Reward Network Activations of Win Versus Loss in a Monetary Gambling Task
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. The Monetary Gambling Task (MGT)
2.3. Behavioral Scores Extracted from Task Performance
2.4. Neuroimaging Protocol
2.4.1. Structural and Functional MRI Acquisition
2.4.2. fMRI Preprocessing
2.4.3. Subject-Level BOLD Response: Activation Detection Between Win and Loss
2.4.4. Group-Level Image Processing of the fMRI Data
2.4.5. Refinement of BOLD Activation Clusters for the Win–Loss Contrast
2.5. Assessment of Impulsivity
2.6. Neuropsychological Assessment
2.6.1. Tower of London Test (TOL)
2.6.2. Visual Span Test (VST)
2.7. Statistical Analyses
3. Results
3.1. The fMRI Activation Clusters for the Win–Loss Contrast
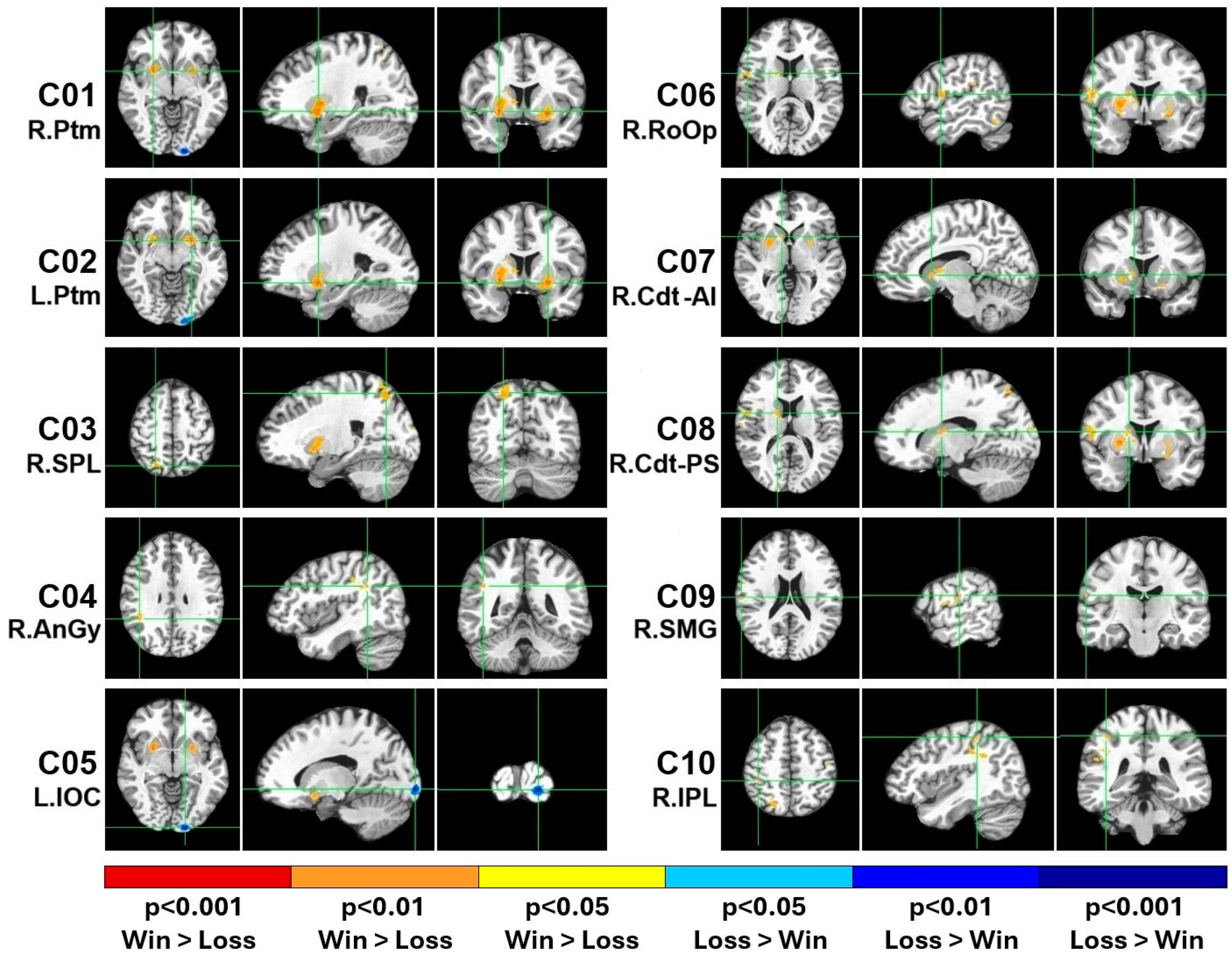
| # | Size | Anatomical Region | Code | Direction | BA | MNI | Mean | SD | SE |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1781 | R. Putamen | R. Ptm | Win > Loss | 49 | 27,5,−6 | 62.87 | 63.99 | 11.68 |
| 2 | 1426 | L. Putamen | L. Ptm | Win > Loss | 49 | −24,5,−9 | 67.61 | 62.27 | 11.37 |
| 3 | 878 | R. Superior Parietal Lobule | R. SPL | Win > Loss | 7 | 23,−68,56 | 80.96 | 120.22 | 21.95 |
| 4 | 663 | R. Angular Gyrus | R. AnGy | Win > Loss | 39 | 44,−47,30 | 61.00 | 64.65 | 11.80 |
| 5 | 640 | L. Inferior Occipital Cortex | L. IOC | Loss > Win | 18 | −15,−100,−6 | −97.03 | 77.14 | 14.08 |
| 6 | 444 | R. Rolandic Operculum | R. RoOp | Win > Loss | 6 | 56,2,12 | 55.53 | 66.63 | 12.16 |
| 7 | 333 | R. Caudate (anterior–inferior) | R. Cdt (A-I) | Win > Loss | 48 | 11,12,0 | 53.03 | 81.01 | 14.79 |
| 8 | 239 | R. Caudate (posterior–superior) | R. Cdt (P-S) | Win > Loss | 48 | 17,1,14 | 54.47 | 100.90 | 18.42 |
| 9 | 100 | R. Supramarginal Gyrus | R. SMG | Win > Loss | 40 | 63,−18,20 | 75.13 | 97.02 | 17.71 |
| 10 | 100 | R. Inferior Parietal Lobule | R. IPL | Win > Loss | 40 | 42,−37,51 | 63.64 | 109.55 | 20.00 |
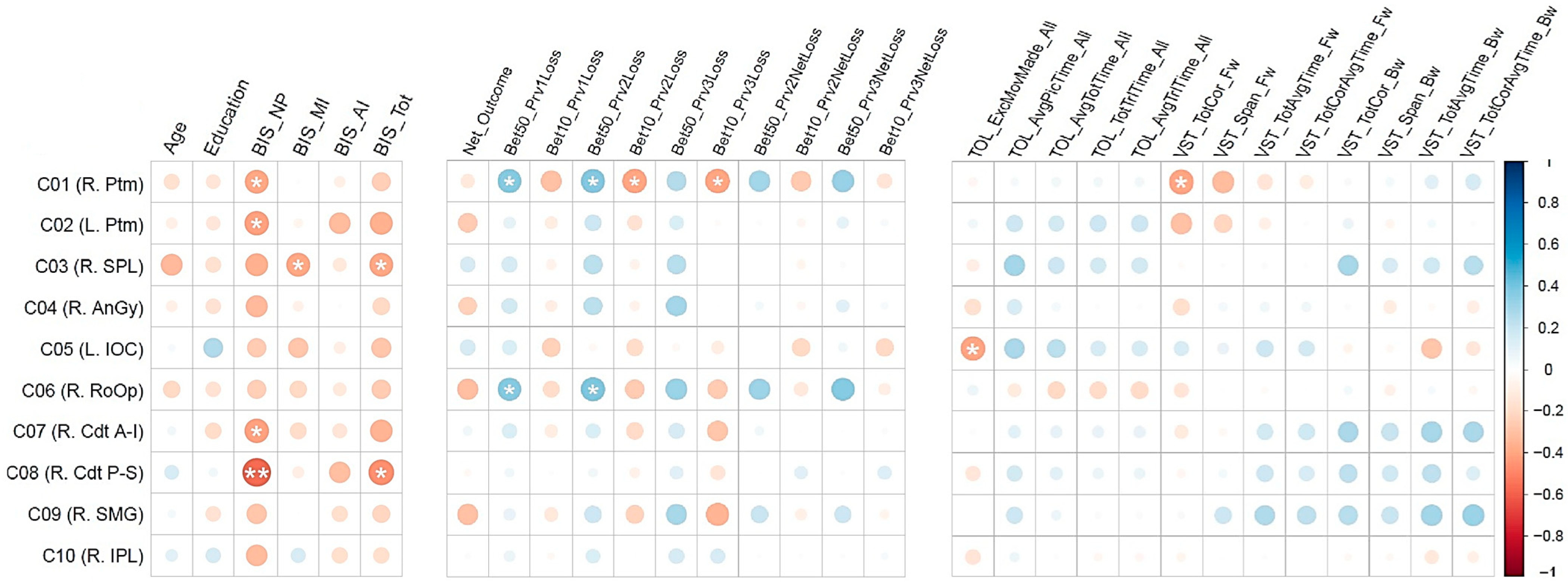
3.2. Correlations Between the fMRI Activation Clusters and Other Variables
- (i)
- Negative correlation of BIS non-planning with fMRI activation cluster 1 (R. Ptm; r = −0.3844, p < 0.05), cluster 2 (L. Ptm; r = −0.4057, p < 0.05), cluster 7 (R. Cdt A-I; r = −0.4073, p < 0.05), and cluster 8 (R. Cdt P-S; r = −0.5603, p < 0.01);
- (ii)
- Negative correlation of BIS motor impulsivity with cluster 3 (R. SPL; r = −0.3885, p < 0.05);
- (iii)
- Negative correlation of BIS total impulsivity with cluster 3 (R. SPL; r = −0.3851, p < 0.05) and cluster 8 (R. Cdt P-S; r = −0.4504).
- (i)
- Positive correlations between the number of bets with 50 tokens after a loss during the previous trial with fMRI activation cluster 1 (R. Ptm; r = 0.3700, p < 0.05) and cluster 6 (R. RoOp; r = 0.3617, p < 0.05);
- (ii)
- Positive correlations between the number of bets with 50 tokens after two consecutive losses during previous trials with fMRI activation cluster 1 (R. Ptm; r = 0.3754, p < 0.05) and cluster 6 (R. RoOp; r = 0.3896, p < 0.05);
- (iii)
- Negative correlations of fMRI activation cluster 1 (R. Ptm) with the number of bets with 10 tokens after consecutively losing during the previous two trials (r = −0.3903, p < 0.05) as well as with the number of bets with 10 tokens after consecutively losing during the previous three trials (r = −0.3943, p < 0.05).
4. Discussion
4.1. Neural Substrates of the Win–Loss Contrast
4.1.1. The Regions Activated During Reward Processing
4.1.2. Correlations Across the fMRI Activation Clusters
4.2. Associations Between the Reward Regions and Behavioral Features
4.2.1. Associations Between the Reward Regions and Impulsivity
4.2.2. Associations Between the Reward Regions and Gambling Performance
4.2.3. Associations Between the Reward Regions and Neuropsychological Scores
4.2.4. Clinical Implications
4.2.5. Limitations and Suggestions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MGT | Monetary Gambling Task |
| BOLD | Blood Oxygenation Level-Dependent |
| MRI | Magnetic Resonance Imaging |
| fMRI | Functional Magnetic Resonance Imaging |
| BIS | Barratt Impulsiveness Scale |
| TOL | Tower of London Test |
| VST | Visual Span Test |
| MPRAGE | Magnetization-Prepared Rapid Gradient Echo |
| ART | Automatic Registration Toolbox |
| sPCA | Sparse Principal Component Analysis |
References
- Admon, R., & Pizzagalli, D. A. (2015). Dysfunctional reward processing in depression. Current Opinion in Psychology, 4, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Allen, T. J., Moeller, F. G., Rhoades, H. M., & Cherek, D. R. (1998). Impulsivity and history of drug dependence. Drug and Alcohol Dependence, 50(2), 137–145. [Google Scholar] [CrossRef] [PubMed]
- Andreou, C., Kleinert, J., Steinmann, S., Fuger, U., Leicht, G., & Mulert, C. (2015). Oscillatory responses to reward processing in borderline personality disorder. World Journal of Biological Psychiatry, 16(8), 575–586. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, B. A., Bachman, A. H., & Helpern, J. A. (2001). A quantitative comparison of motion detection algorithms in fMRI. Magnetic Resonance Imaging, 19(7), 959–963. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, B. A., Bappal, A., D’Angelo, D., Ashtari, M., Lencz, T., Szeszko, P. R., Butler, P. D., Javitt, D. C., Lim, K. O., Hrabe, J., Nierenberg, J., Branch, C. A., & Hoptman, M. J. (2005). Brain morphometry using diffusion-weighted magnetic resonance imaging: Application to schizophrenia. Neuroreport, 16(13), 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, B. A., Braun, M., Hutton, B. F., Kanno, I., & Iida, H. (1995). A fully automatic multimodality image registration algorithm. Journal of Computer Assisted Tomography, 19(4), 615–623. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, B. A., Choi, S. J., Hossein-Zadeh, G. A., Porjesz, B., Tanabe, J. L., Lim, K. O., Bilder, R., Helpern, J. A., & Begleiter, H. (2002). Functional magnetic resonance imaging of brain activity in the visual oddball task. Brain Research: Cognitive Brain Research, 14(3), 347–356. [Google Scholar] [CrossRef] [PubMed]
