgery, underscores the potential interaction between medical interventions and MPs exposure. The prevalence of these synthetic particles, particularly those of PA and PET origins, raises questions about their potential impact on postoperative recovery and cardiovascular health. Furthermore, the dynamic alterations in MPs characteristics emphasize the intricate relationship between medical procedures and the body’s response to plastic pollution. As research in this domain continues to unfold, it becomes imperative to comprehensively address the implications of MPs in the context of medical practices and their broader consequences on human well-being and the environment.
2.3. MPs in Human Heart
MPs in the human heart have emerged as a concerning and relatively novel topic of research in recent. One study published identified MPs in human heart for the first time [32]. To present conclusive evidence of MPs’ presence within the human heart, Yang et al. [32] conducted a study involving 15 patients undergoing cardiac surgeries in China. These individuals, devoid of any thoracic surgery or trauma history, generously contributed their tissue samples. Thirteen patients exhibited normal alanine aminotransferase (ALT) and serum creatinine (Scr) values, while two showed anomalies in ALT and Scr levels. In their investigation, Yang and his team meticulously gathered five distinct types of normal tissue samples: pericardia, epicardial adipose tissue (EAT), pericardial adipose tissue (PAT), myocardia, and left atrial appendage (LAA), based on surgical type and patients’ nutritional status (Figure 1).
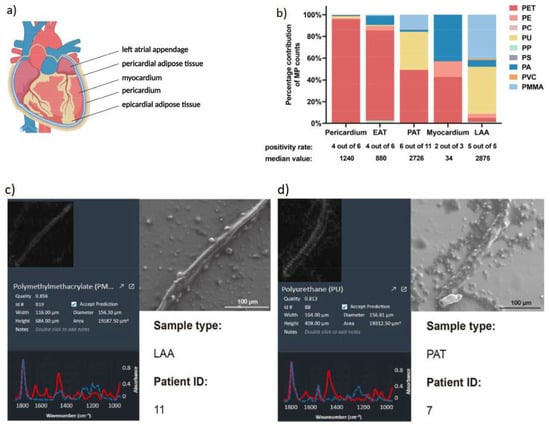
Figure 1.
(a) Part of the heart collected and analyzed for MPs; (b) distribution MPs based on sizes on different heart parts analyzed; (c,d) representative samples of MPs found under SEM (adapted with permission from [32]).
The researchers successfully identified nine categories of MPs (Table 1), with PET (77%) and PU (12%) prevailing as the most common, constituting nearly 90% of the overall MPs content. Curiously, PE was present across all tissue samples, despite comprising only 1% of the total microplastic count. Interestingly, MPs were absent in two pericardium samples, one myocardium sample, two EAT samples, and five PAT samples. Furthermore, PET dominated as the primary microplastic type in the pericardium (96%), EAT (83%), PAT (49%), and myocardium (43%, equivalent to PA), while PU was prominent in the LAA (43%). Notably, the pericardium, PAT, and LAA exhibited the greatest variety of MPs types (7 types), followed by EAT (6 types). Most MPs detected in heart samples possessed a diameter below 100 μm (Figure 1b), with a general diameter range spanning from 20 to 469 μm. Moreover, scanning electron microscopy (SEM) images disclosed diverse in vivo MPs shapes, encompassing threads and rods, often accompanied by varying degrees of fracture and surface roughness (Figure 1c,d).
The implications of these findings remain substantial, as they underscore the pervasive presence of MPs within the human cardiovascular system. Understanding the sources, potential health effects, and routes of entry for these microscopic plastic particles into our bodies holds significant importance for both human health and environmental preservation endeavors.
However, a recent overview of research on the effects of MPs (MPs) and NPs (NPs) on the cardiac physiology of aquatic species was published [47]. When eaten at 120 mg/mL, PS-NPs had a negative impact on heart rate in zebrafish larvae and embryos from maternal and/or co-parental exposure groups due to their interaction with the cardiac sarcomeres [48]. In a different research on zebrafish larvae, PS-NP was only localised in the pericardium at the highest dose of 10 ppm [49]. In this study, after 24 h of egg fertilization, PS-NPs that had been exposed to water gathered in the yolk sac and moved to the developing heart, gastrointestinal system, gallbladder, liver, pancreas, and brain in addition to other organs. Notably, exposed groups displayed significant bradycardia in comparison to controls even at the lowest PS-NP doses of 0.1 ppm [49]. Another research [50] confirmed that marine medaka pups exposed to 20 g/L PS-MPs from their parents had a considerable reduction in heart rate. Along with hypothetical particle interactions with cardiac sarcomeres, the oxidative state produced by MPs may also have an impact on heart rate. However, even at low doses (10 g/L; 100 and 1000 g/L were also tested), tachycardia (medical term for a heart rate >100 beats per minute) was observed in goldfish larvae exposed to PS-MPs and -NPs combination after 7 days of treatment [51]. These contrasting effects on aberrant heart rate may be caused by this organ’s vulnerability to the oxidative stress brought on by the body’s entrance of toxic plastics. These studies suggest that MPs can interfere with normal cardiac function, potentially contributing to cardiovascular diseases in humans. Studies are required to fill this knowledge gap.
2.4. MPs in Human Placenta
During pregnancy, a crucial organ called the placenta develops in the uterus [52]. It starts forming about seven to ten days after conception when a fertilized egg implants in the uterine wall [52]. Throughout pregnancy, the placenta continuously expands to support the growing baby, providing oxygen and nutrients while also eliminating waste products from the baby’s blood [53]. However, placenta faces a plastic problem as it has been reported to be contaminated by MPs [33]. In Ragusa et al. [33], MPs were detected in human placenta. Using µRaman, they examined placentas collected from consenting patients, with an average weight of 23.3 ± 5.7 g. Approximately 600 g of the placenta was used for analysis. They identified 12 MP pieces, primarily composed of PP, with sizes ranging from approximately 5 to 10 µm in the placentas of four women. Interestingly, MPs were found in various sections of the placenta, including the maternal side, fetal side, and chorioamniotic membranes, suggesting that these particles may reach placenta tissues at all levels once inside the human body. In a different research, PVC (43.27%), PP (14.55%), and PBS (10.90%) were the three primary polymer types (from 11 types) discovered in the placenta, according to Zhu et al. [38]. These MPs had diameters ranging from 20.34 to 307.29 µm, with the majority (80.29%) being less than 100 µm, and an abundance of 2.70 ± 2.65 particles/g on average, with a range of 0.28 to 9.55 particles/g.
