Impact of Poor Sleep Quality on Task Switching and Reconfiguration Process Among University Students
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design and Procedure
2.3. Statistical Analysis
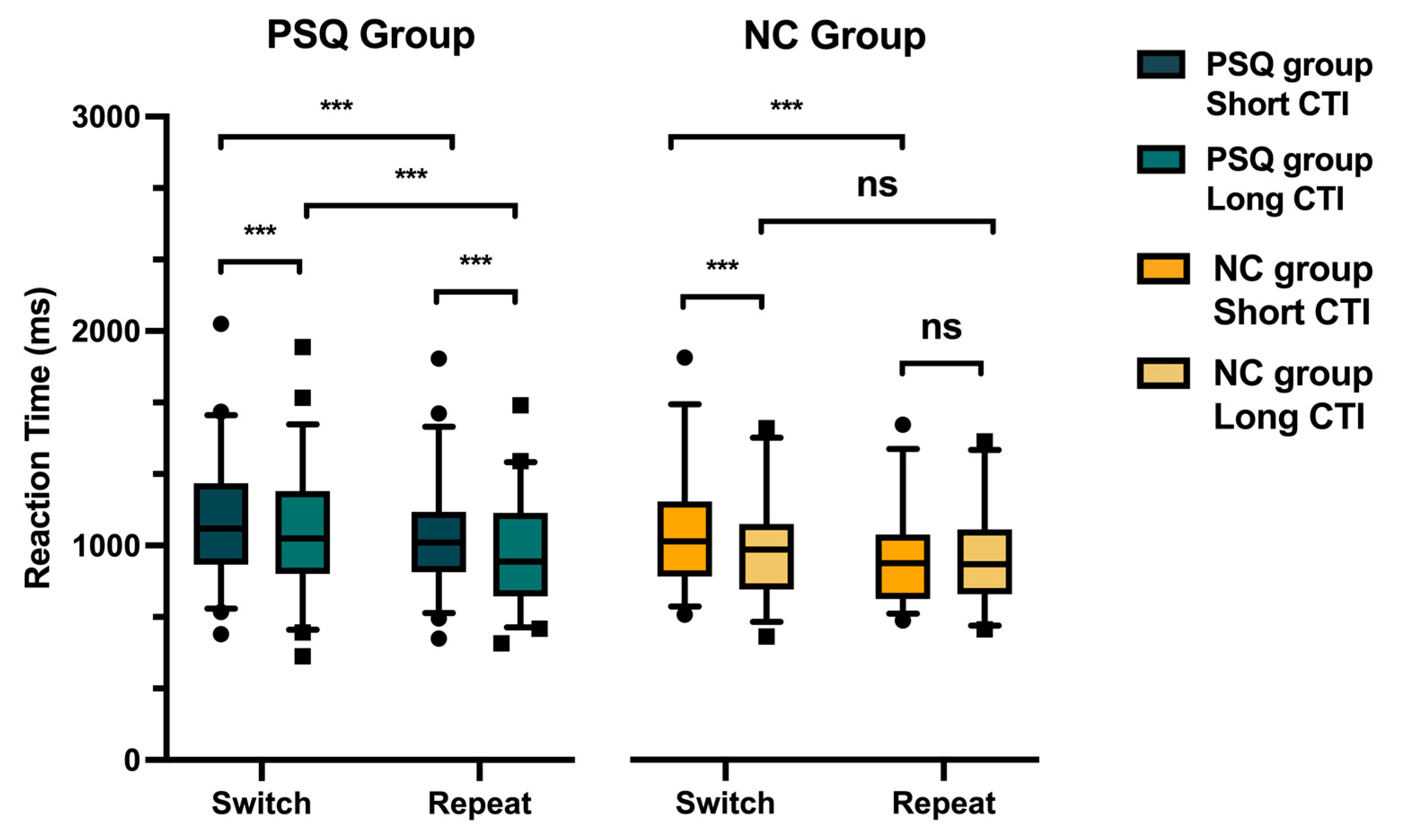
3. Results
3.1. Accuracy Rate
3.2. RT Switching Costs
3.3. Reaction Time (RT)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PSQ | Poor sleep quality |
| NC | Normal control |
| CTI | Cue-to-target interval |
| RT | Reaction time |
| PSQI | Pittsburgh Sleep Quality Index |
References
- Aiyegbusi, O. L., Hughes, S. E., Turner, G., Rivera, S. C., McMullan, C., Chandan, J. S., Haroon, S., Price, G., Davies, E. H., Nirantharakumar, K., Sapey, E., Calvert, M. J., & on behalf of the TLC Study Group. (2021). Symptoms, complications and management of long COVID: A review. Journal of the Royal Society of Medicine, 114(9), 428–442. [Google Scholar] [CrossRef] [PubMed]
- Alsameen, M., DiFrancesco, M. W., Drummond, S. P. A., Franzen, P. L., & Beebe, D. W. (2021). Neuronal activation and performance changes in working memory induced by chronic sleep restriction in adolescents. Journal of Sleep Research, 30(5), e13304. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, J., Junghanns, K., Broocks, A., Riemann, D., & Hohagen, F. (2002). Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of Psychosomatic Research, 53(3), 737–740. [Google Scholar] [CrossRef]
- Ballesio, A., Aquino, M. R. J. V., Kyle, S. D., Ferlazzo, F., & Lombardo, C. (2019). Executive functions in insomnia disorder: A systematic review and exploratory meta-analysis. Frontiers in Psychology, 10, 101. [Google Scholar] [CrossRef]
- Becker, S. P., Jarrett, M. A., Luebbe, A. M., Garner, A. A., Burns, G. L., & Kofler, M. J. (2018). Sleep in a large, multi-university sample of college students: Sleep problem prevalence, sex differences, and mental health correlates. Sleep Health, 4(2), 174–181. [Google Scholar] [CrossRef]
- Benitez, A., & Gunstad, J. (2012). Poor sleep quality diminishes cognitive functioning independent of depression and anxiety in healthy young adults. The Clinical Neuropsychologist, 26(2), 214–223. [Google Scholar] [CrossRef] [PubMed]
- Bliwise, D. L., Friedman, L., & Yesavage, J. A. (1993). Depression as a confounding variable in the estimation of habitual sleep time. Journal of Clinical Psychology, 49(4), 471–477. [Google Scholar] [CrossRef]
- Bortolato, B., Carvalho, A. F., & McIntyre, R. S. (2014). Cognitive dysfunction in major depressive disorder: A state-of-the-art clinical review. CNS & Neurological Disorders—Drug Targets, 13(10), 1804–1818. [Google Scholar] [CrossRef]
- Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [Google Scholar] [CrossRef]
- Cellai, M., & O’kEefe, J. B. (2020). Characterization of prolonged COVID-19 symptoms in an outpatient telemedicine clinic. Open Forum Infectious Diseases, 7(10), ofaa420. [Google Scholar] [CrossRef]
- Chen, G., Xia, L., Wang, F., Li, X., & Jiao, C. (2016). Patients with chronic insomnia have selective impairments in memory that are modulated by cortisol. Psychophysiology, 53(10), 1567–1576. [Google Scholar] [CrossRef]
