Spontaneous Subdural Hematoma and Behavioral Changes Due to a Dural Arteriovenous Fistula. A Case Report and Literature Review
Abstract
1. Introduction
2. Method
3. Clinical Case
3.1. Laboratory Studies
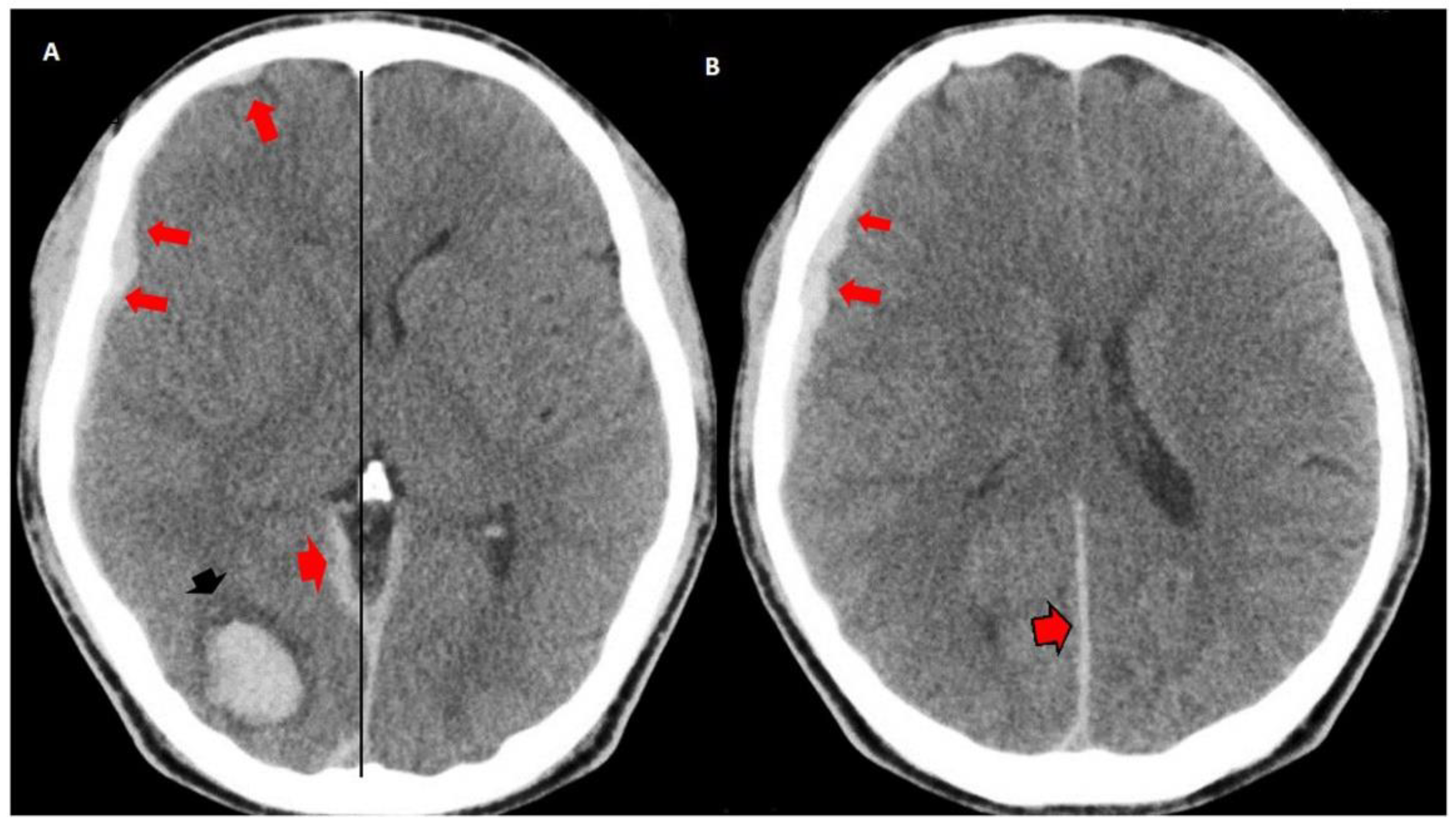
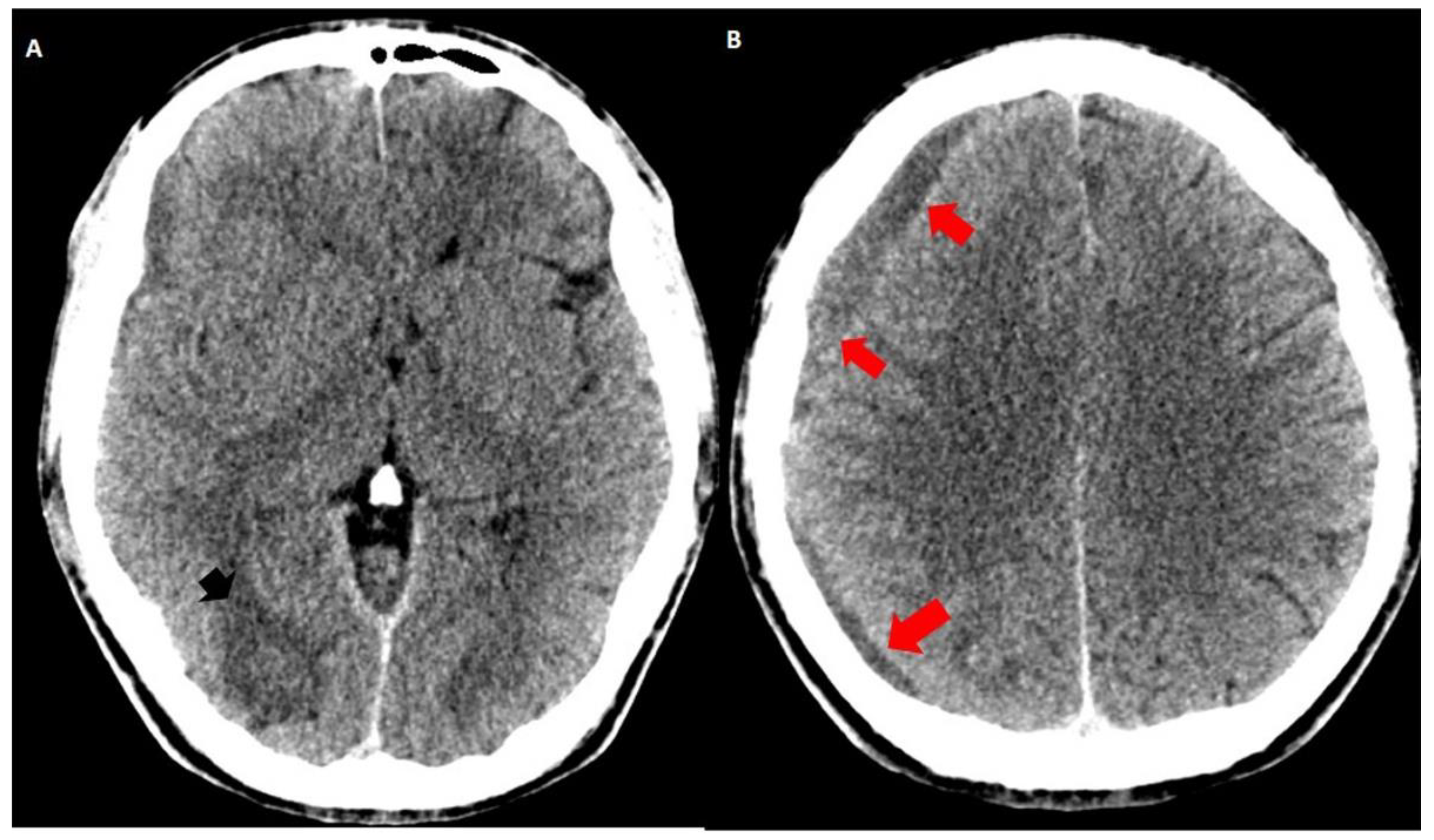
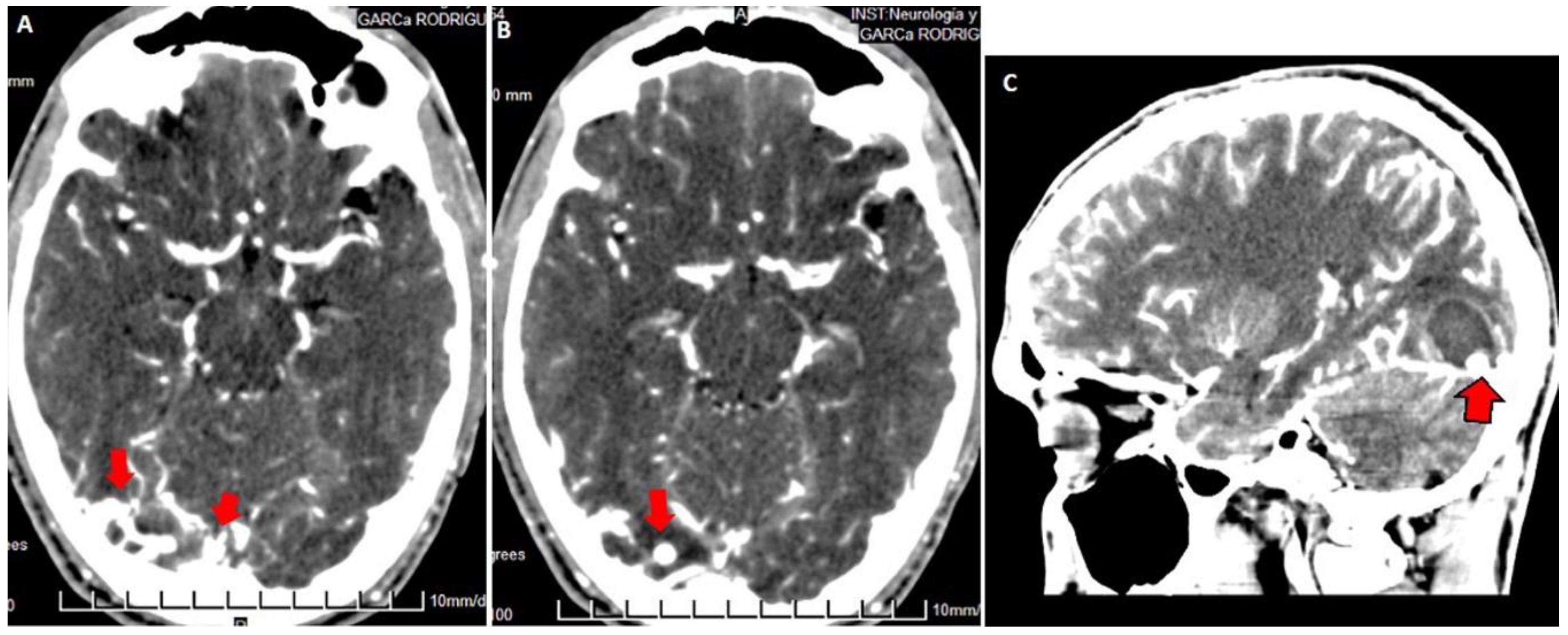
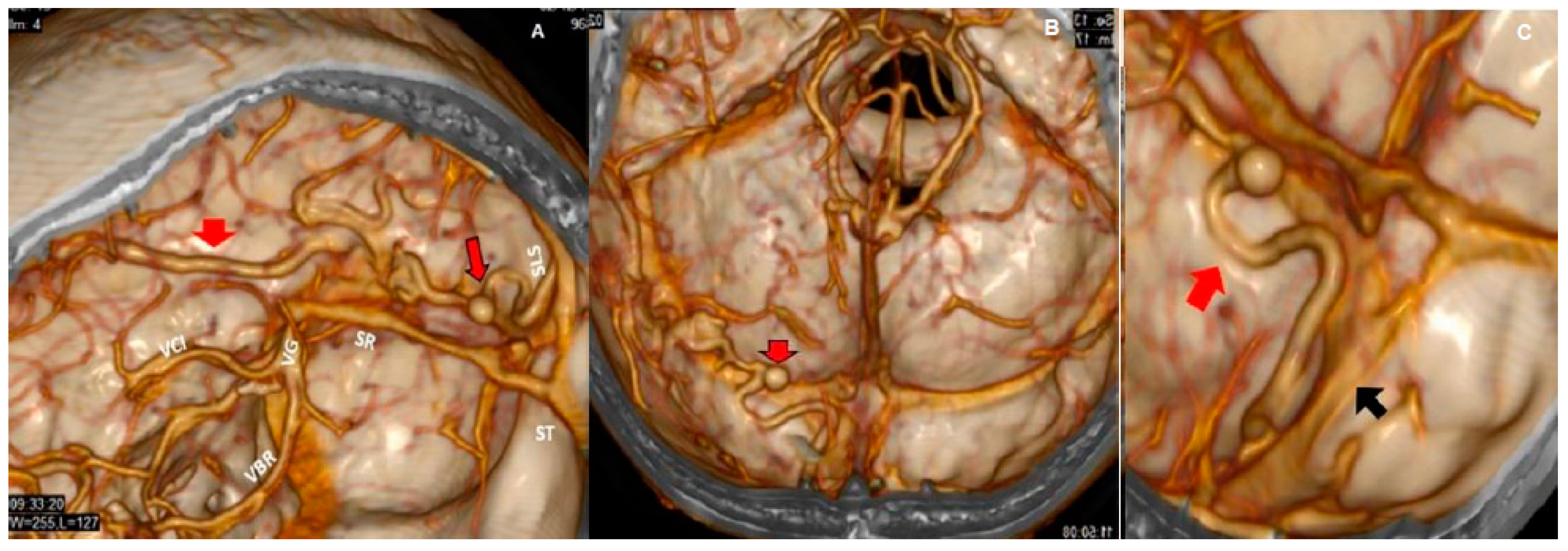
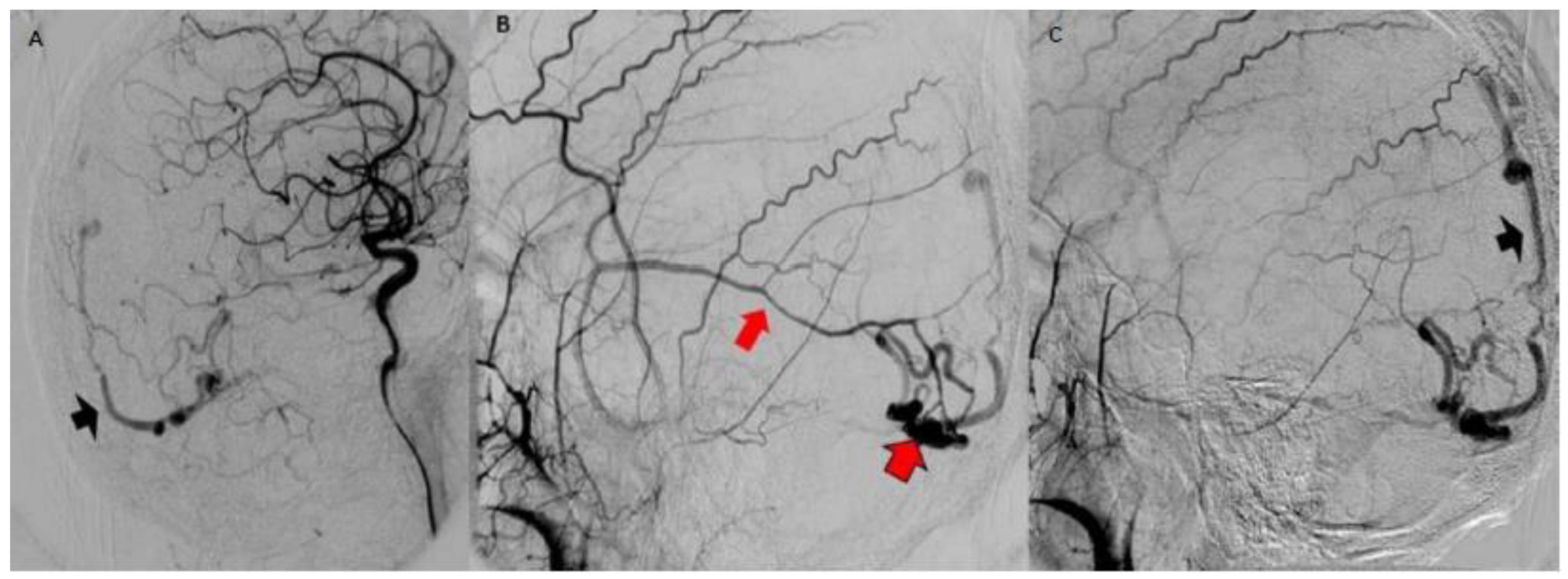
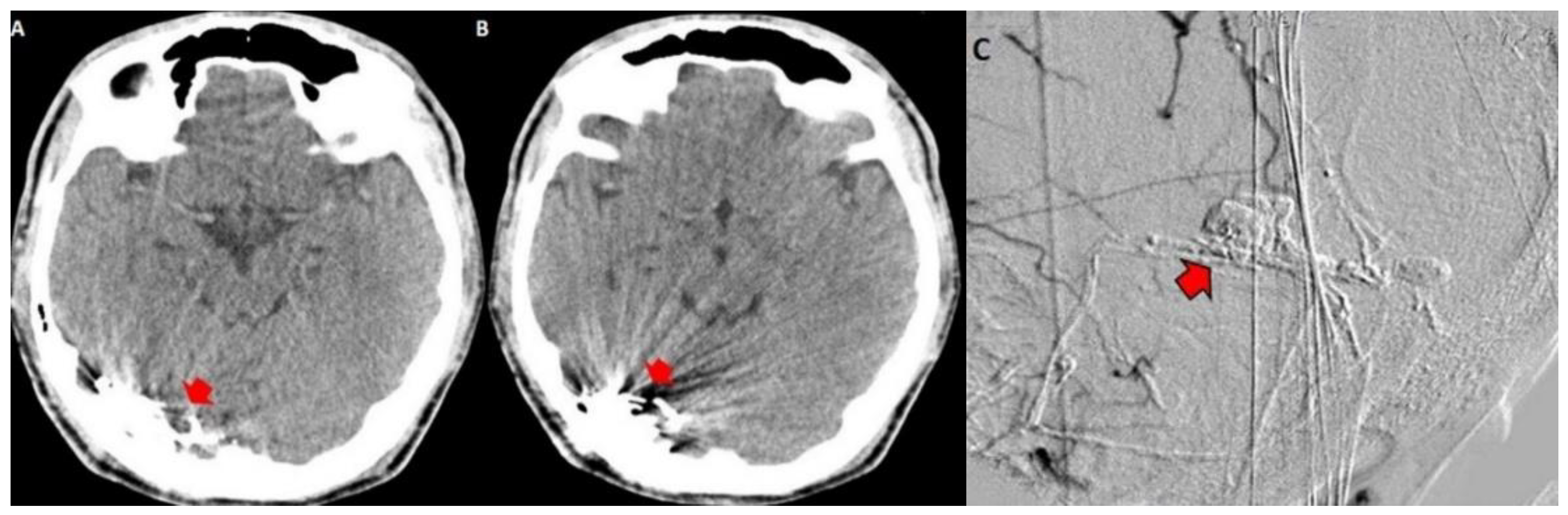
3.2. Imaging Studies
4. Discussion and Literature Review
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chung, S.; Kim, J.; Kim, J.; Lee, S.; Kwon, S.; Lee, M.; Suh, D. Intracranial dural arteriovenous fistulas: Analysis of 60 patients. Cerebrovasc. Dis. 2002, 13, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.J.; Han, M.H.; Kang, H.S.; Chang, K.H. MR imaging findings of intracranial dural arteriovenous fistulas: Relations with venous drainage patterns. Am. J. Neuroradiol. 2005, 26, 2500–2507. [Google Scholar] [PubMed]
- Quintanilla, J.O.; Pérez, R.F.O.; Herrera, V.C.; García, A.G.; Jarrín, I.G.; Deyá, A.M. 31 Congreso Nacional SERAM. In Proceedings of the Fístulas Arteriovenosas Durales: Historia Natural, diagnóstico, caracterización y tratamiento, Granada, Spain, 24–28 May 2012. [Google Scholar]
- Cognard, C.; Gobin, Y.P.; Pierot, L.; Bailly, A.L.; Houdart, E.; Casasco, A.; Chiras, J.; Merland, J.J. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995, 194, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Borden, J.A.; Wu, J.K.; Shucart, W.A. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J. Neurosurg. 1995, 82, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.D.; Wiebers, D.O.; Torner, J.C.; O’Fallon, W.M. Incidence and prevalence of intracranial vascular malformations in Olmsted County, Minnesota, 1965 to 1992. Neurology 1996, 46, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Satomi, J.; Satoh, K. Epidemiology and etiology of dural arteriovenous fistula. Brain Nerve 2008, 60, 883–886. [Google Scholar] [PubMed]
- Kobayashi, A.; Al-Shahi Salman, R. Prognosis and treatment of intracranial dural arteriovenous fistulae: A systematic review and meta-analysis. Int. J. Stroke 2014, 9, 670–677. [Google Scholar] [CrossRef]
