Consumer Neuroscience and Digital/Social Media Health/Social Cause Advertisement Effectiveness
Abstract
:1. Introduction
Hypotheses
2. Materials and Methods
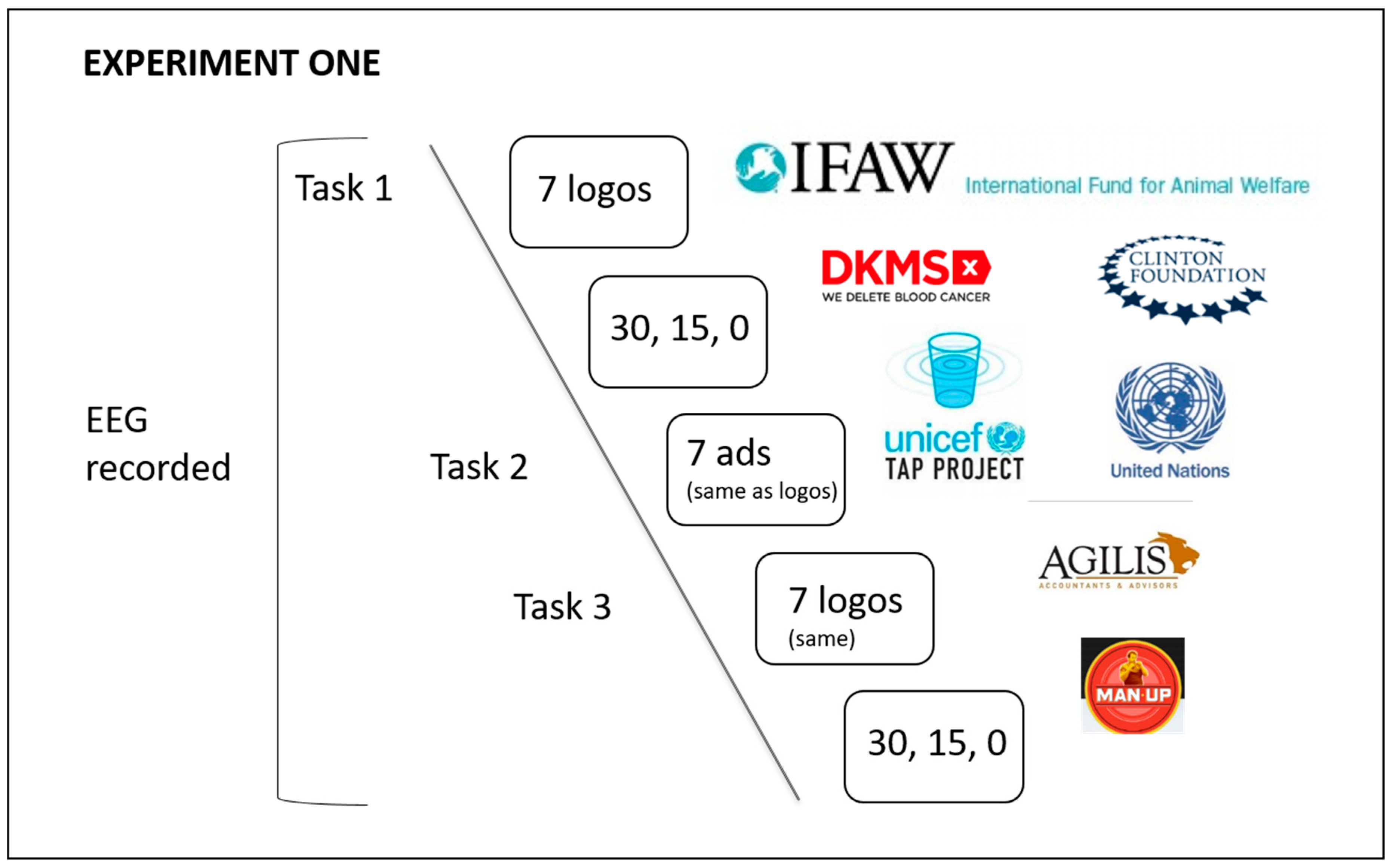
2.1. Method
2.2. Part 1: EEG study
2.3. Part 2: Qualtrics Psychometric Online Survey
3. Results
3.1. EEG Study
3.2. Online Survey
4. Discussion
5. Conclusions
Note
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Anterior Cingulate |
| BA | Brodmann Areas |
| IPL | Inferior Parietal Lobule |
| LH | Left Hemisphere |
| M(C) | Medial (central) |
| MFG | Medial Frontal Gyrus |
| MidFG | Middle Frontal Gyrus |
| OG | Orbital Gyrus |
| RH | Right Hemisphere |
Appendix A
| AD | AD TIME | ||||||||||||
| MANUP | 60 s | AMP | BVA TIME | AD TIME 10 s (10,000 ms) | MPC-HC | sLORETA MAP | BA | HEMI-SPHERE | LOBE | BRAIN REGION | PEAK | ||
| EPOCH | THETA BAND | LOR | uV | Posn sec | AD S1: 0:00–10:00 | FRAME | uAmm2 | TALAIRACH COORDINATES | |||||
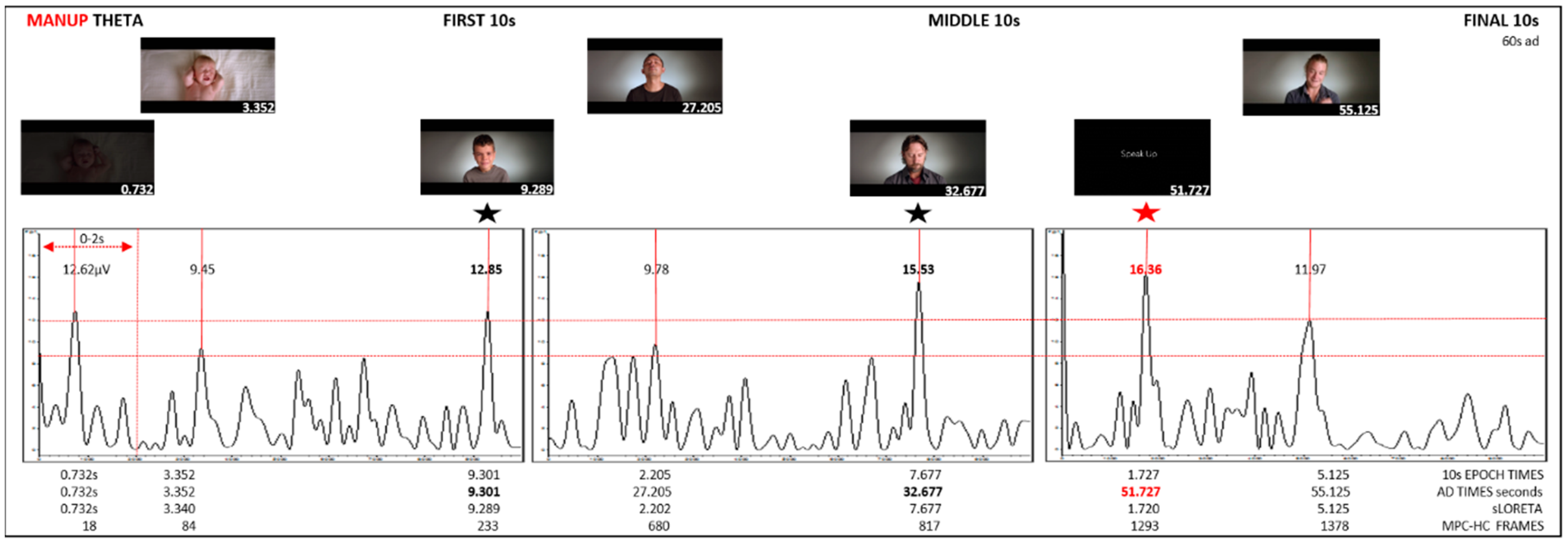
| S1 0–10 s | 0.732 | 12.85 | 0.732 | 0.732 | 18 | 0.174 | X = −3, Y = −60, Z = 57 | BA7 | Parietal | Precuneus | |||
| 3.34 | 9.45 | 3.352 | 3.352 | 84 | 0.0218 | (X = −3, Y = −52, Z = −6) | BA10 | Frontal | MFG | ||||
| 9.289 | 12.85 | 9.301 | 9.301 | 233 | 0.0271 | (X = −3, Y = −52, Z = −6) | BA10 | LH | Frontal | MFG | highest | ||
| S2 25–35 s | 2.202 | 9.78 | 2.205 | 27.205 | 680 | 0.0191 | (X = −3, Y = 38, Z = −20) | BA11 | Frontal | MFG | |||
| 7.677 | 15.53 | 7.677 | 32.677 | 817 | 0.0393 | (X = −3, Y = 38, Z = −20) | BA11 | M(C) | Frontal | MFG | highest | ||
| S3 50–60 s | 1.72 | 16.36 | 1.727 | 51.727 | 1293 | 0.0461 | (X = −38, Y = 52, Z = −6) | BA10 | LH | Frontal | MidFG | highest | |
| 5.125 | 11.97 | 5.125 | 55.125 | 1378 | 0.0231 | (X = −3, Y = 38, Z = −20) | BA11 | Frontal | MFG | ||||
| IFAW | 54 s | AMP | BVA TIME | AD TIME | MPC-HC | sLORETA MAP | BA | HEMI-SPHERE | LOBE | BRAIN REGION | PEAK | ||
| EPOCH | THETA BAND | LOR | uV | Posn sec | AD 0:00–10:00 | FRAME | uAmm2 | TALAIRACH COORDINATES | |||||
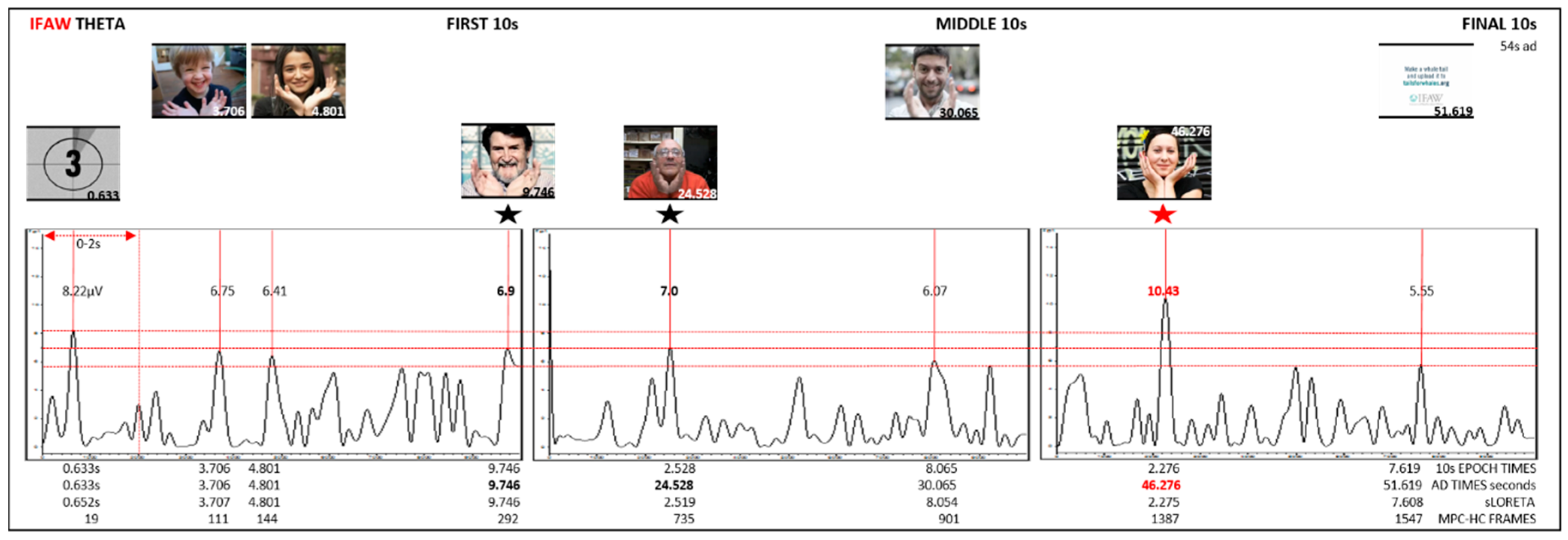
| S1 0–10 s | 0.652 | 8.22 | 0.633 | 0.633 | 19 | 0.0233 | (X = −3, Y = 52, Z = −6) | BA10 | Frontal | MFG | |||
| 3.707 | 6.75 | 3.706 | 3.706 | 111 | 0.0195 | (X = −3, Y = 45, Z = −20) | BA11 | Frontal | OG | ||||
| 4.801 | 6.41 | 4.801 | 4.801 | 144 | 0.0156 | (X = −3, Y = 38, Z = −20) | BA11 | Frontal | MFG | ||||
| 9.746 | 6.9 | 9.746 | 9.746 | 292 | 0.0132 | (X = -3, Y = 38, Z = −20) | BA11 | M(C) | Frontal | MFG | highest | ||
| S2 22–32 s | 2.519 | 7 | 2.532 | 24.532 | 735 | 0.0169 | (X = −3, Y = 38, Z = −20) | BA11 | M(C) | Frontal | MFG | highest | |
| 8.054 | 6.07 | 8.065 | 30.065 | 901 | 0.0147 | (X = −3, Y = 52, Z = 1) | BA10 | Limbic | AC | ||||
| 9.232 | 5.74 | 9.232 | 31.232 | 936 | 0.0208 | (X = 60, Y = −39, Z = 29) | BA40 | Parietal | IPL | ||||
| S3 44–54 s | 0.493 | 5.06 | 0.491 | 44.491 | 1333 | 0.0455 | (X = 60, Y = −39, Z = 29) | BA40 | Parietal | IPL | |||
| 2.275 | 10.43 | 2.276 | 46.276 | 1387 | 0.0183 | (X = −3, Y = 38, Z = −20) | BA11 | M(C) | Frontal | MFG | highest | ||
| 4.999 | 5.55 | 4.999 | 48.999 | 1469 | 0.0473 | (X = 60, Y = −39, Z = 29) | BA40 | Parietal | IPL | ||||
| 7.608 | 5.76 | 7.619 | 51.619 | 1547 | 0.0171 | (X = −3, Y = 45, Z = −20) | BA11 | Frontal | OG | ||||
| AGILIS | 89 s/1:29 m | AMP | BVA TIME | AD TIME | MPC-HC | SLORETA MAP | BA | HEMI-SPHERE | LOBE | BRAIN REGION | PEAK | ||
| EPOCH | THETA BAND | LOR | uV | Posn sec | AD S1 0:00–10:00 | FRAME | uAmm2 | TALAIRACH COORDINATES | |||||
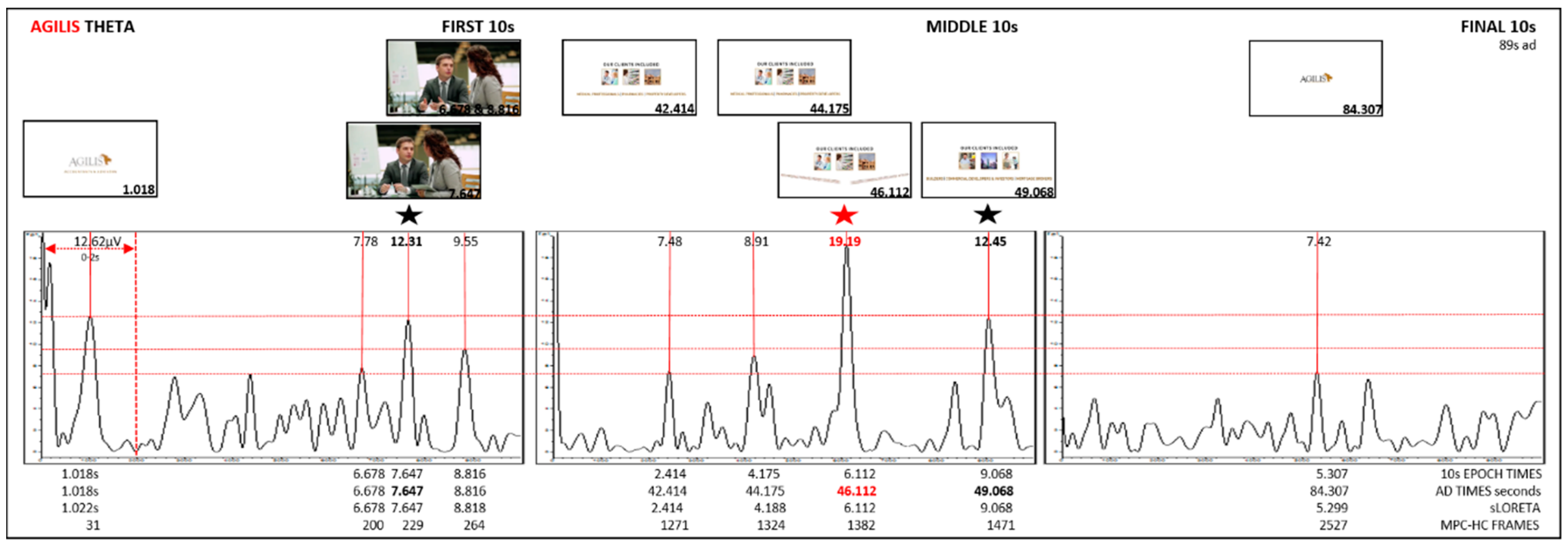
| S1 0–10 s | 1.022 | 12.62 | 1.018 | 1.018 | 31 | 0.0297 | (X = −3, Y = 45, Z = −20) | BA11 | Frontal | OG | |||
| 7.647 | 12.31 | 7.647 | 7.647 | 229 | 0.039 | (X = 32, Y = 59, Z = −6) | BA10 | RH | Frontal | MidFG | highest | ||
| 8.818 | 9.55 | 8.816 | 8.816 | 264 | 0.0191 | (X = −3, Y = 38, Z = −20) | BA11 | Frontal | MFG | ||||
| S2 40–50 s | 2.414 | 7.48 | 2.414 | 42.414 | 1271 | 0.0139 | (X = −3, Y = 38, Z = −20) | BA11 | Frontal | MFG | |||
| 4.188 | 8.91 | 4.175 | 44.175 | 1324 | 0.0237 | (X = −3, Y = 45, Z = −20) | BA11 | Frontal | OG | ||||
| 6.112 | 19.19 | 6.112 | 46.112 | 1382 | 0.0497 | (X = −3, Y = 38, Z = −20) | BA11 | M(C) | Frontal | MFG | highest | ||