- Cho, M., Quach, J., Anderson, P., Mensah, F., Wake, M., & Roberts, G. (2015). Poor sleep and lower working memory in grade 1 children: Cross-sectional, population-based study. Academic Pediatrics, 15(1), 111–116. [Google Scholar] [CrossRef]
- Cooper, P. S., Karayanidis, F., McKewen, M., McLellan-Hall, S., Wong, A. S., Skippen, P., & Cavanagh, J. F. (2019). Frontal theta predicts specific cognitive control-induced behavioural changes beyond general reaction time slowing. NeuroImage, 189, 130–140. [Google Scholar] [CrossRef]
- Couyoumdjian, A., Sdoia, S., Tempesta, D., Curcio, G., Rastellini, E., De Gennaro, L., & Ferrara, M. (2010). The effects of sleep and sleep deprivation on task-switching performance. Journal of Sleep Research, 19(1-Part-I), 64–70. [Google Scholar] [CrossRef]
- Craven, J., McCartney, D., Desbrow, B., Sabapathy, S., Bellinger, P., Roberts, L., & Irwin, C. (2022). Effects of acute sleep loss on physical performance: A systematic and meta-analytical review. Sports Medicine, 52(11), 2669–2690. [Google Scholar] [CrossRef] [PubMed]
- Dajani, D. R., & Uddin, L. Q. (2015). Demystifying cognitive flexibility: Implications for clinical and developmental neuroscience. Trends in Neurosciences, 38(9), 571–578. [Google Scholar] [CrossRef] [PubMed]
- Davis, H. E., Assaf, G. S., McCorkell, L., Wei, H., Low, R. J., Re’EM, Y., Redfield, S., Austin, J. P., & Akrami, A. (2021). Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, H., Alrohaily, L., Alsaedi, S. L., Aljohani, S. M., Jan, R. A., Alharthi, N. N., Garah, R. A., Alrohaily, L. S., & Aljohani Sr, S. M. (2023). Impact of COVID-19 on the lifestyle of students of Taibah university, Madinah. Cureus, 15(8), e43371. [Google Scholar] [CrossRef]
- Fernandez-Mendoza, J., & Vgontzas, A. N. (2013). Insomnia and its impact on physical and mental health. Current Psychiatry Reports, 15(12), 418. [Google Scholar] [CrossRef]
- Fila-Witecka, K., Senczyszyn, A., Kołodziejczyk, A., Ciułkowicz, M., Maciaszek, J., Misiak, B., Szcześniak, D., & Rymaszewska, J. (2021). Lifestyle changes among polish university students during the COVID-19 pandemic. International Journal of Environmental Research and Public Health, 18(18), 9571. [Google Scholar] [CrossRef]
- Fintor, E., Stephan, D. N., & Koch, I. (2019). The interplay of crossmodal attentional preparation and modality compatibility in cued task switching. The Quarterly Journal of Experimental Psychology, 72(4), 955–965. [Google Scholar] [CrossRef] [PubMed]
- Fortier-Brochu, É., & Morin, C. M. (2014). Cognitive impairment in individuals with insomnia: Clinical significance and correlates. Sleep, 37(11), 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Galioto, R., Lechner, W. V., Meister, J., Wright, M., Gunstad, J., & Spitznagel, M. B. (2015). Body mass index moderates the association between sleep quality and vigilance on a test of inhibitory control. The Clinical Neuropsychologist, 29(6), 863–875. [Google Scholar] [CrossRef]
