The Complex Role Played by the Default Mode Network during Sexual Stimulation: A Cluster-Based fMRI Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
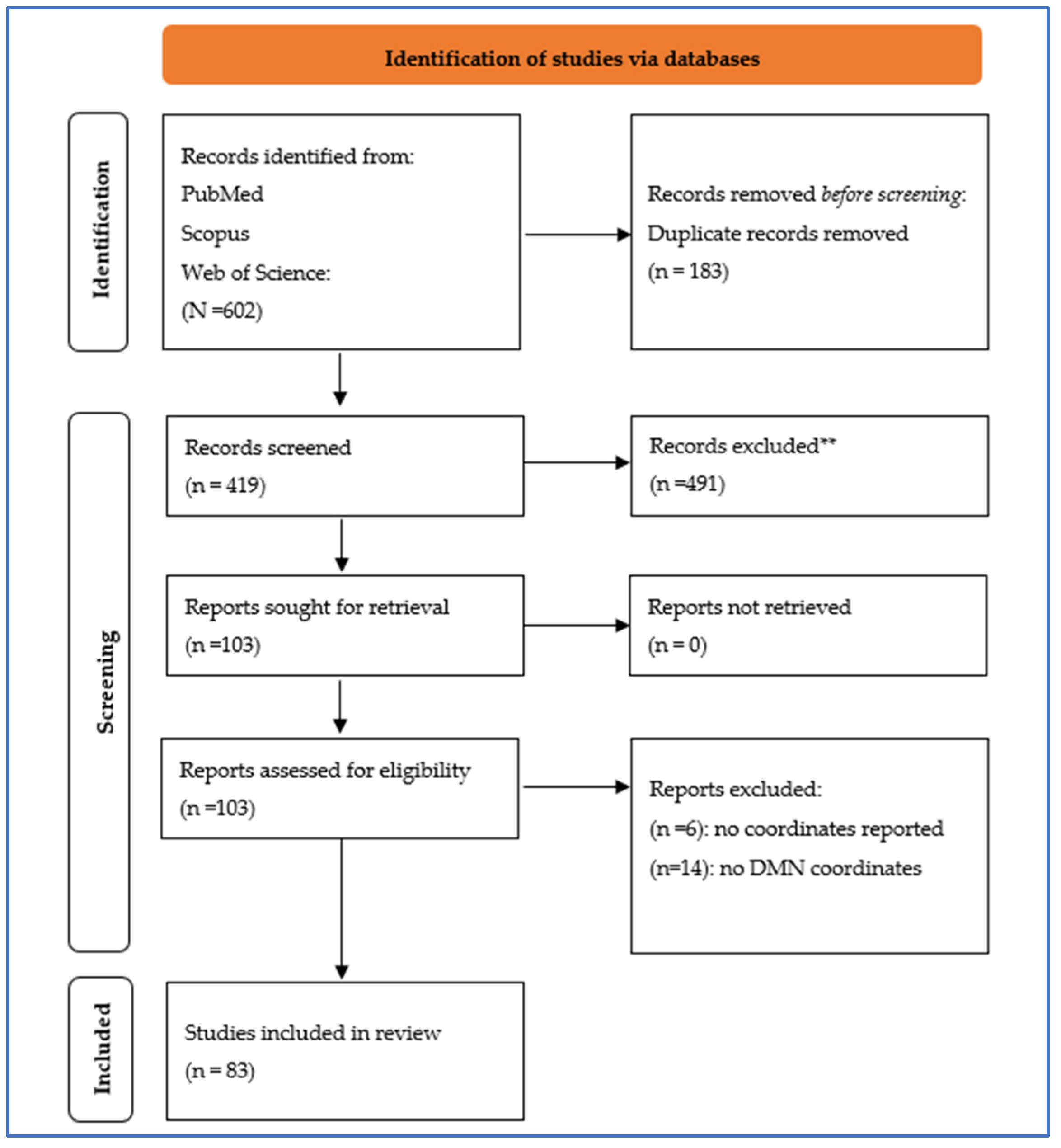
2.1. Systematic Review Protocol, Study Selection, and Study Inclusion
2.2. ALE fMRI Meta-Analysis
- (i)
- Differences between videos and pictures, highlighting the DMN’s involvement in responses to dynamic and static stimuli.
- (ii)
- Similarly, differences in the DMN nodes between heterosexuals and homosexuals and between heterosexuals and transsexuals;
- (iii)
- Differences between heterosexual healthy subjects and pedophiles;
- (iv)
- Differences between heterosexual healthy men and patients affected by sexual dysfunctions, such as psychogenic erectile dysfunction or premature ejaculation.