| 9.068 | 12.45 | 9.068 | 49.068 | 1471 | 0.0341 | (X = −38, Y = 52, Z = −6) | BA10 | Frontal | MidFG | ||||
| S3 79–89 s | 1:29 | 5.299 | 7.42 | 5.307 | 84.307 = 1:24:307 | 2527 | 0.016 | (X = −3, Y = 52, Z = −6) | BA10 | M(C)LH/RH | Frontal | MFG | highest |
| MANUP THETA ALPHA | Paired Samples T-Test | Paired Differences (2-tailed) | ||||||||
| THETA-ALPHA | Mean | Alpha ↓ Theta ↑ | Std. Deviation | Std. Error Mean | 95% Confidence Interval of Difference | t | df | Sig. | ||
| Frontal Electrodes | Lower | Upper | (2-tailed) | |||||||
| Pair 1 | FzmuTH* - FzmuALPH† | 1.239 | Alpha ↓ Theta ↑ | 11.550 | 1.826 | −2.455 | 4.933 | 0.679 | 39 | 0.501 |
| Pair 3 | FpzmuTH - FpzmuALPH | 8.578 | Alpha ↓ Theta ↑ | 5.942 | 0.939 | 6.678 | 10.479 | 9.131 | 39 | 0.000 |
| Pair 4 | Fp1muTH - Fp1muALPH | 8.603 | Alpha ↓ Theta ↑ | 6.036 | 0.954 | 6.673 | 10.534 | 9.015 | 39 | 0.000 |
| Pair 5 | AF3muTH - AF3muALPH | 6.123 | Alpha ↓ Theta ↑ | 8.101 | 1.281 | 3.532 | 8.714 | 4.780 | 39 | 0.000 |
| Pair 6 | F7muTH - F7muALPH | 2.175 | Alpha ↓ Theta ↑ | 9.369 | 1.481 | −0.822 | 5.171 | 1.468 | 39 | 0.150 |
| Pair 7 | F5muTH - F5muALPH | 2.153 | Alpha ↓ Theta ↑ | 9.738 | 1.540 | −0.962 | 5.267 | 1.398 | 39 | 0.170 |
| Pair 8 | F3muTH - F3muALPH | 2.200 | Alpha ↓ Theta ↑ | 10.494 | 1.659 | −1.156 | 5.556 | 1.326 | 39 | 0.193 |
| Pair 9 | F1muTH - F1muALPH | 2.196 | Alpha ↓ Theta ↑ | 11.290 | 1.785 | −1.415 | 5.807 | 1.230 | 39 | 0.226 |
| Pair 13 | Fp2muTH - Fp2muALPH | 8.211 | Alpha ↓ Theta ↑ | 6.009 | 0.950 | 6.290 | 10.133 | 8.643 | 39 | 0.000 |
| Pair 14 | AF4muTH - AF4muALPH | 5.316 | Alpha ↓ Theta ↑ | 8.253 | 1.305 | 2.676 | 7.955 | 4.074 | 39 | 0.000 |
| Pair 15 | F2muTH - F2muALPH | 0.590 | Alpha ↓ Theta ↑ | 11.535 | 1.824 | −3.099 | 4.279 | 0.324 | 39 | 0.748 |
| Pair 16 | F4muTH - F4muALPH | 0.742 | Alpha ↓ Theta ↑ | 10.749 | 1.700 | −2.696 | 4.180 | 0.437 | 39 | 0.665 |
| Pair 17 | F6muTH - F6muALPH | 0.671 | Alpha ↓ Theta ↑ | 9.105 | 1.440 | −2.241 | 3.583 | 0.466 | 39 | 0.644 |
| Pair 18 | F8muTH - F8muALPH | 1.243 | Alpha ↓ Theta ↑ | 8.218 | 1.299 | −1.385 | 3.872 | 0.957 | 39 | 0.345 |
| IFAW THETA ALPHA | Paired Samples T-Test | Paired Differences (2-tailed) | ||||||||
| THETA-ALPHA | Mean | Alpha ↓ Theta ↑ | Std. Deviation | Std. Error Mean | 95% Confidence Interval of Difference | t | df | Sig. | ||
| Frontal electrodes | Lower | Upper | (2-tailed) | |||||||
| Pair 1 | FpzifTH - FpzifALPH | 7.666 | Alpha ↓ Theta ↑ | 8.781 | 1.388 | 4.858 | 10.475 | 5.522 | 39 | 0.000 |
| Pair 3 | FzifTH - FzifALPH | 0.921 | Alpha ↓ Theta ↑ | 12.858 | 2.033 | −3.191 | 5.033 | 0.453 | 39 | 0.653 |
| Pair 4 | Fp1ifTH - Fp1ifALPH | 7.584 | Alpha ↓ Theta ↑ | 8.294 | 1.311 | 4.931 | 10.236 | 5.783 | 39 | 0.000 |
| Pair 5 | AF3ifTH - AF3ifALPH | 5.067 | Alpha ↓ Theta ↑ | 10.143 | 1.604 | 1.824 | 8.311 | 3.160 | 39 | 0.003 |
| Pair 6 | F7ifTH - F7ifALPH | 1.574 | Alpha ↓ Theta ↑ | 10.461 | 1.654 | −1.772 | 4.919 | 0.951 | 39 | 0.347 |
| Pair 7 | F5ifTH - F5ifALPH | 1.591 | Alpha ↓ Theta ↑ | 10.829 | 1.712 | −1.873 | 5.054 | 0.929 | 39 | 0.359 |
| Pair 8 | F3ifTH - F3ifALPH | 1.376 | Alpha ↓ Theta ↑ | 11.709 | 1.851 | −2.368 | 5.121 | 0.743 | 39 | 0.462 |
| Pair 9 | F1ifTH - F1ifALPH | 1.319 | Alpha ↓ Theta ↑ | 12.455 | 1.969 | −2.664 | 5.302 | 0.670 | 39 | 0.507 |
| Pair 13 | Fp2ifTH - Fp2ifALPH | 7.237 | Alpha ↓ Theta ↑ | 8.833 | 1.397 | 4.412 | 10.062 | 5.182 | 39 | 0.000 |
| Pair 14 | AF4ifTH - AF4ifALPH | 4.460 | Alpha ↓ Theta ↑ | 10.381 | 1.641 | 1.140 | 7.780 | 2.717 | 39 | 0.010 |
| Pair 15 | F2ifTH - F2ifALPH | 0.315 | Alpha ↓ Theta ↑ | 12.283 | 1.942 | −3.613 | 4.244 | 0.162 | 39 | 0.872 |
| Pair 16 | F4ifTH - F4ifALPH | 0.579 | Alpha ↓ Theta ↑ | 11.256 | 1.780 | −3.021 | 4.179 | 0.325 | 39 | 0.747 |
| Pair 17 | F6ifTH - F6ifALPH | 0.652 | Alpha ↓ Theta ↑ | 9.616 | 1.520 | −2.423 | 3.727 | 0.429 | 39 | 0.670 |
| Pair 18 | F8ifTH - F8ifALPH | 2.016 | Alpha ↓ Theta ↑ | 10.830 | 1.712 | −1.448 | 5.479 | 1.177 | 39 | 0.246 |
| Pair 22 | P7ifTH - P7ifALPH | 0.816 | Alpha ↓ Theta ↑ | 13.042 | 2.062 | −3.355 | 4.987 | 0.396 | 39 | 0.694 |