- Garcia, A., Del Angel, J., Borrani, J., Ramirez, C., & Valdez, P. (2021). Sleep deprivation effects on basic cognitive processes: Which components of attention, working memory, and executive functions are more susceptible to the lack of sleep. Sleep Science, 14(2), 107–118. [Google Scholar][Green Version]
- Gerhard, A., Raeder, V., Pernice, H. F., Boesl, F., Schroeder, M., Richter, J., Endres, M., Prüß, H., Hahn, K., Audebert, H. J., & Franke, C. (2023). Neurological symptoms after COVID-19 vaccination: A report on the clinical presentation of the first 50 patients. Zeitschrift für Neurologie, 270(10), 4673–4677. [Google Scholar] [CrossRef]
- Gobin, C. M., Banks, J. B., Fins, A. I., & Tartar, J. L. (2015). Poor sleep quality is associated with a negative cognitive bias and decreased sustained attention. Journal of Sleep Research, 24(5), 535–542. [Google Scholar] [CrossRef]
- Grant, M. M., Thase, M. E., & Sweeney, J. A. (2001). Cognitive disturbance in outpatient depressed younger adults: Evidence of modest impairment. Biological Psychiatry, 50(1), 35–43. [Google Scholar] [CrossRef]
- Honn, K., Hinson, J., Whitney, P., & Van Dongen, H. (2019). Cognitive flexibility: A distinct element of performance impairment due to sleep deprivation. Accident Analysis & Prevention, 126, 191–197. [Google Scholar] [CrossRef]
- Houghton, G., Pritchard, R., & Grange, J. A. (2009). The role of cue–target translation in backward inhibition of attentional set. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35(2), 466–476. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S., & Cheng, P. (2006). Task reconfiguration and carryover in task switching: An event-related potential study. Brain Research, 1084(1), 132–145. [Google Scholar] [CrossRef]
- Huang, Z., & Kämpfen, F. (2021). The association between depressive symptoms and self-reported sleep difficulties among college students: Truth or reporting bias? PLoS ONE, 16(2), e0246370. [Google Scholar] [CrossRef]
- Hübner, M., Dreisbach, G., Haider, H., & Kluwe, R. H. (2003). Backward inhibition as a means of sequential task-set control: Evidence for reduction of task competition. Journal of Experimental Psychology: Learning, Memory, and Cognition, 29(2), 289–297. [Google Scholar] [CrossRef]
- Johansson, F., Rozental, A., Edlund, K., Côté, P., Sundberg, T., Onell, C., Rudman, A., & Skillgate, E. (2023). Associations between procrastination and subsequent health outcomes among university students in Sweden. JAMA Network Open, 6(1), e2249346. [Google Scholar] [CrossRef] [PubMed]
- Karayanidis, F., Coltheart, M., Michie, P. T., & Murphy, K. (2003). Electrophysiological correlates of anticipatory and poststimulus components of task switching. Psychophysiology, 40(3), 329–348. [Google Scholar] [CrossRef]
- Kiesel, A., Steinhauser, M., Wendt, M., Falkenstein, M., Jost, K., Philipp, A. M., & Koch, I. (2010). Control and interference in task switching—A review. Psychological Bulletin, 136(5), 849–874. [Google Scholar] [CrossRef]