- Arsalidou, M., Vijayarajah, S., & Sharaev, M. (2020). Basal ganglia lateralization in different types of reward. Brain Imaging and Behavior, 14(6), 2618–2646. [Google Scholar] [CrossRef] [PubMed]
- Baker, S. C., Rogers, R. D., Owen, A. M., Frith, C. D., Dolan, R. J., Frackowiak, R. S., & Robbins, T. W. (1996). Neural systems engaged by planning: A PET study of the Tower of London task. Neuropsychologia, 34(6), 515–526. [Google Scholar] [CrossRef] [PubMed]
- Baskin-Sommers, A. R., & Foti, D. (2015). Abnormal reward functioning across substance use disorders and major depressive disorder: Considering reward as a transdiagnostic mechanism. International Journal of Psychophysiology, 98(2), 227–239. [Google Scholar] [CrossRef] [PubMed]
- Beck, A., Schlagenhauf, F., Wustenberg, T., Hein, J., Kienast, T., Kahnt, T., Schmack, K., Hagele, C., Knutson, B., Heinz, A., & Wrase, J. (2009). Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry, 66(8), 734–742. [Google Scholar] [CrossRef] [PubMed]
- Bentivegna, F., Papachristou, E., & Flouri, E. (2024). A scoping review on self-regulation and reward processing measured with gambling tasks: Evidence from the general youth population. PLoS ONE, 19(4), e0301539. [Google Scholar] [CrossRef] [PubMed]
- Berch, D. B., Krikorian, R., & Huha, E. M. (1998). The Corsi block-tapping task: Methodological and theoretical considerations. Brain and Cognition, 38(3), 317–338. [Google Scholar] [CrossRef] [PubMed]
- Bialaszek, W., Gaik, M., McGoun, E., & Zielonka, P. (2015). Impulsive people have a compulsion for immediate gratification-certain or uncertain. Frontiers in Psychology, 6, 515. [Google Scholar] [CrossRef]
- Blaszczynski, A., Steel, Z., & McConaghy, N. (1997). Impulsivity in pathological gambling: The antisocial impulsivist. Addiction, 92(1), 75–87. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M. M., Sabatinelli, D., Lang, P. J., Fitzsimmons, J. R., King, W., & Desai, P. (2003). Activation of the visual cortex in motivated attention. Behavioral Neuroscience, 117(2), 369–380. [Google Scholar] [CrossRef] [PubMed]
- Brovelli, A., Nazarian, B., Meunier, M., & Boussaoud, D. (2011). Differential roles of caudate nucleus and putamen during instrumental learning. Neuroimage, 57(4), 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Ryan, N., Rubia, K., & Conrod, P. J. (2011). Response inhibition and reward response bias mediate the predictive relationships between impulsivity and sensation seeking and common and unique variance in conduct disorder and substance misuse. Alcoholism: Clinical and Experimental Research, 35(1), 140–155. [Google Scholar] [CrossRef] [PubMed]
- Cavina-Pratesi, C., Kentridge, R. W., Heywood, C. A., & Milner, A. D. (2010). Separate processing of texture and form in the ventral stream: Evidence from FMRI and visual agnosia. Cerebral Cortex, 20(2), 433–446. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R. A., & Potenza, M. N. (2003). Neurodevelopment, impulsivity, and adolescent gambling. Journal of Gambling Studies, 19(1), 53–84. [Google Scholar] [CrossRef] [PubMed]
- Chau, D. T., Roth, R. M., & Green, A. I. (2004). The neural circuitry of reward and its relevance to psychiatric disorders. Current Psychiatry Reports, 6(5), 391–399. [Google Scholar] [CrossRef] [PubMed]
- Chelazzi, L., Perlato, A., Santandrea, E., & Della Libera, C. (2013). Rewards teach visual selective attention. Vision Research, 85, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Cherek, D. R., & Lane, S. D. (1999). Effects of d,l-fenfluramine on aggressive and impulsive responding in adult males with a history of conduct disorder. Psychopharmacology, 146(4), 473–481. [Google Scholar] [CrossRef] [PubMed]
- Coffey, S. F., Gudleski, G. D., Saladin, M. E., & Brady, K. T. (2003). Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Experimental and Clinical Psychopharmacology, 11(1), 18–25. [Google Scholar] [CrossRef] [PubMed]
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173. [Google Scholar] [CrossRef] [PubMed]
- Crockford, D. N., Goodyear, B., Edwards, J., Quickfall, J., & el-Guebaly, N. (2005). Cue-induced brain activity in pathological gamblers. Biological Psychiatry, 58(10), 787–795. [Google Scholar] [CrossRef] [PubMed]
- Davis, H. P., & Keller, F. (2002). Colorado Assessment Tests (CATs), Version 1.2. Colorado Assessment Tests. [Google Scholar]
- Dawe, S., Gullo, M. J., & Loxton, N. J. (2004). Reward drive and rash impulsiveness as dimensions of impulsivity: Implications for substance misuse. Addictive Behaviors, 29(7), 1389–1405. [Google Scholar] [CrossRef] [PubMed]
- Dawe, S., & Loxton, N. J. (2004). The role of impulsivity in the development of substance use and eating disorders [Review]. Neuroscience & Biobehavioral Reviews, 28(3), 343–351. [Google Scholar] [CrossRef]
- de Wit, H. (2009). Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addiction Biology, 14(1), 22–31. [Google Scholar] [CrossRef] [PubMed]