3. Results
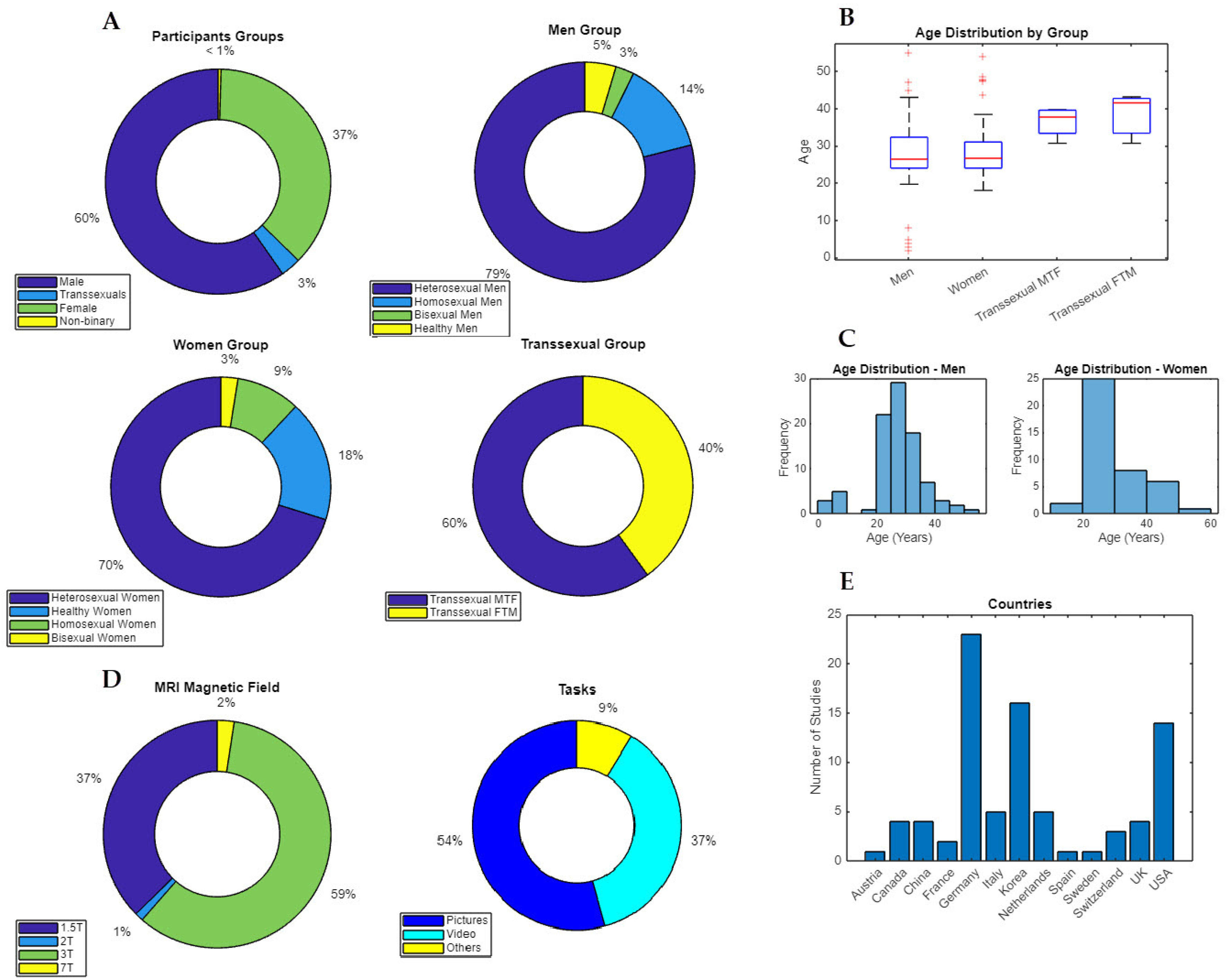
3.1. Principal Characteristics of the Included Studies
3.2. Brain Results
4. Discussion
5. Conclusions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- PICO Worksheet and Search Strategy Protocol
- 1. Define your question using PICO by identifying the patient/problem, intervention, comparison group, and outcome:
- Patient/Problem: Healthy adults; patients with sexual dysfunction; sexual offenders; pedophiles.
- Intervention: fMRI.
- Comparison: Studies using different types of sexual stimulation tasks.
