Prefrontal-Dependent and Gender-Specific Modulation of Guilt Emotion on Human Early Visual Perception
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Stimuli
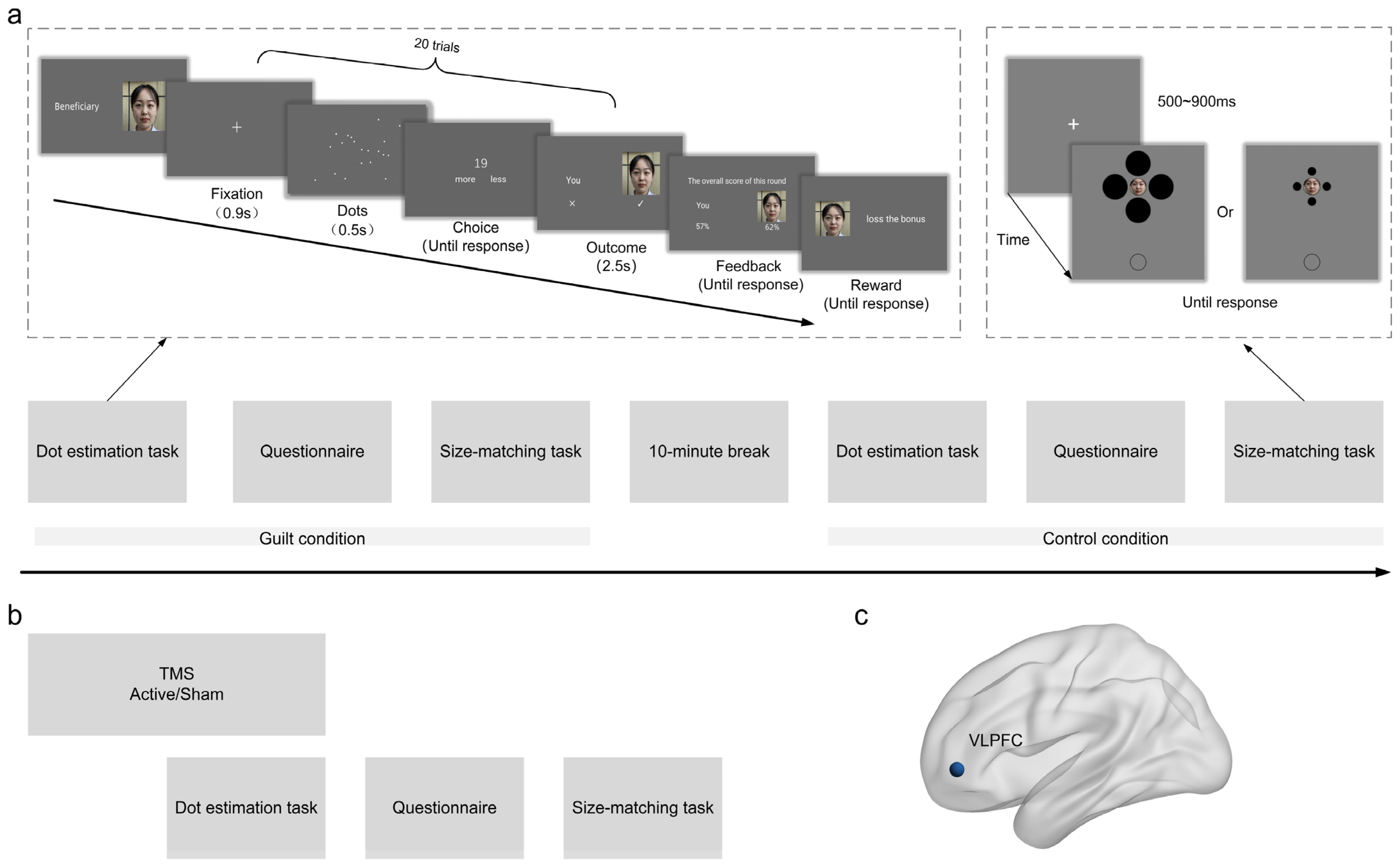
2.3. Procedure
2.4. Dot Estimation Task
2.5. Size-Matching Task
2.6. Repetitive Transcranial Magnetic Stimulation (rTMS)
2.7. Data Analysis
3. Results
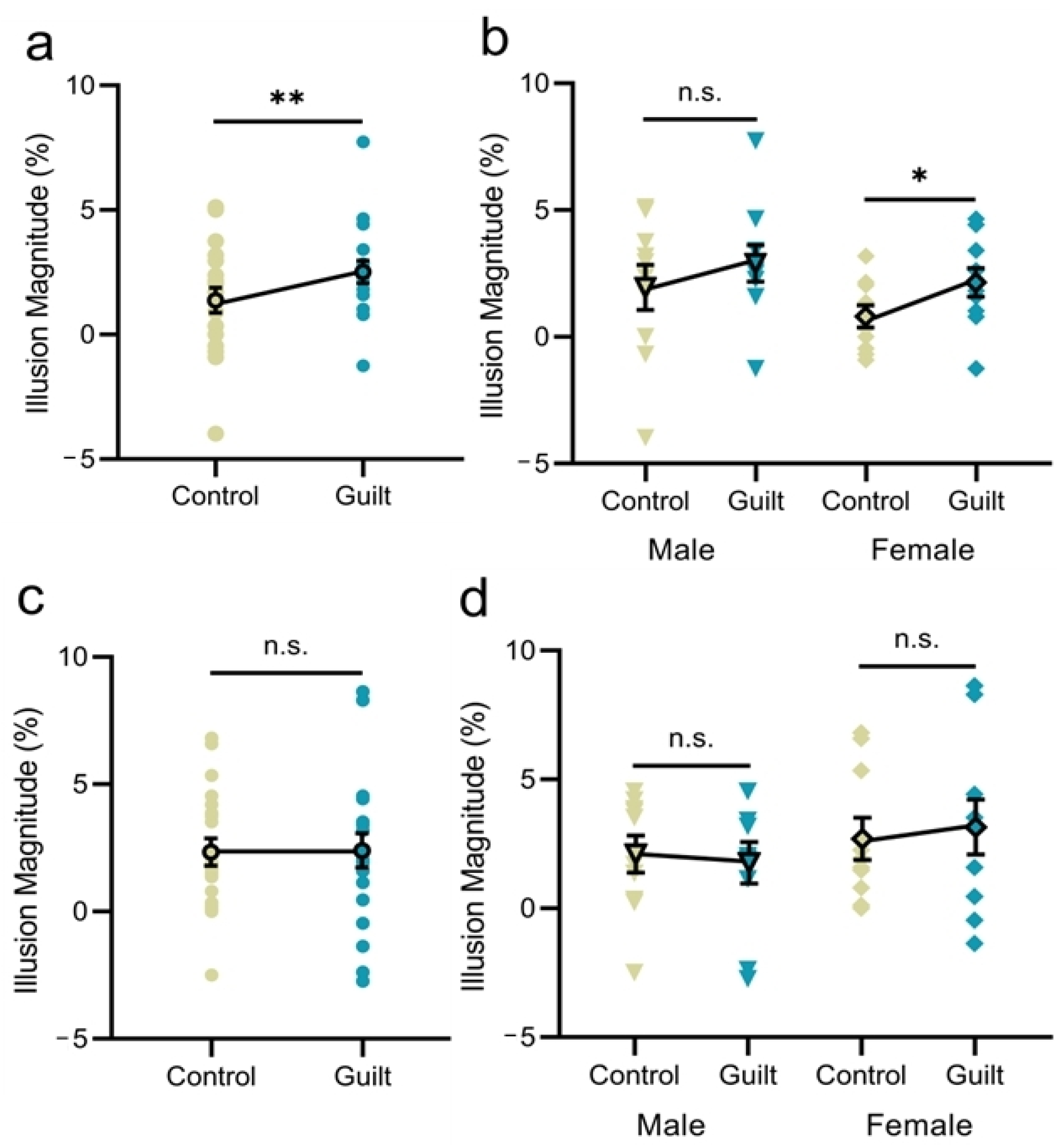
3.1. Experiment 1
3.2. Experiment 2
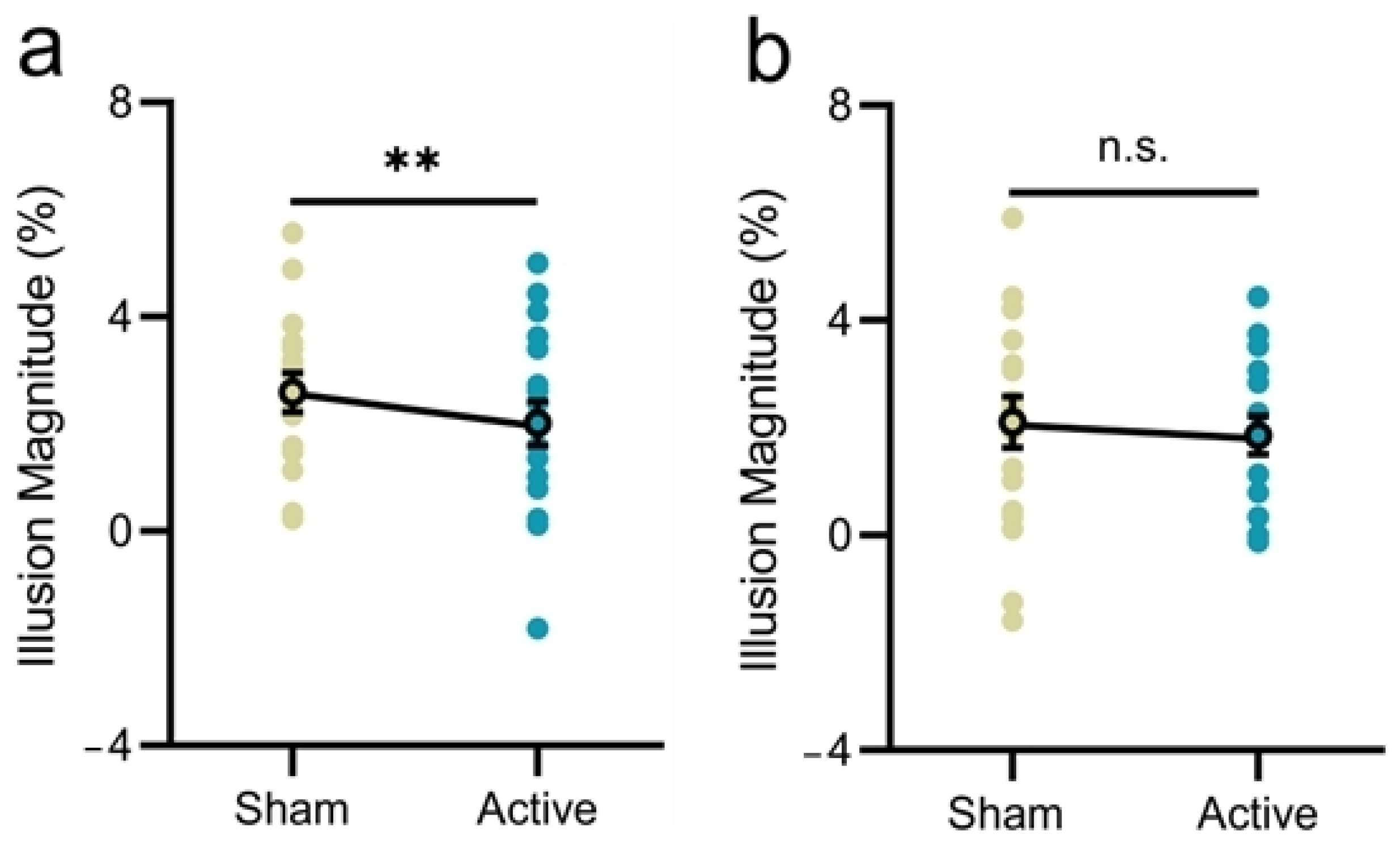
3.3. Experiment 3
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Achterberg, M., van Duijvenvoorde, A. C. K., van der Meulen, M., Bakermans-Kranenburg, M. J., & Crone, E. A. (2018). Heritability of aggression following social evaluation in middle childhood: An fMRI study. Human Brain Mapping, 39(7), 2828–2841. [Google Scholar] [CrossRef]
- Amodio, D. M., Devine, P. G., & Harmon-Jones, E. (2007). A dynamic model of guilt implications for motivation and self-regulation in the context of prejudice. Psychological Science, 18(6), 524–530. [Google Scholar] [CrossRef]
- Benetti-McQuoid, J., & Bursik, K. (2005). Individual differences in experiences of and responses to guilt and shame: Examining the lenses of gender and gender role. Sex Roles, 53, 133–142. [Google Scholar] [CrossRef]
- Birditt, K. S., & Fingerman, K. L. (2003). Age and gender differences in adults’ descriptions of emotional reactions to interpersonal problems. The Journals of Gerontology: Series B, 58(4), 237–245. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra, B. R., & Zeelenberg, R. (2009). Emotion improves and impairs early vision. Psychological Science, 20(6), 707–713. [Google Scholar] [CrossRef] [PubMed]
- Brainard, D. H. (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436. [Google Scholar] [CrossRef]
- Bressler, S. L., Tang, W., Sylvester, C. M., Shulman, G. L., & Corbetta, M. (2008). Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. Journal of Neuroscience, 28(40), 10056–10061. [Google Scholar] [CrossRef]
- Cai, A., Lou, Y., Long, Q., & Yuan, J. (2016). The sex differences in regulating unpleasant emotion by expressive suppression: Extraversion matters. Frontiers in Psychology, 7, 1011. [Google Scholar] [CrossRef]
