Sex Differences in Salivary Oxytocin and Cortisol Concentration Changes during Cooking in a Small Group
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethics Statement
2.3. Assessment
2.4. Saliva Collection and Analysis
2.5. Salivary cortisol assay
2.6. Statistical Analysis
3. Results
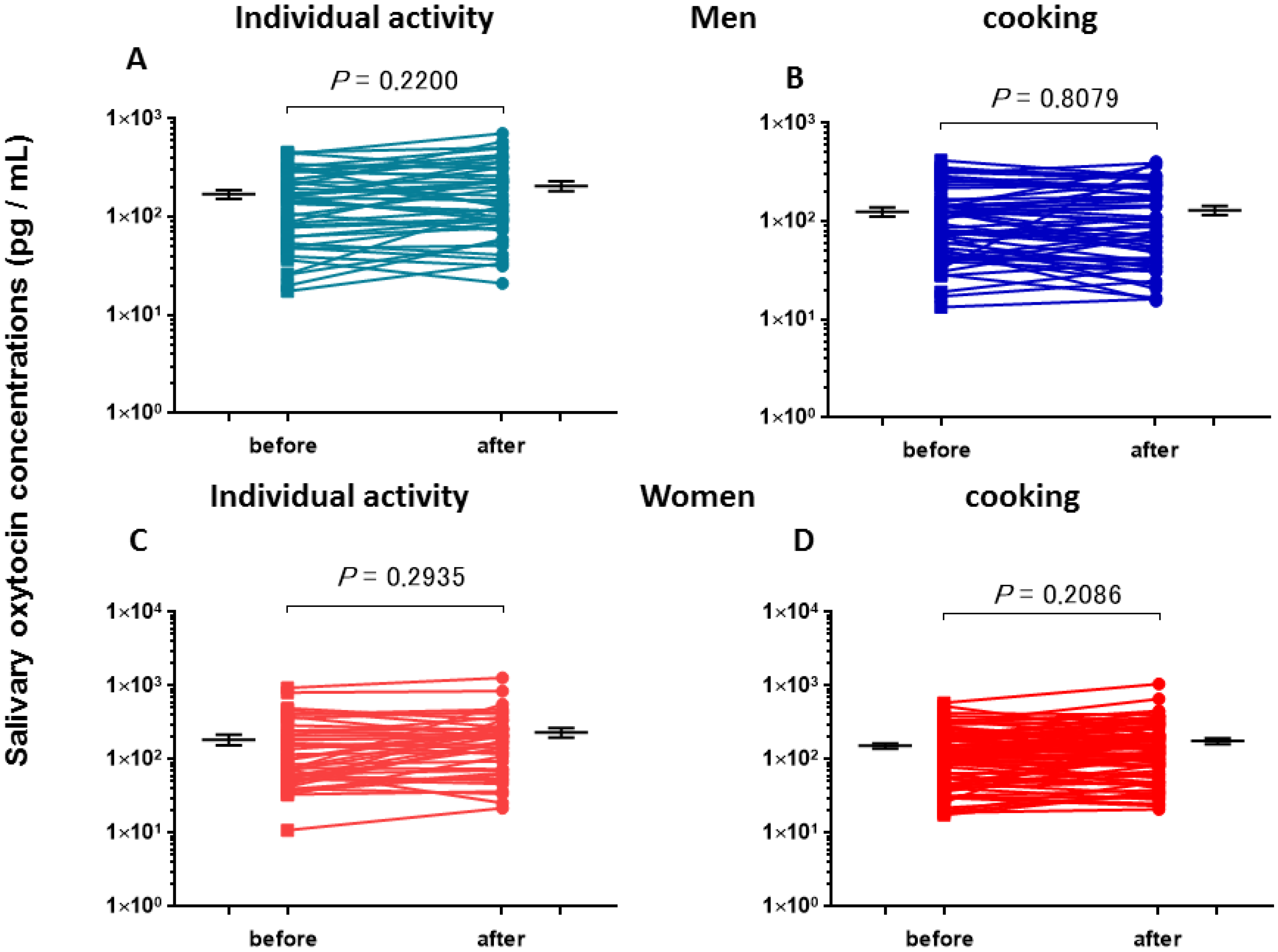
3.1. Salivary Oxytocin Concentrations
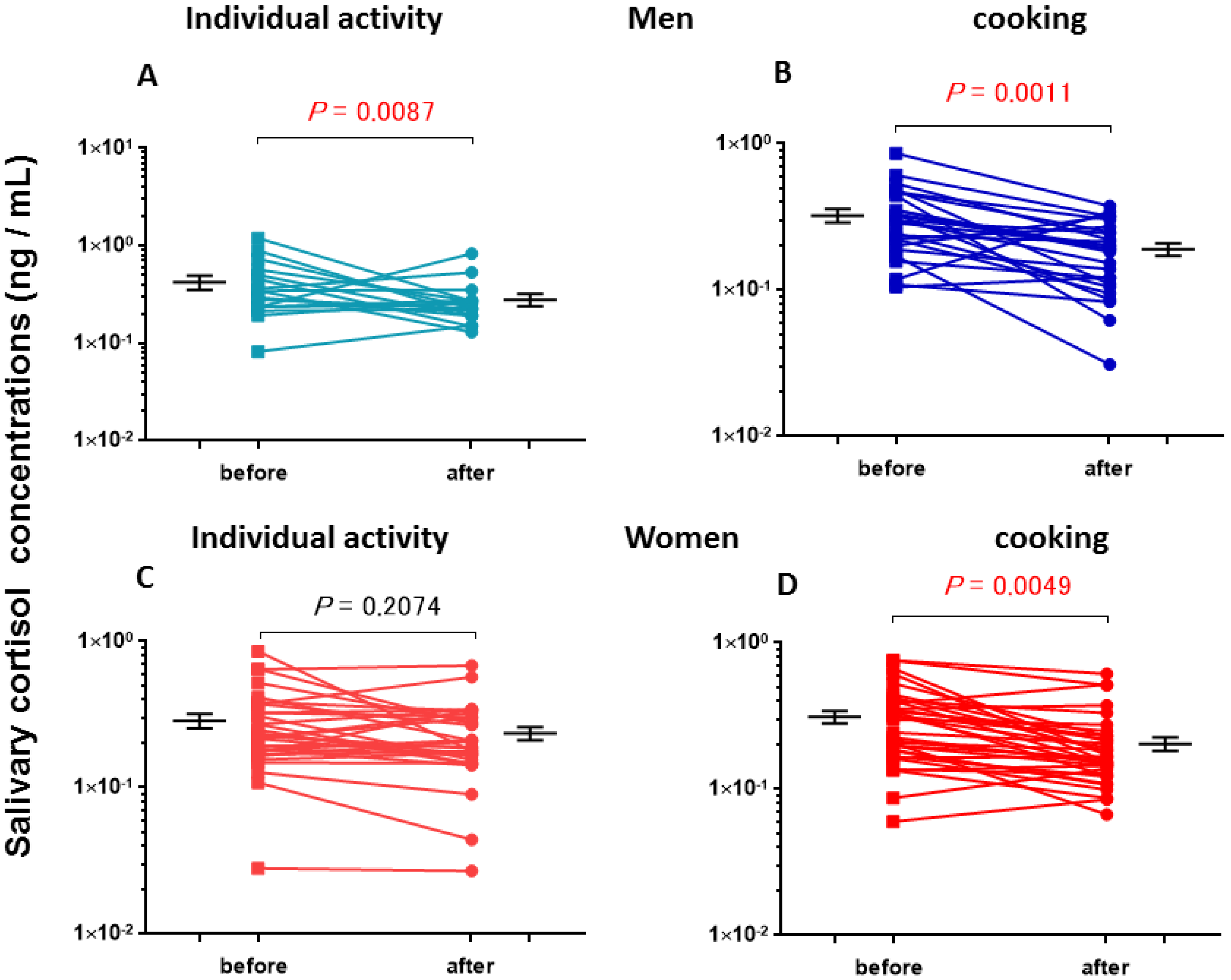
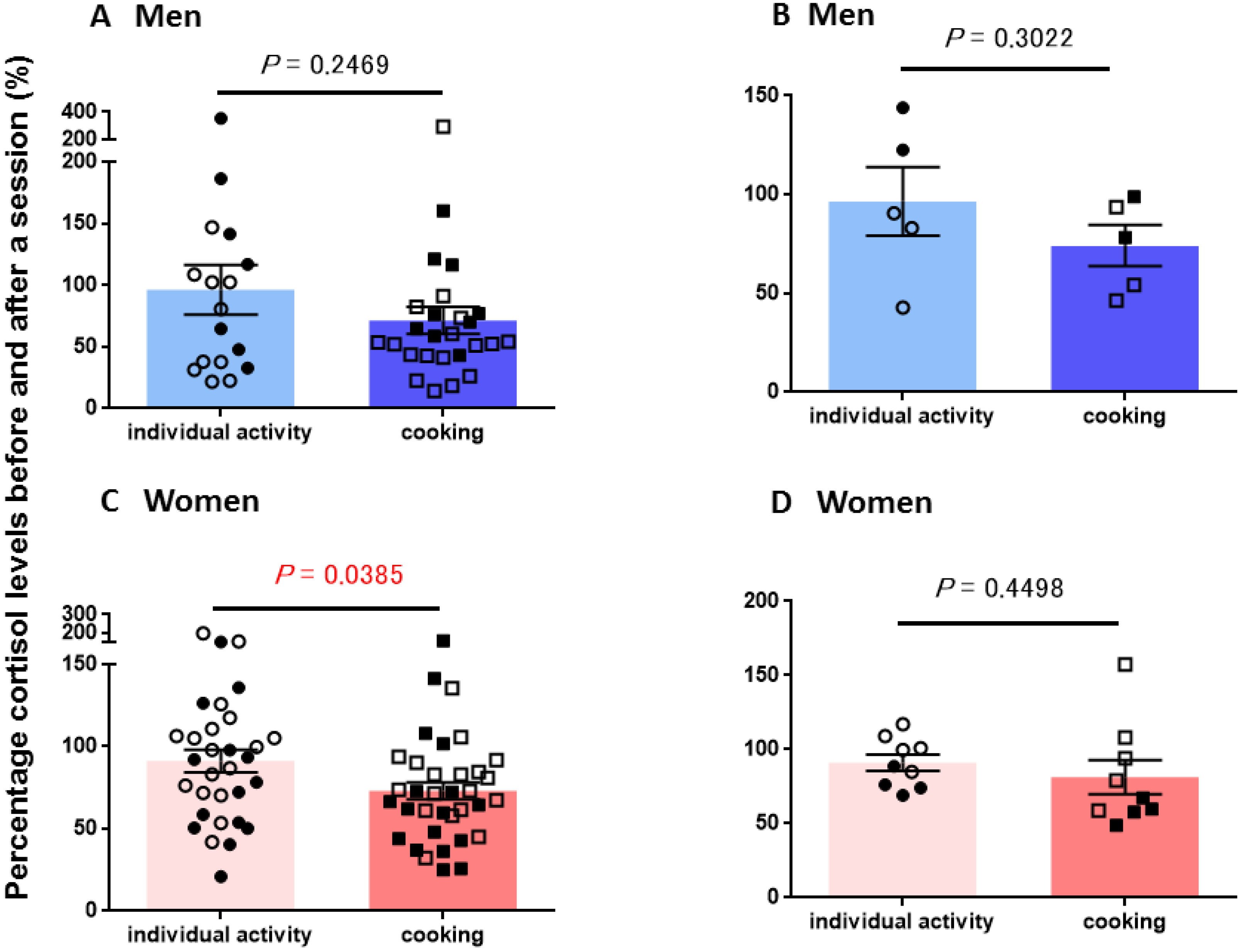
3.2. Salivary Cortisol Concentrations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Donaldson, Z.R.; Young, L.J. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 2008, 322, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Macbeth, A.H.; Pagani, J.H.; Young, W.S. Oxytocin: The great facilitator of life. Prog. Neurobiol. 2009, 88, 127–151. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.D.; Landgraf, R. Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012, 35, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Young, L.J. Oxytocin, social cognition and psychiatry. Neuropsychopharmacology 2015, 40, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Higashida, H.; Yokoyama, S.; Huang, J.J.; Liu, L.; Ma, W.J.; Akther, S.; Higashida, C.; Kikuchi, M.; Minabe, Y.; Munesue, T. Social memory, amnesia, and autism: Brain oxytocin secretion is regulated by NAD+ metabolites and single nucleotide polymorphisms of CD38. Neurochem. Int. 2012, 61, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Higashida, H.; Yuhi, T.; Akther, S.; Amina, S.; Zhong, J.; Liang, M.; Nishimura, T.; Liu, H.X.; Lopatina, O. Oxytocin release via activation of TRPM2 and CD38 in the hypothalamus during hyperthermia in mice: Implication for autism spectrum disorder. Neurochem. Int. 2018, 119, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R. The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron 2010, 65, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Kanat, M.; Heinrichs, M.; Domes, G. Oxytocin and the social brain: Neural mechanisms and perspectives in human research. Brain Res. 2014, 1580, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Feh, E. Oxytocin increases trust in humans. Nature 2005, 35, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Deuse, L.; Wudarczyk, O.; Rademacher, L.; Kaleta, P.; Karges, W.; Kacheva, S.; Gründer, G.; Lammertz, S.E. Peripheral oxytocin predicts higher-level social cognition in men regardless of empathy quotient. Pharmacopsychiatry 2018. [Google Scholar] [CrossRef] [PubMed]
- Nishizato, M.; Fujisawa, T.X.; Kosaka, H.; Tomoda, A. Developmental changes in social attention and oxytocin levels in infants and children. Sci. Rep. 2017, 7, 2540. [Google Scholar] [CrossRef] [PubMed]
