Using Tangible User Interfaces (TUIs): Preliminary Evidence on Memory and Comprehension Skills in Children with Autism Spectrum Disorder
Abstract
1. Introduction
1.1. Autism Spectrum Disorder (ASD)
1.2. Traditional Interventions for Autism Spectrum Disorder
1.3. The Role of Technology
1.4. Tangible User Interfaces (TUIs) as a Novel Approach
2. Materials and Methods
2.1. Participants
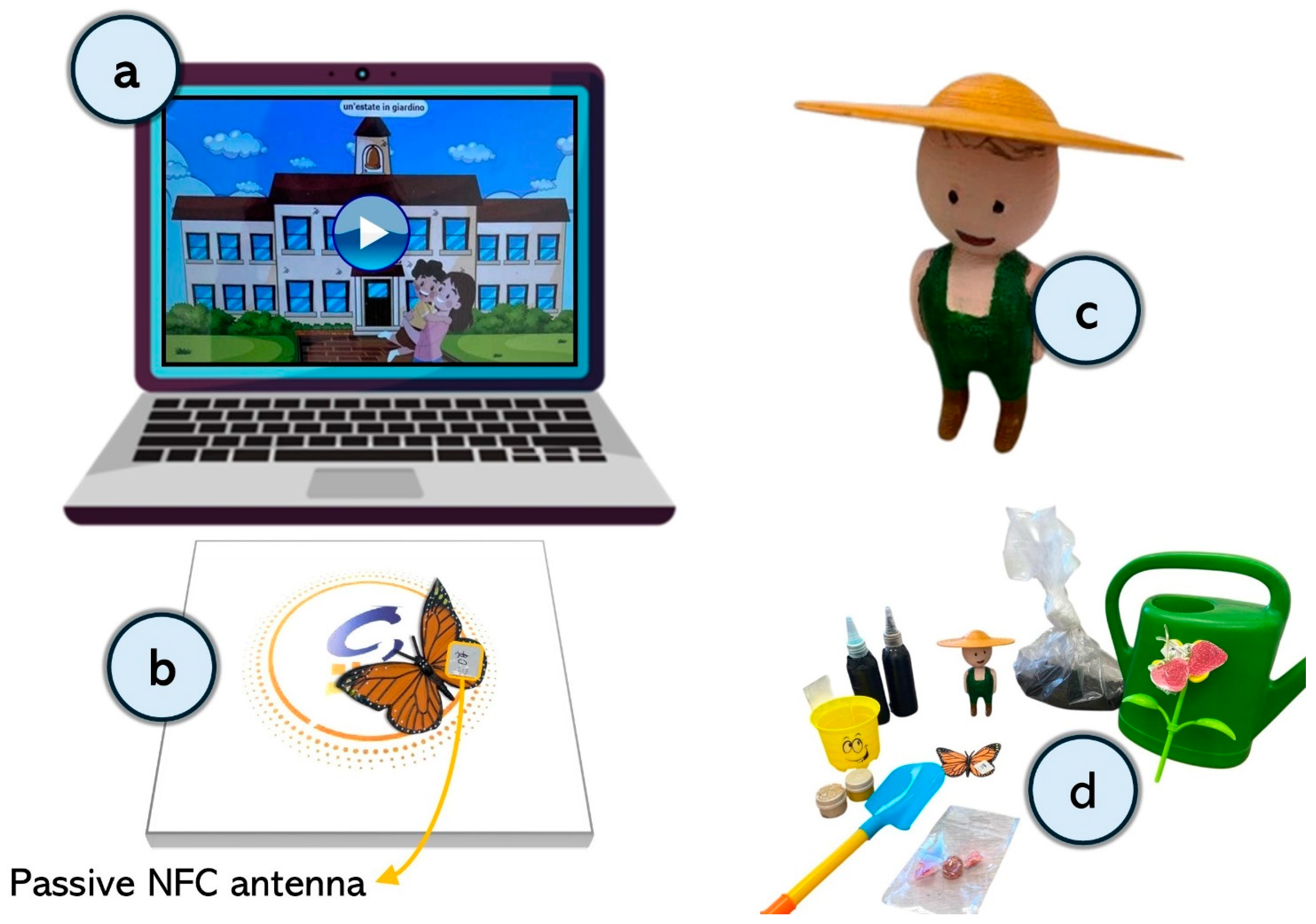
2.2. Apparatus and Stimuli
2.3. Neuropsychological Testing
2.4. Procedure
2.5. Ethical Approval
2.6. Statistical Analysis
3. Results
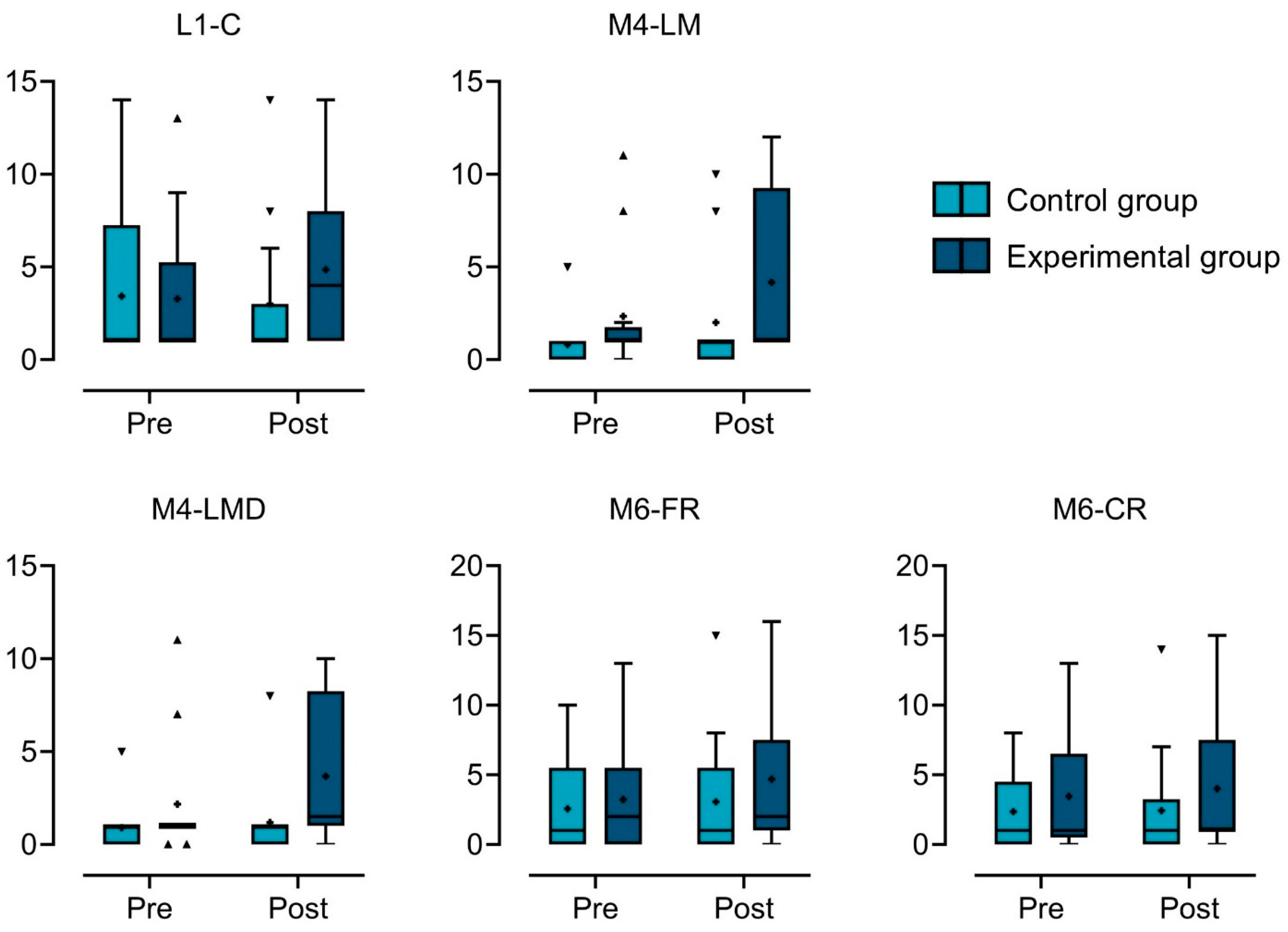
3.1. Descriptive Statistics
3.2. Results from ART LMM Analysis
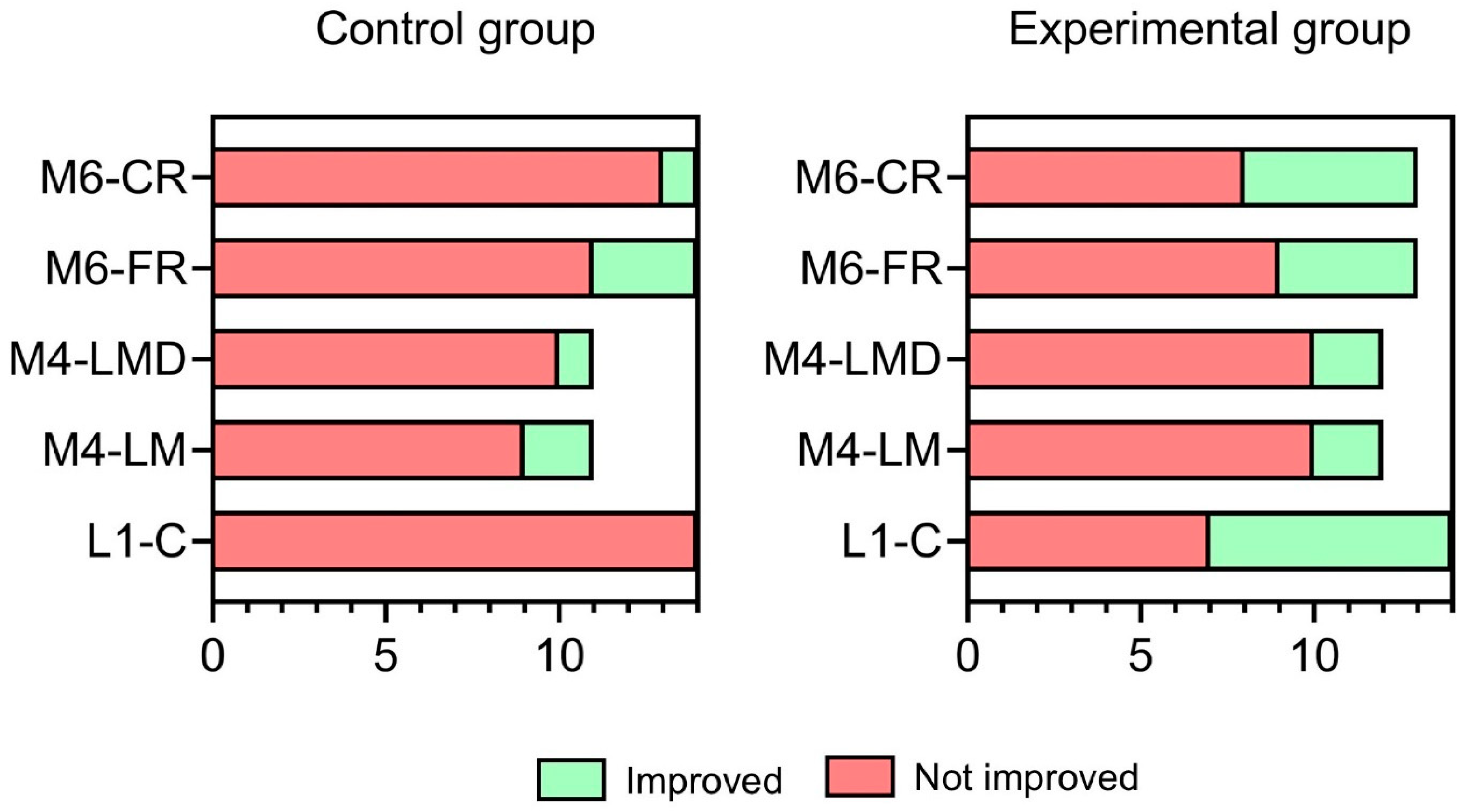
3.3. Proportion of Improvement in NEPSY-II
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Mahmud, A., & Soysa, A. I. (2020). POMA: A tangible user interface to improve social and cognitive skills of Sri Lankan children with ASD. International Journal of Human-Computer Studies, 144, 102486. [Google Scholar] [CrossRef]
- Andrzejewski, J. A., Greenberg, T., & Carlson, J. M. (2019). Neural correlates of aversive anticipation: An activation likelihood estimate meta-analysis across multiple sensory modalities. Cognitive, Affective & Behavioral Neuroscience, 19(6), 1379–1390. [Google Scholar] [CrossRef]
- Bara, F., Gentaz, E., & Valente, D. (2018). The effect of tactile illustrations on comprehension of storybooks by three children with visual impairments: An exploratory study. Journal of Visual Impairment & Blindness, 112(6), 759–765. [Google Scholar] [CrossRef]
- Barros, F., & Soares, S. C. (2020). Giving meaning to the social world in autism spectrum disorders: Olfaction as a missing piece of the puzzle? Neuroscience and Biobehavioral Reviews, 116, 239–250. [Google Scholar] [CrossRef]
- Beccaluva, E., Riccardi, F., Gianotti, M., Barbieri, J., & Garzotto, F. (2022). VIC—A tangible user interface to train memory skills in children with intellectual disability. International Journal of Child-Computer Interaction, 32, 100376. [Google Scholar] [CrossRef]
- Boccia, M., Barbetti, S., Piccardi, L., Guariglia, C., Ferlazzo, F., Giannini, A. M., & Zaidel, D. W. (2016). Where does brain neural activation in aesthetic responses to visual art occur? Meta-analytic evidence from neuroimaging studies. Neuroscience and Biobehavioral Reviews, 60, 65–71. [Google Scholar] [CrossRef]
- Bottema-Beutel, K., & Crowley, S. (2021). Pervasive undisclosed conflicts of interest in applied behavior analysis autism literature. Frontiers in Psychology, 12, 676303. [Google Scholar] [CrossRef]
- Boucher, J., Mayes, A., & Bigham, S. (2012). Memory in autistic spectrum disorder. Psychological Bulletin, 138(3), 458–496. [Google Scholar] [CrossRef]
- Boudjarane, M. A., Grandgeorge, M., Marianowski, R., Misery, L., & Lemonnier, É. (2017). Perception of odors and tastes in autism spectrum disorders: A systematic review of assessments. Autism Research: Official Journal of the International Society for Autism Research, 10(6), 1045–1057. [Google Scholar] [CrossRef]
- Bowler, D. M., Gardiner, J. M., & Berthollier, N. (2004). Source memory in adolescents and adults with Asperger’s syndrome. Journal of Autism and Developmental Disorders, 34(5), 533–542. [Google Scholar] [CrossRef]