- Cavalera, C., & Pepe, A. (2014). Social emotions and cognition: Shame, guilt and working memory. Procedia-Social Behavioral Sciences, 112, 457–464. [Google Scholar] [CrossRef]
- Chen, L., Wu, B., Yu, H., & Sperandio, I. (2024). Network dynamics underlying alterations in apparent object size. Brain Communications, 6(1), fcae006. [Google Scholar] [CrossRef]
- Chen, L., Yuan, X., Xu, Q., Wang, Y., & Jiang, Y. (2016). Subliminal impending collision increases perceived object size and enhances pupillary light reflex. Frontiers in Psychology, 7, 1897. [Google Scholar] [CrossRef]
- Chick, C. F., Rolle, C. E., Trivedi, H. M., Monuszko, K. A., & Etkin, A. (2020). Transcranial magnetic stimulation demonstrates a role for the ventrolateral prefrontal cortex in emotion perception. Psychiatry Research, 284, 112515. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T. R., Panter, A., & Turan, N. (2012). Guilt proneness and moral character. Current Directions in Psychological Science, 21(5), 355–359. [Google Scholar] [CrossRef]
- Cross, S. E., & Madson, L. (1997). Models of the self: Self-construals and gender. Psychological Bulletin, 122(1), 5–37. [Google Scholar] [CrossRef]
- Else-Quest, N. M., Higgins, A., Allison, C. M., & Morton, L. C. (2012). Gender differences in self-conscious emotional experience: A meta-analysis. Psychological Bulletin, 138(5), 947–981. [Google Scholar] [CrossRef] [PubMed]
- Ester, E. F., Sprague, T. C., & Serences, J. T. (2015). Parietal and frontal cortex encode stimulus-specific mnemonic representations during visual working memory. Neuron, 87(4), 893–905. [Google Scholar] [CrossRef]
- Gilead, M., Katzir, M., Eyal, T., & Liberman, N. (2016). Neural correlates of processing “self-conscious” versus “basic” emotions. Neuropsychologia, 81, 207–218. [Google Scholar] [CrossRef]
- Henderson, S. E., Vallejo, A. I., Ely, B. A., Kang, G., Roy, A. K., Pine, D. S., Stern, E. R., & Gabbay, V. (2014). The neural correlates of emotional face-processing in adolescent depression: A dimensional approach focusing on anhedonia and illness severity. Psychiatry Research: Neuroimaging, 224(3), 234–241. [Google Scholar] [CrossRef]