- Dulac, C.; O’Connell, L.A.; Wu, Z. Neural control of maternal and paternal behaviors. Science 2014, 345, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.; Monakhov, M.; Pratt, M.; Ebstein, R.P. Oxytocin pathway genes: Evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol. Psychiatry 2016, 79, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Burkett, J.P.; Andari, E.; Johnson, Z.V.; Curry, D.C.; de Waal, F.B.; Young, L.J. Oxytocin-dependent consolation behavior in rodents. Science 2016, 351, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Higashida, H.; Furuhara, K.; Yamauchi, A.M.; Deguchi, K.; Harashima, A.; Munesue, S.; Lopatina, O.; Gerasimenko, M.; Salmina, A.B.; Zhang, J.S.; et al. Intestinal transepithelial permeability of oxytocin into the blood is dependent on the receptor for advanced glycation end products in mice. Sci. Rep. 2017, 7, 7883. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.S.; Wang, Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatry 2014, 76, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, X.J.; Zhang, H.F.; Han, S.P.; Han, J.S. The role of the oxytocin/arginine vasopressin system in animal models of autism spectrum disorder. Adv. Anat. Embryol. Cell Biol. 2017, 224, 135–158. [Google Scholar] [PubMed]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011, 12, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.M.; Fallon, D.; Hill, M.; Frazier, J.A. The role of oxytocin in psychiatric disorders: A review of biological and therapeutic research findings. Harv. Rev. Psychiatry 2013, 21, 219–247. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.D.; Slattery, D.A. Oxytocin in general anxiety and social fear: A translational approach. Biol. Psychiatry 2016, 79, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Munesue, T.; Yokoyama, S.; Nakamura, K.; Anitha, A.; Yamada, K.; Hayashi, K.; Asaka, T.; Liu, H.X.; Jin, D.; Koizumi, K.; et al. Two genetic variants of CD38 in subjects with autism spectrum disorder and controls. Neurosci. Res. 2010, 67, 181–191. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.; Feifel, D. Oxytocin’s role in anxiety: A critical appraisal. Brain Res. 2014, 1580, 22–56. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Tempesta, B.; Micioni Di Bonaventura, M.V.; Gaetani, S. From autism to eating disorders and more: The role of oxytocin in neuropsychiatric disorders. Front. Neurosci. 2016, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Munesue, T.; Nakamura, H.; Kikuchi, M.; Miura, Y.; Takeuch, N.; Anme, T.; Nanba, E.; Adachi, K.; Tsubouchi, K.; Sai, Y.; et al. Oxytocin for male subjects with autism spectrum disorder and comorbid intellectual disabilities: A randomized pilot study. Front. Psychiatry 2016, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, H.; Okamoto, Y.; Munesue, T.; Yamasue, H.; Inohara, K.; Fujioka, T.; Anme, T.; Orisaka, M.; Ishitobi, M.; Jung, M.; et al. Oxytocin efficacy is modulated by dosage and oxytocin receptor genotype in young adults with high-functioning autism: A 24-week randomized clinical trial. Transl. Psychiatry 2016, 6, e872. [Google Scholar] [CrossRef] [PubMed]
- DeMay, M.M.; Song, Y.J.C.; Hickie, I.B.; Guastella, A.J. A review of the safety, efficacy and mechanisms of delivery of nasal oxytocin in children: Therapeutic potential for Autism and Prader-Willi Syndrome, and recommendations for future research. Paediatr. Drugs 2017, 19, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kuroda, M.; Kuwabara, H.; Aoki, Y.; Iwashiro, N.; Tatsunobu, N.; Takao, H.; Nippashi, Y.; Kawakubo, Y.; Kunimatsu, A.; et al. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain 2015, 138, 3400–3412. [Google Scholar] [CrossRef] [PubMed]