- Killgore, W. D. (2010). Effects of sleep deprivation on cognition. Progress in Brain Research, 185, 105–129. [Google Scholar] [CrossRef]
- Kiriş, N. (2022). Effects of partial sleep deprivation on prefrontal cognitive functions in adolescents. Sleep and Biological Rhythms, 20(4), 499–508. [Google Scholar] [CrossRef]
- Koch, I., Prinz, W., & Allport, A. (2005). Involuntary retrieval in alphabet-arithmetic tasks: Task-mixing and task-switching costs. Psychological Research, 69(4), 252–261. [Google Scholar] [CrossRef]
- Köhler, S., Soons, L. M., Tange, H., Deckers, K., & van Boxtel, M. P. (2023). Sleep quality and cognitive decline across the adult age range: Findings from the maastricht aging study (MAAS). Journal of Alzheimer’s Disease, 96(3), 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Kray, J. (2006). Task-set switching under cue-based versus memory-based switching conditions in younger and older adults. Brain Research, 1105(1), 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G., Fisicaro, F., Cantone, M., Pennisi, M., Cosentino, F. I. I., Lanuzza, B., Tripodi, M., Bella, R., Paulus, W., & Ferri, R. (2023). Repetitive transcranial magnetic stimulation in primary sleep disorders. Sleep Medicine Reviews, 67, 101735. [Google Scholar] [CrossRef] [PubMed]
- Lemma, S., Gelaye, B., Berhane, Y., Worku, A., & Williams, M. A. (2012). Sleep quality and its psychological correlates among university students in Ethiopia: A cross-sectional study. BMC Psychiatry, 12(1), 237. [Google Scholar] [CrossRef]
- Li, J., Cao, Y., Ou, S., Jiang, T., Wang, L., & Ma, N. (2024). The effect of total sleep deprivation on working memory: Evidence from diffusion model. Sleep, 47(2), zsae006. [Google Scholar] [CrossRef]
- Li, L., Lok, K., Mei, S., Cui, X., Li, L., Ng, C. H., Ungvari, G. S., Ning, Y., An, F., & Xiang, Y. (2019). Sleep duration and self-rated health in Chinese university students. Sleep and Breathing, 23(4), 1351–1356. [Google Scholar] [CrossRef]
- Meiran, N. (1996). Reconfiguration of processing mode prior to task performance. Journal of Experimental Psychology: Learning, Memory, and Cognition, 22(6), 1423–1442. [Google Scholar] [CrossRef]
- Mollayeva, T., Thurairajah, P., Burton, K., Mollayeva, S., Shapiro, C. M., & Colantonio, A. (2016). The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Medicine Reviews, 25, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Monsell, S. (2003). Task switching. Trends in Cognitive Sciences, 7(3), 134–140. [Google Scholar] [CrossRef]
- Nakashima, A., Bouak, F., Lam, Q., Smith, I., & Vartanian, O. (2018). Task switching following 24 h of total sleep deprivation: A functional MRI study. NeuroReport, 29(2), 123–127. [Google Scholar] [CrossRef]
- Nicholson, R., Karayanidis, F., Poboka, D., Heathcote, A., & Michie, P. T. (2005). Electrophysiological correlates of anticipatory task-switching processes. Psychophysiology, 42(5), 540–554. [Google Scholar] [CrossRef] [PubMed]
- Paelecke-Habermann, Y., Pohl, J., & Leplow, B. (2005). Attention and executive functions in remitted major depression patients. Journal of Affective Disorders, 89(1–3), 125–135. [Google Scholar] [CrossRef]