- Brang, D., Plass, J., Sherman, A., Stacey, W. C., Wasade, V. S., Grabowecky, M., Ahn, E., Towle, V. L., Tao, J. X., Wu, S., Issa, N. P., & Suzuki, S. (2022). Visual cortex responds to sound onset and offset during passive listening. Journal of Neurophysiology, 127(6), 1547–1563. [Google Scholar] [CrossRef] [PubMed]
- Brown, H. M., Oram-Cardy, J., & Johnson, A. (2013). A meta-analysis of the reading comprehension skills of individuals on the autism spectrum. Journal of Autism and Developmental Disorders, 43(4), 932–955. [Google Scholar] [CrossRef] [PubMed]
- Callan, D. E., Jones, J. A., Munhall, K., Callan, A. M., Kroos, C., & Vatikiotis-Bateson, E. (2003). Neural processes underlying perceptual enhancement by visual speech gestures. Neuroreport, 14(17), 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Calvert, G. A. (2001). Crossmodal processing in the human brain: Insights from functional neuroimaging studies. Cerebral Cortex, 11(12), 1110–1123. [Google Scholar] [CrossRef]
- Cappe, C., Morel, A., Barone, P., & Rouiller, E. M. (2009). The thalamocortical projection systems in primate: An anatomical support for multisensory and sensorimotor interplay. Cerebral Cortex, 19(9), 2025–2037. [Google Scholar] [CrossRef]
- Cardillo, R., & Mammarella, I. C. (2015). L’utilità della NEPSY-II per la valutazione neuropsicologica nella psicopatologia dello sviluppo. In Psicologia clinica dello sviluppo (pp. 553–560). Il Mulino. [Google Scholar] [CrossRef]
- Cheung, M., Chan, A. S., Sze, S. L., Leung, W. W., & To, C. Y. (2010). Verbal memory deficits in relation to organization strategy in high- and low-functioning autistic children. Research in Autism Spectrum Disorders, 4(4), 764–771. [Google Scholar] [CrossRef]
- Cnaan, A., Laird, N. M., & Slasor, P. (1997). Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Statistics in Medicine, 16(20), 2349–2380. [Google Scholar] [CrossRef]
- Cohen, J. (2013). Statistical power analysis for the behavioral sciences (2nd ed.). Routledge. [Google Scholar] [CrossRef]
- Dalton, P., Doolittle, N., Nagata, H., & Breslin, P. A. (2000). The merging of the senses: Integration of subthreshold taste and smell. Nature Neuroscience, 3(5), 431–432. [Google Scholar] [CrossRef]
- Dautenhahn, K., & Werry, I. (2004). Towards interactive robots in autism therapy: Background, motivation and challenges. Pragmatics & Cognition, 12(1), 1–35. [Google Scholar] [CrossRef]
- Di Fuccio, R. (2022). I sensi nel digitale. Le tangible user interfaces innovano la pratica pedagogica. Progedit–Progetti Editoriali srl. [Google Scholar]
- Di Fuccio, R., & Mastroberti, S. (2018). Tangible user interfaces for multisensory storytelling at school: A study of acceptability. QWERTY-Interdisciplinary Journal of Technology, Culture and Education, 13(1), 20–32. [Google Scholar]
- Di Fuccio, R., Ponticorvo, M., Ferrara, F., & Miglino, O. (2016). Digital and multisensory storytelling: Narration with smell, taste and touch. Springer International Publishing. [Google Scholar] [CrossRef]
- Dionne-Dostie, E., Paquette, N., Lassonde, M., & Gallagher, A. (2015). Multisensory integration and child neurodevelopment. Brain Sciences, 5(1), 32–57. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L. J., Muller, K. E., Wolfinger, R. D., Qaqish, B. F., & Schabenberger, O. (2008). An R2 statistic for fixed effects in the linear mixed model. Statistics in Medicine, 27(29), 6137–6157. [Google Scholar] [CrossRef] [PubMed]