- Gross, B.; Du, R. Natural history of cerebral arteriovenous malformations: A meta-analysis. J. Neurosurg. 2013, 118, 437–443. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Sugiu, K.; Hishikawa, T.; Haruma, J.; Tokunaga, K.; Date, I.; Kuwayama, N.; Sakai, N. Epidemiology of Dural Arteriovenous Fistula in Japan: Analysis of Japanese Registry of Neuroendovascular Therapy (JR-NET2). Neurol. Med. Chir. (Tokyo) 2014, 54 (Suppl. 2), 63–71. [Google Scholar] [CrossRef]
- Hou, K.; Ji, T.; Guo, Y.; Xu, B.; Xu, K.; Yu, J. Current Status of Endovascular Treatment for Dural Arteriovenous Fistulas in the Superior Sagittal Sinus Region: A Systematic Review of the Literature. World Neurosurg. 2019, 122, 133–143. [Google Scholar] [CrossRef]
- Piippo, A.; Niemelä, M.; van Popta, J.; Kangasniemi, M.; Rinne, J.; Jääskeläinen, J.; Hernesniemi, J. Characteristics and long-term outcome of 251 patients with dural arteriovenous fistulas in a defined population. J. Neurosurg. 2013, 118, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, F.; Iaconetta, G.; Sardo, L.; Briganti, F. Dural arteriovenous malformation associated with recurrent subdural haematoma and intracranial hypertension. Br. J. Neurosurg. 2001, 15, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Oishi, M.; Mizutani, T.; Maejima, S.; Mori, T. Dural arteriovenous fistula on the convexity presenting with pure acute subdural hematoma. Acta Neurol. Belg. 2010, 110, 190–192. [Google Scholar] [PubMed]
- Li, C.; Wang, Y.; Li, Y.; Jiang, C.; Yang, X.; Wu, Z. Clinical and Angioarchitectural Risk Factors Associated with Intracranial Hemorrhage in Dural Arteriovenous Fistulas: A Single-Center Retrospective Study. PLoS ONE 2015, 10, e0131235. [Google Scholar] [CrossRef] [PubMed]
- De Aguiar, G.B.; Veiga, J.C.; Silva, J.M.; Conti, M.L. Spontaneous acute subdural hematoma: A rare presentation of a dural intracranial fistula. J. Clin. Neurosci. 2016, 25, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, S.; Ishihara, S.; Yamane, F.; Kanazawa, R.; Ishihara, H. Dural arteriovenous fistula presenting as an acute subdural hemorrhage that subsequently progressed to a chronic subdural hemorrhage: Case report. Minim. Invasive Neurosurg. 2009, 52, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H.; Lopes, M.; Janosevic, V.; Sichez, J.P.; Faillot, T.; Capelle, L.; Ismail, M.; Bitar, A.; Arthuis, F.; Fohanno, D. Early rebleeding from intracranial dural arteriovenous fistulas: Report of 20 cases and review of the literature. J. Neurosurg. 1999, 90, 78–84. [Google Scholar] [CrossRef]