| Pair 23 | P5if - P5ifALPH | 0.884 | Alpha ↓ Theta ↑ | 14.553 | 2.301 | −3.771 | 5.538 | 0.384 | 39 | 0.703 |
| Pair 26 | PO7if - PO7ifALPH | 1.684 | Alpha ↓ Theta ↑ | 13.501 | 2.135 | −2.634 | 6.002 | 0.789 | 39 | 0.435 |
| Pair 27 | PO5if - PO5ifALPH | 1.645 | Alpha ↓ Theta ↑ | 13.634 | 2.156 | −2.715 | 6.006 | 0.763 | 39 | 0.450 |
| Pair 28 | PO3ifTH - PO3ifALPH | 1.859 | Alpha ↓ Theta ↑ | 13.171 | 2.082 | −2.353 | 6.071 | 0.893 | 39 | 0.378 |
| Pair 29 | P2ifTH - P2ifALPH | 0.109 | Alpha ↓ Theta ↑ | 15.614 | 2.469 | −4.885 | 5.103 | 0.044 | 39 | 0.965 |
| Pair 30 | P4ifTH - P4ifALPH | 1.394 | Alpha ↓ Theta ↑ | 14.853 | 2.349 | −3.356 | 6.144 | 0.594 | 39 | 0.556 |
| Pair 31 | P6ifTH - P6ifALPH | 2.557 | Alpha ↓ Theta ↑ | 13.711 | 2.168 | −1.828 | 6.942 | 1.179 | 39 | 0.245 |
| Pair 32 | P8ifTH - P8ifALPH | 3.392 | Alpha ↓ Theta ↑ | 14.629 | 2.313 | −1.286 | 8.071 | 1.466 | 39 | 0.151 |
| Pair 33 | PO4ifTH - PO4ifALPH | 3.418 | Alpha ↓ Theta ↑ | 13.815 | 2.184 | −1.001 | 7.836 | 1.565 | 39 | 0.126 |
| Pair 34 | PO6ifTH - PO6ifALPH | 3.586 | Alpha ↓ Theta ↑ | 13.669 | 2.161 | −0.785 | 7.958 | 1.659 | 39 | 0.105 |
| Pair 35 | PO8ifTH - PO8ifALPH | 3.301 | Alpha ↓ Theta ↑ | 13.260 | 2.097 | −0.940 | 7.541 | 1.574 | 39 | 0.123 |
| AGILIS THETA ALPHA | Paired Samples T-Test | Paired Differences (2-tailed) | ||||||||
| THETA-ALPHA | Mean | Alpha ↓ Theta ↑ | Std. Deviation | Std. Error Mean | 95% Confidence Interval of Difference | t | df | Sig. | ||
| Frontal electrodes | Lower | Upper | (2-tailed) | |||||||
| Pair 1 | FpzagTH - FpzagALPH | 8.023 | Alpha ↓ Theta ↑ | 6.205 | 0.981 | 6.038 | 10.007 | 8.177 | 39 | 0.000 |
| Pair 3 | FzagTH - FzagALPH | 1.474 | Alpha ↓ Theta ↑ | 10.590 | 1.674 | −1.913 | 4.860 | 0.880 | 39 | 0.384 |
| Pair 4 | Fp1AagTH - Fp1agALPH | 7.921 | Alpha ↓ Theta ↑ | 6.180 | 0.977 | 5.945 | 9.897 | 8.106 | 39 | 0.000 |
| Pair 5 | AF3agTH - AF3agALPH | 5.551 | Alpha ↓ Theta ↑ | 7.925 | 1.253 | 3.016 | 8.085 | 4.429 | 39 | 0.000 |
| Pair 6 | F7agTH - F7agALPH | 2.773 | Alpha ↓ Theta ↑ | 6.658 | 1.053 | 0.644 | 4.903 | 2.635 | 39 | 0.012 |
| Pair 7 | F5agTH - F5agALPH | 2.453 | Alpha ↓ Theta ↑ | 7.747 | 1.225 | −0.025 | 4.930 | 2.003 | 39 | 0.052 |
| Pair 8 | F3agTH - F3agALPH | 1.945 | Alpha ↓ Theta ↑ | 9.353 | 1.479 | −1.046 | 4.937 | 1.315 | 39 | 0.196 |
| Pair 9 | F1agTH - F1agALPH | 1.916 | Alpha ↓ Theta ↑ | 10.193 | 1.612 | −1.343 | 5.176 | 1.189 | 39 | 0.242 |
| Pair 13 | Fp2agTH - Fp2agALPH | 7.707 | Alpha ↓ Theta ↑ | 6.085 | 0.962 | 5.761 | 9.653 | 8.011 | 39 | 0.000 |
| Pair 14 | AF4agTH - AF4agALPH | 5.294 | Alpha ↓ Theta ↑ | 7.682 | 1.215 | 2.837 | 7.751 | 4.359 | 39 | 0.000 |
| Pair 15 | F2agTH - F2agALPH | 1.134 | Alpha ↓ Theta ↑ | 10.152 | 1.605 | −2.113 | 4.380 | 0.706 | 39 | 0.484 |
| Pair 16 | F4agTH - F4agALPH | 1.353 | Alpha ↓ Theta ↑ | 8.879 | 1.404 | −1.486 | 4.193 | 0.964 | 39 | 0.341 |
| Pair 17 | F6agTH - F6agALPH | 1.833 | Alpha ↓ Theta ↑ | 6.958 | 1.100 | −0.392 | 4.058 | 1.666 | 39 | 0.104 |
| Pair 18 | F8agTH - F8agALPH | 2.982 | Alpha ↓ Theta ↑ | 7.180 | 1.135 | 0.686 | 5.278 | 2.627 | 39 | 0.012 |
References
- Ferrier, A.; Ward, B.; Palermo, J. Behavior Change: Why action advertising works harder than passive advertising. In Proceedings of the Society for Consumer Psychology: Proceedings of the 2012 Annual Conference, Las Vegas, NV, USA, 16–18 February 2012. [Google Scholar]
- Sigurdarson, J. Action-based advertising: The effectos of behaviour change on attitudes. Master’s Thesis, Copenhagen Business School, Copenhagen, Denmark, 2014. [Google Scholar]
- Harris, J.M.; Ciorciari, J.; Gountas, J. Public health social media communications and consumer neuroscience. Cogent Psychol. 2018, 5, 1434058. [Google Scholar] [CrossRef]
- Republic of Everyone. Tails for Whales. Available online: http://republicofeveryone.com (accessed on 1 September 2015).
- Droga5. World Humanitarian Day. 19 August 2012. Available online: http://www.whd-iwashere.org/ (accessed on 12 April 2019).
- Ferrier, A. The Advertising Effect; Oxford University Press: South Melbourne, Australia, 2014. [Google Scholar]
- Grönroos, C. On defining marketing: Finding a new roadmap for marketing. Mark. Theory 2006, 6, 395–417. [Google Scholar] [CrossRef]