- Hilgetag, C. C., Théoret, H., & Pascual-Leone, A. (2001). Enhanced visual spatial attention ipsilateral to rTMS-induced ‘virtual lesions’ of human parietal cortex. Nature Neuroscience, 4(9), 953–957. [Google Scholar] [CrossRef]
- Hsu, D. T., Anjali, S., Mohammad, A. M., Scott, A. L., Brian, J. M., & Tiffany, M. L. (2020). Common neural responses to romantic rejection and acceptance in healthy adults. Social Neuroscience, 15(5), 571–583. [Google Scholar] [CrossRef]
- Hu, X., Feng, B., Chen, L., & Luo, W. (2023). Threat shapes visual context sensitivity selectively through low-spatial-frequency channels. Cognition, 230, 105305. [Google Scholar] [CrossRef] [PubMed]
- Kashima, Y., Yamaguchi, S., Kim, U., Choi, S. C., Gelfand, M. J., & Yuki, M. (1995). Culture, gender, and self: A perspective from individualism-collectivism research. Journal of Personality and Social Psychology, 69(5), 925–937. [Google Scholar] [CrossRef] [PubMed]
- Keding, T. J., & Herringa, R. J. (2016). Paradoxical prefrontal–amygdala recruitment to angry and happy expressions in pediatric posttraumatic stress disorder. Neuropsychopharmacology, 41, 2903–2912. [Google Scholar] [CrossRef]
- Keener, E., Strough, J., & Didonato, L. (2012). Gender differences and similarities in strategies for managing conflict with friends and romantic partners. Sex Roles, 67, 83–97. [Google Scholar] [CrossRef]
- Leibovich, T., Cohen, N., & Henik, A. (2016). Itsy bitsy spider?: Valence and self-relevance predict size estimation. Biological Psychology, 121, 138–145. [Google Scholar] [CrossRef]
- Lewis, H. B. (1985). Depression vs. paranoia: Why are there sex differences in mental illness? Journal of Personality, 53(2), 150–178. [Google Scholar] [CrossRef]
- Li, S., Xie, H., Zheng, Z., Chen, W., Xu, F., Hu, X., & Zhang, D. (2022). The causal role of the bilateral ventrolateral prefrontal cortices on emotion regulation of social feedback. Human Brain Mapping, 43(9), 2898–2910. [Google Scholar] [CrossRef]
- Lourenco, S. F., Longo, M. R., & Pathman, T. (2011). Near space and its relation to claustrophobic fear. Cognition, 119(3), 448–453. [Google Scholar] [CrossRef] [PubMed]
