Micronucleus Induction in Vicia faba Root Tips by Crude Oil-Polluted Soil from Ecuadorian Amazon
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Studied Area
2.2. Root Germination and Exposure Protocol
2.3. Micronuclei Assay
2.4. Statistical Analysis
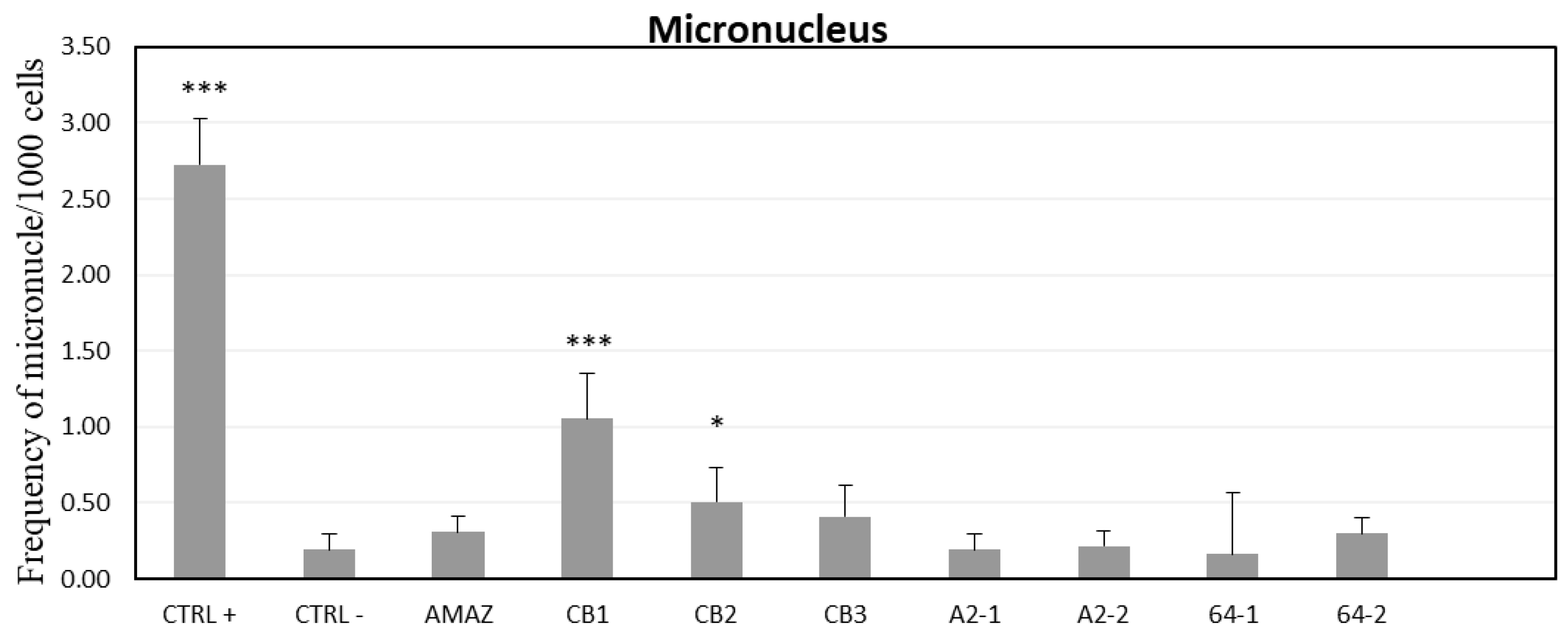
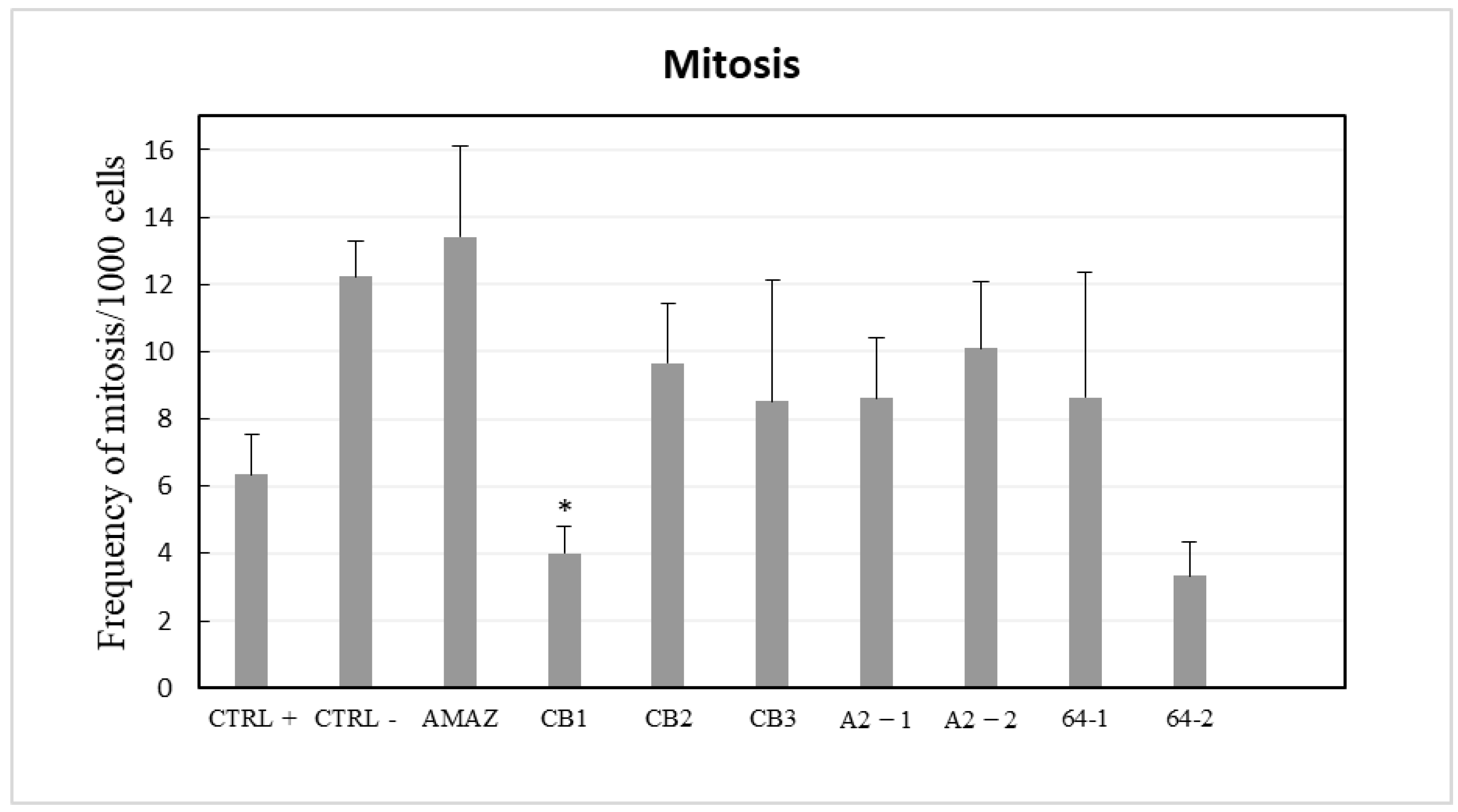
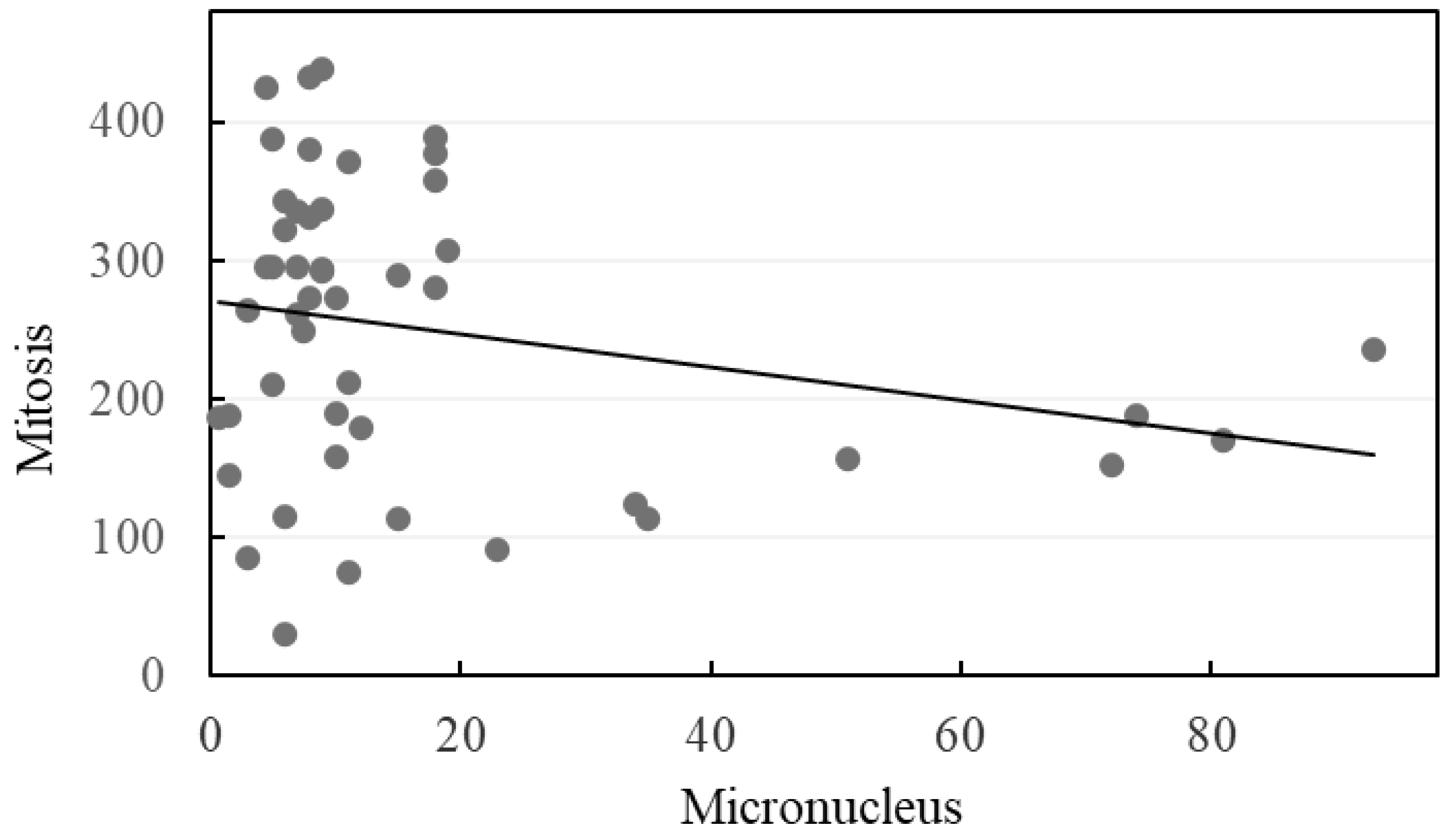
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ter Steege, H.; Prado, P.I.; de Lima, R.A.F.; Pos, E.; de Souza Coelho, L.; de Andrade Lima Filho, D.; Salomão, R.P.; Amaral, I.L.; de Almeida Matos, F.D.; Castilho, C.V.; et al. Biased-Corrected Richness Estimates for the Amazonian Tree Flora. Sci. Rep. 2020, 10, 10130. [Google Scholar] [CrossRef] [PubMed]
- Hurtig, A.K.; San Sebastian, M.; Soto, A.; Shingre, A.; Zambrano, D.; Guerrero, W. Pesticide Use among Farmers in the Amazon Basin of Ecuador. Arch. Environ. Health: Int. J. 2010, 58, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Kulmann-Leal, B.; Kaminski, V.L.; Valverde-Villegas, J.M.; da Veiga, A.B.G.; Spilki, F.R.; Fearnside, P.M.; Caesar, L.; Giatti, L.L.; Wallau, G.L.; et al. Beyond Diversity Loss and Climate Change: Impacts of Amazon Deforestation on Infectious Diseases and Public Health. An. Acad. Bras. Cienc. 2020, 92, e20191375. [Google Scholar] [CrossRef]