- Dichter, G. S., Damiano, C. A., & Allen, J. A. (2012). Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: Animal models and clinical findings. Journal of Neurodevelopmental Disorders, 4(1), 19. [Google Scholar] [CrossRef] [PubMed]
- Diekhof, E. K., Nerenberg, L., Falkai, P., Dechent, P., Baudewig, J., & Gruber, O. (2012). Impulsive personality and the ability to resist immediate reward: An fMRI study examining interindividual differences in the neural mechanisms underlying self-control. Human Brain Mapping, 33(12), 2768–2784. [Google Scholar] [CrossRef] [PubMed]
- Dobrossy, M. D., Ramanathan, C., Ashouri Vajari, D., Tong, Y., Schlaepfer, T., & Coenen, V. A. (2021). Neuromodulation in Psychiatric disorders: Experimental and Clinical evidence for reward and motivation network Deep Brain Stimulation: Focus on the medial forebrain bundle. European Journal of Neuroscience, 53(1), 89–113. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M., Nelson, E. E., McClure, E. B., Monk, C. S., Munson, S., Eshel, N., Zarahn, E., Leibenluft, E., Zametkin, A., Towbin, K., Blair, J., Charney, D., & Pine, D. S. (2004). Choice selection and reward anticipation: An fMRI study. Neuropsychologia, 42(12), 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Flannery, J. S., Riedel, M. C., Bottenhorn, K. L., Poudel, R., Salo, T., Hill-Bowen, L. D., Laird, A. R., & Sutherland, M. T. (2020). Meta-analytic clustering dissociates brain activity and behavior profiles across reward processing paradigms. Cognitive, Affective & Behavioral Neuroscience, 20(2), 215–235. [Google Scholar] [CrossRef]
- Garcia-Garcia, I., Horstmann, A., Jurado, M. A., Garolera, M., Chaudhry, S. J., Margulies, D. S., Villringer, A., & Neumann, J. (2014). Reward processing in obesity, substance addiction and non-substance addiction. Obesity Reviews, 15(11), 853–869. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R. (2003). Underlying processes in the poor response inhibition of children with attention-deficit/hyperactivity disorder. Journal of Attention Disorders, 6(3), 111–122. [Google Scholar] [CrossRef] [PubMed]
- Goudriaan, A. E., de Ruiter, M. B., van den Brink, W., Oosterlaan, J., & Veltman, D. J. (2010). Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: An fMRI study. Addiction Biology, 15(4), 491–503. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T., Dresler, T., Ehlis, A. C., Plichta, M. M., Heinzel, S., Polak, T., Lesch, K. P., Breuer, F., Jakob, P. M., & Fallgatter, A. J. (2009). Neural response to reward anticipation is modulated by Gray’s impulsivity. Neuroimage, 46(4), 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Haruno, M., & Kawato, M. (2006). Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. Journal of Neurophysiology, 95(2), 948–959. [Google Scholar] [CrossRef] [PubMed]
- Hege, M. A., Stingl, K. T., Kullmann, S., Schag, K., Giel, K. E., Zipfel, S., & Preissl, H. (2015). Attentional impulsivity in binge eating disorder modulates response inhibition performance and frontal brain networks. International Journal of Obesity (London), 39(2), 353–360. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J. F. (2003). Interpreting the magnitudes of correlation coefficients. American Psychologist, 58(1), 78–79. [Google Scholar] [CrossRef] [PubMed]
- Hesselbrock, M. N., & Hesselbrock, V. M. (1992). Relationship of family history, antisocial personality disorder and personality traits in young men at risk for alcoholism. Journal of Studies on Alcohol, 53(6), 619–625. [Google Scholar] [CrossRef] [PubMed]
- Hollunder, B., Rajamani, N., Siddiqi, S. H., Finke, C., Kuhn, A. A., Mayberg, H. S., Fox, M. D., Neudorfer, C., & Horn, A. (2022). Toward personalized medicine in connectomic deep brain stimulation. Progress in Neurobiology, 210, 102211. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, S., Yang, C., & Tan, A. (2015). Basal ganglia circuit loops, dopamine and motivation: A review and enquiry. Behavioural Brain Research, 290, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Jarbo, K., & Verstynen, T. D. (2015). Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. Journal of Neuroscience, 35(9), 3865–3878. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K., Zhao, G., Feng, Q., Guan, S., Im, H., Zhang, B., Wang, P., Jia, X., Zhu, H., Zhu, Y., Wang, H., & Wang, Q. (2024). The computational and neural substrates of individual differences in impulsivity under loss framework. Human Brain Mapping, 45(11), e26808. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S., Davis, B., Jomier, M., & Gerig, G. (2004). Unbiased diffeomorphic atlas construction for computational anatomy. Neuroimage, 23(Suppl. S1), S151–S160. [Google Scholar] [CrossRef] [PubMed]
- Kaas, J. H. (2017). Evolution of visual cortex in primates. In J. H. Kaas (Ed.), Evolution of nervous systems (pp. 187–201). Academic Press. [Google Scholar] [CrossRef]
- Kahneman, D., & Tversky, A. (1979). Prospect theory: An analysis of decision under risk. Econometrica, 47(2), 263–291. [Google Scholar] [CrossRef]
- Kalivas, P. W., & Volkow, N. D. (2005). The neural basis of addiction: A pathology of motivation and choice. American Journal of Psychiatry, 162(8), 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Kamarajan, C., Ardekani, B. A., Pandey, A. K., Kinreich, S., Pandey, G., Chorlian, D. B., Meyers, J. L., Zhang, J., Bermudez, E., Kuang, W., Stimus, A. T., & Porjesz, B. (2022). Differentiating individuals with and without alcohol use disorder using resting-state fMRI functional connectivity of reward network, neuropsychological performance, and impulsivity measures. Behavioral Sciences (Basel), 12(5), 128. [Google Scholar] [CrossRef] [PubMed]