- Mevorach, C., Humphreys, G. W., & Shalev, L. (2006). Effects of saliency, not global dominance, in patients with left parietal damage. Neuropsychologia, 44(2), 307–319. [Google Scholar] [CrossRef]
- Miller, A. B., Prinstein, M. J., Munier, E., Machlin, L. S., & Sheridan, M. A. (2019). Emotion reactivity and regulation in adolescent girls following an interpersonal rejection. Journal of Cognitive Neuroscience, 31(2), 249–261. [Google Scholar] [CrossRef]
- Moll, J., De Oliveira-Souza, R., Eslinger, P. J., Bramati, I. E., Mourao-Miranda, J., Andreiuolo, P. A., & Pessoa, L. (2002). The neural correlates of moral sensitivity: A functional magnetic resonance imaging investigation of basic and moral emotions. Journal of Neuroscience, 22(7), 2730–2736. [Google Scholar] [CrossRef]
- Morey, R. A., Mccarthy, G., Selgrade, E. S., Seth, S., Nasser, J. D., & Labar, K. S. (2012). Neural systems for guilt from actions affecting self versus others. NeuroImage, 60, 683–692. [Google Scholar] [CrossRef]
- Notzon, S., Steinberg, C., Zwanzger, P., & Junghöfer, M. (2018). Modulating emotion perception: Opposing effects of inhibitory and excitatory prefrontal cortex stimulation. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(4), 329–336. [Google Scholar] [CrossRef] [PubMed]
- Park, D., Kim, T., & Lee, S. H. (2021). Strong correspondence between prefrontal and visual representations during emotional perception. Human Brain Mapping, 42(7), 2115–2127. [Google Scholar] [CrossRef] [PubMed]
- Pelli, D. (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10(4), 437–442. [Google Scholar] [CrossRef] [PubMed]
- Phelps, E. A., Ling, S., & Carrasco, M. (2006). Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychological Science, 17(4), 292–299. [Google Scholar] [CrossRef]
- Phillips, M. L., & Swartz, H. A. (2014). A critical appraisal of neuroimaging studies of bipolar disorder: Toward a new conceptualization of underlying neural circuitry and a road map for future research. The American Journal of Psychiatry, 171(8), 829–843. [Google Scholar] [CrossRef]
- Ross, E. D. (2021). Differential hemispheric lateralization of emotions and related display behaviors: Emotion-type hypothesis. Brain Sciences, 11(8), 1034. [Google Scholar] [CrossRef]