- Yatawara, C.J.; Einfeld, S.L.; Hickie, I.B.; Davenport, T.A.; Guastella, A.J. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: A randomized clinical crossover trial. Mol. Psychiatry 2016, 21, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Benner, S.; Yamasue, H. Clinical potential of oxytocin in autism spectrum disorder: Current issues and future perspectives. Behav. Pharmacol. 2018, 29, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Domes, G.; Sibold, M.; Schulze, L.; Lischke, A.; Herpertz, S.C.; Heinrichs, M. Intranasal oxytocin increases covert attention to positive social cues. Psychol. Med. 2013, 43, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Guastella, A.J.; Gray, K.M.; Rinehart, N.J.; Alvares, G.A.; Tonge, B.J.; Hickie, I.B.; Keating, C.M.; Cacciotti-Saija, C.; Einfeld, S.L. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: A randomized controlled trial. J. Child Psychol. Psychiatry 2015, 56, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Liu, H.X.; Hirai, H.; Torashima, T.; Nagai, T.; Lopatina, O.; Shnayder, N.A.; Yamada, K.; Noda, M.; Seike, T.; et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 2007, 446, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Valstad, M.; Alvares, G.A.; Egknud, M.; Matziorinis, A.M.; Andreassen, O.A.; Westlye, L.T.; Quintana, D.S. The correlation between central and peripheral oxytocin concentrations: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2017, 78, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Crockford, C.; Deschner, T.; Ziegler, T.E.; Wittig, R.M. Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: A review. Front. Behav. Neurosci. 2014, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- De Jong, T.R.; Beiderbeckm, D.I.; Neumannm, I.D. Measuring virgin female aggression in the female intruder test (FIT): Effects of oxytocin, estrous cycle, and anxiety. PLoS ONE 2014, 9, e91701. [Google Scholar] [CrossRef] [PubMed]
- Rutigliano, G.; Rocchetti, M.; Paloyelis, Y.; Gilleen, J.; Sardella, A.; Cappucciati, M.; Palombini, E.; Dell’Osso, L.; Caverzasi, E.; Politi, P.; et al. Peripheral oxytocin and vasopressin: Biomarkers of psychiatric disorders? A comprehensive systematic review and preliminary meta-analysis. Psychiatry Res. 2016, 241, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, J.; Chatzittofis, A.; Hellström, C.; Nordström, P.; Uvnäs-Moberg, K.; Asberg, M. Low CSF oxytocin reflects high intent in suicide attempters. Psychoneuroendocrinology 2012, 37, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Kagerbauer, S.M.; Martin, J.; Schuster, T.; Blobner, M.; Kochs, E.F.; Landgraf, R. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J. Neuroendocrinol. 2013, 25, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.S.; Berquist, S.W.; Trujillo, T.H.; Garner, J.P.; Hannah, S.L.; Hyde, S.A.; Sumiyoshi, R.D.; Jackson, L.P.; Moss, J.K.; Strehlow, M.C.; et al. Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Mol. Psychiatry 2015, 20, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Yuhi, T.; Furuhara, K.; Ohta, S.; Shimizu, Y.; Higashida, H. Salivary oxytocin concentrations in seven boys with autism spectrum disorder received massage from their mothers: A pilot study. Front. Psychiatry 2015, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Yuhi, T.; Kyuta, H.; Mori, H.A.; Murakami, C.; Furuhara, K.; Okuno, M.; Takahashi, M.; Fuji, D.; Higashida, H. Salivary oxytocin concentration changes during a group drumming intervention for maltreated school children. Brain Sci. 2017, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Koven, N.S.; Max, L.K. Basal salivary oxytocin level predicts extra- but not intra-personal dimensions of emotional intelligence. Psychoneuroendocrinology 2014, 44, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Huffmeijer, R.; Alink, L.R.; Tops, M.; Grewen, K.M.; Light, K.C.; Bakermans-Kranenburg, M.J.; Ijzendoorn, M.H. Salivary levels of oxytocin remain elevated for more than two hours after intranasal oxytocin administration. Neuro Endocrinol. Lett. 2012, 33, 21–25. [Google Scholar] [PubMed]
- Javor, A.; Riedl, R.; Kindermann, H.; Brandstätter, W.; Ransmayr, G.; Gabriel, M. Correlation of plasma and salivary oxytocin in healthy young men—Experimental evidence. Neuro Endocrinol. Lett. 2014, 35, 470–473. [Google Scholar] [PubMed]
- Lunsky, Y.P.; Hastings, R.; Weiss, J.A.M.; Palucka, A.; Hutton, S.; White, K. Comparative effects of mindfulness and support and information group interventions for parents of adults with autism spectrum disorder and other developmental disabilities. J. Autism Dev. Disord. 2017, 47, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Schladt, T.M.; Nordmann, G.C.; Emilius, R.; Kudielka, B.M.; de Jong, T.R.; Neumann, I.D. Choir versus solo singing: Effects on mood, and salivary oxytocin and cortisol concentrations. Front. Hum. Neurosci. 2017, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Altenmüller, E.; Schlaug, G. Apollo’s gift: New aspects of neurologic music therapy. Prog. Brain Res. 2015, 217, 237–252. [Google Scholar] [PubMed]
- Daykin, N.; de Viggiani, N.; Pilkington, P.; Moriarty, Y. Music making for health, well-being and behaviour change in youth justice settings: A systematic review. Health Promot. Int. 2013, 28, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, G.; Bongard, S.; Rohrmann, S.; Hodapp, V.; Grebe, D. Effects of choir singing or listening on secretory immunoglobulin A, cortisol, and emotional state. J. Behav. Med. 2004, 27, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Roden, I.; Kreutz, G.; Bongard, S. Effects of a school-based instrumental music program on verbal and visual memory in primary school children: A longitudinal study. Front. Psychol. 2012, 3, 572. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.; Clément, S.; Narme, P.; Schiaratura, L.; Ehrlé, N. Efficacy of musical interventions in dementia: Methodological requirements of nonpharmacological trials. Ann. N. Y. Acad. Sci. 2015, 1337, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Perkins, R.; Ascenso, S.; Atkins, L.; Fancourt, D.; Williamon, A. Making music for mental health: How group drumming mediates recovery. Psychol. Well Being 2016, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Fancourt, D.; Perkins, R.; Ascenso, S.; Carvalho, L.A.; Steptoe, A.; Williamon, A. Effects of group drumming interventions on anxiety, depression, social resilience and inflammatory immune response among mental health service users. PLoS ONE 2016, 11, e0151136. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zhou, Y.; Yang, S.; Thomas, W.K.S.; Smith, G.D.; Yang, Z.; Yuan, L.; Chung, J.W. Effect of music intervention on apathy in nursing home residents with dementia. Geriatr. Nurs. 2018, 39, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Daykin, N.; Mansfield, L.; Meads, C.; Julier, G.; Tomlinson, A.; Payne, A.; Grigsby, D.L.; Lane, J.; D’Innocenzo, G.; Burnett, A.; et al. What works for wellbeing? A systematic review of wellbeing outcomes for music and singing in adults. Perspect. Public Health 2018, 138, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Cevasco, A.M. Effects of the therapist’s nonverbal behavior on participation and affect of individuals with Alzheimer’s disease during group music therapy sessions. J. Music Ther. 2010, 47, 282–299. [Google Scholar] [CrossRef] [PubMed]
- Bittman, B.B.; Berk, L.S.; Felten, D.L.; Westengard, J.; Simonton, O.C.; Pappas, J.; Ninehouser, M. Composite effects of group drumming music therapy on modulation of neuroendocrine-immune parameters in normal subjects. Altern. Ther. Health Med. 2001, 7, 38–47. [Google Scholar] [PubMed]
- Van Ijzendoorn, M.H.; Bhandari, R.; van der Veen, R.; Grewen, K.M.; Bakermans-Kranenburg, M.J. Elevated salivary levels of oxytocin persist more than 7 h after intranasal administration. Front. Neurosci. 2012, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen-Rufi, M.C.; Vink, A.C.; Wolterbeek, R.; Achterberg, W.P.; Roos, R.A. The effect of music therapy in patients with Huntington’s Disease: A randomized controlled trial. J. Huntingt. Dis. 2017, 6, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Baum, C.; Edwards, D.F. Cognitive performance in senile dementia of the Alzheimer’s type: The Kitchen Task Assessment. Am. J. Occup. Ther. 1993, 47, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Fjellström, C.; Starkenberg, A.; Wesslén, A.; Licentiate, M.S.; Tysén Bäckström, A.C.; Faxén-Irving, G.; OmegAD Study Group. To be a good food provider: An exploratory study among spouses of persons with Alzheimer’s disease. Am. J. Alzheimers Dis. Other Dement. 2010, 25, 521–526. [Google Scholar]
- Cerasa, A.; Arcuri, F.; Pignataro, L.M.; Serra, S.; Messina, D.; Carozzo, S.; Biafora, A.; Ceraudo, C.; Abbruzzino, L.; Pignolo, L.; et al. The cooking therapy for cognitive rehabilitation of cerebellar damage: A case report and a review of the literature. J. Clin. Neurosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Majlesi, A.R.; Ekström, A. Baking together-the coordination of actions in activities involving people with dementia. J. Aging Stud. 2016, 38, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Farmer, N.; Touchton-Leonard, K.; Ross, A. Psychosocial benefits of cooking interventions: A systematic review. Health Educ. Behav. 