- Strong, E.K., Jr. Theories of selling. J. Appl. Psychol. 1925, 9, 75. [Google Scholar] [CrossRef]
- Dry July Foundation. Dry July. Available online: https://www.dryjuly.com/ (accessed on 12 April 2019).
- FebFast. Pause for a Cause. Available online: https://www.febfast.org.au/ (accessed on 12 April 2019).
- 100 Day Challenge. Available online: https://www.100dc.com.au/ (accessed on 12 April 2019).
- Ahlert, D.; Kenning, P.; Plassmann, H. A Window to the Consumer’s Mind: Application of Functional Brain Imaging Techniques to Advertising Research. In International Advertising and Communication; Diehl, S., Terlutter, R., Eds.; Springer: Berlin, Germany, 2006; pp. 163–178. [Google Scholar]
- Ciorciari, J. Bioelectrical Signals: The Electroencephalogram. In Physiology, Biophysics and Biomedical Engineering; Wood, A., Ed.; Taylor & Francis Inc.: Washington, DC, USA, 2012. [Google Scholar]
- Micu, A.; Plummer, J.T. Measurable Emotions: How Television Ads Really Work—Patterns of Reactions to Commercials Can Demonstrate Advertising Effectiveness. J. Advert. Res. 2010, 50, 137–153. [Google Scholar] [CrossRef]
- Shapiro, S.; MacInnis, D.J. Understanding program-induced mood effects: Decoupling arousal from valence. J. Advert. 2002, 31, 15–26. [Google Scholar] [CrossRef]
- Lim, W.M. Demystifying neuromarketing. J. Bus. Res. 2018, 91, 205–220. [Google Scholar] [CrossRef]
- Eser, Z.; Isin, F.B.; Tolon, M. Perceptions of marketing academics, neurologists, and marketing professionals about neuromarketing. J. Mark. Manag. 2011, 27, 854–868. [Google Scholar] [CrossRef]
- Kenning, P. What advertisers can do and cannot do with neuroscience. Int. J. Advert. 2008, 27, 472–473. [Google Scholar]
- Javor, A.; Koller, M.; Lee, N.; Chamberlain, L.; Ransmayr, G. Neuromarketing and consumer neuroscience: Contributions to neurology. BMC Neurol. 2013, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Kenning, P.; Linzmajer, M. Consumer neuroscience: An overview of an emerging discipline with implications for consumer policy. J. Für Verbrauch. Und Lebensm. 2011, 6, 111–125. [Google Scholar] [CrossRef]
- Venkatraman, V.; Dimoka, A.; Pavlou, P.A.; Vo, K.; Hampton, W.; Bollinger, B.; Hershfield, H.E.; Ishihara, M.; Winer, R.S. Predicting advertising success beyond traditional measures: New insights from neurophysiological methods and market response modeling. J. Mark. Res. 2015, 52, 436–452. [Google Scholar] [CrossRef]
- Ohme, R.; Reykowska, D.; Wiener, D.; Choromanska, A. Application of frontal EEG asymmetry to advertising research. J. Econ. Psychol. 2010, 31, 785–793. [Google Scholar] [CrossRef]
- Plassmann, H.; Ambler, T.; Braeutigam, S.; Kenning, P. What can advertisers learn from neuroscience? Int. J. Advert. 2007, 26, 151–175. [Google Scholar] [CrossRef]
- Vecchiato, G.; Astolfi, L.; De Vico Fallani, F.; Toppi, J.; Aloise, F.; Bez, F.; Wei, D.; Kong, W.; Dai, J.; Cincotti, F. On the use of EEG or MEG brain imaging tools in neuromarketing research. Comput. Intell. Neurosci. 2011, 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Vecchiato, G.; Cherubino, P.; Trettel, A.; Babiloni, F. Neuroelectrical Brain Imaging Tools for the Study of the Efficacy of TV Advertising Stimuli and Their Application to Neuromarketing; Springer: Berlin, Germany, 2013. [Google Scholar] [CrossRef]
- Stead, M.; Gordon, R.; Angus, K.; McDermott, L. A systematic review of social marketing effectiveness. Health Educ. 2007, 107, 126–191. [Google Scholar] [CrossRef]
- The Economist Intelligence Unit. Industry Report, Healthcare: Australia; The Economist Intelligence Unit: London, UK, March 2014. [Google Scholar]
- Eurostat. Health care expenditure by provider [hlth_sha11_hp]; Eurostat, Ed.; Eurostat: Luxembourg, 2018. [Google Scholar]
- Australian Institute of Health and Welfare. Health expenditure Australia 2016-17; Australian Institute of Health and Welfare, Ed.; Australian Institute of Health and Welfare: Canberra, Australia, 2018; Volume Cat. no. HWE 74.
- Stoye, G. UK Health Spending; Institute for Fiscal Studies, Nuffield Foundation: London, UK, 2017. [Google Scholar]
- Ford, G.; Lewis, J.; Cooper, J. UK Health Accounts: 2016; Office for National Statistics: Newport, UK, 2018.