The immunological mechanism of self-tolerance has to be reconsidered in light of the presence of MPs in the placenta tissue. The placenta serves as the link between the foetus and its surroundings [53]. Through a series of intricate reactions, embryos and foetuses must constantly adjust to the maternal environment and, in turn, the external one. This set of reactions depend heavily on the ability to distinguish between self and non-self, a system that can be compromised by the presence of MPs. In fact, it has been suggested that MPs, if ingested by humans, may accumulate and cause localised toxicity by triggering and/or increasing immunological responses, thereby weakening the body’s defences against infections and changing how energy reserves are used [20,54]. Further studies are required to explicitly identify the toxicity these MPs pose within the placenta and risks to the foetus. In a research by Enyoh et al. [55], the toxicity of 10 different kinds of NPs on the placenta was investigated utilising in silico and machine learning techniques. The placenta plays a critical role in metabolic and excretion processes through its enzymatic system, hence the researchers concentrated on evaluating the inhibition of placental enzymes by these NPs. The soluble epoxide hydrolase, uracil phosphoribosyltransferase, beta 1,3-glucuronyltransferase I, sulfotransferase, N-acetyltransferase 2, and cytochrome P450 1A1 were among the human enzymes involved in placental function that the NPs were docked onto in the research. The binding affinity-based toxicity was then contrasted with control substances. Artificial Neural Networks were utilised to predict toxicity based on these reactivity descriptors after density functional theory analysis of the NPs was carried out to determine their global reactivity descriptors. According to the findings, the most hazardous polymers are PC, PET, and PS, which all exhibit the greatest levels of toxicity to all enzymes. A fractional factorial design technique and a fixed effects model were used to evaluate the impact of NPs in a composite system. The simulation’s findings indicated that a combination of NPs may seriously endanger the placenta by blocking important enzymes. This work sheds light on the possible dangers of exposure to plastic particles during pregnancy and their effects on placental function by highlighting the potential toxicity of certain NPs to placental enzymes.
2.5. MPs in Breast Milk
Breast milk often called as the ‘liquid gold’, not only due to its yellow or orange color, but also as it’s a vital source of nutrition and protection for the new born baby [56]. Breast milk contains millions of live cells, significant amount of protein, over two hundred complex sugars called oligosaccharides, and more than forty enzymes that are critical micronutrients for the growth and development of a baby [57].
Recently, Ragusa et al. [34] have found MPs in the milk produced by a female human breast, which is apparently a matter of great concern. In their study breast milk of 34 women were collected after a week of delivery, each sample contained on an average of 4.16 gm of milk and was stored at −20 °C. Surprisingly the MP particles were found in 26 out of 34 women samples. MPs are further classified in terms of shape, size, colour, dimensions and chemical compositions. In terms of shape and colour, nearly all MPs were found as blue or orange/yellow spherical or irregular fragments with dimensions ranging between 2 and 12 µm. Within the identified polymer matrices, the most abundant ones were PE (38%), PP (38%), and PVC (17%).
For a new born early years are both crucial and sensitive, it’s where the physical and mental growth are at peak and the only source of immunity and nutrition to them is their mother’s milk. When the very elixir of their lives begins to sustain impurities such as MPs, there occurs a need for urgent attention. However, the adverse effects are yet to be discovered, but that does not necessarily decrease the risk level. In light of these, Enyoh et al. [58] conducted a computational simulation to assess the risks posed by small plastic particles (including PS, PVC, PUR, PMMA, PET, PE, PP, PCP, and PC) in breast milk to newborns, potentially affecting the levels of secretory immunoglobulin A (SIgA). SIgA is a crucial antibody that plays a vital role in protecting against diseases and contributes to the development of the infant’s immune system [59]. The study revealed that PC (polycarbonate) was found to be considerably toxic, whereas the other plastic particles showed moderate toxicity. These findings highlight the potential risks associated with the presence of these plastic particles in breast milk, impacting the immunity of infants.
Understanding how MPs enter breast milk and the potential risks they pose will be essential in developing strategies to reduce exposure and protect infant health. As such, future perspectives in this area include conducting comprehensive studies on the presence and concentration of MPs in breast milk from different populations, investigating any potential short-term or long-term health impacts on breastfeeding infants, and exploring ways to mitigate MPs exposure for both lactating mothers and infants.
2.6. MPs in Sputum
The mucus or phlegm that is coughed up from the respiratory tract, especially the lungs and bronchi, is known as sputum. Saliva, nasal secretions, and materials made by the respiratory system, such as mucus and cells, are all included in its composition [60]. Depending on the underlying illness or infection, the colour, consistency, and smell of sputum might change [60]. As a possible route for human MP intake, sputum is now being examined for the existence of MPs [35]. Huang et al. [35] tested human sputum for MPs (20-500 µm) using samples collected from 22 patients with diverse respiratory conditions to ascertain whether people unwittingly inhale MPs. Participants had to submit a questionnaire and sputum samples for examination. To determine if MPs were present in the respiratory tract, sputum samples were analyzed using an FTIR microscope and a laser infrared imaging spectrometer. According to their findings, 21 distinct MP types were found in the sputum samples, with PUR being the most prevalent. Polyester, chlorinated polyethylene, and alkyd varnish were next, making up 78.36% of all of the MPs found. MPs are abundant in sputum samples, according to the results of this study, indicating that inhalation may be a key way for plastics to enter the human body. Furthermore, with statistical significance (p < 0.05), it was shown that the levels of specific types of MPs identified in the respiratory tract were related to a number of characteristics, including smoking and invasive medical procedures, among others. These results offer important new information on MPs exposure and give crucial information for evaluating the possible dangers of MPs to human health.