- Kitazono, M.; Yamane, K.; Toyota, A.; Okita, S.; Kumano, K.; Hashimoto, N. A case of dural arteriovenous fistula associated with subcortical and subdural hemorrhage. No Shinkei Geka. Neurol. Surg. 2010, 38, 757–762. [Google Scholar]
- Saito, A.; Kawaguchi, T.; Sasaki, T.; Nishijima, M. A Case of Dural Arteriovenous Fistula Presenting as Acute Subdural Hematoma. Case Rep. Neurol. 2014, 6, 122–125. [Google Scholar] [CrossRef]
- Kominato, Y.; Matsui, K.; Hata, Y.; Matsui, K.; Kuwayama, N.; Ishizawa, S.; Takizawa, H. Acute subdural hematoma due to arteriovenous malformation primarily in dura mater: A case report. Leg. Med. 2004, 6, 256–260. [Google Scholar] [CrossRef]
- Paredes, I.; Martinez-Perez, R.; Munarriz, P.M.; Castaño-León, A.M.; Campollo, J.; Alén, J.F.; Lobato, R.D.; Lagares, A. Fístulas durales arteriovenosas intracraneales. Experiencia con 81 casos y revisión de la literatura. Neurocirugía 2013, 24, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Hasumi, T.; Fukushima, T.; Haisa, T.; Yonemitsu, T.; Waragai, M. Focal dural arteriovenous fistula (DAVF) presenting with progressive cognitive impairment including amnesia and alexia. Intern. Med. 2007, 46, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Van Munster, C.E.; van den Ber, R.; Weinstein, H.C. A Dural Fistula as a Treatable Cause of Cognitive Impairment. Neurohospitalist 2014, 4, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.B.; Maia, O., Jr.; Correa, J.L.; Siqueira, S.B.; Christoph Dde, H.; Landeiro, J.A. Dural arteriovenous fistula presenting as thalamic dementia. Arquivos de Neuro-Psiquiatria 2008, 66, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.; Bagley, L.; Galetta, S.; Glosser, G.; Lieberman, A.; Trojanowski, J.; Sinson, G.; Stecker, M.; Zager, E.; Raps, E.; et al. Dementia resulting from dural arteriovenous fistulas: The pathologic findings of venous hypertensive encephalopathy. Am. J. Neuroradiol. 1998, 19, 1267–1273. [Google Scholar]
- Magot, A.; Desal, H.; Wiertlewski, S.; Houdart, E.; Vercelletto, M.; Al Hammad Ibrahim, M.; Guillon, B. Dural arteriovenous fistula. A rare cause of treatable dementia. Rev. Neurol. 2004, 160, 425–433. [Google Scholar] [CrossRef]
- Racine, C.A.; Lawton, M.T.; Hetts, S.W.; Josephson, S.A. Neuropyschological profile of reversible cognitive impairment in a patient with a dural arteriovenous fistula. Neurocase 2008, 14, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Kanazawa, R.; Maeshima, S.; Osawa, A.; Ochiai, I.; Uemiya, N.; Kohyama, S.; Yamane, F.; Ishihara, S. A case of curable dementia treated by effective endovascular embolization for dural arteriovenous fistula. Case Rep. Neurol. 2014, 6, 116–121. [Google Scholar] [CrossRef]