- Ruff, C. C., Bestmann, S., Blankenburg, F., Bjoertomt, O., Josephs, O., Weiskopf, N., Deichmann, R., & Driver, J. (2008). Distinct causal influences of parietal versus frontal areas on human visual cortex: Evidence from concurrent TMS–fMRI. Cerebral Cortex, 18(4), 817–827. [Google Scholar] [CrossRef]
- Russell, L. H., Gould, K. L., & Fergus, T. A. (2017). Self-construal and gender interact to cause social evaluative concerns. Personality and Individual Differences, 109, 51–55. [Google Scholar] [CrossRef]
- Shen, M., Zhu, C., Liao, H., Zhang, H., Zhou, J., & Gao, Z. (2018). Guilt leads to enhanced facing-the-viewer bias. PLoS ONE, 13(4), e0195590. [Google Scholar] [CrossRef] [PubMed]
- Shiban, Y., Fruth, M. B., Pauli, P., Kinateder, M., Reichenberger, J., & Mühlberger, A. (2016). Treatment effect on biases in size estimation in spider phobia. Biological Psychology, 121, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Silver, M. A., & Kastner, S. (2009). Topographic maps in human frontal and parietal cortex. Trends in Cognitive Sciences, 13(11), 488–495. [Google Scholar] [CrossRef] [PubMed]
- Sloan, D. M. E., & Kornstein, S. G. (2003). Gender differences in depression and response to antidepressant treatment. Psychiatric Clinics, 26(3), 581–594. [Google Scholar] [CrossRef]
- Stuijfzand, S., de Wied, M., Kempes, M., Van de Graaff, J., Branje, S. J. T., & Meeus, W. H. J. (2016). Gender differences in empathic sadness towards persons of the same-versus other-sex during adolescence. Sex Roles, 75, 434–446. [Google Scholar] [CrossRef]
- Vagnoni, E., Lourenco, S. F., & Longo, M. R. (2012). Threat modulates perception of looming visual stimuli. Current Biology, 22(19), R826–R827. [Google Scholar] [CrossRef]
- Van Ulzen, N. R., Semin, G. R., Oudejans, R. R. D., & Beek, P. J. (2008). Affective stimulus properties influence size perception and the Ebbinghaus illusion. Psychological Research, 72, 304–310. [Google Scholar] [CrossRef]
- Vasey, M. W., Vilensky, M. R., Heath, J. H., Harbaugh, C. N., Buffington, A. G., & Fazio, R. H. (2012). It was as big as my head, I swear!: Biased spider size estimation in spider phobia. Journal of Anxiety Disorders, 26(1), 20–24. [Google Scholar] [CrossRef]