- Palmer, C. A., & Alfano, C. A. (2017). Sleep and emotion regulation: An organizing, integrative review. Sleep Medicine Reviews, 31, 6–16. [Google Scholar] [CrossRef]
- Pavlova, M. K., & Latreille, V. (2019). Sleep disorders. The American Journal of Medicine, 132(3), 292–299. [Google Scholar] [CrossRef] [PubMed]
- Percze, A. R., Nagy, A., Polivka, L., Barczi, E., Czaller, I., Kovats, Z., Varga, J. T., Ballai, J. H., Muller, V., & Horvath, G. (2023). Fatigue, sleepiness and sleep quality are SARS-CoV-2 variant independent in patients with long COVID symptoms. Inflammopharmacology, 31(6), 2819–2825. [Google Scholar] [CrossRef]
- Poulsen, C., Luu, P., Davey, C., & Tucker, D. M. (2005). Dynamics of task sets: Evidence from dense-array event-related potentials. Cognitive Brain Research, 24(1), 133–154. [Google Scholar] [CrossRef] [PubMed]
- Rångtell, F. H., Karamchedu, S., Andersson, P., Liethof, L., Búcaro, M. O., Lampola, L., Schiöth, H. B., Cedernaes, J., & Benedict, C. (2019). A single night of sleep loss impairs objective but not subjective working memory performance in a sex-dependent manner. Journal of Sleep Research, 28(1), e12651. [Google Scholar] [CrossRef]
- Rogers, R. D., & Monsell, S. (1995). Costs of a predictible switch between simple cognitive tasks. Journal of Experimental Psychology: General, 124(2), 207–231. [Google Scholar] [CrossRef]
- Rubin, O., & Meiran, N. (2005). On the origins of the task mixing cost in the cuing task-switching paradigm. Journal of Experimental Psychology: Learning, Memory, and Cognition, 31(6), 1477–1491. [Google Scholar] [CrossRef]
- Schapkin, S. A., Gajewski, P. D., & Freude, G. (2014). Age differences in memory-based task switching with and without cues. Journal of Psychophysiology, 28(3), 187–201. [Google Scholar] [CrossRef]
- Schlarb, A. A., Friedrich, A., & Claßen, M. (2017). Sleep problems in university students—An intervention. Neuropsychiatric Disease and Treatment, 13, 1989–2001. [Google Scholar] [CrossRef] [PubMed]
- Schmickler, J. M., Blaschke, S., Robbins, R., & Mess, F. (2023). Determinants of sleep quality: A cross-sectional study in university students. International Journal of Environmental Research and Public Health, 20(3), 2019. [Google Scholar] [CrossRef]
- Schmitter-Edgecombe, M., & Langill, M. (2006). Costs of a predictable switch between simple cognitive tasks following severe closed-head injury. Neuropsychology, 20(6), 675–684. [Google Scholar] [CrossRef]
- Schmitz, F., & Krämer, R. J. (2023). Task switching: On the relation of cognitive flexibility with cognitive capacity. Journal of Intelligence, 11(4), 68. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D. W., & Logan, G. D. (2007). Task switching versus cue switching: Using transition cuing to disentangle sequential effects in task-switching performance. Journal of Experimental Psychology: Learning, Memory, and Cognition, 33(2), 370–378. [Google Scholar] [CrossRef]