- Zenteno, M.; Lee, A.; Satyarthee, G.; Pinilla, G.; Agrawal, A.; Moscote-Salazar, L. Cognitive Improvement After Endovascular Treatment in a Case of Intracranial Dural Fistula With Concomitant Dementia. MAMC J. Med. Sci. 2018, 4, 32–37. [Google Scholar]
- Enofe, I.; Thacker, I.; Shamim, S. Dural arteriovenous fistula as a treatable dementia. Proc. (Bayl. Univ. Med. Cent.) 2017, 30, 215–217. [Google Scholar] [CrossRef]
- Fujii, H.; Nagano, Y.; Hosomi, N.; Matsumoto, M. Dural arteriovenous fistula presenting with progressive dementia and parkinsonism. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef]
- Martínez-Burbano, B.; Correa, E.P.; Sánchez, C.J. Evolutionary History of Multiple Dural Fistula. J. Investig. Med. High Impact Case Rep. 2016, 4, 1–11. [Google Scholar] [CrossRef]
- Matsuda, S.; Waragai, M.; Shinotoh, H.; Takahashi, N.; Takagi, K.; Hattori, T. Intracranial dural arteriovenous fistula (DAVF) presenting progressive dementia and parkinsonism. J. Neurol. Sci. 1999, 165, 43–47. [Google Scholar] [CrossRef]
- Pu, J.; Si, X.; Ye, R.; Zhang, B. Straight sinus dural arteriovenous fistula presenting with reversible parkinsonism A case report and literature review. Medicine 2017, 96, 49. [Google Scholar] [CrossRef] [PubMed]
- Soderman, M.; Pavic, L.; Edner, G.; Holmin, S.; Andersson, T. Natural history of dural arteriovenous shunts. Stroke 2008, 39, 1735–1739. [Google Scholar] [CrossRef]
- Xu, K.; Ji, T.; Li, C.; Yu, J. Current status of endovascular treatment for dural arteriovenous fistulae in the anterior cranial fossa: A systematic literature review. Int. J. Med. Sci. 2019, 16, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, R.; Albuquerque, L.; Ferro, J.M.; Sousa, R.; Sequeira, P.; Campos, J. Rapidly progressive cognitive impairment, ataxia, and myoclonus: An unusual presentation of a dural arteriovenous fistula. J. Stroke Cereb. Dis. 2012, 21, e613–e615. [Google Scholar] [CrossRef]
- Gopinath, M.; Nagesh, C.; Santhosh, K.; Jayadevan, E. Dementia and Parkinsonism-a Rare Presentation of Intracranial Dural Arteriovenous Fistulae. Neurointervention 2017, 12, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Takeuchi, T.; Kabeya, R.; Ando, K.; Tosaki, F. Transverse-sigmoid sinus dural arteriovenous fistula presenting with parkinsonism. Neurol. Med. Chir. 2013, 53, 224–227. [Google Scholar] [CrossRef]