- Wang, A., Chen, L., & Jiang, Y. (2021). Anodal occipital transcranial direct current stimulation enhances perceived visual size illusions. Journal of Cognitive Neuroscience, 33(3), 528–535. [Google Scholar] [CrossRef]
- Ward, S., & King, L. A. (2018). Gender differences in emotion explain women’s lower immoral intentions and harsher moral condemnation. Personality Social Psychology Bulletin, 44, 653–669. [Google Scholar] [CrossRef]
- Wu, B., Feng, B., Han, X., Chen, L., & Luo, W. (2023). Intrinsic excitability of human right parietal cortex shapes the experienced visual size illusions. Cerebral Cortex, 33(10), 6345–6353. [Google Scholar] [CrossRef] [PubMed]
- Wyczesany, M., Ligeza, T. S., & Grzybowski, S. J. (2015). Effective connectivity during visual processing is affected by emotional state. Brain Imaging and Behavior, 9, 717–728. [Google Scholar] [CrossRef]
- Yokoyama, C., Kaiya, H., Kumano, H., Kinou, M., Umekage, T., Yasuda, S., Takei, K., Nishikawa, M., Sasaki, T., Nishimura, Y., Hara, N., Inoue, K., Kaneko, Y., Suzuki, S., Tanii, H., Okada, M., & Okazaki, Y. (2015). Dysfunction of ventrolateral prefrontal cortex underlying social anxiety disorder: A multi-channel NIRS study. Neuroimage Clinical, 8, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Yu, H., Duan, Y., & Zhou, X. (2017). Guilt in the eyes: Eye movement and physiological evidence for guilt-induced social avoidance. Journal of Experimental Social Psychology, 71, 128–137. [Google Scholar] [CrossRef]
- Yuan, J., Luo, Y., Yan, J., Meng, X., Yu, F., & Li, H. (2009). Neural correlates of the females’ susceptibility to negative emotions: An insight into gender-related prevalence of affective disturbances. Human Brain Mapping, 30(11), 3676–3686. [Google Scholar] [CrossRef]
- Zhao, W., Yang, J., & Hu, Z. (2023). Guilt-inducing interaction with others modulates subsequent attentional orienting via their gaze. Scientific Reports, 13, 5348. [Google Scholar] [CrossRef]