2018, 45, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Jung, N.H. The effect of exercise combined with a cognitive-enhancement group training program on cognition and depression in the community-dwelling elderly. J. Phys. Ther. Sci. 2018, 30, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Hellhammer, D.H.; Wüst, S.; Kudielka, B.M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009, 34, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Kumazaki, H.; Warren, Z.; Corbett, B.A.; Yoshikawa, Y.; Matsumoto, Y.; Higashida, H.; Yuhi, T.; Ikeda, T.; Ishiguro, H.; Kikuchi, M. Android Robot-Mediated Mock Job Interview Sessions for Young Adults with Autism Spectrum Disorder: A Pilot Study. Front. Psychiatry 2017, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.; Kemp, T.; Pan, Y.; Yang, Z.; Giuliano, A.R.; Pinto, L.A. Evaluation of HPV-16 and HPV-18 specific antibody measurements in saliva collected in oral rinses and merocel® sponges. Vaccine 2018, 36, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- MacLean, E.; Gesquiere, L.; Gee, N.; Levy, K.; Martin, W.L.; Carter, C.S. Validation of salivary oxytocin and vasopressin as biomarkers in domestic dogs. J. Neurosci. Methods 2017, 293, 67–76. [Google Scholar] [CrossRef] [PubMed]
- MacLean, E.L.; Gesquiere, L.R.; Gee, N.R.; Levy, K.; Martin, W.L.; Carter, C.S. Effects of affiliative human-animal interaction on dog salivary and plasma oxytocin and vasopressin. Front. Psychol. 2017, 8, 1606. [Google Scholar] [CrossRef] [PubMed]
- Leng, G.; Sabatier, N. Measuring oxytocin and vasopressin: Bioassays, immunoassays and random numbers. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.A.; Blair, R.J. Deficits in facial affect recognition among antisocial populations: A meta-analysis. Neurosci. Biobehav. Rev. 2008, 32, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Domes, G.; Steiner, A.; Porges, S.W.; Heinrichs, M. Oxytocin differentially modulates eye gaze to naturalistic social signals of happiness and anger. Psychoneuroendocrinology 2013, 38, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, M.; Jeung, H.; Schmitt, R.; Boll, S.; Freitag, C.M.; Bertsch, K.; Herpertz, S.C. Oxytocin improves facial emotion recognition in young adults with antisocial personality disorder. Psychoneuroendocrinology 2017, 85, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Adam, E.K.; Kumari, M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology 2009, 34, 1423–1436. [Google Scholar] [CrossRef] [PubMed]