- Jagtap, S.A.; Nair, S.S.; Jain, N.; Nair, M.D. Rapidly progressive dementia, parkinsonism and myoclonus: An unusual presentation of dural arteriovenous fistula. Neurol. India 2014, 62, 107–110. [Google Scholar] [CrossRef]
- Netravathi, M.; Pal, P.K.; Bharath, R.D.; Ravishankar, S. Intracranial dural arteriovenous fistula presenting as parkinsonism and cognitive dysfunction. J. Clin. Neurosci. 2011, 18, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Waragai, M.; Takeuchi, H.; Fukushima, T.; Haisa, T.; Yonemitsu, T. MRI and SPECT studies of dural arteriovenous fistulas presenting as pure progressive dementia with leukoencephalopathy: A cause of treatable dementia. Eur. J. Neurol. 2006, 13, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Qi, J.; Cen, Z.; Hu, H.; Jiang, B.; Lou, W. Two cases of dural arteriovenous fistula presenting with parkinsonism and progressive cognitive dysfunction. J. Neurol. Sci. 2014, 343, 211–214. [Google Scholar]
- Chahbazian, K.; Theaudin, M.; Lehmann, P.; Sachet, M.; Adams, D.; Saliou, G. Reversible pseudo-Creutzfeldt-Jakob syndrome related to cerebral dural arteriovenous fistula. J. Am. Geriatr. Soc. 2014, 62, 2024–2026. [Google Scholar] [CrossRef] [PubMed]
- Morparia, N.; Miller, G.; Rabinstein, A.; Lanzino, G.; Kumar, N. Cognitive decline and hypersomnolence: Thalamic manifestations of a tentorial dural arteriovenous fistula (dAVF). Neurocrit. Care 2012, 17, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Quiali, L.; Wanchao, S.; Zhiguo, S.; Yujun, Z.; Chen, L.; Zhenlin, L. Diagnosis and treatment of a dural arteriovenous fistula presenting with progressive parkinsonism and dementia: A case report and literature review. Exp. Ther. Med. 2015, 9, 523–526. [Google Scholar]
- Holekamp, T.F.; Mollman, M.E.; Murphy, R.K.; Kolar, G.R.; Kramer, N.M.; Derdeyn, C.P.; Moran, C.J.; Perrin, R.J.; Rich, K.M.; Lanzino, G.; et al. Dural arteriovenous fistula-induced thalamic dementia: Report of 4 cases. J. Neurosurg. 2016, 124, 1752–1765. [Google Scholar] [CrossRef] [PubMed]
- Greenough, G.P.; Mamourian, A.; Harbaugh, R.E. Venous Hypertension Associated with a Posterior Fossa Dural Arteriovenous Fistula: Another Cause of Bithalamic Lesions on MR Images. Am. J. Neuroradiol. 1999, 20, 145–147. [Google Scholar] [PubMed]