- Kamarajan, C., Ardekani, B. A., Pandey, A. K., Kinreich, S., Pandey, G., Chorlian, D. B., Meyers, J. L., Zhang, J., Bermudez, E., Stimus, A. T., & Porjesz, B. (2020). Random forest classification of alcohol use disorder using fMRI functional connectivity, neuropsychological functioning, and impulsivity measures. Brain Sciences, 10(2), 115. [Google Scholar] [CrossRef] [PubMed]
- Kamarajan, C., Rangaswamy, M., Tang, Y., Chorlian, D. B., Pandey, A. K., Roopesh, B. N., Manz, N., Saunders, R., Stimus, A. T., & Porjesz, B. (2010). Dysfunctional reward processing in male alcoholics: An ERP study during a gambling task. Journal of Psychiatric Research, 44(9), 576–590. [Google Scholar] [CrossRef] [PubMed]
- Kastner, S., & Ungerleider, L. G. (2000). Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience, 23, 315–341. [Google Scholar] [CrossRef] [PubMed]
- Klein, A., Andersson, J., Ardekani, B. A., Ashburner, J., Avants, B., Chiang, M. C., Christensen, G. E., Collins, D. L., Gee, J., Hellier, P., Song, J. H., Jenkinson, M., Lepage, C., Rueckert, D., Thompson, P., Vercauteren, T., Woods, R. P., Mann, J. J., & Parsey, R. V. (2009). Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage, 46(3), 786–802. [Google Scholar] [CrossRef] [PubMed]
- Koay, C. G., Carew, J. D., Alexander, A. L., Basser, P. J., & Meyerand, M. E. (2006). Investigation of anomalous estimates of tensor-derived quantities in diffusion tensor imaging. Magnetic Resonance in Medicine, 55(4), 930–936. [Google Scholar] [CrossRef] [PubMed]
- Krebs, R. M., Boehler, C. N., Roberts, K. C., Song, A. W., & Woldorff, M. G. (2012). The involvement of the dopaminergic midbrain and cortico-striatal-thalamic circuits in the integration of reward prospect and attentional task demands. Cerebral Cortex, 22(3), 607–615. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K. A., Allen, J. S., & Chanen, A. M. (2010). Impulsivity in borderline personality disorder: Reward-based decision-making and its relationship to emotional distress [Research Support, Non-U.S. Gov’t]. Journal of Personality Disorders, 24(6), 786–799. [Google Scholar] [CrossRef] [PubMed]
- Liu, X., Hairston, J., Schrier, M., & Fan, J. (2011). Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews, 35(5), 1219–1236. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, J. J., 3rd, Hanlon, C. A., Marshalek, P. J., Rezai, A. R., & Krinke, L. (2020). Transcranial magnetic stimulation, deep brain stimulation, and other forms of neuromodulation for substance use disorders: Review of modalities and implications for treatment. Journal of the Neurological Sciences, 418, 117149. [Google Scholar] [CrossRef] [PubMed]
- McClure, S. M., York, M. K., & Montague, P. R. (2004). The neural substrates of reward processing in humans: The modern role of FMRI. Neuroscientist, 10(3), 260–268. [Google Scholar] [CrossRef] [PubMed]
- McGuire, K. L., Amsalem, O., Sugden, A. U., Ramesh, R. N., Fernando, J., Burgess, C. R., & Andermann, M. L. (2022). Visual association cortex links cues with conjunctions of reward and locomotor contexts. Current Biology, 32(7), 1563–1576.e8. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, D., Davenport, D., Talhelm, T., & Li, Y. (2024). The motivating effect of monetary over psychological incentives is stronger in WEIRD cultures. Nature Human Behaviour, 8(3), 456–470. [Google Scholar] [CrossRef] [PubMed]
- Miedl, S. F., Fehr, T., Meyer, G., & Herrmann, M. (2010). Neurobiological correlates of problem gambling in a quasi-realistic blackjack scenario as revealed by fMRI. Psychiatry Research, 181(3), 165–173. [Google Scholar] [CrossRef] [PubMed]
- Milner, B. (1971). Interhemispheric differences in the localization of psychological processes in man. British Medical Bulletin, 27(3), 272–277. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K., Kawatani, J., Tajima, K., Sasaki, A. T., Yoneda, T., Komi, M., Hirai, T., Tomoda, A., Joudoi, T., & Watanabe, Y. (2016). Low putamen activity associated with poor reward sensitivity in childhood chronic fatigue syndrome. Neuroimage Clin, 12, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Moeller, F. G., Barratt, E. S., Dougherty, D. M., Schmitz, J. M., & Swann, A. C. (2001). Psychiatric aspects of impulsivity. American Journal of Psychiatry, 158(11), 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Muehlberg, C., Goerg, S., Rullmann, M., Hesse, S., Sabri, O., Wawrzyniak, M., Classen, J., Fricke, C., & Rumpf, J. J. (2024). Motor learning is modulated by dopamine availability in the sensorimotor putamen. Brain Commun, 6(6), fcae409. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E. J., & Carlezon, W. A., Jr. (2006). The mesolimbic dopamine reward circuit in depression. Biological Psychiatry, 59(12), 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Numssen, O., Bzdok, D., & Hartwigsen, G. (2021). Functional specialization within the inferior parietal lobes across cognitive domains. Elife, 10, e63591. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, J. P., Cockburn, J., & Pauli, W. M. (2017). Learning, reward, and decision making. Annual Review of Psychology, 68, 73–100. [Google Scholar] [CrossRef] [PubMed]
- OpenStax & Lumen Learning. (2024). Reinforcement and punishment. In General psychology. Pressbooks. Available online: https://pressbooks.online.ucf.edu/lumenpsychology/ (accessed on 20 March 2025).