- Elkin, L. A., Kay, M., Higgins, J. J., & Wobbrock, J. O. (2021, October 10–14). An aligned rank transform procedure for multifactor contrast tests. The 34th Annual ACM Symposium on User Interface Software and Technology (pp. 754–768), Virtual. [Google Scholar] [CrossRef]
- Escobedo, L., Ibarra, C., Hernandez, J., Alvelais, M., & Tentori, M. (2014). Smart objects to support the discrimination training of children with autism. Personal and Ubiquitous Computing, 18(6), 1485–1497. [Google Scholar] [CrossRef]
- Fails, J. A., Druin, A., Guha, M. L., Chipman, G., Simms, S., & Churaman, W. (2005). Child’s play: A comparison of desktop and physical interactive environments (pp. 48–55). Association for Computing Machinery. [Google Scholar] [CrossRef]
- Fani-Panagiota, R. (2015). Teaching strategies for children with autism. Journal of Physical Education and Sport, 15(1), 148. [Google Scholar]
- Faras, H., Al Ateeqi, N., & Tidmarsh, L. (2010). Autism spectrum disorders. Annals of Saudi Medicine, 30(4), 295–300. [Google Scholar] [CrossRef]
- Farr, W., Yuill, N., & Raffle, H. (2010). Social benefits of a tangible user interface for children with Autistic Spectrum Conditions. Autism, 14(3), 237–252. [Google Scholar] [CrossRef]
- Froesel, M., Cappe, C., & Ben Hamed, S. (2021). A multisensory perspective onto primate pulvinar functions. Neuroscience and Biobehavioral Reviews, 125, 231–243. [Google Scholar] [CrossRef]
- Garcia-Ruiz, M. A., Kapralos, B., & Rebolledo-Mendez, G. (2021). An overview of olfactory displays in education and training. Multimodal Technologies and Interaction, 5(10), 64. [Google Scholar] [CrossRef]
- Gotham, K., Risi, S., Pickles, A., & Lord, C. (2007). The autism diagnostic observation schedule: REVISED algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders, 37(4), 613–627. [Google Scholar] [CrossRef]
- Herz, R. S. (2016). The role of odor-evoked memory in psychological and physiological health. Brain Sciences, 6(3), 22. [Google Scholar] [CrossRef]
- Hirota, T., & King, B. H. (2023). Autism spectrum disorder: A review. JAMA, 329(2), 157–168. [Google Scholar] [CrossRef] [PubMed]
- Iacoboni, M. (2008). The role of premotor cortex in speech perception: Evidence from fMRI and rTMS. Journal of Physiology, Paris, 102(1–3), 31–34. [Google Scholar] [CrossRef]
- Ilardi, C. R., Federico, G., La Marra, M., Amato, R., Iavarone, A., Soricelli, A., Santangelo, G., & Chieffi, S. (2025). Deficits in reaching movements under visual interference as a novel diagnostic marker for mild cognitive impairment. Scientific Reports, 15(1), 1901. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H., & Ullmer, B. (1997). Tangible bits: Towards seamless interfaces between people, bits and atoms. In Proceedings of the ACM SIGCHI conference on human factors in computing systems (pp. 234–241). Association for Computing Machinery. [Google Scholar] [CrossRef]
- Kaplan, H., Clopton, M., Kaplan, M., Messbauer, L., & McPherson, K. (2006). Snoezelen multi-sensory environments: Task engagement and generalization. Research in Developmental Disabilities, 27(4), 443–455. [Google Scholar] [CrossRef]
- Korkman, M., Kirk, U., & Kemp, S. (2012). NEPSY—Second edition [dataset]. APA PsycTests. [Google Scholar] [CrossRef]
- Kumazaki, H., Muramatsu, T., Fujisawa, T. X., Miyao, M., Matsuura, E., Okada, K., Kosaka, H., Tomoda, A., & Mimura, M. (2016). Assessment of olfactory detection thresholds in children with autism spectrum disorders using a pulse ejection system. Molecular Autism, 7(1), 6. [Google Scholar] [CrossRef]