- Maurice, L.; López, F.; Becerra, S.; Jamhoury, H.; le Menach, K.; Dévier, M.H.; Budzinski, H.; Prunier, J.; Juteau-Martineau, G.; Ochoa-Herrera, V.; et al. Drinking Water Quality in Areas Impacted by Oil Activities in Ecuador: Associated Health Risks and Social Perception of Human Exposure. Sci. Total Environ. 2019, 690, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Maestripieri, N.; Saqalli, M. Assessing Health Risk Using Regional Mappings Based on Local Perceptions: A Comparative Study of Three Different Hazards. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 721–735. [Google Scholar] [CrossRef]
- Arellano, P.; Tansey, K.; Balzter, H.; Tellkamp, M. Plant Family-Specific Impacts of Petroleum Pollution on Biodiversity and Leaf Chlorophyll Content in the Amazon Rainforest of Ecuador. PLoS ONE 2017, 12, e0169867. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.I.; Arévalo-Jaramillo, A.P.; Espinosa, C.I.; Bailon-Moscoso, N. Is the Anemia in Men an Effect of the Risk of Crude Oil Contamination? Toxicol. Rep. 2022, 9, 480–486. [Google Scholar] [CrossRef]
- San Sebastián, M.; Armstrong, B.; Stephens, C. [Health of Women Living near Oil Wells and Oil Production Stations in the Amazon Region of Ecuador]. Rev. Panam. Salud Publica 2001, 9, 375–384. [Google Scholar] [CrossRef]
- Webb, J.; Coomes, O.T.; Mergler, D.; Ross, N.A. Levels of 1-Hydroxypyrene in Urine of People Living in an Oil Producing Region of the Andean Amazon (Ecuador and Peru). Int. Arch. Occup. Environ. Health 2018, 91, 105–115. [Google Scholar] [CrossRef]
- Vasco-Viteri, S.; Cabrera, M.; Pérez-González, A.; Hauser-Davis, R.A.; Moulatlet, G.M.; Capparelli, M.V. Metal Bioaccumulation and Genotoxicity in Oreochromis Niloticus Reared in Farming Pools Influenced by Mining Activities in Napo, in the Ecuadorian Amazonia. Chemosphere 2023, 335, 139157. [Google Scholar] [CrossRef]