- Abrahams, J.M.; Bagley, L.J.; Flamm, E.S.; Hurst, R.W.; Sinson, G.P. Alternative management considerations for ethmoidal dural arteriovenous fistulas. Surg. Neurol. 2002, 58, 410–416. [Google Scholar] [CrossRef]
- Chan, H.; Cheng, K.; Lo, M.; Chan, C.; Cheung, Y. A treatable case of dementia intracranial dural arteriovenous fistula. Hong Kong Med. J. 2006, 12, 74–76. [Google Scholar]
- Kajitani, M.; Yagura, H.; Kawahara, M.; Hirano, M.; Ueno, S.; Fujimoto, K.; Sakaki, T.; Taoka, T.; Nakagawa, H.; Kichikawa, K. Treatable fluctuating Parkinsonism and dementia in a patient with a dural arteriovenous fistula. Mov. Disord. 2007, 22, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, Y.; Liu, A.; Lv, M.; Jiang, C.; Wu, Z. Endovascular embolization of dural arteriovenous fistulas of the anterior cranial fossa: Three case reports. Neurol. Res. 2008, 30, 852–859. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Baccin, C.E.; Rabinov, J.D.; Pryor, J.C.; Buonanno, F.S.; Hirsch, J.A. Reversible parkinsonism after treatment of dural arteriovenous fistula. J. Neuroimaging 2009, 19, 183–184. [Google Scholar] [CrossRef]
- Wilson, M.; Doran, M.; Enevoldson, T.P.; Larner, A.J. Cognitive profiles associated with intracranial dural arteriovenous fistula. Age Ageing 2010, 39, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, E.; Ishibashi, S.; Miki, K.; Yoshino, Y.; Nemoto, S.; Mizusawa, H. Teaching NeuroImages: Reversible cognitive impairment with bithalamic lesions caused by a dural arteriovenous fistula. Neurology 2013, 81, e38–e39. [Google Scholar] [CrossRef]
- Labeyrie, M.A.; Lenck, S.; Saint-Maurice, J.P.; Bresson, D.; Houdart, E. Dural arteriovenous fistulas presenting with reversible dementia are associated with a specific venous drainage. Eur. J. Neurol. 2014, 21, 545–547. [Google Scholar] [CrossRef]
- Pasi, M.; Nappini, S.; Salvadori, E.; Mangiafico, S.; Limbucci, N.; Pantoni, L. Rapidly progressive cognitive impairment in a patient with high flow dural arteriovenous fistulas, cerebral sinus thrombosis and protein S deficiency. J. Clin. Neurosci. 2014, 21, 1654–1656. [Google Scholar] [CrossRef] [PubMed]
- Imazeki, R.; Amari, K.; Sekiguchi, T.; Mochizuki, T.; Shimizu, S.; Yamamoto, M.; Takizawa, S.; Johkura, K. Rapidly progressive dementia caused by a superior sagittal sinus dural arteriovenous fistula: A case report. Tokai J. Exp. Clin. Med. 2015, 40, 22–26. [Google Scholar] [PubMed]