2.7. MPs in Testis and Semen
The male reproductive system is a complex network of organs and tissues responsible for the production, storage, and distribution of sperm, which significantly impacts fertility and overall reproductive health [61]. This system comprises two main components: the testes, responsible for sperm cell production through spermatogenesis, and semen, which includes sperm cells and other seminal fluid components necessary for their survival and motility [61]. Recently, researchers have turned their attention to investigating the potential presence and harmful effects of MPs (MPs) within the male reproductive system. A study by Zhao et al. [36] reported the discovery of MPs in human testis and semen. In this study, two groups of samples were collected: the first group included 5 semen samples analyzed using Py-GC/MS for MP analysis, and the second group comprised 25 semen samples and 6 testis samples analyzed using LD-IR for MP analysis. The results of the LD-IR analysis indicated that semen had an average MP abundance of 0.23 ± 0.45 (ranging from 0 to 2.06) particles per milliliter. This abundance was lower compared to the MP levels found in sputum samples [35]. Similarly, using Py-GC/MS analysis, MPs were detected in semen samples from 5 individuals, with an average abundance of 15.34 ± 23.31 (ranging from 0.098 to 56.188) mg/mL. Regarding morphology, the identified MPs in semen comprised approximately 29% fibers, 29% fragments, 29% films, and 13% subspherical particles, with sizes ranging between 21.76 and 286.71 (average of 96.19 ± 74.17) micrometers. Notably, 67% and 80.6% of the particles found in human testis and semen, respectively, were MPs with sizes between 20 and 100 micrometers. Among the MPs identified, PVC and PE were more prevalent in testis than PS. Additionally, the MPs found in the testis were significantly smaller than those observed in semen, aligning with the hypothesis proposed by Zhao et al. [36] that larger MPs are expelled or found in the external contact fraction, while smaller MPs tend to accumulate in vivo. These findings provide important insights into the presence and characteristics of MPs in human semen and testis. The prevalence of specific MP types and their size distributions in these reproductive samples warrant further investigation to understand their potential implications for male reproductive health and fertility. Animal studies have demonstrated that exposure to MPs can lead to decreased sperm quality, altered hormone levels, testicular inflammation, and impaired fertility [62]. For instance, the Nrf2/HO-1/NF-κB be activated by exposure to polystyrene MPs (PS-MPs) and disrupt the testicular tissue of mice, leading to poor sperm quality [63]. Additionally, PS-MPs can drastically inhibit follicle formation in rats and result in granulosa cell death, which reduces ovarian reserve capacity and harms the ovaries via activating the Wnt/β-Catenin pathway [64]. It has also been demonstrated that accumulation of PS-MPs of 0.5, 4, and 10 μm may occur in mouse testicular tissue, leading to the shedding of seminiferous epithelial cells and rupturing of the blood-testis barrier.
According to earlier research, exposure to PS-MPs can also cause aberrant growth of germ cells in male Japanese medaka [65] and lower the number of sperm in oysters [66]. Mammals’ sperm quality and quantity are declining, and this is particularly true of mice [67]. There is, however, a paucity of information and research about MPs on male reproduction and its underlying harmful mechanism as a new persistent pollutant. Multiple signaling pathways are engaged with MPs, and as strongly confirmed by other research [68,69], oxidative stress is seen as a primary and prominent harmful mechanism. According to Wang et al. [50], oxidative stress is characterized by rising levels of malondialdehyde (MDA) and falling levels of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-PX), which is a relative oxygen species (ROS) producer. In the meantime, it has been shown that exposure to PS-MPs in mice testis increases ROS and MDA levels while decreasing glutathione (GSH) levels, indicating that the p38 mitogen-activated protein kinases (MAPK) pathway is activated [70]. Furthermore, prior research has unequivocally confirmed the direct link between Bric-a-Brac/Tramtrack/Broad-complex protein (BTB) deficiency and p38 pathway activation. BTB are a family of transcription factors or co-regulators involved in gene regulation Betaine has protective effects on BTB through downregulating the expressions of p38 MAPK phosphorylation [71], and BTB might be damaged by activating the TGF-3/p38 MAPK pathway in rats [72]. It is yet unknown, nevertheless, whether MPs exposure through the p38 MAPK pathway might cause BTB impairment. Male reproduction and spermatogenesis depend on the health of the BTB ultrastructure. Tight junctions (TJs), gap junctions (GJs), basal ectoplasmic specialization (ESs), and desmosomes make up the BTB, which is a physical barrier created by Sertoli cells (SCs) between blood arteries and testicular seminiferous tubules [73]. In addition to serving as a source of nutrients, BTB also serves as a crucial physical barrier and immune-privileged location that can block the entry of hazardous and damaging pollutants, creating a favorable microenvironment for spermatogenesis [73]. For maintaining male reproductive function, proper spermatogenesis is unquestionably important. Therefore, spermatogenesis can be disturbed by the disruption of BTB normal structure, which can then result in a reproductive problem in male mammals. Literature on the impact of MPs on BTB is still only occasionally documented, though. According to a newly released study, mice’s BTB could potentially be damaged by 0.5 µm PS-MPs absorbed by Sertoli cells [70]. However, the chemical process underlying it is still not fully understood. Moreover, the physical and chemical properties of MPs, such as their small size, large surface area, and ability to adsorb and release toxic chemicals, raise concerns about their potential to disrupt hormonal balance and interfere with cellular processes within the testes. As the scientific community continues to investigate the impacts of MPs on male reproductive health, it is crucial to explore the mechanisms through which MPs exert their effects and identify potential long-term consequences. Further research is needed to determine the extent of human exposure to MPs, understand their distribution and accumulation patterns within the male reproductive system, and elucidate the mechanisms by which they may impact sperm function, fertility, and overall reproductive health.