- Galarza, E.; Moulatlet, G.M.; Rico, A.; Cabrera, M.; Pinos-Velez, V.; Pérez-González, A.; Capparelli, M.V. Human Health Risk Assessment of Metals and Metalloids in Mining Areas of the Northeast Andean Foothills of the Ecuadorian Amazon. Integr. Environ. Assess. Manag. 2023, 19, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Mestanza-Ramón, C.; Jiménez-Oyola, S.; Montoya, A.V.G.; Vizuete, D.D.C.; D’Orio, G.; Cedeño-Laje, J.; Straface, S. Assessment of Hg Pollution in Stream Waters and Human Health Risk in Areas Impacted by Mining Activities in the Ecuadorian Amazon. Environ. Geochem. Health 2023, 45, 7183–7197. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Rivas, F.; Paul, N.; Spiegel, J.; Henderson, S.B.; Parrott, L.; Delgado-Ron, J.A.; Echeverri, A.; van den Bosch, M. Mapping Potential Population-Level Pesticide Exposures in Ecuador Using a Modular and Scalable Geospatial Strategy. Geohealth 2023, 7, e2022GH000775. [Google Scholar] [CrossRef] [PubMed]
- Guamán-Ortiz, L.M.; Romero-Benavides, J.C.; Suarez, A.I.; Torres-Aguilar, S.; Castillo-Veintimilla, P.; Samaniego-Romero, J.; Ortiz-Diaz, K.; Bailon-Moscoso, N. Cytotoxic Property of Grias Neuberthii Extract on Human Colon Cancer Cells: A Crucial Role of Autophagy. Evid. Based Complement. Alternat Med. 2020, 2020, 1565306. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, P. Medicinal Plants of the Achuar (Jivaro) of Amazonian Ecuador: Ethnobotanical Survey and Comparison with Other Amazonian Pharmacopoeias. J. Ethnopharmacol. 2015, 164, 78–88. [Google Scholar] [CrossRef]