- Lai, J.; Heran, M.K.S.; Stoessl, A.J.; Gooderham, P.A. Reversible Parkinsonism and rapidly progressive dementia due to dural arteriovenous fistula: Case series and literature review. Mov. Disord. Clin. Pract. 2017, 4, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Tsang, A.C.O.; Hilditch, C.; Nicholson, P.; Krings, T.; Brinjikji, W. Intracranial Dural Arteriovenous Fistula as a Reversible Cause of Dementia: Case Series and Literature Review. World Neurosurg. 2019, 121, e543–e553. [Google Scholar] [CrossRef]
| Source | Total Patients | Cognitive Impairment | Dementia | Parkinsonism | Behavioral and Mood Changes | Time and Spatial Disorientation | Hemorrhage | Treatment | Evolution |
|---|---|---|---|---|---|---|---|---|---|
| Hurst et al., 1998 [26] | 40/5 | X | 4 ET 1 ET and S | 1 Death 4 Improved | |||||
| Matsuda S., et al., 1999 [34] | 3 | X | X | ET | 2 Improved 1 Not improved | ||||
| Greenough et al., 1999 [49] | 1 | X | S | Improved | |||||
| Abrahams., et al., 2002 [50] | 1 | X | ET | Improved | |||||
| Magot A., et al., 2004 [27] | 2 | X | X | ET | Improved | ||||
| Kwon., et al., 2005 [2] | 27/1 | X | X | ET | Improved | ||||
| Chan H Y., et al., 2006 [51] | 1 | X | X | X | X | X | ET | Improved | |
| Waragai M., et al., 2006 [43] | 2 | X | ET | Improved | |||||
| Kajitani M et al., 2007 [52] | 1 | X | X | X | ET | Improved | |||
| Hasumi T et al., 2007 [23] | 1 | X | X | ET | Improved | ||||
| Gonçalves MB et al., 2008 [25] | 1 | X | X | ET | Improved | ||||
| Racine CA, et al., 2008 [28] | 1 | X | ET and S | Improved | |||||
| Lv et al., 2008 [53] | 3/1 | X | ET | Improved | |||||
| Nogueira RG., et al., 2009 [54] | 1 | X | ET and S | Improved | |||||
| Wilson M, et al., 2010 [55] | 3 | X | ET | Improved | |||||
| Netravathi M, et al., 2011 [42] | 2 | X | X | ET | Not improved | ||||
| Geraldes et al., 2012 [38] | 1 | X | X | ET | Improved | ||||
| Morparia N.., et al., 2012 [46] | 1 | X | ET | Improved | |||||
| Hattori T., et al., 2013 [40] | 1 | X | X | ET | Improved | ||||
| Iwasawa E., et al., 2013 [56] | 1 | X | ET | Improved | |||||
| Jagtap SA., et al., 2014 [41] | 1 | X | X | NE | Death | ||||
| Labeyrie MA., et al., 2014 [57] | 45/8 | X | X | ET | Improved | ||||
| Chahbazian K., et al., 2014 [45] | 1 | X | X | ET | Improved | ||||
| van Munster CE., et al., 2014 [24] | 1 | X | X | ET | Improved | ||||
| Pasi M., et al., 2014 [58] | 1 | X | ET | Improved | |||||