- Department of Finance. Campaign Advertising by Australian Government Departments and Agencies Annual Report 2016-17; Department of Finance, Ed.; Department of Finance: Canberra, Australia, 2017; p. 40.
- Kantar Media. Healthcare Marketing; Kantar Media: Chicago, IL, USA, 17 October 2016. [Google Scholar]
- Hornik, R. Public Health Communication: EVIDENCE for Behavior Change; Routledge: Abingdon, UK, 2002. [Google Scholar]
- Lim, M.S.; Wright, C.J.; Carrotte, E.R.; Pedrana, A.E. Reach, engagement, and effectiveness: A systematic review of evaluation methodologies used in health promotion via social networking sites. Health Promot. J. Aust. 2016, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Kubacki, K. The four Es of social marketing: Ethicality, expensiveness, exaggeration and effectiveness. J. Soc. Mark. 2015, 5, 83–99. [Google Scholar] [CrossRef]
- Mehta, A. Advertising attitudes and advertising effectiveness. J. Advert. Res. 2000, 40, 67–72. [Google Scholar] [CrossRef]
- Strecher, V.J.; McClure, J.; Alexander, G.; Chakraborty, B.; Nair, V.; Konkel, J.; Greene, S.; Couper, M.; Carlier, C.; Wiese, C. The role of engagement in a tailored web-based smoking cessation program: Randomized controlled trial. J. Med Internet Res. 2008, 10, e36. [Google Scholar] [CrossRef]
- Kong, W.; Zhao, X.; Hu, S.; Vecchiato, G.; Babiloni, F. Electronic evaluation for video commercials by impression index. Cogn. Neurodyn. 2013, 7, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Quester, P.; Neal, C.; Pettigrew, S. Consumer Behaviour: Implications for Marketing Strategy, 5th ed.; McGraw-Hill Irwin: New York City, NY, USA, 2007. [Google Scholar]
- Heath, R. Seducing the Subconscious: The Psychology of Emotional Influence in Advertising; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Teixeira, T.S.; Wedel, M.; Pieters, R. Moment-to-moment optimal branding in TV commercials: Preventing avoidance by pulsing. Mark. Sci. 2010, 29, 783–804. [Google Scholar] [CrossRef]
- Silberstein, R.; Schier, M.; Pipingas, A.; Ciorciari, J.; Wood, S.; Simpson, D. Steady-state visually evoked potential topography associated with a visual vigilance task. Brain Topogr. 1990, 3, 337–347. [Google Scholar] [CrossRef]
- Silberstein, R.; Nield, G. Brain activity correlates of consumer brand choice shift associated with television advertising. Int. J. Advert. 2008, 27, 359–380. [Google Scholar] [CrossRef]
- Rossiter, J.R.; Silberstein, R.S.; Harris, P.G.; Nield, G. Brain-imaging detection of visual scene encoding in long-term memory for TV commercials. J. Advert. Res. 2001, 41, 13–22. [Google Scholar] [CrossRef]
- Roca, M.; Torralva, T.; Gleichgerrcht, E.; Woolgar, A.; Thompson, R.; Duncan, J.; Manes, F. The role of Area 10 (BA10) in human multitasking and in social cognition: A lesion study. Neuropsychologia 2011, 49, 3525–3531. [Google Scholar] [CrossRef] [PubMed]
- Deppe, M.; Schwindt, W.; Kugel, H.; Plassmann, H.; Kenning, P. Nonlinear responses within the medial prefrontal cortex reveal when specific implicit information influences economic decision making. J. Neuroimaging 2005, 15, 171–182. [Google Scholar] [CrossRef]
- Rogers, R.D.; Owen, A.M.; Middleton, H.C.; Williams, E.J.; Pickard, J.D.; Sahakian, B.J.; Robbins, T.W. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J. Neurosci. 1999, 19, 9029–9038. [Google Scholar] [CrossRef]
- Ioannides, A.A.; Liu, L.; Theofilou, D.; Dammers, J.; Burne, T.; Ambler, T.; Rose, S. Real time processing of affective and cognitive stimuli in the human brain extracted from MEG signals. Brain Topogr. 2000, 13, 11–19. [Google Scholar] [CrossRef]
- Silberstein, R.; Ciorciari, J.; Pipingas, A. Steady-state visually evoked potential topography during the Wisconsin card sorting test. Electroencephalogr. Clin. Neurophysiol. 1995, 96, 24–35. [Google Scholar] [CrossRef]
- Davidson, R.J.; Jackson, D.C.; Kalin, N.H. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychol. Bull. 2000, 126, 890. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J.; Tomarken, A.J. Laterality and emotion: An electrophysiological approach. Handb. Neuropsychol. 1989, 3, 419–441. [Google Scholar]
- Davidson, R.J.; Ekman, P.; Saron, C.D.; Senulis, J.A.; Friesen, W.V. Approach-withdrawal and cerebral asymmetry: Emotional expression and brain physiology: I. J. Personal. Soc. Psychol. 1990, 58, 330. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Vecchiato, G.; Toppi, J.; Astolfi, L.; Fallani, F.D.V.; Cincotti, F.; Mattia, D.; Bez, F.; Babiloni, F. Spectral EEG frontal asymmetries correlate with the experienced pleasantness of TV commercial advertisements. Med Biol. Eng. Comput. 2011, 49, 579–583. [Google Scholar] [CrossRef]
- Vecchiato, G.; Kong, W.; Giulio Maglione, A.; Wei, D. Understanding the impact of TV commercials. IEEE Pulse 2012, 3, 42. [Google Scholar] [CrossRef]
- Davidson, R.J.; Irwin, W. The functional neuroanatomy of emotion and affective style. Trends Cogn. Sci. 1999, 3, 11–21. [Google Scholar] [CrossRef]
- Ohme, R.; Matukin, M. A small frog that makes a big difference: Brain wave testing of TV advertisements. IEEE Pulse 2012, 3, 28–33. [Google Scholar] [CrossRef]
- Belanche, D.; Flavián, C.; Pérez-Rueda, A. Understanding interactive online advertising: Congruence and product involvement in highly and lowly arousing, skippable video ads. J. Interact. Mark. 2017, 37, 75–88. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Esslen, M.; Kochi, K.; Lehmann, D. Functional imaging with low-resolution brain electromagnetic tomography (LORETA): A review. Methods Find. Exp. Clin. Pharmacol. 2002, 24, 91–95. [Google Scholar]
- Liu, Y.; Shrum, L. What is interactivity and is it always such a good thing? Implications of definition, person, and situation for the influence of interactivity on advertising effectiveness. J. Advert. 2002, 31, 53–64. [Google Scholar] [CrossRef]
- Hanna, R.; Rohm, A.; Crittenden, V.L. We’re all connected: The power of the social media ecosystem. Bus. Horiz. 2011, 54, 265–273. [Google Scholar] [CrossRef]
- O’Reilly, T.; Battelle, J. Web Squared: Web 2.0 Five Years on; Web 2.0 summit; O’Reilly Media, Inc.: Newton, MA, USA, 2009. [Google Scholar]
- Lustria, M.L.A. Can interactivity make a difference? Effects of interactivity on the comprehension of and attitudes toward online health content. J. Am. Soc. Inf. Sci. Technol. 2007, 58, 766–776. [Google Scholar] [CrossRef]
- Garretson, R. Future tense: The global CMO. A Report from the Economist Intelligence Unit; Sponsored by Google; Economist Intelligence Unit: London, UK, 2008; p. 2010. [Google Scholar]
- Cassell, M.; Jackson, C.; Cheuvront, B. Health communication on the Internet: An effective channel for health behavior change? J. Health Commun. 1998, 3, 71–79. [Google Scholar]
- Reading, N.; Bellman, S.; Varan, D.; Winzar, H. Effectiveness of telescopic advertisements delivered via personal video recorders. J. Advert. Res. 2006, 46, 217–227. [Google Scholar] [CrossRef]
- Treleaven-Hassard, S.; Gold, J.; Bellman, S.; Schweda, A.; Ciorciari, J.; Critchley, C.; Varan, D. Using the P3a to gauge automatic attention to interactive television advertising. J. Econ. Psychol. 2010, 31, 777–784. [Google Scholar] [CrossRef]
- Belanche, D.; Flavián, C.; Pérez-Rueda, A. User adaptation to interactive advertising formats: The effect of previous exposure, habit and time urgency on ad skipping behaviors. Telemat. Inform. 2017, 34, 961–972. [Google Scholar] [CrossRef]