- Webb, J.; Coomes, O.T.; Ross, N.; Mergler, D. Mercury Concentrations in Urine of Amerindian Populations near Oil Fields in the Peruvian and Ecuadorian Amazon. Environ. Res. 2016, 151, 344–350. [Google Scholar] [CrossRef]
- Haghsheno, H.; Arabani, M. Geotechnical Properties of Oil-Polluted Soil: A Review. Environ. Sci. Pollut. Res. 2022, 29, 32670–32701. [Google Scholar] [CrossRef]
- Borah, G.; Deka, H. Crude Oil Associated Heavy Metals (HMs) Contamination in Agricultural Land: Understanding Risk Factors and Changes in Soil Biological Properties. Chemosphere 2023, 310, 136890. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Liu, Y.; Ma, M.; Zhang, T.; Zheng, X.; Zong, M. Ecological Risk Assessment of Heavy Metals in Soils Surrounding Oil Waste Disposal Areas. Environ. Monit. Assess. 2016, 188, 125. [Google Scholar] [CrossRef]
- Nwaichi, E.O.; Wegwu, M.O.; Nwosu, U.L. Distribution of Selected Carcinogenic Hydrocarbon and Heavy Metals in an Oil-Polluted Agriculture Zone. Environ. Monit. Assess. 2014, 186, 8697–8706. [Google Scholar] [CrossRef]
- Barraza, F.; Schreck, E.; Lévêque, T.; Uzu, G.; López, F.; Ruales, J.; Prunier, J.; Marquet, A.; Maurice, L. Cadmium Bioaccumulation and Gastric Bioaccessibility in Cacao: A Field Study in Areas Impacted by Oil Activities in Ecuador. Environ. Pollut. 2017, 229, 950–963. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, N.; Gill, B.S.; Grover, I.S.; Murin, A.; Osiecka, R.; Sandhu, S.S.; Andersson, H.C. Vicia Faba Chromosomal Aberration Assay. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1994, 310, 231–247. [Google Scholar] [CrossRef] [PubMed]