- Zhu, R., Feng, C., Zhang, S., Mai, X., & Liu, C. (2019). Differentiating guilt and shame in an interpersonal context with univariate activation and multivariate pattern analyses. NeuroImage, 14(1), 476–486. [Google Scholar] [CrossRef]
- Zhu, R., Wu, H., Xu, Z., Tang, H., Shen, X., Mai, X., & Liu, C. (2017). Early distinction between shame and guilt processing in an interpersonal context. Social Neuroscience, 14(1), 53–66. [Google Scholar] [CrossRef]
| Emotion | Experiment1 | Experiment2 | Experiment3 | |||||
|---|---|---|---|---|---|---|---|---|
| Guilt | Control | Guilt | Control | Sham | Active | |||
| Guilt | Control | Guilt | Control | |||||
| Guilt | 5.05 ± 2.01 | 2.25 ± 1.55 | 5.30 ± 1.13 | 2.65 ± 1.23 | 5.33 ± 1.94 | 1.28 ± 0.58 | 4.11 ± 2.03 | 1.50 ± 0.62 |
| Shame | 3.95 ± 2.24 | 2.15 ± 1.35 | 3.95 ± 1.93 | 2.20 ± 1.06 | 3.50 ± 2.18 | 1.28 ± 0.58 | 3.17 ± 2.15 | 1.56 ± 0.71 |
| Anger | 1.60 ± 1.14 | 1.50 ± 0.83 | 2.45 ± 1.47 | 1.50 ± 0.89 | 2.17 ± 1.54 | 1.22 ± 0.43 | 1.56 ± 1.34 | 1.39 ± 0.61 |
| Pride | 2.60 ± 1.88 | 3.75 ± 1.94 | 2.25 ± 1.21 | 4.25 ± 1.25 | 2.78 ± 1.40 | 3.28 ± 2.14 | 3.06 ± 1.47 | 3.61 ± 2.00 |
| Sadness | 3.40 ± 1.60 | 1.80 ± 1.28 | 3.50 ± 1.67 | 1.65 ± 0.93 | 3.00 ± 1.88 | 1.22 ± 0.55 | 2.61 ± 1.85 | 1.33 ± 0.59 |
| Happiness | 3.00 ± 1.81 | 4.55 ± 1.82 | 2.80 ± 1.51 | 5.25 ± 1.41 | 2.94 ± 1.39 | 4.11 ± 1.88 | 4.11 ± 1.18 | 4.44 ± 1.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Chen, L. Prefrontal-Dependent and Gender-Specific Modulation of Guilt Emotion on Human Early Visual Perception. Behav. Sci. 2025, 15, 333. https://doi.org/10.3390/bs15030333
Sun M, Chen L. Prefrontal-Dependent and Gender-Specific Modulation of Guilt Emotion on Human Early Visual Perception. Behavioral Sciences. 2025; 15(3):333. https://doi.org/10.3390/bs15030333
Chicago/Turabian StyleSun, Mingyang, and Lihong Chen. 2025. "Prefrontal-Dependent and Gender-Specific Modulation of Guilt Emotion on Human Early Visual Perception" Behavioral Sciences 15, no. 3: 333. https://doi.org/10.3390/bs15030333
APA StyleSun, M., & Chen, L. (2025). Prefrontal-Dependent and Gender-Specific Modulation of Guilt Emotion on Human Early Visual Perception. Behavioral Sciences, 15(3), 333. https://doi.org/10.3390/bs15030333