- Sen, A., & Tai, X. Y. (2023). Sleep duration and executive function in adults. Current Neurology and Neuroscience Reports, 23(11), 801–813. [Google Scholar] [CrossRef]
- Shantakumari, N., Elawaddlly, S. H. S., Kanawati, A. J. A., Abufanas, A. S., Dakak, A., Ibham, F. M., & Bani, I. (2024). Sleep quality and its daytime effects among university students in the UAE. Oman Medical Journal, 39(2), e612. [Google Scholar] [CrossRef]
- Slama, H., Chylinski, D. O., Deliens, G., Leproult, R., Schmitz, R., & Peigneux, P. (2018). Sleep deprivation triggers cognitive control impairments in task-goal switching. Sleep, 41(2), zsx200. [Google Scholar] [CrossRef]
- Snyder, H. R. (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139(1), 81–132. [Google Scholar] [CrossRef] [PubMed]
- Stepan, M. E., Wilckens, K. A., Hostler, D., Wallace, M. L., & Franzen, P. L. (2022). Physical exertion partially mitigates task-switching deficits from sleep loss. Journal of Occupational and Environmental Medicine, 64(10), e622–e628. [Google Scholar] [CrossRef] [PubMed]
- Stern, E. R., Wager, T. D., Egner, T., Hirsch, J., & Mangels, J. A. (2007). Preparatory neural activity predicts performance on a conflict task. Brain Research, 1176, 92–102. [Google Scholar] [CrossRef]
- Stiver, J., Fusco-Gessick, B., Moran, E., Crook, C., & Zimmerman, M. E. (2021). Variable objective sleep quality is related to worse spatial learning and memory in young adults. Sleep Medicine, 84, 114–120. [Google Scholar] [CrossRef]
- Stordal, K. I., Lundervold, A. J., Egeland, J., Mykletun, A., Asbjørnsen, A., Landrø, N. I., Roness, A., Rund, B. R., Sundet, K., Oedegaard, K. J., & Lund, A. (2004). Impairment across executive functions in recurrent major depression. Nordic Journal of Psychiatry, 58(1), 41–47. [Google Scholar] [CrossRef]
- Tsuno, N., Besset, A., & Ritchie, K. (2005). Sleep and depression. The Journal of Clinical Psychiatry, 66(10), 1254–1269. [Google Scholar] [CrossRef]
- Vandierendonck, A., Liefooghe, B., & Verbruggen, F. (2010). Task switching: Interplay of reconfiguration and interference control. Psychological Bulletin, 136(4), 601–626. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y., Wen, Q., Luo, S., Tang, L., Zhan, S., Cao, J., Wang, S., & Chen, Q. (2025). Phenome-wide analysis of diseases in relation to objectively measured sleep traits and comparison with subjective sleep traits in 88,461 adults. Health Data Science, 5, 0161. [Google Scholar] [CrossRef]
- Weeda, W. D., van der Molen, M. W., Barceló, F., & Huizinga, M. (2014). A diffusion model analysis of developmental changes in children’s task switching. Journal of Experimental Child Psychology, 126, 178–197. [Google Scholar] [CrossRef]
- Wilckens, K. A., Hall, M. H., Erickson, K. I., Germain, A., Nimgaonkar, V. L., Monk, T. H., & Buysse, D. J. (2017). Task switching in older adults with and without insomnia. Sleep Medicine, 30, 113–120. [Google Scholar] [CrossRef]
- Wylie, G., & Allport, A. (2000). Task switching and the measurement of “switch costs”. Psychological Research, 63(3–4), 212–233. [Google Scholar] [CrossRef] [PubMed]