- Packard, M. G., & Knowlton, B. J. (2002). Learning and memory functions of the Basal Ganglia. Annual Review of Neuroscience, 25, 563–593. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. K., Ardekani, B. A., Byrne, K. N., Kamarajan, C., Zhang, J., Pandey, G., Meyers, J. L., Kinreich, S., Chorlian, D. B., Kuang, W., Stimus, A. T., & Porjesz, B. (2022). Statistical nonparametric fMRI maps in the analysis of response inhibition in abstinent individuals with history of alcohol use disorder. Behavioral Sciences (Basel), 12(5), 121. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. K., Ardekani, B. A., Kamarajan, C., Zhang, J., Chorlian, D. B., Byrne, K. N., Pandey, G., Meyers, J. L., Kinreich, S., Stimus, A., & Porjesz, B. (2018). Lower prefrontal and hippocampal volume and diffusion tensor imaging differences reflect structural and functional abnormalities in abstinent individuals with alcohol use disorder. Alcoholism: Clinical and Experimental Research, 42(10), 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Parent, A., & Hazrati, L. N. (1995). Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Research: Brain Research Reviews, 20(1), 91–127. [Google Scholar] [CrossRef] [PubMed]
- Parsons, L. M., Fox, P. T., Downs, J. H., Glass, T., Hirsch, T. B., Martin, C. C., Jerabek, P. A., & Lancaster, J. L. (1995). Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature, 375(6526), 54–58. [Google Scholar] [CrossRef] [PubMed]
- Patton, J. H., Stanford, M. S., & Barratt, E. S. (1995). Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology, 51(6), 768–774. [Google Scholar] [CrossRef] [PubMed]
- Peters, S. K., Dunlop, K., & Downar, J. (2016). Cortico-striatal-thalamic loop circuits of the salience network: A central pathway in psychiatric disease and treatment [review]. Frontiers in Systems Neuroscience, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Petry, N. M. (2001). Substance abuse, pathological gambling, and impulsiveness. Drug and Alcohol Dependence, 63(1), 29–38. [Google Scholar] [CrossRef] [PubMed]
- Pujara, M., & Koenigs, M. (2014). Mechanisms of reward circuit dysfunction in psychiatric illness: Prefrontal-striatal interactions. Neuroscientist, 20(1), 82–95. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. (2024). R: A language and environment for statistical computing. R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 20 March 2025).
- Rangaswamy, M., Porjesz, B., Ardekani, B. A., Choi, S. J., Tanabe, J. L., Lim, K. O., & Begleiter, H. (2004). A functional MRI study of visual oddball: Evidence for frontoparietal dysfunction in subjects at risk for alcoholism. Neuroimage, 21(1), 329–339. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, I. A., Antonsen, I. K., Berntsen, E. M., Xu, J., Lagopoulos, J., & Haberg, A. K. (2006). Brain activation measured using functional magnetic resonance imaging during the tower of London task. Acta Neuropsychiatrica. Officieel Wetenschappelijk Orgaan van Het IGBP (Interdisciplinair Genootschap voor Biologische Psychiatrie), 18(5), 216–225. [Google Scholar] [CrossRef] [PubMed]
- Reise, S. P., Moore, T. M., Sabb, F. W., Brown, A. K., & London, E. D. (2013). The barratt impulsiveness Scale-11: Reassessment of its structure in a community sample. Psychological Assessment, 25(2), 631–642. [Google Scholar] [CrossRef] [PubMed]
- Richards, J. M., Plate, R. C., & Ernst, M. (2013). A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: The impact of task design and implications for understanding neurodevelopment. Neuroscience & Biobehavioral Reviews, 37(5), 976–991. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E. T., Huang, C. C., Lin, C. P., Feng, J., & Joliot, M. (2020). Automated anatomical labelling atlas 3. Neuroimage, 206, 116189. [Google Scholar] [CrossRef] [PubMed]
- Rovelli, K., & Allegretta, R. A. (2023). Framing decision-making: The role of executive functions, cognitive bias and reward. Neuropsychological Trends, 33(33), 37–50. [Google Scholar] [CrossRef]
- Rubio, G., Jimenez, M., Rodriguez-Jimenez, R., Martinez, I., Iribarren, M. M., Jimenez-Arriero, M. A., Ponce, G., & Avila, C. (2007). Varieties of impulsivity in males with alcohol dependence: The role of Cluster-B personality disorder. Alcoholism: Clinical and Experimental Research, 31(11), 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Sefcsik, T., Nemeth, D., Janacsek, K., Hoffmann, I., Scialabba, J., Klivenyi, P., Ambrus, G. G., Haden, G., & Vecsei, L. (2009). The role of the putamen in cognitive functions—A case study. Learning & Perception, 1(2), 215–227. [Google Scholar] [CrossRef]
- Shallice, T. (1982). Specific impairments of planning. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 298(1089), 199–209. [Google Scholar] [CrossRef] [PubMed]
- Shu, S. Y., Song, C., Wu, Y., Mo, L., Guo, Z., Liu, S. H., & Bao, X. (2009). Learning and memory deficits caused by a lesion in the medial area of the left putamen in the human brain. CNS Spectrums, 14(9), 473–476. [Google Scholar] [CrossRef] [PubMed]
- Silani, G., Lamm, C., Ruff, C. C., & Singer, T. (2013). Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. Journal of Neuroscience, 33(39), 15466–15476. [Google Scholar] [CrossRef] [PubMed]
- Sjöstrand, K., Lund, T. E., Madsen, K. H., & Larsen, R. (2006). Sparse PCA—A new method for unsupervised analyses of fMRI data. Proceedings of the International Society of Magnetic Resonance in Medicine (ISMRM), 14, 2845. Available online: https://cds.ismrm.org/protected/06MProceedings/PDFfiles/02845.pdf (accessed on 2 February 2010).