| Fujii H., et al., 2014 [32] | 1 | X | X | ET | Improved | ||||
| Yoshihara., et al., 2014 [29] | 1 | X | X | X | ET | Improved | |||
| Imazeki R., et al., 2015 [59] | 1 | X | ET and S | Improved | |||||
| Chen MA., et al., 2015 [47] | 1 | X | X | ET and S | Improved | ||||
| Martínez Burbano, et al., 2016 [33] | 1 | X | X | X | NE | Death | |||
| Holekamp T F., et al., 2016 [48] | 4 | X | X | X | 2 ET 2 ET and S | 3 Improved 1 Death | |||
| Pu J., et al., 2017 [35] | 1 | X | X | ET | Improved | ||||
| Lai J., et al., 2017 [60] | 2 | X | X | X | ET | Improved | |||
| Gopinath M., et al., 2017 [39] | 1 | X | X | ET | Improved | ||||
| Enofe I., et al., 2017 [31] | 1 | X | ET | Improved | |||||
| Zenteno M., et al., 2018 [30] | 1 | X | X | ET | Improved | ||||
| Brito A., et al., 2019 [61] | 389/6 | X | ET | Improved | |||||
| Current case report 2019 | 1 | X | X | ET |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Díaz, Z.M.; Llíbre-Guerra, J.C.; Arteche-Prior, M.; de la Paz-Bermúdez, T.; Águila-Ruiz, A.F.; Figueroa-García, L.M.; Robinson-Agramonte, M.d.l.Á. Spontaneous Subdural Hematoma and Behavioral Changes Due to a Dural Arteriovenous Fistula. A Case Report and Literature Review. Behav. Sci. 2019, 9, 63. https://doi.org/10.3390/bs9060063
Hernández-Díaz ZM, Llíbre-Guerra JC, Arteche-Prior M, de la Paz-Bermúdez T, Águila-Ruiz AF, Figueroa-García LM, Robinson-Agramonte MdlÁ. Spontaneous Subdural Hematoma and Behavioral Changes Due to a Dural Arteriovenous Fistula. A Case Report and Literature Review. Behavioral Sciences. 2019; 9(6):63. https://doi.org/10.3390/bs9060063
Chicago/Turabian StyleHernández-Díaz, Zenaida Milagros, Juan Carlos Llíbre-Guerra, Marianela Arteche-Prior, Tania de la Paz-Bermúdez, Angel Francisco Águila-Ruiz, Luisa María Figueroa-García, and María de los Ángeles Robinson-Agramonte. 2019. "Spontaneous Subdural Hematoma and Behavioral Changes Due to a Dural Arteriovenous Fistula. A Case Report and Literature Review" Behavioral Sciences 9, no. 6: 63. https://doi.org/10.3390/bs9060063
APA StyleHernández-Díaz, Z. M., Llíbre-Guerra, J. C., Arteche-Prior, M., de la Paz-Bermúdez, T., Águila-Ruiz, A. F., Figueroa-García, L. M., & Robinson-Agramonte, M. d. l. Á. (2019). Spontaneous Subdural Hematoma and Behavioral Changes Due to a Dural Arteriovenous Fistula. A Case Report and Literature Review. Behavioral Sciences, 9(6), 63. https://doi.org/10.3390/bs9060063