- Landa, R. J., & Goldberg, M. C. (2005). Language, social, and executive functions in high functioning autism: A continuum of performance. Journal of Autism and Developmental Disorders, 35(5), 557–573. [Google Scholar] [CrossRef]
- Leaf, J. B., Cihon, J. H., Leaf, R., McEachin, J., Liu, N., Russell, N., Unumb, L., Shapiro, S., & Khosrowshahi, D. (2022). Concerns about ABA-based intervention: An evaluation and recommendations. Journal of Autism and Developmental Disorders, 52(6), 2838–2853. [Google Scholar] [CrossRef]
- Lehr, R. G. (2001). Some practical considerations and a crude formula for estimating sample size for McNemar’s test. Drug Information Journal: DIJ/Drug Information Association, 35(4), 1227–1233. [Google Scholar] [CrossRef]
- Leonard, A., Mitchell, P., & Parsons, S. (2002, September 18–20). Finding a place to sit: A preliminary investigation into the effectiveness of virtual environments for social skills training for people with autistic spectrum disorders. 4th International Conference on Disability, Virtual Reality & Associated Technologies, Veszprém, Hungary. [Google Scholar]
- Lindgren, K. A., Folstein, S. E., Tomblin, J. B., & Tager-Flusberg, H. (2009). Language and reading abilities of children with autism spectrum disorders and specific language impairment and their first-degree relatives. Autism Research: Official Journal of the International Society for Autism Research, 2(1), 22–38. [Google Scholar] [CrossRef]
- Lord, C., Elsabbagh, M., Baird, G., & Veenstra-Vanderweele, J. (2018). Autism spectrum disorder. The Lancet, 392(10146), 508–520. [Google Scholar] [CrossRef]
- Lord, C., Risi, S., Lambrecht, L., Cook, E. H., Leventhal, B. L., DiLavore, P. C., Pickles, A., & Rutter, M. (2000). The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. [Google Scholar] [CrossRef]
- Lord, C., Rutter, M., DiLavore, P., Risi, S., Gotham, K., Bishop, S. L., Lord, C., Luyster, R. J., Gotham, K., & Guthrie, W. (2012). Autism diagnostic observation schedule, second edition (ADOS-2) (2nd ed.). Pearson. [Google Scholar]
- Maenner, M. J. (2023). Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2020. MMWR Surveillance Summaries, 72, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Martin, G. N., & Daniel, N. (2014). Autism spectrum disorders and chemoreception: Dead-end or fruitful avenue of inquiry? Frontiers in Psychology, 5, 42. [Google Scholar] [CrossRef]
- McCrimmon, A., & Rostad, K. (2014). Test review: Autism diagnostic observation schedule, second edition (ADOS-2) manual (Part II): Toddler module. Journal of Psychoeducational Assessment, 32(1), 88–92. [Google Scholar] [CrossRef]
- McDougal, E., Riby, D. M., & Hanley, M. (2020). Profiles of academic achievement and attention in children with and without autism spectrum disorder. Research in Developmental Disabilities, 106, 103749. [Google Scholar] [CrossRef] [PubMed]
- McNemar, Q. (1947). Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika, 12(2), 153–157. [Google Scholar] [CrossRef]
- Meister, I. G., Wilson, S. M., Deblieck, C., Wu, A. D., & Iacoboni, M. (2007). The essential role of premotor cortex in speech perception. Current Biology: CB, 17(19), 1692–1696. [Google Scholar] [CrossRef] [PubMed]
- Miglino, O., Di Ferdinando, A., Massimiliano, C., Angelo, R., & Carlo, R. (2013). STELT (Smart Technologies to Enhance Learning and Teaching): Una piattaforma per realizzare ambienti di realtà aumentata per apprendere, insegnare e giocare. In Sistemi intelligenti (pp. 397–404). Il Mulino. [Google Scholar]