- Dumbrell, D.; Steele, R. #worldhealthday 2014: The anatomy of a global public health twitter campaign. In Proceedings of the 48th Annual Hawaii International Conference on System Sciences HICSS 2015, Kauai, HI, USA, 5–8 January 2015. [Google Scholar]
- Gold, J.; Pedrana, A.E.; Stoove, M.A.; Chang, S.; Howard, S.; Asselin, J.; Ilic, O.; Batrouney, C.; Hellard, M.E. Developing health promotion interventions on social networking sites: Recommendations from The FaceSpace Project. J. Med Internet Res. 2012, 14, e30. [Google Scholar] [CrossRef] [PubMed]
- Voorveld, H.A.; van Noort, G.; Muntinga, D.G.; Bronner, F. Engagement with social media and social media advertising: The differentiating role of platform type. J. Advert. 2018, 47, 38–54. [Google Scholar] [CrossRef]
- Dolan, R.; Conduit, J.; Fahy, J.; Goodman, S. Social media engagement behaviour: A uses and gratifications perspective. J. Strateg. Mark. 2016, 24, 261–277. [Google Scholar] [CrossRef]
- Hollebeek, L.D.; Glynn, M.S.; Brodie, R.J. Consumer brand engagement in social media: Conceptualization, scale development and validation. J. Interact. Mark. 2014, 28, 149–165. [Google Scholar] [CrossRef]
- Brodie, R.J.; Ilic, A.; Juric, B.; Hollebeek, L. Consumer engagement in a virtual brand community: An exploratory analysis. J. Bus. Res. 2013, 66, 105–114. [Google Scholar] [CrossRef]
- Hollebeek, L. Exploring customer brand engagement: Definition and themes. J. Strateg. Mark. 2011, 19, 555–573. [Google Scholar] [CrossRef]
- D. Hollebeek, L.; Chen, T. Exploring positively-versus negatively-valenced brand engagement: A conceptual model. J. Prod. Brand Manag. 2014, 23, 62–74. [Google Scholar] [CrossRef]
- Davis Mersey, R.; Malthouse, E.C.; Calder, B.J. Engagement with online media. J. Media Bus. Stud. 2010, 7, 39–56. [Google Scholar] [CrossRef]
- Calder, B.J.; Malthouse, E.C.; Schaedel, U. An experimental study of the relationship between online engagement and advertising effectiveness. J. Interact. Mark. 2009, 23, 321–331. [Google Scholar] [CrossRef]
- Calder, B.J.; Isaac, M.S.; Malthouse, E.C. How to capture consumer experiences: A context-specific approach to measuring engagement: Predicting consumer behavior across qualitatively different experiences. J. Advert. Res. 2016, 56, 39–52. [Google Scholar] [CrossRef]
- Plassmann, H.; Venkatraman, V.; Huettel, S.; Yoon, C. Consumer neuroscience: Applications, challenges, and possible solutions. J. Mark. Res. 2015, 52, 427–435. [Google Scholar] [CrossRef]
- Gountas, S.; Ciorciari, J.; Gountas, G.; Huddle, S. An investigation of the EEG correlates associated with viewing alcohol and drug-related behaviours on social media. In Proceedings of the 17th World Congress of Psychophysiology, Hiroshima, Japan, 23–27 September 2014. [Google Scholar]
- Knutson, B.; Bossaerts, P. Neural antecedents of financial decisions. J. Neurosci. 2007, 27, 8174–8177. [Google Scholar] [CrossRef]
- Couwenberg, L.E.; Boksem, M.A.; Dietvorst, R.C.; Worm, L.; Verbeke, W.J.; Smidts, A. Neural responses to functional and experiential ad appeals: Explaining ad effectiveness. Int. J. Res. Mark. 2016, 34, 355–366. [Google Scholar] [CrossRef]
- Peirce, J. PsychoPy—Psychophysics software in Python. J. Neurosci. Methods 2007, 162, 8–13. [Google Scholar] [CrossRef]
- Peirce, J. Generating stimuli for neuroscience using PsychoPy. Front. Neuroinform. 2009, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Mayr, S.; Erdfelder, E.; Buchner, A.; Faul, F. A short tutorial of GPower. Tutor. Quant. Methods Psychol. 2007, 3, 51–59. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155. [Google Scholar] [CrossRef]
- Mostafa, M.M. The persistence of memory: An fMRI investigation of the brain processing of Surrealistic imagery in advertising. J. Mark. Commun. 2013, 19, 341–359. [Google Scholar] [CrossRef]
- Rhodes, G. The evolutionary psychology of facial beauty. Annu. Rev. Psychol. 2006, 57, 199–226. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, R.; Gangestad, S.W. Human facial beauty. Hum. Nat. 1993, 4, 237–269. [Google Scholar] [CrossRef]
- Zubcevic, N.; Mavondo, F.; Luxton, S. Achieving at university and beyond: Does it help to be good looking? Asia Pac. J. Mark. Logist. 2012, 24, 785–804. [Google Scholar] [CrossRef]
- Winkielman, P.; Halberstadt, J.; Fazendeiro, T.; Catty, S. Prototypes are attractive because they are easy on the mind. Psychol. Sci. 2006, 17, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, L.T.; Jankowitsch, J.M.; Langlois, J.H. Beauty is in the ease of the beholding: A neurophysiological test of the averageness theory of facial attractiveness. Cogn. Affect. Behav. Neurosci. 2014, 14, 1061–1076. [Google Scholar] [CrossRef]
- ManUp.org.au. Making of the Man Up Campaign Ad. Available online: https://www.youtube.com/watch?v=ze1FeFO8wqo (accessed on 12 April 2019).
- Mulligan, K.; Scherer, K.R. Toward a working definition of emotion. Emot. Rev. 2012, 4, 345–357. [Google Scholar] [CrossRef]
- LeDoux, J. A neuroscientist’s perspective on debates about the nature of emotion. Emot. Rev. 2012, 4, 375–379. [Google Scholar] [CrossRef]
- Adams, R.B., Jr.; Kleck, R.E. Perceived gaze direction and the processing of facial displays of emotion. Psychol. Sci. 2003, 14, 644–647. [Google Scholar] [CrossRef]
- Deppe, M.; Schwindt, W.; Pieper, A.; Kugel, H.; Plassmann, H.; Kenning, P.; Deppe, K.; Ringelstein, E.B. Anterior cingulate reflects susceptibility to framing during attractiveness evaluation. Neuroreport 2007, 18, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Posner, J.; Russell, J.A.; Peterson, B.S. The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev. Psychopathol. 2005, 17, 715–734. [Google Scholar] [CrossRef]
- Baker, W.E.; Honea, H.; Russell, C.A. Do not wait to reveal the brand name: The effect of brand-name placement on television advertising effectiveness. J. Advert. 2004, 33, 77–85. [Google Scholar] [CrossRef]
- Nelson-Field, K. The Client was Right: Make the Logo Bigger. In Proceedings of the Mumbrella MSIX 2018, Powerhouse Museum, Sydney, Australia, 9 November 2018. [Google Scholar]
- Horton, T. 10 Ted Minutes: What Science has Taught Me. In Proceedings of the Mumbrell MSIX 2018, Powerhouse Musuem, Sydney, Australia, 9 November 2018. [Google Scholar]
- Pynta, P.; Seixas, S.A.; Nield, G.E.; Hier, J.; Millward, E.; Silberstein, R.B. The Power of Social Television: Can Social Media Build Viewer Engagement?: A New Approach to Brain Imaging of Viewer Immersion. J. Advert. Res. 2014, 54, 71–80. [Google Scholar] [CrossRef]
- Kanwisher, N.; McDermott, J.; Chun, M.M. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997, 17, 4302–4311. [Google Scholar] [CrossRef] [PubMed]
- Rossion, B.; Schiltz, C.; Crommelinck, M. The functionally defined right occipital and fusiform “face areas” discriminate novel from visually familiar faces. Neuroimage 2003, 19, 877–883. [Google Scholar] [CrossRef]
- Farrow, T.F.; Zheng, Y.; Wilkinson, I.D.; Spence, S.A.; Deakin, J.W.; Tarrier, N.; Griffiths, P.D.; Woodruff, P.W. Investigating the functional anatomy of empathy and forgiveness. Neuroreport 2001, 12, 2433–2438. [Google Scholar] [CrossRef] [PubMed]
- Psychology Board of Australia. Registrant Data—Psychology Board Australia; Psychology Board of Australia: Sydney, Australia, 19 April 2018; p. 11.