- Stanford, M. S., Mathias, C. W., Dougherty, D. M., Lake, S. L., Anderson, N. E., & Patton, J. H. (2009). Fifty years of the barratt impulsiveness scale: An update and review. Personality and Individual Differences, 47(5), 385–395. [Google Scholar] [CrossRef]
- Streiner, D. L., & Norman, G. R. (2011). Correction for multiple testing: Is there a resolution? Chest, 140(1), 16–18. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G. M., & Feinn, R. (2012). Using effect size-or why the P value is not enough. Journal of Graduate Medical Education, 4(3), 279–282. [Google Scholar] [CrossRef] [PubMed]
- Sutoko, S., Atsumori, H., Obata, A., Funane, T., Kandori, A., Shimonaga, K., Hama, S., Yamawaki, S., & Tsuji, T. (2020). Lesions in the right Rolandic operculum are associated with self-rating affective and apathetic depressive symptoms for post-stroke patients. Scientific Reports, 10(1), 20264. [Google Scholar] [CrossRef] [PubMed]
- Swann, A. C., Bjork, J. M., Moeller, F. G., & Dougherty, D. M. (2002). Two models of impulsivity: Relationship to personality traits and psychopathology. Biological Psychiatry, 51(12), 988–994. [Google Scholar] [CrossRef] [PubMed]
- Swann, A. C., Lijffijt, M., Lane, S. D., Steinberg, J. L., & Moeller, F. G. (2009). Trait impulsivity and response inhibition in antisocial personality disorder [research support, N.I.H., extramural]. Journal of Psychiatric Research, 43(12), 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R. (1996). Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society Series B: Statistical Methodology, 58(1), 267–288. [Google Scholar] [CrossRef]
- Tomasi, D., & Volkow, N. D. (2014). Functional connectivity of substantia nigra and ventral tegmental area: Maturation during adolescence and effects of ADHD. Cerebral Cortex, 24(4), 935–944. [Google Scholar] [CrossRef] [PubMed]
- Toni, I., Thoenissen, D., & Zilles, K. (2001). Movement preparation and motor intention. Neuroimage, 14(1), S110–S117. [Google Scholar] [CrossRef] [PubMed]
- Ungerleider, L. G., Courtney, S. M., & Haxby, J. V. (1998). A neural system for human visual working memory. Proceedings of the National Academy of Sciences of the United States of America, 95(3), 883–890. [Google Scholar] [CrossRef] [PubMed]
- van Reekum, R., Links, P. S., & Fedorov, C. (1994). Impulsivity in borderline personality disorder. In K. R. Silk (Ed.), Biological and neurobehavioral studies of borderline personality disorder (pp. 1–22). American Psychiatric Association. [Google Scholar]
- Wang, J., Yang, Y., Fan, L., Xu, J., Li, C., Liu, Y., Fox, P. T., Eickhoff, S. B., Yu, C., & Jiang, T. (2015). Convergent functional architecture of the superior parietal lobule unraveled with multimodal neuroimaging approaches. Human Brain Mapping, 36(1), 238–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, K. S., Smith, D. V., & Delgado, M. R. (2016). Using fMRI to study reward processing in humans: Past, present, and future. Journal of Neurophysiology, 115(3), 1664–1678. [Google Scholar] [CrossRef] [PubMed]
- Wang, P., Chen, S., Deng, K., Zhang, B., Im, H., Feng, J., Liu, L., Yang, Q., Zhao, G., He, Q., Chen, C., Wang, H., & Wang, Q. (2023). Distributed attribute representation in the superior parietal lobe during probabilistic decision-making. Human Brain Mapping, 44(17), 5693–5711. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y., FitzGerald, T. H., & Friston, K. J. (2013). Working memory and anticipatory set modulate midbrain and putamen activity. Journal of Neuroscience, 33(35), 14040–14047. [Google Scholar] [CrossRef] [PubMed]
- Zald, D. H., Boileau, I., El-Dearedy, W., Gunn, R., McGlone, F., Dichter, G. S., & Dagher, A. (2004). Dopamine transmission in the human striatum during monetary reward tasks. Journal of Neuroscience, 24(17), 4105–4112. [Google Scholar] [CrossRef] [PubMed]
- Zald, D. H., & Treadway, M. T. (2017). Reward processing, neuroeconomics, and psychopathology. Annual Review of Clinical Psychology, 13(1), 471–495. [Google Scholar] [CrossRef] [PubMed]
- Zou, H., Hastie, T., & Tibshirani, R. (2006). Sparse principal component analysis. Journal of Computational and Graphical Statistics, 15(2), 265–286. [Google Scholar] [CrossRef]