- White, P.A.; Claxton, L.D. Mutagens in Contaminated Soil: A Review. Mutat. Res./Rev. Mutat. Res. 2004, 567, 227–345. [Google Scholar] [CrossRef] [PubMed]
- Marcato-Romain, C.E.; Pinelli, E.; Pourrut, B.; Silvestre, J.; Guiresse, M. Assessment of the Genotoxicity of Cu and Zn in Raw and Anaerobically Digested Slurry with the Vicia Faba Micronucleus Test. Mutat. Res. 2009, 672, 113–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duan, C.; Hu, B.; Guo, T.; Luo, M.; Xu, X.; Chang, X.; Wen, C.; Meng, L.; Yang, L.; Wang, H. Changes of Reliability and Efficiency of Micronucleus Bioassay in Vicia Faba after Exposure to Metal Contamination for Several Generations. Environ. Exp. Bot. 2000, 44, 83–92. [Google Scholar] [CrossRef]
- Klein, P.; Chauvey, L.; Kallerhoff, J.; Pinelli, E.; Morard, M.; Silvestre, J. A Tool Derived from the Vicia Faba Micronucleus Assay, to Assess Genotoxicity, Cytotoxicity or Biostimulation of Novel Compounds Used in Agriculture. Agronomy 2021, 11, 321. [Google Scholar] [CrossRef]
- Ministerio de Energía y Recursos Naturales no Renovables del Ecuador. Informe Anual Del Potencial Hidrocarburífero Del Ecuador 2018. Available online: https://www.recursosyenergia.gob.ec/wp-content/uploads/2019/11/Informe-Anual-del-Potencial-Hidrocarburi%CC%81fero-del-Ecuador-2018.pdf (accessed on 30 January 2023).