2.8. MPs in Urine
Human urine is a liquid waste product excreted by the kidneys as part of the body’s process of filtering and eliminating waste materials from the bloodstream. It is a transparent to pale-yellow fluid that varies in color depending on an individual’s hydration levels and dietary intake. Urine is composed of approximately 95% water, making it an essential component of the body’s waste removal system [74]. The characteristics of urine, such as color, odor, and frequency of urination, can provide valuable insights into an individual’s hydration status and overall health [74].
There is a paucity of studies investigating the presence of MPs in human urine and their potential implications for human health. A notable study by Pironti et al. [37] shed light on this topic by examining urine samples from six volunteers residing in various cities in southern Italy. The researchers utilized µRaman to analyze the samples and identify MPs. The results of the analysis revealed the existence of four pigmented microplastic fragments, ranging in size from 4 to 15 micrometers, with irregular shapes [37]. The researchers further investigated the morphology and chemical composition of these MPs. Specifically, they identified the presence of polyethylene vinyl acetate (PVA), polyvinyl chloride (PVC), PP, and polyethylene (PE) MPs in the samples. Interestingly, one female sample contained both PVA and PVC MPs, while three male samples contained PP and PE MPs (Figure 2).
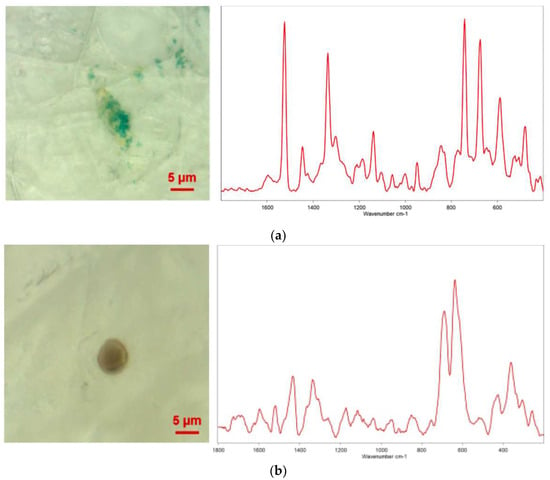
Figure 2.
Samples of MPs under the microscope and µRaman spectra found in urine of (a) male and (b) female (Adapted with permission from [37]).
These findings offer preliminary evidence suggesting that MPs may be able to traverse the gastrointestinal tract and be expelled from the body through biological processes, ultimately appearing in urine. Such research is crucial as it provides valuable insights into the potential pathways of MPs exposure in humans and opens up new avenues for investigating their health implications. Further studies are warranted to comprehensively understand the extent and impact of microplastic presence in human urine.
3. Plausible Pathways of MPs to Different Organs and Bodily Fluids
An overview of the plausible pathways of MPs to different organs and bodily fluids is presented in Figure 3. MPs may enter the circulation by a variety of routes, such as ingesting, inhaling, and cutaneous absorption. Once within the body, they may build up in the digestive system. Given that MPs are frequently present in seafood, drinking water, and even the air we breathe, studies indicate that ingestion is the primary method by which they enter the body [75]. Smaller particles can enter the bloodstream once they reach the gastrointestinal tract through: (1) permeability, where MPs may pass through the intestinal epithelium due to its porous nature, as MPs have been observed in human colectomy samples [76]; (2) MPs may be transported through the lymphatic system, which is involved in immune cell transport and the absorption of fats [77]; and (3) from the liver, which turns nitrogenous waste into urea, a less harmful compound. The circulation receives urea once liver cells release it [78]. As a result, MPs could enter the bloodstream. From the bloodstream, MPs can potentially be distributed to various organs and tissues, such as breast milk, heart, placenta, sputum, semen, testis and urine (Figure 3).
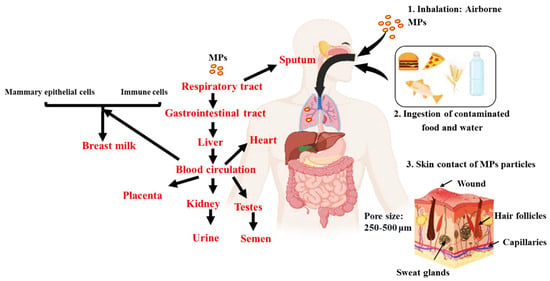
Figure 3.
An overview of the plausible pathways of MPs to different organs and bodily fluids. Intake of MPs are possible via inhalation, ingestion and skin contact. Once in the body, they are absorbed into the gastrointestinal tract from which it is transported to the bloodstream. The bloodstream then circulates it to the different internal organs and then potentially excreted via the urine and semen.
According to one theory, MPs move from the circulation into breast milk via two different paths, each of which depends on immune cells and mammary epithelial cells, with the latter being particularly important for inhaled particles [34]. The major cells in charge of generating and secreting breast milk are mammary epithelial cells. These cells go through particular physiological changes during lactation in order to produce and transport different substances, such as proteins, lipids, and carbohydrates, into breast milk [79]. According to the theory, during this secretion phase MPs can be picked up by mammary epithelial cells and then released into the breast milk. One potential explanation is that MPs may be recognised by certain receptors on the surface of mammary epithelial cells once they have entered the circulation or interstitial fluid surrounding the mammary glands. When the MPs are taken up by vesicles and carried into the cytoplasm of the cell, a mechanism known as receptor-mediated endocytosis may be used by the cells to internalise the MPs [80]. Once within the cell, the MPs could be contained in milk secretory vesicles and discharged during lactation into the mother’s milk. Passive diffusion is another possible process. MPs could diffuse into the cells and then be delivered to milk secretory vesicles if their size and chemical makeup enable them to pass past the cellular membranes of mammary epithelial cells. The second route involves immune cells such as macrophages, which are found in breast tissue and assist in immunological defence by phagocytosing and eliminating foreign substances and debris from the lungs [81]. Including MPs, these foreign particles allow for engulfment and internalisation. After being taken up by immune cells in the lungs, MPs can then move via the lymphatic system or circulation until they reach the breast tissue, where they may be released into breast milk during lactation.