- Muratori, F., Tonacci, A., Billeci, L., Catalucci, T., Igliozzi, R., Calderoni, S., & Narzisi, A. (2017). Olfactory processing in male children with autism: Atypical odor threshold and identification. Journal of Autism and Developmental Disorders, 47(10), 3243–3251. [Google Scholar] [CrossRef]
- Ojanen, V., Möttönen, R., Pekkola, J., Jääskeläinen, I. P., Joensuu, R., Autti, T., & Sams, M. (2005). Processing of audiovisual speech in Broca’s area. NeuroImage, 25(2), 333–338. [Google Scholar] [CrossRef]
- Parés, N., Carreras, A., Durany, J., Ferrer, J., Freixa, P., Gómez, D., Kruglanski, O., Parés, R., Ribas, J. I., Soler, M., & Sanjurjo, À. (2005, June 8–10). Promotion of creative activity in children with severe autism through visuals in an interactive multisensory environment. Proceedings of the 2005 Conference on Interaction Design and Children (pp. 110–116), Boulder, CO, USA. [Google Scholar] [CrossRef]
- Parisi, A., Bellinzona, F., Di Lernia, D., Repetto, C., De Gaspari, S., Brizzi, G., Riva, G., & Tuena, C. (2022). Efficacy of Multisensory Technology in Post-Stroke Cognitive Rehabilitation: A Systematic Review. Journal of Clinical Medicine, 11(21), 6324. [Google Scholar] [CrossRef]
- Ponticorvo, M., Di Fuccio, R., Ferrara, F., Rega, A., & Miglino, O. (2019). Multisensory Educational Materials: Five Senses to Learn. In T. Di Mascio, P. Vittorini, R. Gennari, F. De la Prieta, S. Rodríguez, M. Temperini, R. Azambuja Silveira, E. Popescu, & L. Lancia (Eds.), Methodologies and intelligent systems for technology enhanced learning, 8th international conference (pp. 45–52). Springer International Publishing. [Google Scholar] [CrossRef]
- Puce, A., Syngeniotis, A., Thompson, J. C., Abbott, D. F., Wheaton, K. J., & Castiello, U. (2003). The human temporal lobe integrates facial form and motion: Evidence from fMRI and ERP studies. NeuroImage, 19(3), 861–869. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Aguiar, L. R., & Álvarez-Rodríguez, F. J. (2021). Teaching emotions in children with autism spectrum disorder through a computer program with tangible interfaces. IEEE Revista Iberoamericana de Tecnologías Del Aprendizaje, 16(4), 365–371. [Google Scholar] [CrossRef]
- Ross, L. A., Molholm, S., Butler, J. S., Bene, V. A. D., & Foxe, J. J. (2022). Neural correlates of multisensory enhancement in audiovisual narrative speech perception: A fMRI investigation. NeuroImage, 263, 119598. [Google Scholar] [CrossRef]
- Rossiter, M., & Garcia, P. A. (2010). Digital storytelling: A new player on the narrative field. New Directions for Adult and Continuing Education, 126, 37–48. [Google Scholar] [CrossRef]
- Rozenkrantz, L., Zachor, D., Heller, I., Plotkin, A., Weissbrod, A., Snitz, K., Secundo, L., & Sobel, N. (2015). A mechanistic link between olfaction and autism spectrum disorder. Current Biology: CB, 25(14), 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Russo, N., Foxe, J. J., Brandwein, A. B., Altschuler, T., Gomes, H., & Molholm, S. (2010). Multisensory processing in children with autism: High-density electrical mapping of auditory-somatosensory integration. Autism Research: Official Journal of the International Society for Autism Research, 3(5), 253–267. [Google Scholar] [CrossRef]