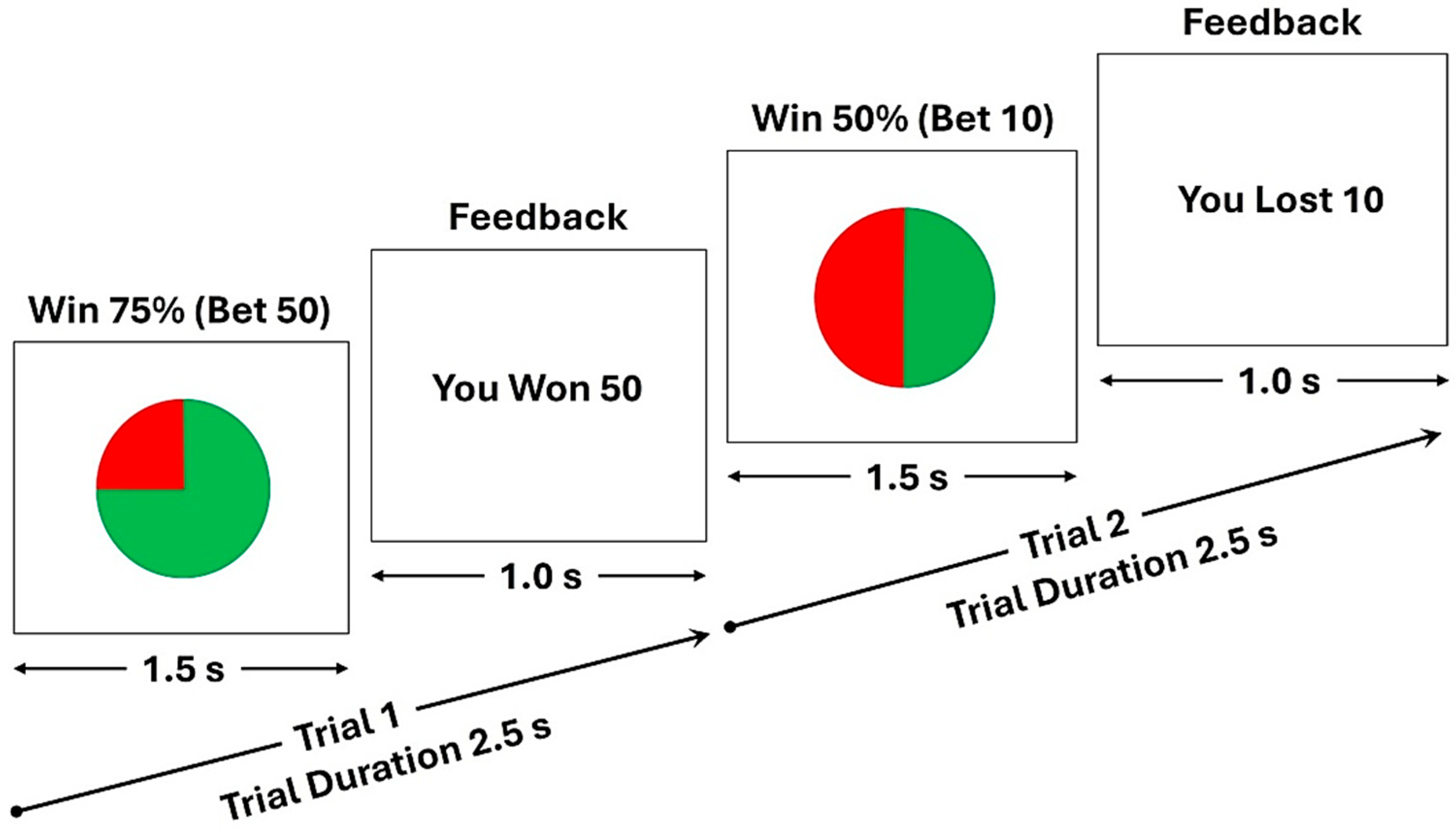

| Trial Type | Chance of Winning | Outcome | Number of Trials | Trial Probability (%) |
|---|---|---|---|---|
| 1 | 50% | Win | 40 | 16.67% |
| 2 | 50% | Loss | 40 | 16.67% |
| 3 | 75% | Win | 60 | 25.00% |
| 4 | 75% | Loss | 20 | 8.33% |
| 5 | 25% | Win | 20 | 8.33% |
| 6 | 25% | Loss | 60 | 25.00% |
| Variable Name | Variable Description | Min | Max | Mean | SD | SE |
|---|---|---|---|---|---|---|
| Net_Outcome | Total tokens won or lost at the end of the task | 0 | 1790 | 1187.67 | 386.00 | 70.47 |
| Bet50_Prv1Loss | # 50 tokens after a loss during the previous trial | 16 | 90 | 60.77 | 14.75 | 2.69 |
| Bet10_Prv1Loss | # 10 tokens after a loss during the previous trial | 21 | 103 | 56.13 | 16.05 | 2.93 |
| Bet50_Prv2Loss | # 50 tokens after two consecutive losses during the previous trials | 6 | 45 | 30.83 | 8.63 | 1.58 |
| Bet10_Prv2Loss | # 10 tokens after two consecutive losses during the previous trials | 12 | 54 | 27.93 | 8.98 | 1.64 |
| Bet50_Prv3Loss | # 50 tokens after three consecutive losses during the previous trials | 3 | 24 | 15.03 | 5.17 | 0.94 |
| Bet10_Prv3Loss | # 10 tokens after three consecutive losses during the previous trials | 4 | 29 | 13.73 | 5.85 | 1.07 |
| Bet50_Prv2NetLoss | # 50 tokens after the net outcome of loss during the previous two trials | 23 | 99 | 65.5 | 16.21 | 2.96 |
| Bet10_Prv2NetLoss | # 10 tokens after the net outcome of loss during the previous two trials | 26 | 125 | 60.93 | 18.00 | 3.29 |
| Bet50_Prv3NetLoss | # 50 tokens after the net outcome of loss during the previous three trials | 11 | 78 | 51.33 | 13.66 | 2.49 |
| Bet10_Prv3NetLoss | # 10 tokens after the net outcome of loss during the previous three trials | 27 | 90 | 47.47 | 12.55 | 2.29 |
| Variable Name | Variable Description | Min | Max | Mean | SD | SE |
|---|---|---|---|---|---|---|
| BIS_NP | Non-Planning | 13 | 34 | 19.80 | 4.61 | 0.84 |
| BIS_MI | Motor Impulsivity | 14 | 27 | 19.30 | 3.28 | 0.60 |
| BIS_AI | Attentional Impulsivity | 8 | 21 | 12.57 | 3.19 | 0.58 |
| BIS_Tot | Total Impulsivity | 39 | 72 | 51.67 | 8.60 | 1.57 |
| Variable Name | Variable Description | Min | Max | Mean | SD | SE |
|---|---|---|---|---|---|---|
| TOL_ExcMovMade | Excess moves made | 0.00 | 29.00 | 7.83 | 6.66 | 1.24 |
| TOL_AvgPicTime | Average pickup time | 1.47 | 5.45 | 2.81 | 0.96 | 0.18 |
| TOL_AvgTotTime | Average total time | 2.58 | 8.80 | 4.72 | 1.64 | 0.30 |
| TOL_TotTrlTime | Total trial time | 241.65 | 788.01 | 404.24 | 139.05 | 25.82 |
| TOL_AvgTrlTime | Average trial time | 11.51 | 37.52 | 19.25 | 6.62 | 1.23 |
| Variable Name | Variable Description | Min | Max | Mean | SD | SE |
|---|---|---|---|---|---|---|
| VST_TotCor_Fw | Total correct scores for forward trials | 5.00 | 14.00 | 10.21 | 2.78 | 0.52 |
| VST_Span_Fw | Span for forward trials | 4.00 | 8.00 | 6.83 | 1.37 | 0.25 |
| VST_TotAvgTime_Fw | Total average time for forward trials | 9.99 | 49.11 | 28.31 | 10.53 | 1.96 |
| VST_TotCorAvgTime_Fw | Total correct average time for forward trials | 14.90 | 49.11 | 32.48 | 8.07 | 1.50 |
| VST_TotCor_Bw | Total correct scores for backward trials | 5.00 | 14.00 | 8.31 | 1.87 | 0.35 |
| VST_Span_Bw | Span for backward trials | 4.00 | 8.00 | 5.52 | 0.95 | 0.18 |
| VST_TotAvgTime_Bw | Total average time for backward trials | 9.84 | 56.48 | 17.79 | 10.01 | 1.86 |
| VST_TotCorAvgTime_Bw | Total correct average time for backward trials | 14.73 | 56.48 | 27.16 | 10.66 | 1.98 |
| Variable Set | Variable | C01 R. Ptm | C02 L. Ptm | C03 R. SPL | C04 R. AnGy | C05 L. IOC | C06 R. RoOp | C07 R. Cdt (AI) | C08 R. Cdt (PS) | C09 R. SMG | C10 R. IPL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographic variables | Age | −0.1686 | −0.0885 | −0.3225 | −0.0864 | 0.0366 | −0.2015 | 0.0519 | 0.1424 | 0.0452 | 0.1024 |
| Education | −0.1406 | −0.1360 | −0.1668 | −0.1591 | 0.2654 | −0.1568 | −0.1810 | 0.0538 | −0.1594 | 0.1576 | |
| Impulsivity scores | BIS_NP | −0.3844 * | −0.4057 * | −0.3534 | −0.3291 | −0.2510 | −0.2459 | −0.4072 | −0.5603 ** | −0.2798 | −0.3155 |
| BIS_MI | −0.0127 | −0.0581 | −0.3885 * | −0.0754 | −0.2758 | −0.2030 | −0.1888 | −0.0924 | −0.0088 | 0.1499 | |