It is important to remember that the actual processes may be more complicated than those proposed, and that the concept of MPs translocating to breast milk through these routes is still an active area of research. The MPs’ precise dimensions, make-up, and interactions with diverse cells and tissues might have an impact on the translocation process. The quantity and presence of MPs in breast milk may also depend on additional variables such exposure levels, length of exposure, and individual variances.
In the case of the heart, it may take place as a result of MPs penetrating the blood channel endothelial cells. Additionally, MPs can cause oxidative stress and inflammation, which can cause the blood vessel barrier to rupture and let MPs into the heart tissue [32]. MPs can cross the placental barrier after entering the mother’s circulation and go to the foetal side [55]. During pregnancy, a unique structure called the placental barrier divides the maternal blood supply from the foetal blood supply [55]. Its primary job is to defend against hazardous chemicals while facilitating the flow of gases, nutrients, and other vital substances. Syncytiotrophoblast, the placenta’s outermost layer, foetal endothelial cells, and other supporting tissues make up the placental barrier’s many layers [82]. Theoretically, there are two ways that MPs might traverse the placental barrier: (1) through microscopic holes or breaches in the barrier, which may also be created by inflammation and barrier disruption; (2) by endocytosis, in which MPs are taken up by cells and transported over the barrier; and (3) immunological cells can also transfer MPs through the barrier as part of immunological reactions. To completely comprehend the methods and degree of microplastic transfer to the placenta, more studies are necessary.
However, factors such as their elimination by renal filtration or biliary excretion, or their deposit in organs such as the liver, spleen, or other fenestrated capillaries and sinusoids, dictate the fate of plastic particles inside the body [30]. Sputum can also be used for elimination in the respiratory system. Nitrogenous waste must first be changed by the liver into a less dangerous molecule before being released from liver cells into the circulation and finally to the kidney [78]. Although the renal excretion may be a viable pathway for the removal of MPs, it is known that the glomerular filtration barrier only permits the transit of particles with sizes of 10 nm. The mechanism for this may involve exocytosis and endocytosis close to the tubular epithelial cells after leaving the glomerulus via the efferent artery and entering the peritubular capillaries, before the MPs are excreted into the urine [37]. MPs can also pass through the renal tubule system, though this is less common [37].
As the testes have a rich blood supply, it is possible that some MPs circulating in the blood could reach the testicular tissue which include a barrier (blood-testis barrier) (Figure 4). The testes are protected by this specialized barrier, which also helps to maintain a stable internal environment for spermatogenesis and protects developing sperm from harmful substances in the blood. However, some studies have suggested that certain toxicants may be able to disturb this barrier [83] and thus form a pathway of MPs reaching the testes.
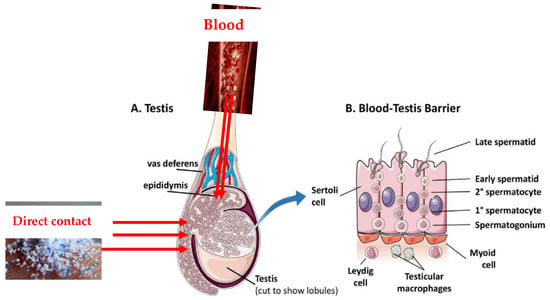
Figure 4.
Plausible pathways of MPs into the testes. (A) is showing the specific location of the blood-testes barriers while (B) is unraveling the cellular composition and intricate structure of the blood-testis barrier, while exploring the supportive role of neighboring cells in maintaining barrier function and balance (Adapted with permission and modification from ref. [83]).
The lymphatic system is another potential pathway through which MPs could reach the testes. The lymphatic system is a network of vessels and organs that helps to transport lymph, a fluid containing white blood cells, throughout the body [84]. MPs may be taken up by immune cells in the lymphatic system and transported to different tissues, including the testes. However, in some cases, MPs may come into direct contact with the male reproductive system. For example, certain lifestyle or occupational exposures may lead to direct contact with MPs. Once MPs are in the testes, they might then find their way into the semen during the process of sperm maturation and secretion and through unknown mechanisms.
It is important to emphasize that the pathways described above are hypothetical and require further investigation and scientific evidence to be fully confirmed. As research progresses, it will be essential to explore the mechanisms by which MPs reach the semen and evaluate their potential impacts on male reproductive health. It is important to keep in mind that intrusive medical procedures might provide MPs direct access to the bloodstream and tissues. This is a potential source of contamination. There are MPs in the air in the operating theatre, according to recent research [85,86], which suggests that MPs may immediately descend to the surface of patients’ viscera through air [31]. It is necessary to look at these prospective sources’ roles more thoroughly.
4. Conclusions and Future Perspectives
The ubiquitous presence of MPs in human organs and bodily fluids has ignited significant concerns, underscoring the need for comprehensive research and proactive measures. This review has delved into the diverse pathways through which MPs infiltrate critical human physiological systems, including the heart, placenta, testes, and various bodily fluids. It has highlighted the fact that ingestion, inhalation, and dermal absorption serve as portals for MPs’ entry, potentially having profound health implications. Furthermore, the toxicological aspect, such as the capacity of MPs to adsorb and release toxic chemicals, accentuates the urgency of understanding their interaction with cellular processes. However, the multifaceted challenges presented by MPs necessitate holistic risk assessments, standardized methodologies, and public education to drive change.
Moving forward, an array of significant future directions emerge. Identifying the sources of MPs in the environment and tracing their pathways into the human body is crucial for developing effective strategies for prevention and reduction. Furthermore, it is imperative to delve deeper into the mechanisms through which MPs interact with biological systems and toxic substances, to accurately assess potential health impacts. Longitudinal studies evaluating the chronic consequences of MPs exposure on organ function and human health are crucial for informed decision-making. The establishment of comprehensive risk assessment frameworks, encompassing exposure routes and toxicological interactions, will facilitate evidence-based policies and regulations and establishing safe exposure limits. Additionally, fostering public awareness campaigns is pivotal to incite behavioral shifts towards responsible plastic consumption and waste management. In addressing the intricate challenges posed by MPs’ intrusion, interdisciplinary collaborations between environmental, medical, and policy sectors will be instrumental in safeguarding human health and preserving the integrity of our environment.