- Salimi, M., Tabasi, F., Nazari, M., Ghazvineh, S., Salimi, A., Jamaati, H., & Raoufy, M. R. (2021). The olfactory bulb modulates entorhinal cortex oscillations during spatial working memory. The Journal of Physiological Sciences: JPS, 71(1), 21. [Google Scholar] [CrossRef] [PubMed]
- Scheliga, S., Kellermann, T., Lampert, A., Rolke, R., Spehr, M., & Habel, U. (2023). Neural correlates of multisensory integration in the human brain: An ALE meta-analysis. Reviews in the Neurosciences, 34(2), 223–245. [Google Scholar] [CrossRef]
- Shumway, S., Farmer, C., Thurm, A., Joseph, L., Black, D., & Golden, C. (2012). The ADOS calibrated severity score: Relationship to phenotypic variables and stability over time. Autism Research: Official Journal of the International Society for Autism Research, 5(4), 267–276. [Google Scholar] [CrossRef]
- Silver, M., & Oakes, P. (2001). Evaluation of a new computer intervention to teach people with autism or Asperger syndrome to recognize and predict emotions in others. Autism: The International Journal of Research and Practice, 5(3), 299–316. [Google Scholar] [CrossRef]
- Sitdhisanguan, K., Chotikakamthorn, N., Dechaboon, A., & Out, P. (2012). Using tangible user interfaces in computer-based training systems for low-functioning autistic children. Personal and Ubiquitous Computing, 16(2), 143–155. [Google Scholar] [CrossRef]
- Sorokowska, A., Sorokowski, P., Karwowski, M., Larsson, M., & Hummel, T. (2019). Olfactory perception and blindness: A systematic review and meta-analysis. Psychological Research, 83(8), 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Stein, B. E., & Stanford, T. R. (2008). Multisensory integration: Current issues from the perspective of the single neuron. Nature Reviews. Neuroscience, 9(4), 255–266. [Google Scholar] [CrossRef]
- Stevenson, R. A., Segers, M., Ncube, B. L., Black, K. R., Bebko, J. M., Ferber, S., & Barense, M. D. (2018). The cascading influence of multisensory processing on speech perception in autism. Autism: The International Journal of Research and Practice, 22(5), 609–624. [Google Scholar] [CrossRef] [PubMed]
- Sweigert, J. R., St John, T., Begay, K. K., Davis, G. E., Munson, J., Shankland, E., Estes, A., Dager, S. R., & Kleinhans, N. M. (2020). Characterizing olfactory function in children with autism spectrum disorder and children with sensory processing dysfunction. Brain Sciences, 10(6), 362. [Google Scholar] [CrossRef]
- Tager-Flusberg, H., & Joseph, R. M. (2003). Identifying neurocognitive phenotypes in autism. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358(1430), 303–314. [Google Scholar] [CrossRef]
- Tárraga-Mínguez, R., Gómez-Marí, I., & Sanz-Cervera, P. (2020). Interventions for improving reading comprehension in children with ASD: A systematic review. Behavioral Sciences (Basel, Switzerland), 11(1), 3. [Google Scholar] [CrossRef]
- The Peacock Hill Working Group, Zabel, R. K., Kauffman, J. M., Lloyd, J. W., Cook, L., Cullinan, D., Epstein, M. H., Hallahan, D. P., Nelson, C. M., Polsgrove, L., Sabornie, E. J., Strain, P. S., & Walker, H. M. (1991). Problems and Promises in Special Education and Related Services for Children and Youth with Emotional or Behavioral Disorders. Behavioral Disorders, 16(4), 299–313. [Google Scholar] [CrossRef]
- Tyll, S., Budinger, E., & Noesselt, T. (2011). Thalamic influences on multisensory integration. Communicative & Integrative Biology, 4(4), 378–381. [Google Scholar] [CrossRef]