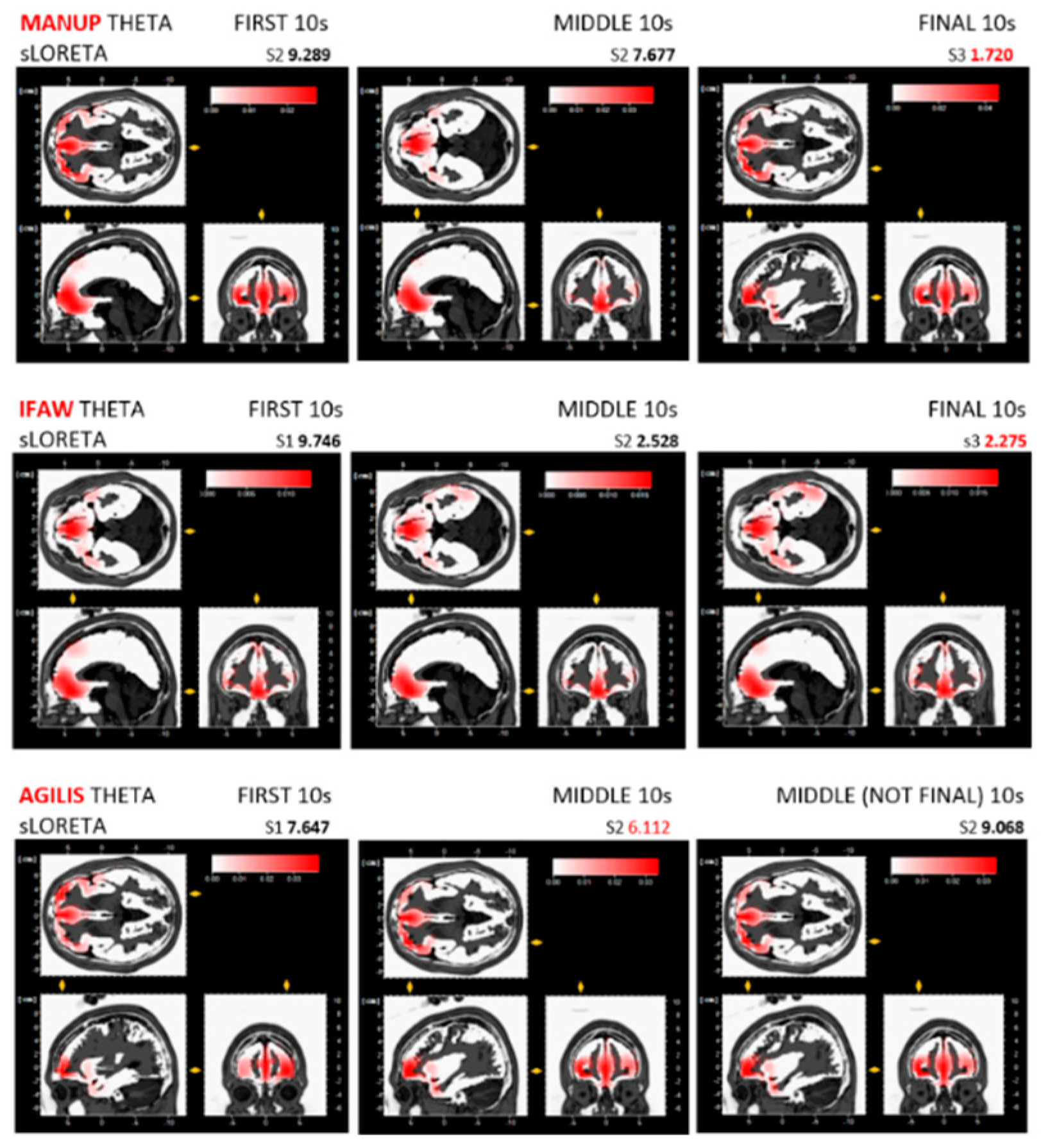
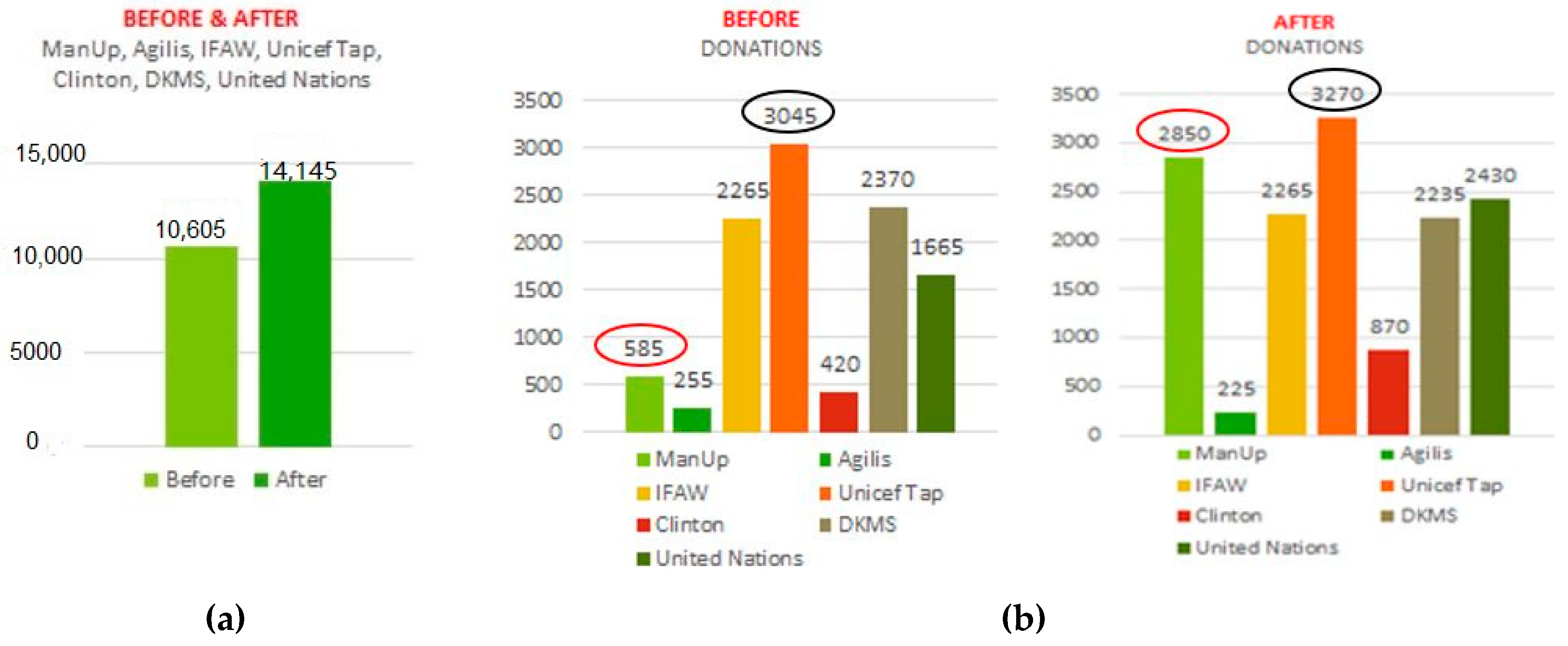
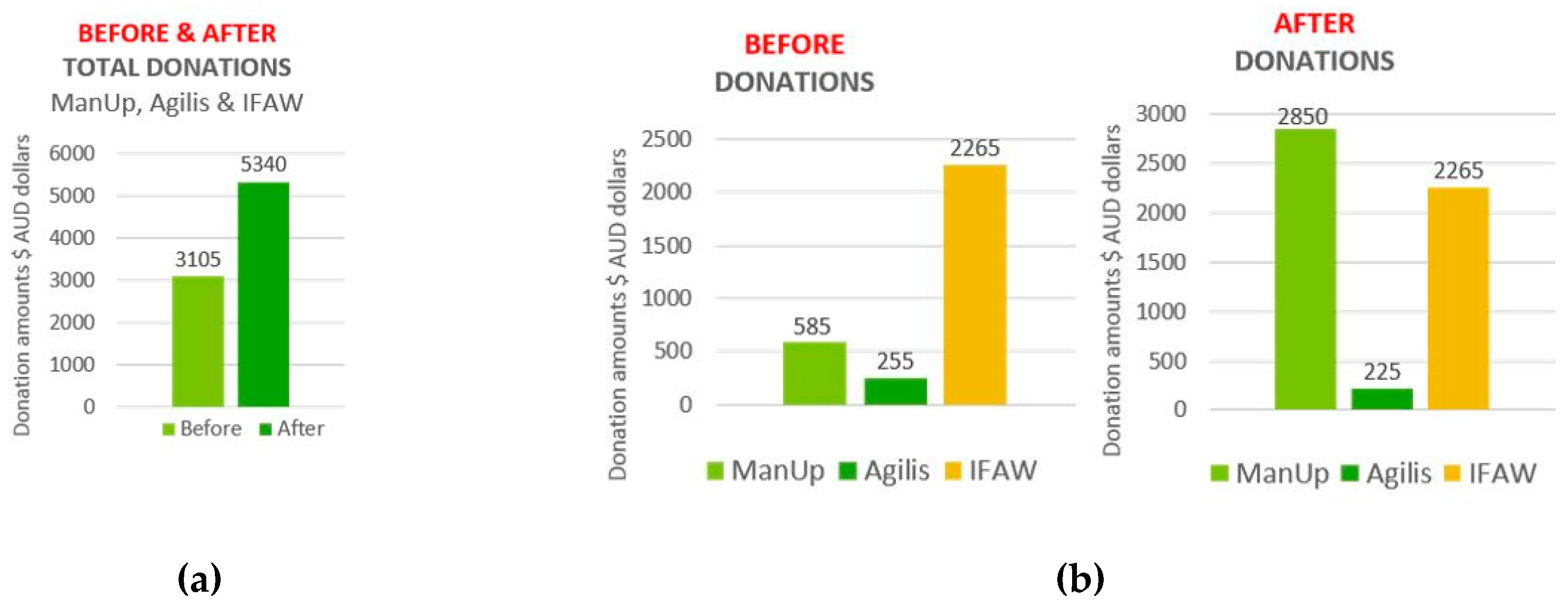
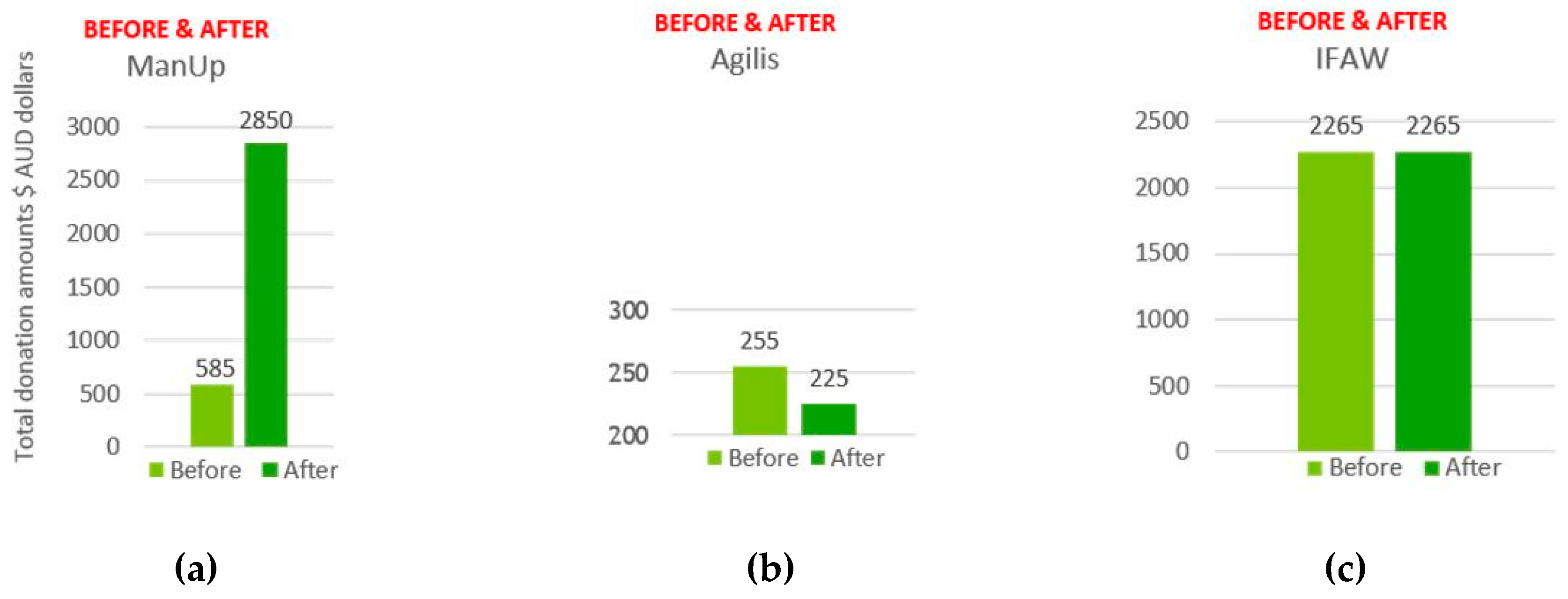
| Brain Region/Lobe | Central Electrodes | Left Hemisphere Electrodes | Right Hemisphere Electrodes |
|---|---|---|---|
| anterior frontal | FpZ | Fp1, AF3 | Fp2, AF4 |
| frontal | Fz | F7, F5, F3, F1 | F2, F4, F6, F8 |
| fronto-central | FCZ | FC3, FC1 | FC2, FC4 |
| left frontal temporal | FC5 | FC6 | |
| parietal | P7, P5, P3, P1 | P2, P4, P6, P8 | |
| parieto-occipital | PO7, PO5, PO3 | PO4, PO6, PO8 |
| Ad | Grand Average Amplitudes µV | 0–2 s | FIRST 10 s | MIDDLE 10 s | FINAL 10 s | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ManUp | theta average ↑ µV | 12.85 | 9.45 | 12.85 | X | 9.78 | 15.53 | X | 16.36 * BA10, LH | 11.97 |
| alpha average ↓ µV | 0.535 | 1.87 | 0.728 | X | 0.180 | 0.0139 | X | 0.837 | 1.19 | |
| IFAW | theta average ↑ µV | 8.22 | 6.75 | 6.41 | 6.9 | 7.0 | 6.07 | X | 10.43 * BA11, M | 5.55 |
| alpha average ↓ µV | 0.618 | 0.261 | 0.150 | 0.102 | 0.560 | 0.152 | X | 1.24 | 0.833 | |
| Agilis | theta average ↑ µV | 12.62 | 12.31 | 9.55 | X | 7.48 | 8.91 | 19.19 * BA11, M | 7.42 | X |
| alpha average ↓ µV | 0.272 | 2.65 | 0.793 | X | 0.262 | 1.19 | 1.24 | 0.572 | X | |
| Advertisement 10 s Epochhs | Brodmann Area (BA) | Lobe | Greater Left or Right Hemisphere (LH/RH) Activation/Dominance | Brain Region: Frontal Gyrus |
|---|---|---|---|---|
| FIRST 10 s (S1) | ||||
| ManUp | BA10 | Frontal | LH | Medial |
| IFAW | BA11 | Frontal | neither substantial L/RH dominance | Medial |
| Agilis | BA10 | Frontal | RH | Middle |
| MIDDLE 10 s (S2) | ||||
| ManUp | BA11 | Frontal | neither substantial L/RH dominance | Medial |
| IFAW | BA11 | Frontal | neither substantial L/RH dominance | Medial |
| Agilis | BA11 | Frontal | LH | Medial |
| FINAL 10 s (S3)/MIDDLE 10 s (S2) for Agilis * | ||||
| ManUp | BA10 | Frontal | LH | Middle |
| IFAW | BA11 | Frontal | neither substantial L/RH dominance | Medial |
| Agilis | BA11 | Frontal | LH | Medial |
| 2 TAILED T-TEST | ELECTRODES | N = 40 | MEAN ALPHA ↓ THETA ↑ | |||
|---|---|---|---|---|---|---|
| PAIRS | Paired Differences (theta-alpha average) | Paired Mean | Paired Mean | Paired Mean | Paired Mean Differences | Paired Mean Differences |
| ManUp | IFAW | Agilis | ManUp - Agilis | IFAW - Agilis | ||
| LEFT | ||||||
| Pair 1 | FpzTH * - FpzAPH † | 1.239 | 7.666 | 8.023 | 0.556 | −0.356 |
| Pair 3 | FzTH - FzAPH | 8.578 | 0.921 | 1.474 | −0.234 | −0.552 |
| Pair 4 | Fp1TH - Fp1APH | 8.603 | 7.584 | 7.921 | 0.682 | −0.337 |
| Pair 5 | AF3TH – AF3APH | 6.123 | 5.067 | 5.551 | −5.075 | −0.483 |