- Durango-Cordero, J.; Saqalli, M.; Laplanche, C.; Locquet, M.; Elger, A. Spatial Analysis of Accidental Oil Spills Using Heterogeneous Data: A Case Study from the North-Eastern Ecuadorian Amazon. Sustainability 2018, 10, 4719. [Google Scholar] [CrossRef]
- Cura Di, A.; Gustavino, B.; Caciolli, S.; Mancini, L. Istituto Superiore di Sanità Linea Guida Del Test Dei Micronuclei in Vicia Faba per La Valutazione Di Effetti Mutageni in Acque Dolci e Sedimenti. Rapp. Istisan 2013, 5, 323380. [Google Scholar]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Johann, S.; Goßen, M.; Behnisch, P.A.; Hollert, H.; Seiler, T.B. Combining Different In Vitro Bioassays to Evaluate Genotoxicity of Water-Accommodated Fractions from Petroleum Products. Toxics 2020, 8, 45. [Google Scholar] [CrossRef]
- Fenech, M. The in Vitro Micronucleus Technique. Mutat. Res. 2000, 455, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Arellano, P.; Tansey, K.; Balzter, H.; Boyd, D.S. Detecting the Effects of Hydrocarbon Pollution in the Amazon Forest Using Hyperspectral Satellite Images. Environ. Pollut. 2015, 205, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.; Shiu, W.-Y.; Shiu, W.-Y.; Lee, S.C. Polynuclear Aromatic Hydrocarbons (PAHs) and Related Aromatic Hydrocarbons. In Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals; CRC Press: Boca Raton, FL, USA, 2020; pp. 629–932. [Google Scholar]
- Huesemann, M.H. Predictive Model for Estimating the Extent of Petroleum Hydrocaihon Biodegradation in Contaminated Soils. Environ. Sci. Technol. 1995, 29, 7–18. [Google Scholar] [CrossRef]
- Yang Id, J.-K.; Liang, J.-F.; Xiao, L.-M.; Yang, Y.; Chao, Q.-F. Vertical Stratification of Bacteria and the Chemical Compounds in Crude Oil-Contaminated Soil Layers of the Semi-Deserted Dzungharian Basin. PLoS ONE 2018, 13, e0203919. [Google Scholar] [CrossRef] [PubMed]
- Amro, M.M. Factors Affecting Chemical Remediation of Oil Contaminated Water-Wetted Soil. Chem. Eng. Technol. 2004, 27, 890–894. [Google Scholar] [CrossRef]
- da Silva Correa, H.; Blum, C.T.; Galvão, F.; Maranho, L.T. Effects of Oil Contamination on Plant Growth and Development: A Review. Environ. Sci. Pollut. Res. 2022, 29, 43501–43515. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Mendelssohn, I.A. The Combined Effects of Phytoremediation and Biostimulation in Enhancing Habitat Restoration and Oil Degradation of Petroleum Contaminated Wetlands. Ecol. Eng. 1998, 10, 263–274. [Google Scholar] [CrossRef]