Author Contributions
C.E.E.: Conceptualization, Methodology, Software, Formal analysis, Validation, Visualization, Investigation, Data curation, Project administration, Writing—original draft preparation, Writing—reviewing and Editing. A.D.: Data curation, Formal analysis, Software, Visualization, Writing—original draft preparation. H.K.: Resources, Writing—reviewing and Editing. Q.W.: Resources, Funding acquisition, Writing—reviewing and Editing. M.H.R.: Writing—original draft preparation, Writing—reviewing and Editing, Formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by the Special Funds for Basic Research (B) (No.22H03747, FY2022-FY2024) of Grant-in-Aid for Scientific Research of Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Verla, A.W.; Enyoh, C.E.; Verla, E.N.; Nwarnorh, K.O. Microplastic–Toxic Chemical Interaction: A Review Study on Quantified Levels, Mechanism and Implication. SN Appl. Sci. 2019, 1, 1400. [Google Scholar] [CrossRef]
- Andrady, A.L. MPs in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Dick Vethaak, A.; Lavorante, B.R.B.O.; Lundebye, A.K.; Guilhermino, L. Marine Microplastic Debris: An Emerging Issue for Food Security, Food Safety and Human Health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- United Nations Environment Programme. UNEP Year Book 2011: Emerging Issues in Our Global Environment; UNEP Publications: Nairobi, Kenya, 2011. [Google Scholar]
- Eriksen, M.; Mason, S.; Wilson, S.; Box, C.; Zellers, A.; Edwards, W.; Farley, H.; Amato, S. Microplastic Pollution in the Surface Waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177–182. [Google Scholar] [CrossRef]
- Estahbanati, S.; Fahrenfeld, N.L. Influence of Wastewater Treatment Plant Discharges on Microplastic Concentrations in Surface Water. Chemosphere 2016, 162, 277–284. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Spatial and Temporal Distribution of MPs in Water and Sediments of a Freshwater System (Antuã River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559. [Google Scholar] [CrossRef]
- Norén Fredrik Small Plastic Particles in Coastal Swedish Waters. N-Research 2007, 11, 1–11.
- Mancuso, M.; Conti-Nibali, V.; Porcino, N. Monitoring of anthropogenic microplastic pollution in Antarctic fish (emerald rockcod) from the Terranova Bay after a quarter of century. Sci. Total Environ. 2023, 25, 167244. [Google Scholar] [CrossRef]
- Abidli, S.; Antunes, J.C.; Ferreira, J.L.; Lahbib, Y.; Sobral, P.; Trigui El Menif, N. MPs in Sediments from the Littoral Zone of the North Tunisian Coast (Mediterranean Sea). Estuar. Coast. Shelf Sci. 2018, 205, 1–9. [Google Scholar] [CrossRef]
- Reed, S.; Clark, M.; Thompson, R.; Hughes, K.A. MPs in Marine Sediments near Rothera Research Station, Antarctica. Mar. Pollut. Bull. 2018, 133, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Watteau, F.; Dignac, M.F.; Bouchard, A.; Revallier, A.; Houot, S. Microplastic Detection in Soil Amended With Municipal Solid Waste Composts as Revealed by Transmission Electronic Microscopy and Pyrolysis/GC/MS. Front. Sustain. Food Syst. 2018, 2, 81. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A Simple Method for the Extraction and Identification of Light Density MPs from Soil. Sci. Total Environ. 2018, 616–617, 1056–1065. [Google Scholar] [CrossRef]
- Rabin, M.H.; Wang, Q.; Enyoh, C.E.; Kai, X.; Sheuty, T.F. Distribution, Potential Sources, and Health Risk of MPs (MPs) in Street Dust during and after COVID-19 Lockdown in Bangladesh. Environments 2023, 10, 130. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and Potential Health Impacts of MPs and Microrubbers in Air and Street Dusts from Asaluyeh County, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic Fibers in Atmospheric Fallout: A Source of MPs in the Environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic Contamination of Tap Water, Beer, and Sea Salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Reed, C. Dawn of the Plasticene Age. New Sci. 2015, 225, 28–32. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Shafea, L.; Verla, A.W.; Verla, E.N.; Qingyue, W.; Chowdhury, T.; Paredes, M. MPs Exposure Routes and Toxicity Studies to Ecosystems: An Overview. Environ. Health Toxicol. 2020, 35, e2020004. [Google Scholar] [CrossRef]
- Galloway, T.S. Micro-and Nano-Plastics and Human Health. Mar. Anthropog. Litter 2015, 12, 343–366. [Google Scholar]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of MPs. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.; Kaplan, G.; Madsen, K.L. Air Pollution Effects on the Gut Microbiota: A Link between Exposure and Inflammatory Disease. Gut Microbes 2013, 5, 215–219. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne MPs: Consequences to Human Health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating Human Exposure to Indoor Airborne MPs Using a Breathing Thermal Manikin. Sci. Rep. 2019, 9, 8670. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Verla, A.W.; Verla, E.N.; Ibe, F.C.; Amaobi, C.E. Airborne MPs: A Review Study on Method for Analysis, Occurrence, Movement and Risks. Environ. Monit. Assess. 2019, 191, 1–17. [Google Scholar] [CrossRef]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro (Nano) Plastics: A Threat to Human Health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic Effects of Commonly Used Nanomaterials and MPs on Cerebral and Epithelial Human Cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef]
- Schwabl, P.; Koppel, S.; Konigshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various MPs in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. MPs Detected in Cirrhotic Liver Tissue. eBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, E.; Du, Z.; Peng, Z.; Han, Z.; Li, L.; Zhao, R.; Qin, Y.; Xue, M.; Li, F.; et al. Detection of Various MPs in Patients Undergoing Cardiac Surgery. Environ. Sci. Technol. 2023, 57, 10911–10918. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of MPs in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of MPs in Human Breastmilk. Polymers 2022, 14, 2700. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Huang, X.; Bi, R.; Guo, Q.; Yu, X.; Zeng, Q.; Huang, Z.; Liu, T.; Wu, H.; Chen, Y.; et al. Detection and Analysis of MPs in Human Sputum. Environ. Sci. Technol. 2022, 56, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, L.; Weng, J.; Jin, Z.; Cao, Y.; Jiang, H.; Zhang, Z. Detection and Characterization of MPs in the Human Testis and Semen. Sci. Total Environ. 2023, 877, 162713. [Google Scholar] [CrossRef]
- Pironti, C.; Notarstefano, V.; Ricciardi, M.; Motta, O.; Giorgini, E.; Montano, L. First Evidence of MPs in Human Urine, a Preliminary Study of Intake in the Human Body. Toxics 2023, 11, 40. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, J.; Zuo, R.; Xu, Q.; Qian, Y.; AN, L. Identification of MPs in Human Placenta Using Laser Direct Infrared Spectroscopy. Sci. Total Environ. 2023, 856, 159060. [Google Scholar] [CrossRef]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The Liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Kalra, A.; Yetiskul, E.; Wehrle, C.J.; Tuma, F. Physiology, Liver; St. StatPearls Publishing: Petersburg, FL, USA, 2023. [Google Scholar]
- Ogobuiro, I.; Tuma, F. Physiology, Renal. StatPearls 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538339/ (accessed on 1 November 2023).