- Van der Burg, E., Olivers, C. N. L., Bronkhorst, A. W., & Theeuwes, J. (2008). Pip and pop: Nonspatial auditory signals improve spatial visual search. Journal of Experimental Psychology. Human Perception and Performance, 34(5), 1053–1065. [Google Scholar] [CrossRef]
- Villafuerte, L., Markova, M., & Jorda, S. (2012, May 5–10). Acquisition of social abilities through musical tangible user interface: Children with autism spectrum condition and the reactable. CHI ’12 Extended Abstracts on Human Factors in Computing Systems (pp. 745–760), Austin, TX, USA. [Google Scholar] [CrossRef]
- Vroomen, J., & de Gelder, B. (2000). Sound enhances visual perception: Cross-modal effects of auditory organization on vision. Journal of Experimental Psychology. Human Perception and Performance, 26(5), 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Wicker, B., Monfardini, E., & Royet, J.-P. (2016). Olfactory processing in adults with autism spectrum disorders. Molecular Autism, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Williams, C., Wright, B., Callaghan, G., & Coughlan, B. (2002). Do children with autism learn to read more readily by computer assisted instruction or traditional book methods? A pilot study. Autism: The International Journal of Research and Practice, 6(1), 71–91. [Google Scholar] [CrossRef] [PubMed]
- Williams, D. L., Goldstein, G., & Minshew, N. J. (2006). The profile of memory function in children with autism. Neuropsychology, 20(1), 21–29. [Google Scholar] [CrossRef]
- Wobbrock, J. O., Findlater, L., Gergle, D., & Higgins, J. J. (2011, May 7–12). The aligned rank transform for nonparametric factorial analyses using only anova procedures. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems (pp. 143–146), Vancouver, BC, Canada. [Google Scholar] [CrossRef]
- Wright, T. M., Pelphrey, K. A., Allison, T., McKeown, M. J., & McCarthy, G. (2003). Polysensory interactions along lateral temporal regions evoked by audiovisual speech. Cerebral Cortex, 13(10), 1034–1043. [Google Scholar] [CrossRef]
- Wynn, J. W., & Smith, T. (2003). Generalization Between Receptive and Expressive Language in Young Children with Autism. Behavioral Interventions, 18(4), 245–266. [Google Scholar] [CrossRef]
- Yu, Q., Li, E., Li, L., & Liang, W. (2020). Efficacy of Interventions Based on Applied Behavior Analysis for Autism Spectrum Disorder: A Meta-Analysis. Psychiatry Investigation, 17(5), 432–443. [Google Scholar] [CrossRef]
- Yue, Q. (2023). Maintaining auditory working memory representations beyond sensory cortices makes working memory work. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 43(40), 6714–6716. [Google Scholar] [CrossRef]
- Zhou, G., Olofsson, J. K., Koubeissi, M. Z., Menelaou, G., Rosenow, J., Schuele, S. U., Xu, P., Voss, J. L., Lane, G., & Zelano, C. (2021). Human hippocampal connectivity is stronger in olfaction than other sensory systems. Progress in Neurobiology, 201, 102027. [Google Scholar] [CrossRef]
| Experimental Group (n = 14) | Control Group (n = 14) | p-Value | |
|---|---|---|---|
| Age, years; mean (SD) | 9.14 (2.80) | 9.00 (2.07) | 0.88 a |
| Education, year; mean (SD) | 6.21 (2.75) | 6.00 (2.07) | 0.82 a |
| Functional level (ADOS-2) | |||
| Low functioning, CSS range: 6–10 | 6 | 7 | |
| Moderate functioning, CSS range: 4–5 | 5 | 4 | |
| High functioning, CSS range: 1–3 | 3 | 3 | |
| L1-C, raw score; mean (SD) | 3.29 (3.77) | 3.43 (4.13) | 0.92 a |