- Narme, P.; Clément, S.; Ehrlé, N.; Schiaratura, L.; Vachez, S.; Courtaigne, B.; Munsch, F.; Samson, S. Efficacy of musical interventions in dementia: Evidence from a randomized controlled trial. J. Alzheimers Dis. 2014, 38, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, S.; Ruud, T. Group therapy in public mental health services: Approaches, patients and group therapists. J. Psychiatr. Ment. Health Nurs. 2014, 21, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Woods, B.; O’Philbin, L.; Farrell, E.M.; Spector, A.E.; Orrell, M. Reminiscence therapy for dementia. Cochrane Database Syst. Rev. 2018, 3, CD001120. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.Y.; Lin, L.J. A systematic review of reminiscence therapy for older adults in Taiwan. J. Nurs. Res. 2018, 26, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Swan, K.; Hopper, M.; Wenke, R.; Jackson, C.; Till, T.; Conway, E. Speech-language pathologist interventions for communication in moderate-severe dementia: A systematic review. Am. J. Speech Lang. Pathol. 2018, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, M.D.; Reavell, J.; Lane, D.A.; Mallikarjun, P. Cognitive behavioral therapy for depression, anxiety, and stress in caregivers of dementia patients: A systematic review and meta-analysis. Gerontologist 2018. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, G. Does singing facilitates social bonding? Music Med. 2014, 6, 51–60. [Google Scholar]
- Grape, C.; Sandgren, M.; Hansson, L.O.; Ericson, M.; Theorell, T. Does singing promote well-being? An empirical study of professional and amateur singers during a singing lesson. Integr. Physiol. Behav. Sci. 2003, 38, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Hellhammer, D.H. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 1994, 19, 313–333. [Google Scholar] [CrossRef]
- Carter, C.S.; Pournajafi-Nazarloo, H.; Kramer, K.M.; Ziegler, T.E.; White-Traut, R.; Bello, D.; Schwertz, D. Oxytocin: Behavioral associations and potential as a salivary biomarker. Ann. N. Y. Acad. Sci. 2007, 1098, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Amina, S.; Liang, M.; Akther, S.; Yuhi, T.; Nishimura, T.; Tsuji, C.; Tsuji, T.; Liu, H.X.; Hashii, M.; et al. Cyclic ADP-Ribose and heat regulate oxytocin release via CD38 and TRPM2 in the hypothalamus during social or psychological stress in mice. Front. Neurosci. 2016, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, M.; Hellhammer, D.H.; Pruessner, J.C.; Lupien, S.J. Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosom. Med. 2003, 65, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, S.S.; Kemeny, M.E. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004, 130, 355–391. [Google Scholar] [CrossRef] [PubMed]
- Adam, E.K.; Quinn, M.E.; Tavernier, R.; McQuillan, M.T.; Dahlke, K.A.; Gilbert, K.E. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Clow, A.; Thorn, L.; Evans, P.; Hucklebridge, F. The awakening cortisol response: Methodological issues and significance. Stress 2004, 7, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Holmqvist-Jämsén, S.; Johansson, A.; Santtila, P.; Westberg, L.; von der Pahlen, B.; Simberg, S. Investigating the Role of Salivary Cortisol on Vocal Symptoms. J. Speech Lang. Hear. Res. 2017, 60, 2781–2791. [Google Scholar] [CrossRef] [PubMed]
- Kristenson, M.; Olsson, A.G.; Kucinskiene, Z. Good self-rated health is related to psychosocial resources and a strong cortisol response to acute stress: The LiVicordia study of middle-aged men. Int. J. Behav. Med. 2005, 12, 153–160. [Google Scholar] [CrossRef] [PubMed]