| Pair 6 | F7TH - F7APH | 2.175 | 1.574 | 2.773 | −0.599 | −1.200 |
| Pair 7 | F5TH - F5APH | 2.153 | 1.591 | 2.453 | −0.300 | −0.862 |
| Pair 8 | F3TH - F3APH | 2.200 | 1.376 | 1.945 | 0.254 | −0.569 |
| Pair 9 | F1TH - F1APH | 2.196 | 1.319 | 1.916 | 0.279 | −0.598 |
| RIGHT | ||||||
| Pair 13 | Fp2TH - Fp2APH | 8.211 | 7.237 | 7.707 | 0.504 | −0.470 |
| Pair 14 | AF4TH - AF4APH | 5.316 | 4.460 | 5.294 | 0.022 | −0.835 |
| Pair 15 | F2TH - F2APH | 0.590 | 0.315 | 1.134 | −0.543 | −0.818 |
| Pair 16 | F4TH - F4APH | 0.742 | 0.579 | 1.353 | −0.611 | −0.774 |
| Pair 17 | F6TH - F6APH | 0.671 | 0.652 | 1.833 | −1.162 | −1.181 |
| Pair 18 | F8TH - F8APH | 1.243 | 2.016 | 2.982 | −1.739 | −0.966 |
| Advertisement | Brain Region/Lobe | Central Electrodes | Left Hemisphere Electrodes | Right Hemisphere Electrodes |
|---|---|---|---|---|
| ManUp, IFAW & Agilis | anterior frontal | FpZ, | Fp1, AF3 | Fp2, AF4 |
| ManUp, IFAW & Agilis | frontal | Fz | F7, F5, F3, F1 | F2, F4, F6, F8 |
| IFAW only | parietal | P7, P5, P3, P1 | P2, P4, P6, P8 | |
| IFAW only | parieto-occipital | PO7, PO5, PO3 | PO4, PO6, PO8 |
| Advertisement | Before | After | Variance | Sig. | |||
|---|---|---|---|---|---|---|---|
| N = 153 * | Mean | SD | Mean | SD | Mean | SD | |
| Unicef Tap Project | 1.67 | 0.742 | 1.58 | 0.723 | 0.98 | 0.723 | 0.096 |
| United Nations | 2.27 | 0.78 | 1.94 | 0.771 | 0.333 | −0.009 | 0.000 |
| Agilis | 2.89 | 0.373 | 2.90 | 0.358 | −0.013 | −0.015 | 0.707 |
| DKMS | 1.97 | 0.823 | 2.03 | 0.778 | −0.059 | −0.045 | 0.358 |
| ManUp | 2.75 | 0.532 | 1.76 | 0.698 | 0.987 | 0.166 | 0.000 |
| IFAW | 2.01 | 0.752 | 2.01 | 0.761 | 0.000 | 0.009 | 1.000 |
| Clinton | 2.82 | 0.436 | 2.62 | 0.618 | 0.196 | 0.563 | 0.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, J.M.; Ciorciari, J.; Gountas, J. Consumer Neuroscience and Digital/Social Media Health/Social Cause Advertisement Effectiveness. Behav. Sci. 2019, 9, 42. https://doi.org/10.3390/bs9040042
Harris JM, Ciorciari J, Gountas J. Consumer Neuroscience and Digital/Social Media Health/Social Cause Advertisement Effectiveness. Behavioral Sciences. 2019; 9(4):42. https://doi.org/10.3390/bs9040042
Chicago/Turabian StyleHarris, Joanne M, Joseph Ciorciari, and John Gountas. 2019. "Consumer Neuroscience and Digital/Social Media Health/Social Cause Advertisement Effectiveness" Behavioral Sciences 9, no. 4: 42. https://doi.org/10.3390/bs9040042
APA StyleHarris, J. M., Ciorciari, J., & Gountas, J. (2019). Consumer Neuroscience and Digital/Social Media Health/Social Cause Advertisement Effectiveness. Behavioral Sciences, 9(4), 42. https://doi.org/10.3390/bs9040042