| M4-LM, raw score; mean (SD) | 2.33 (3.45) † | 0.82 (1.47) †† | 0.19 a |
| M4-LMD, raw score; mean (SD) | 2.17 (3.33) † | 0.91 (1.45) †† | 0.21 b |
| M6-FR, raw score; mean (SD) | 3.23 (3.81) ⁋ | 2.57 (3.39) | 0.53 a |
| M6-CR, raw score; mean (SD) | 3.46 (3.93) ⁋ | 2.36 (2.90) | 0.35 a |
| ID | Group | L1-C | M4-LM | M4-LMD | M6-FR | M6-CR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| P1 | EG | 1 | 1 | 1 | 3 | 1 | 2 | 1 | 1 | 1 | 1 |
| P2 | EG | 1 | 2 | 1 | 1 | 1 | 2 | 3 | 1 | 3 | 2 |
| P3 | EG | 13 | 14 | 11 | 11 | 11 | 10 | 13 | 15 | 13 | 15 |
| P4 | EG | 4 | 5 | • | • | • | • | 5 | 5 | 7 | 10 |
| P5 | EG | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| P6 | EG | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| P7 | EG | 1 | 6 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 |
| P8 | EG | 5 | 6 | 1 | 12 | 1 | 10 | 7 | 8 | 7 | 9 |
| P9 | EG | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| P10 | EG | 6 | 8 | 2 | 1 | 1 | 1 | 4 | 4 | 5 | 6 |
| P11 | EG | 1 | 3 | 1 | 10 | 1 | 9 | 1 | 7 | 1 | 4 |
| P12 | EG | 9 | 11 | 8 | 7 | 7 | 6 | 6 | 16 | 6 | 1 |
| P13 | EG | 1 | 8 | • | • | • | • | • | • | • | • |
| P14 | EG | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| P15 | CG | 14 | 14 | 0 | 0 | 1 | 0 | 7 | 15 | 6 | 14 |
| P16 | CG | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| P17 | CG | 2 | 2 | • | • | • | • | 1 | 1 | 1 | 3 |
| P18 | CG | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| P19 | CG | 1 | 2 | • | • | • | • | 5 | 7 | 4 | 4 |
| P20 | CG | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 |
| P21 | CG | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 1 | 2 | 2 |
| P22 | CG | 7 | 6 | • | • | • | • | 7 | 8 | 8 | 1 |
| P23 | CG | 8 | 1 | 5 | 10 | 0 | 8 | 4 | 4 | 4 | 2 |
| P24 | CG | 1 | 1 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 |
| P25 | CG | 8 | 8 | 1 | 8 | 1 | 1 | 10 | 5 | 7 | 7 |
| P26 | CG | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| P27 | CG | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P28 | CG | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, M.; Ilardi, C.R.; Dolce, P.; Rega, A.; Di Fuccio, R.; Rubinacci, F.; Gallucci, M.; Marangolo, P. Using Tangible User Interfaces (TUIs): Preliminary Evidence on Memory and Comprehension Skills in Children with Autism Spectrum Disorder. Behav. Sci. 2025, 15, 267. https://doi.org/10.3390/bs15030267
De Luca M, Ilardi CR, Dolce P, Rega A, Di Fuccio R, Rubinacci F, Gallucci M, Marangolo P. Using Tangible User Interfaces (TUIs): Preliminary Evidence on Memory and Comprehension Skills in Children with Autism Spectrum Disorder. Behavioral Sciences. 2025; 15(3):267. https://doi.org/10.3390/bs15030267
Chicago/Turabian StyleDe Luca, Mariagiovanna, Ciro Rosario Ilardi, Pasquale Dolce, Angelo Rega, Raffaele Di Fuccio, Franco Rubinacci, Maria Gallucci, and Paola Marangolo. 2025. "Using Tangible User Interfaces (TUIs): Preliminary Evidence on Memory and Comprehension Skills in Children with Autism Spectrum Disorder" Behavioral Sciences 15, no. 3: 267. https://doi.org/10.3390/bs15030267
APA StyleDe Luca, M., Ilardi, C. R., Dolce, P., Rega, A., Di Fuccio, R., Rubinacci, F., Gallucci, M., & Marangolo, P. (2025). Using Tangible User Interfaces (TUIs): Preliminary Evidence on Memory and Comprehension Skills in Children with Autism Spectrum Disorder. Behavioral Sciences, 15(3), 267. https://doi.org/10.3390/bs15030267