- Kumar, P.S.; Minhas, P.S.; Govindasamy, V.; Choudhary, R.L. Influence of Moisture Stress on Growth, Development, Physiological Process and Quality of Fruits and Vegetables and Its Management Strategies. In Approaches to Plant Stress and Their Management; Springer: Berlin/Heidelberg, Germany, 2014; pp. 125–148. [Google Scholar]
- Ramirez, M.I.; Arevalo, A.P.; Sotomayor, S.; Bailon-Moscoso, N. Contamination by Oil Crude Extraction—Refinement and Their Effects on Human Health. Environ. Pollut. 2017, 231, 415–425. [Google Scholar] [CrossRef]
- Webb, J.; Coomes, O.T.; Mainville, N.; Mergler, D. Mercury Contamination in an Indicator Fish Species from Andean Amazonian Rivers Affected by Petroleum Extraction. Bull. Environ. Contam. Toxicol. 2015, 95, 279–285. [Google Scholar] [CrossRef]
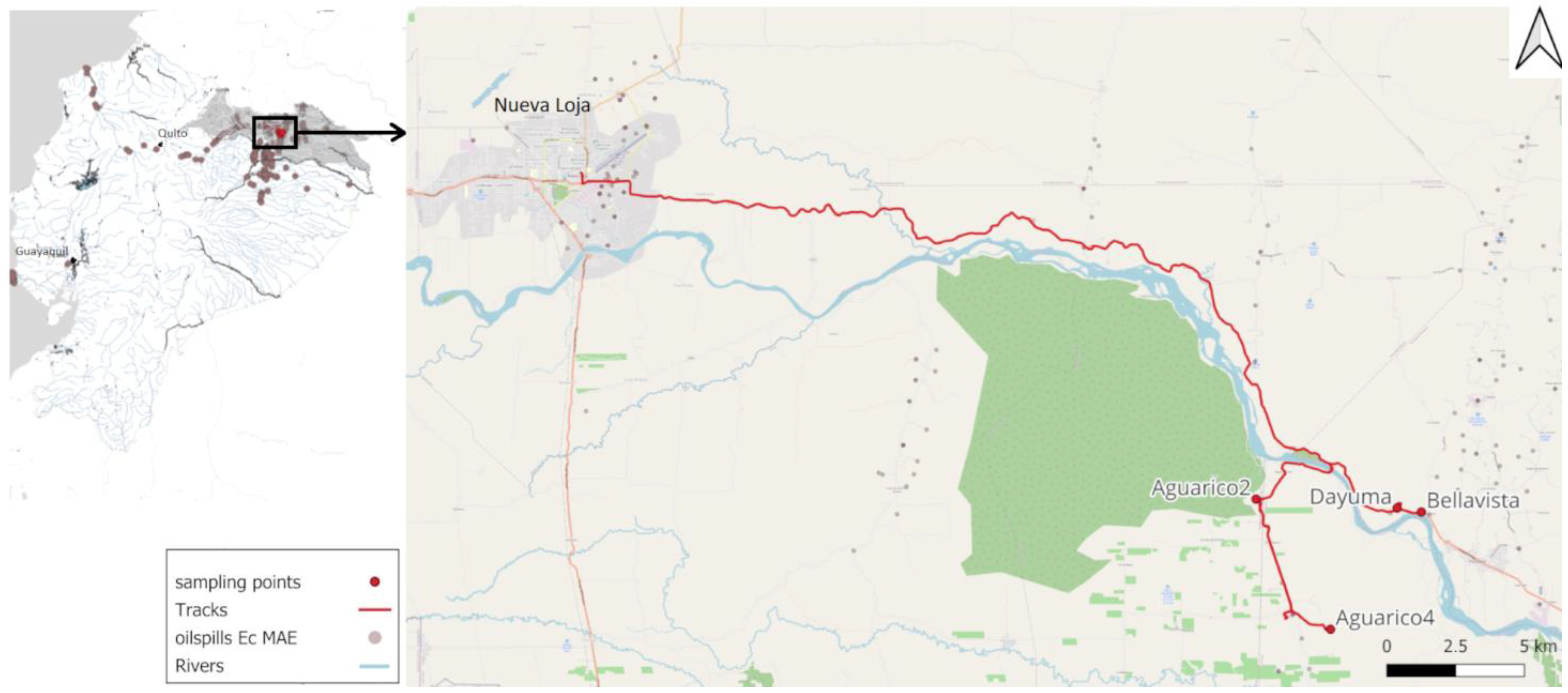

| Area | Point Name | Description | Crude Oil Presence | GPS (Lat, Long) |
|---|---|---|---|---|
| Aguarico 2 | A2-1 | Superficial soil | Yes | −0.02079, −76.66181 |
| A2-2 | Soil from 30 cm deep | Yes | −0.02079, −76.66181 | |
| Dayuma | CB1 | 50 cm deep soil from a cocoa crop field from a small-scale farm | Yes | - |
| CB2 | Superficial soil from a cocoa crop field from a small-scale farm | Yes | - | |
| CB3 | 125 cm deep soil outside the crop field from a small-scale farm. | Yes | - | |
| Aguarico 4 | 64-1 | Superficial wastewater from open-air pool | Yes | −0.06332, −76.63745 |
| 64-2 | Soil from 20 cm deep from the open-air pool | Yes | −0.06332, −76.63745 | |
| Bellavista | Amaz | Superficial soil from a residential area bordering the rainforest | No | −0.02501, −76.6078 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coronel Vargas, G.; Izzotti, A.; Rosano, C.; La Maestra, S. Micronucleus Induction in Vicia faba Root Tips by Crude Oil-Polluted Soil from Ecuadorian Amazon. Environments 2023, 10, 195. https://doi.org/10.3390/environments10110195
Coronel Vargas G, Izzotti A, Rosano C, La Maestra S. Micronucleus Induction in Vicia faba Root Tips by Crude Oil-Polluted Soil from Ecuadorian Amazon. Environments. 2023; 10(11):195. https://doi.org/10.3390/environments10110195
Chicago/Turabian StyleCoronel Vargas, Gabriela, Alberto Izzotti, Camillo Rosano, and Sebastiano La Maestra. 2023. "Micronucleus Induction in Vicia faba Root Tips by Crude Oil-Polluted Soil from Ecuadorian Amazon" Environments 10, no. 11: 195. https://doi.org/10.3390/environments10110195
APA StyleCoronel Vargas, G., Izzotti, A., Rosano, C., & La Maestra, S. (2023). Micronucleus Induction in Vicia faba Root Tips by Crude Oil-Polluted Soil from Ecuadorian Amazon. Environments, 10(11), 195. https://doi.org/10.3390/environments10110195