- Wylie, G. R., Javitt, D. C., & Foxe, J. J. (2004). Don’t think of a white bear: An fMRI investigation of the effects of sequential instructional sets on cortical activity in a task-switching paradigm. Human Brain Mapping, 21(4), 279–297. [Google Scholar] [CrossRef] [PubMed]
- Xie, W., Berry, A., Lustig, C., Deldin, P., & Zhang, W. (2019). Poor sleep quality and compromised visual working memory capacity. Journal of the International Neuropsychological Society, 25(6), 583–594. [Google Scholar] [CrossRef]
- Zhang, B., Lei, S. M., Le, S., Gong, Q., Cheng, S., & Wang, X. (2022). Changes in health behaviors and conditions during COVID-19 pandemic strict campus lockdown among Chinese university students. Frontiers in Psychology, 13, 1022966. [Google Scholar] [CrossRef]
- Zheng, K., Liu, Z., Miao, Z., Xiong, G., Yang, H., Zhong, M., & Yi, J. (2024). Impaired cognitive flexibility in major depressive disorder: Evidences from spatial-temporal ERPs analysis. Journal of Affective Disorders, 365, 406–416. [Google Scholar] [CrossRef] [PubMed]

| Features/Measures | PSQ Group (n = 47) | NC Group (n = 38) | χ2/t | p |
|---|---|---|---|---|
| Male/Female | 16:31 | 19:19 | 2.21 | 0.14 |
| Age (Years) | 19.51 (1.46) | 19.45 (1.31) | 0.21 | 0.84 |
| Wechsler Adult Intelligence Scale | 113.09 (8.84) | 115.08 (9.49) | −1.00 | 0.32 |
| Pittsburgh Sleep Index | 8.72 (1.78) | 2.37 (1.10) | 20.18 | <0.001 |
| Epworth Sleepiness Scale | 8.45 (5.97) | 4.47 (3.05) | 3.97 | <0.001 |
| Beck Depression Inventory | 2.91 (2.62) | 0.42 (0.89) | 6.11 | <0.001 |
| Statistical Outcomes from Group Comparisons | PSQ Group (n = 47) | NC Group (n = 38) |
|---|---|---|
| Accuracy with Short CTI in Switch Trials (%) | 92.96 (6.23) | 91.64 (6.94) |
| Accuracy with Short CTI in Repeat Trials (%) | 93.36 (7.79) | 94.11 (4.68) |
| Accuracy with Long CTI in Switch Trials (%) | 93.22 (6.42) | 92.54 (6.19) |
| Accuracy with Long CTI in Repeat Trials (%) | 95.23 (5.96) | 94.81 (4.66) |
| RT Switching Cost with Short CTI (ms) | 90.40 (139.97) | 124.49 (141.10) |
| RT Switching Cost with Long CTI (ms) | 83.96 (119.86) | 30.47 (82.77) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Sun, Y.; Jia, Y.; Shi, J.; Sun, Y. Impact of Poor Sleep Quality on Task Switching and Reconfiguration Process Among University Students. Behav. Sci. 2025, 15, 1054. https://doi.org/10.3390/bs15081054
Ma S, Sun Y, Jia Y, Shi J, Sun Y. Impact of Poor Sleep Quality on Task Switching and Reconfiguration Process Among University Students. Behavioral Sciences. 2025; 15(8):1054. https://doi.org/10.3390/bs15081054
Chicago/Turabian StyleMa, Shaoyang, Yue Sun, Yunxin Jia, Jinfu Shi, and Yekun Sun. 2025. "Impact of Poor Sleep Quality on Task Switching and Reconfiguration Process Among University Students" Behavioral Sciences 15, no. 8: 1054. https://doi.org/10.3390/bs15081054
APA StyleMa, S., Sun, Y., Jia, Y., Shi, J., & Sun, Y. (2025). Impact of Poor Sleep Quality on Task Switching and Reconfiguration Process Among University Students. Behavioral Sciences, 15(8), 1054. https://doi.org/10.3390/bs15081054