- Soriano, R.M.; Penfold, D.; Leslie, S.W. Anatomy, Abdomen and Pelvis: Kidneys. StatPearls 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482385/ (accessed on 1 November 2023).
- Mebius, R.; Kraal, G. Structure and Function of the Spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Cesta, M.F. Normal Structure, Function, and Histology of the Spleen. Toxicol. Pathol. 2006, 34, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.; Yhome, N.; Muralidaran, Y.; Rajagopal, S.; Mishra, P. Nanoplastics Toxicity Specific to Liver in Inducing Metabolic Dysfunction—A Comprehensive Review. Genes 2023, 14, 590. [Google Scholar] [CrossRef]
- Cheng, H.; Zhenghua, D.; Yinghong, W.; Yudi, W.; Haihong, Z.; Yansong, S.; Huajing, Z.; Yanjie, W.; Hongwen, S. Immunotoxicity responses to polystyrene nanoplastics and their related mechanisms in the liver of zebrafish (Danio rerio) larvae. Environ. Int. 2022, 161, 107128. [Google Scholar] [CrossRef]
- Persiani, E.; Cecchettini, A.; Ceccherini, E.; Gisone, I.; Morales, M.A.; Vozzi, F. MPs: A Matter of the Heart (and Vascular System). Biomedicines 2023, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.A.; Trevisan, R.; Massarsky, A.; Kozal, J.S.; Levin, E.D.; Di Giulio, R.T. Maternal Transfer of NPs to Offspring in Zebrafish (Danio Rerio): A Case Study with Nanopolystyrene. Sci. Total Environ. 2018, 643, 324–334. [Google Scholar] [CrossRef]
- Pitt, J.A.; Kozal, J.S.; Jayasundara, N.; Massarsky, A.; Trevisan, R.; Geitner, N.; Wiesner, M.; Levin, E.D.; Di Giulio, R.T. Uptake, Tissue Distribution, and Toxicity of Polystyrene Nanoparticles in Developing Zebrafish (Danio Rerio). Aquat. Toxicol. 2018, 194, 185–194. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Lu, L.; Zheng, M.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Polystyrene MPs Cause Tissue Damages, Sex-Specific Reproductive Disruption and Transgenerational Effects in Marine Medaka (Oryzias Melastigma). Environ. Pollut. 2019, 254, 113024. [Google Scholar] [CrossRef]
- Acharya, S.; Rumi, S.S.; Hu, Y.; Abidi, N. Microfibers from Synthetic Textiles as a Major Source of MPs in the Environment: A Review. Text. Res. J. 2021, 91, 2136–2156. [Google Scholar] [CrossRef]
- Turco, M.Y.; Moffett, A. Development of the Human Placenta. Development 2019, 146, dev163428. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. What Is the Placenta? Am. J. Obstet. Gynecol. 2015, 213, S6.e1–S6.e4. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Enyoh, C.E.; Duru, C.E.; Ovuoraye, P.E.; Wang, Q. Evaluation of NPs Toxicity to the Human Placenta in Systems. J. Hazard. Mater. 2023, 446, 130600. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Ovuoraye, P.E.; Qingyue, W.; Wang, W. Examining the Impact of NPs and PFAS Exposure on Immune Functions through Inhibition of Secretory Immunoglobin A in Human Breast Milk. J. Hazard. Mater. 2023, 459, 132103. [Google Scholar] [CrossRef] [PubMed]
- Dunne-Castagna, V.P.; Mills, D.A.; Lönnerdal, B. Effects of Milk Secretory Immunoglobulin A on the Commensal Microbiota. Nestle Nutr. Inst. Workshop Ser. 2020, 94, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Sergi, C. Sputum Analysis. In The Pocketbook of Chest Physiotherapy; Jaypee: New Delhi, India, 2023; Volume 43. [Google Scholar] [CrossRef]
- Ostrer, H.; Huang, H.Y.; Masch, R.J.; Shapiro, E. A Cellular Study of Human Testis Development. Sex. Dev. 2007, 1, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yan, M.; Pan, C.; Liu, Z.; Sha, X.; Jiang, C.; Li, L.; Pan, M.; Li, D.; Han, X.; et al. Chronic Exposure to Polystyrene Microplastics Induced Male Reproductive Toxicity and Decreased Testosterone Levels via the LH-Mediated LHR/CAMP/PKA/StAR Pathway. Part. Fibre Toxicol. 2022, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Wang, F.; Liu, T.; Wang, Z. Reproductive Toxicity of Polystyrene Microplastics: In Vivo Experimental Study on Testicular Toxicity in Mice. J. Hazard. Mater. 2021, 405, 124028. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wang, X.; Yang, L.; Zhang, J.; Wang, N.; Xu, F.; Hou, Y.; Zhang, H.; Zhang, L. Polystyrene Microplastics Cause Granulosa Cells Apoptosis and Fibrosis in Ovary through Oxidative Stress in Rats. Toxicology 2021, 449, 152665. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kurobe, T.; Flores, I.; Teh, S.J. Early Warning Signs of Endocrine Disruption in Adult Fish from the Ingestion of Polyethylene with and without Sorbed Chemical Pollutants from the Marine Environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster Reproduction Is Affected by Exposure to Polystyrene MPs. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to Polystyrene MPs Causes Reproductive Toxicity through Oxidative Stress and Activation of the P38 MAPK Signaling Pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Sun, Y.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Long-Term Phototransformation of MPs under Simulated Sunlight Irradiation in Aquatic Environments: Roles of Reactive Oxygen Species. Water Res. 2020, 173, 115564. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Soliman, H.A.M.; Osman, A.G.M.; Sayed, A.E.D.H. Antioxidants and Molecular Damage in Nile Tilapia (Oreochromis Niloticus) after Exposure to MPs. Environ. Sci. Pollut. Res. 2020, 27, 14581–14588. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene MPs Induced Male Reproductive Toxicity in Mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef]
- Jiang, D.; Im, H.-J.; Boleyn, M.E.; England, C.G.; Ni, D.; Kang, L.; Engle, J.W.; Huang, P.; Lan, X.; Cai, W. Efficient Renal Clearance of DNA Tetrahedron Nanoparticles Enables Quantitative Evaluation of Kidney Function. Nano Res. 2019, 12, 637–642. [Google Scholar] [CrossRef]
- Liu, J.; Ren, L.; Wei, J.; Zhang, J.; Zhu, Y.; Li, X.; Jing, L.; Duan, J.; Zhou, X.; Sun, Z. Fine Particle Matter Disrupts the Blood–Testis Barrier by Activating TGF-Β3/P38 MAPK Pathway and Decreasing Testosterone Secretion in Rat. Environ. Toxicol. 2018, 33, 711–719. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. Sertoli-Sertoli and Sertoli-Germ Cell Interactions and Their Significance in Germ Cell Movement in the Seminiferous Epithelium during Spermatogenesis. Endocr. Rev. 2004, 25, 747–806. [Google Scholar] [CrossRef]
- Brouard, V.; Guénon, I.; Bouraima-Lelong, H.; Delalande, C. Differential Effects of Bisphenol A and Estradiol on Rat Spermatogenesis’ Establishment. Reprod. Toxicol. 2016, 63, 49–61. [Google Scholar] [CrossRef]
- Queremel Milani DA, J.I. Urinalysis. In A Medication Guide to Internal Medicine Tests and Procedures; Elsevier: Amsterdam, The Netherlands, 2023; pp. 261–264. [Google Scholar] [CrossRef]
- Maciel, A.T.; Vitorio, D.; Osawa, E.A. Urine biochemistry assessment in the sequential evaluation of renal function: Time to think outside the box. Front Med (Lausanne) 2022, 26, 912877. [Google Scholar] [CrossRef]
- Shuaib Ibrahim, Y.; Tuan Anuar, S.; Azmi, A.A.; Mohd Afiq Wan Mohd Khalik, W.; Lehata, S.; Rabaah Hamzah, S.; Ismail, D.; Feei Ma, Z.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of MPs in Human Colectomy Specimens. JGH Open 2020, 5, 116–121. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to MPs: An Overview on Possible Human Health Effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Physiological and Pathological Interactions between Liver and Kidney. Liver Syst. Dis. 2016, 221–249. [Google Scholar] [CrossRef]
- McManaman, J.L. Lipid Transport in the Lactating Mammary Gland. J. Mammary Gland Biol. Neoplasia 2014, 19, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Severin, E.S.; Posypanova, G.A. [Molecular Physiology of Receptor Mediated Endocytosis and Its Role in Overcoming Multidrug Resistance]. Ross. Fiziol. Zh. Im. IM Sechenova 2011, 97, 553–565. [Google Scholar]
- Hallett, M.B. An Introduction to Phagocytosis. Adv. Exp. Med. Biol. 2020, 1246, 1–7. [Google Scholar] [CrossRef]
- Zhu, D.; Gong, X.; Miao, L.; Fang, J.; Reports, J.Z.-S.C. Efficient Induction of Syncytiotrophoblast Layer II Cells from Trophoblast Stem Cells by Canonical Wnt Signaling Activation. Stem Cell Rep. 2017, 9, 2034–2049. [Google Scholar] [CrossRef]
- Yan Cheng, C.; Mruk, D.D. The Blood-Testis Barrier and Its Implications for Male Contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef]
- Schindell, B.; Webb, A.; Kindrachuk, J. Persistence and Sexual Transmission of Filoviruses. Viruses 2018, 10, 683. [Google Scholar] [CrossRef]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B.; Fricker, G.; Deli, M.A. Detection of Microplastic in Human Placenta and Meconium in a Clinical Setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef]
- Field, D.T.; Green, J.L.; Bennett, R.; Jenner, L.C.; Sadofsky, L.R.; Chapman, E.; Loubani, M.; Rotchell, J.M. MPs in the Surgical Environment. Environ. Int. 2022, 170, 107630. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.J.; Ana, C.B.; Theodore, B.H.; Monique, E.J.; Albert, A.K.; Antonio, R.; Montoro, B.; Joanna, M.; Matthias, R.; Jian, Z.; et al. Potential Artifacts and Control Experiments in Toxicity Tests of Nanoplastic and Microplastic Particles. Environ. Sci. Technol. 2022, 56, 15192–15206. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).