| BIS_AI | −0.0844 | −0.3143 | −0.1279 | −0.0107 | −0.0940 | −0.1215 | −0.1463 | −0.3095 | −0.1738 | −0.1755 | |
| BIS_Tot | −0.2422 | −0.3562 | −0.3851 * | −0.2091 | −0.2746 | −0.2543 | −0.3446 | −0.4504 * | −0.2178 | −0.1770 | |
| Task performance | Net_Outcome | −0.1250 | −0.2576 | 0.1526 | −0.2325 | 0.1594 | −0.3039 | 0.0517 | −0.0319 | −0.2912 | 0.0202 |
| Bet50_Prv1Loss | 0.3700 * | 0.0915 | 0.1586 | 0.1642 | 0.1348 | 0.3617 * | 0.1382 | 0.0521 | 0.0873 | 0.0799 | |
| Bet10_Prv1Loss | −0.2937 | −0.1051 | −0.0691 | −0.0909 | −0.2319 | −0.1873 | −0.1078 | 0.0133 | −0.1279 | −0.0318 | |
| Bet50_Prv2Loss | 0.3754 * | 0.1812 | 0.2366 | 0.2337 | −0.0326 | 0.3896 * | 0.1347 | 0.0513 | 0.1883 | 0.1301 | |
| Bet10_Prv2Loss | −0.3903 * | −0.1510 | −0.0632 | −0.0695 | −0.1868 | −0.2537 | −0.1929 | −0.0736 | −0.2222 | −0.0041 | |
| Bet50_Prv3Loss | 0.2540 | 0.1339 | 0.2415 | 0.2929 | −0.0371 | 0.3139 | 0.1563 | 0.1143 | 0.2803 | 0.1552 | |
| Bet10_Prv3Loss | −0.3943 * | −0.0221 | 0.0013 | −0.0020 | −0.1044 | −0.2551 | −0.2749 | −0.1478 | −0.3456 | 0.1476 | |
| Bet50_Prv2NetLoss | 0.2952 | 0.0196 | −0.0084 | 0.0484 | 0.0049 | 0.3052 | 0.0501 | −0.0094 | 0.1951 | 0.0100 | |
| Bet10_Prv2NetLoss | −0.2676 | −0.0495 | −0.0523 | −0.0499 | −0.2094 | −0.1299 | −0.0098 | 0.1107 | −0.0841 | 0.0173 | |
| Bet50_Prv3NetLoss | 0.3246 | 0.0931 | 0.0230 | 0.1136 | 0.0553 | 0.3604 | 0.0022 | −0.0122 | 0.1900 | 0.0138 | |
| Bet10_Prv3NetLoss | −0.1418 | 0.0355 | 0.0016 | 0.0317 | −0.2043 | −0.0929 | 0.0215 | 0.1228 | −0.0544 | 0.0315 | |
| Neuropsychological scores | TOL_ExcMovMade | −0.0468 | 0.0523 | −0.0938 | −0.1615 | −0.3927 * | 0.0679 | −0.0179 | −0.1394 | −0.0035 | −0.1466 |
| TOL_AvgPicTime | 0.0411 | 0.1848 | 0.2992 | 0.1470 | 0.2767 | −0.1186 | 0.1002 | 0.1636 | 0.1843 | 0.0613 | |
| TOL_AvgTotTime | 0.0533 | 0.1626 | 0.1840 | 0.0473 | 0.2300 | −0.2029 | 0.0916 | 0.1049 | 0.0622 | −0.0133 | |
| TOL_TotTrlTime | 0.0514 | 0.1852 | 0.1691 | 0.0168 | 0.1580 | −0.1979 | 0.0893 | 0.0756 | 0.0297 | −0.0447 | |
| TOL_AvgTrlTime | 0.0514 | 0.1852 | 0.1691 | 0.0168 | 0.1580 | −0.1979 | 0.0894 | 0.0756 | 0.0297 | −0.0447 | |
| VST_TotCor_Fw | −0.3981 * | −0.2994 | −0.0212 | −0.1768 | 0.1453 | −0.1415 | −0.1193 | −0.0483 | 0.0324 | −0.0508 | |
| VST_Span_Fw | −0.3102 | −0.2193 | 0.0114 | 0.0489 | 0.0826 | 0.0098 | −0.0375 | 0.0441 | 0.1885 | 0.0089 | |
| VST_TotAvgTime_Fw | −0.1477 | −0.0876 | 0.0234 | 0.0592 | 0.1884 | −0.0288 | 0.1645 | 0.1863 | 0.2613 | −0.0152 | |
| VST_TotCorAvgTime_Fw | −0.1073 | 0.0129 | 0.0125 | 0.0592 | 0.1609 | −0.0083 | 0.1710 | 0.1496 | 0.2279 | 0.0012 | |
| VST_TotCor_Bw | 0.0308 | 0.0717 | 0.2810 | 0.0232 | −0.0563 | 0.0489 | 0.2737 | 0.2256 | 0.2482 | −0.0321 | |
| VST_Span_Bw | 0.0492 | −0.0505 | 0.1533 | −0.1092 | −0.0339 | −0.0772 | 0.2028 | 0.1715 | 0.1973 | −0.0333 | |
| VST_TotAvgTime_Bw | 0.1166 | 0.0429 | 0.1718 | −0.0298 | −0.2722 | 0.0345 | 0.2830 | 0.2251 | 0.2971 | −0.1202 | |
| VST_TotCorAvgTime_Bw | 0.1548 | 0.0176 | 0.2422 | −0.0960 | −0.1317 | −0.0426 | 0.2714 | 0.1209 | 0.3142 | −0.0829 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamarajan, C.; Ardekani, B.A.; Pandey, A.K.; Pandey, G.; Kinreich, S.; Kuang, W.; Meyers, J.L.; Porjesz, B. Reward Network Activations of Win Versus Loss in a Monetary Gambling Task. Behav. Sci. 2025, 15, 994. https://doi.org/10.3390/bs15080994
Kamarajan C, Ardekani BA, Pandey AK, Pandey G, Kinreich S, Kuang W, Meyers JL, Porjesz B. Reward Network Activations of Win Versus Loss in a Monetary Gambling Task. Behavioral Sciences. 2025; 15(8):994. https://doi.org/10.3390/bs15080994
Chicago/Turabian StyleKamarajan, Chella, Babak A. Ardekani, Ashwini K. Pandey, Gayathri Pandey, Sivan Kinreich, Weipeng Kuang, Jacquelyn L. Meyers, and Bernice Porjesz. 2025. "Reward Network Activations of Win Versus Loss in a Monetary Gambling Task" Behavioral Sciences 15, no. 8: 994. https://doi.org/10.3390/bs15080994
APA StyleKamarajan, C., Ardekani, B. A., Pandey, A. K., Pandey, G., Kinreich, S., Kuang, W., Meyers, J. L., & Porjesz, B. (2025). Reward Network Activations of Win Versus Loss in a Monetary Gambling Task. Behavioral Sciences, 15(8), 994. https://doi.org/10.3390/bs15080994