| Men (Married) | Women (Married) | |
|---|---|---|
| Number of participants | 9 (2) | 9 (4) |
| Each year | ||
| 2015 | 3 (1) | 4 (3) |
| 2016 | 4 (2) | 6 (4) |
| 2017 | 5 (2) | 9 (4) |
| Age (years ± s.e.m. (range)) a | 27.6 ± 5.3 (21–69) | 33.9 ± 3.9 (21–50) |
| Education (years ± s.e.m. (range)) b | 16.4 ± 0.96 (15–22) | 16.1 ± 0.54 (14–20) |
| Ethnicity | ||
| Japanese | 8 (1) | 6 (3) |
| Russian | 1 (1) | 2 (0) |
| Chinese | 0 | 1 (1) |
| Men | Women | ||
|---|---|---|---|
| OT concentrations (pg/mL ± s.e.m.) | |||
| Individual activities | before | 170 ± 18 (46) a,b | 185 ± 31 (44) a,b |
| after | 207 ± 24 (46) b,c | 234 ± 35 (44) b,c | |
| Cooking | before | 125 ± 13 (55) a,b,c | 153 ± 12 (90) a,b |
| after | 120 ± 14 (55) b,c | 178 ± 16 (90) b | |
| CORT concentrations (ng/dL ± s.e.m.) | |||
| Individual activities | before | 420 ± 69 (17) d,e,f | 286 ± 31 (31) d,e |
| after | 278 ± 41 (17) e | 235 ± 24 (31) e,f | |
| Cooking | before | 330 ± 34 (26) d,e,g | 312± 49 (35) d,e,h |
| after | 190 ± 18 (26) e,f,g | 204 ± 21 (35) e,f,h |
| Men | Women | |
|---|---|---|
| OT level changed (%±s.e.m.) a | ||
| Individual activities c (Married b) | 148 ± 22 (22) | 153 ± 20 (23) |
| Individual activities c (Single b) | 128 ± 12 (24) | 205 ± 45 (21) |
| Cooking c (Married b) | 102± 12 (22) | 137 ± 12 (55) |
| Cooking c (Single b) | 108 ± 8 (38) | 152 ± 32 (35) |
| CORT level changed (%±s.e.m.) d | ||
| Individual activities c (Married b) | 135 ± 42 (7) | 82 ± 10 (14) |
| Individual activities c (Single b) | 69 ± 14 (10) | 103 ± 9 (17) |
| Cooking c (Married b) | 87 ± 12 (9) | 60 ± 8 (15) |
| Cooking c (Single b) | 63 ± 15 (17) | 82 ± 6 (20) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuhi, T.; Ise, K.; Iwashina, K.; Terao, N.; Yoshioka, S.; Shomura, K.; Maehara, T.; Yazaki, A.; Koichi, K.; Furuhara, K.; et al. Sex Differences in Salivary Oxytocin and Cortisol Concentration Changes during Cooking in a Small Group. Behav. Sci. 2018, 8, 101. https://doi.org/10.3390/bs8110101
Yuhi T, Ise K, Iwashina K, Terao N, Yoshioka S, Shomura K, Maehara T, Yazaki A, Koichi K, Furuhara K, et al. Sex Differences in Salivary Oxytocin and Cortisol Concentration Changes during Cooking in a Small Group. Behavioral Sciences. 2018; 8(11):101. https://doi.org/10.3390/bs8110101
Chicago/Turabian StyleYuhi, Teruko, Kosuke Ise, Kei Iwashina, Naoya Terao, Satoshi Yoshioka, Keijiro Shomura, Toshikatsu Maehara, Akari Yazaki, Kana Koichi, Kazumi Furuhara, and et al. 2018. "Sex Differences in Salivary Oxytocin and Cortisol Concentration Changes during Cooking in a Small Group" Behavioral Sciences 8, no. 11: 101. https://doi.org/10.3390/bs8110101
APA StyleYuhi, T., Ise, K., Iwashina, K., Terao, N., Yoshioka, S., Shomura, K., Maehara, T., Yazaki, A., Koichi, K., Furuhara, K., Cherepanov, S. M., Gerasimenko, M., Shabalova, A. A., Hosoki, K., Kodama, H., Zhu, H., Tsuji, C., Yokoyama, S., & Higashida, H. (2018). Sex Differences in Salivary Oxytocin and Cortisol Concentration Changes during Cooking in a Small Group. Behavioral Sciences, 8(11), 101. https://doi.org/10.3390/bs8110101