- Outcome: Topographic alteration of DMN during sexual stimulation.
- Write out your question
- 2. Type of question/problem: Alteration and involvement of DMN subsystems in imaging studies about human sexual behavior.
- Circle one: Therapy/Prevention/Diagnosis/Etiology/Prognosis.
- 3. Type of studies/publications to include in the search:
- Check all that apply:
- □ Meta-analysis □ Systematic review
- □ Clinical practice guidelines □ Randomized controlled trial
- □ Research studies or articles □ Case report or series
- □ Research report or other grey literature
- 4. List main topics and alternate terms from your PICO question that can be used for your search: “Sexuality”; “Sexual behavior”; “Sexual arousal”
- 5. Write out your search strategy: sexual arousal: “sexual arousal”[MeSH Terms] OR (“sexual”[All Fields] AND “arousal”[All Fields]) OR “sexual arousal”[All Fields]; fMRI: “magnetic resonance imaging”[MeSH Terms] OR (“magnetic”[All Fields] AND “resonance”[All Fields] AND “imaging”[All Fields]) OR “magnetic resonance imaging”[All Fields] OR “fMRI”[All Fields]
- 6. List any limits that may apply to your search:
- Gender: Female; Male.
- Age: Adult.
- Year(s) of publication: no limits; Language(s): English
- 7. List the databases you will search: PubMed, Scopus, and Web of Science.
References
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef]
- Imperatori, C.; Della Marca, G.; Brunetti, R.; Carbone, G.A.; Massullo, C.; Valenti, E.M.; Amoroso, N.; Maestoso, G.; Contardi, A.; Farina, B. Default Mode Network alterations in alexithymia: An EEG power spectra and connectivity study. Sci. Rep. 2016, 6, 36653. [Google Scholar] [CrossRef][Green Version]
- Raichle, M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Crittenden, B.M.; Mitchell, D.J.; Duncan, J. Recruitment of the default mode network during a demanding act of executive control. Elife 2015, 4, e06481. [Google Scholar] [CrossRef]
- Yeshurun, Y.; Nguyen, M.; Hasson, U. The default mode network: Where the idiosyncratic self meets the shared social world. Nat. Rev. Neurosci. 2021, 22, 181–192. [Google Scholar] [CrossRef]
- Hasson, U.; Yang, E.; Vallines, I.; Heeger, D.J.; Rubin, N. A hierarchy of temporal receptive windows in human cortex. J. Neurosci. 2008, 28, 2539–2550. [Google Scholar] [CrossRef]
- Lerner, Y.; Honey, C.J.; Silbert, L.J.; Hasson, U. Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J. Neurosci. 2011, 31, 2906–2915. [Google Scholar] [CrossRef]
- Hasson, U.; Chen, J.; Honey, C.J. Hierarchical process memory: Memory as an integral component of information processing. Trends Cogn. Sci. 2015, 19, 304–313. [Google Scholar] [CrossRef]
- Vessel, E.A.; Isik, A.I.; Belfi, A.M.; Stahl, J.L.; Starr, G.G. The default-mode network represents aesthetic appeal that generalizes across visual domains. Proc. Natl. Acad. Sci. USA 2019, 116, 19155–19164. [Google Scholar] [CrossRef]
- D’Argembeau, A.; Collette, F.; Van der Linden, M.; Laureys, S.; Del Fiore, G.; Degueldre, C.; Luxen, A.; Salmon, E. Self-referential reflective activity and its relationship with rest: A PET study. Neuroimage 2005, 25, 616–624. [Google Scholar] [CrossRef]
- D’Argembeau, A.; Ruby, P.; Collette, F.; Degueldre, C.; Balteau, E.; Luxen, A.; Maquet, P.; Salmon, E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J. Cogn. Neurosci. 2007, 19, 935–944. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Reidler, J.S.; Sepulcre, J.; Poulin, R.; Buckner, R.L. Functional-anatomic fractionation of the brain’s default network. Neuron 2010, 65, 550–562. [Google Scholar] [CrossRef]
- Lee, T.W.; Xue, S.W. Functional connectivity maps based on hippocampal and thalamic dynamics may account for the default-mode network. Eur. J. Neurosci. 2018, 47, 388–398. [Google Scholar] [CrossRef]
- Hassabis, D.; Maguire, E.A. Deconstructing episodic memory with construction. Trends Cogn. Sci. 2007, 11, 299–306. [Google Scholar] [CrossRef]
- Rolls, E.T. Spatial coordinate transforms linking the allocentric hippocampal and egocentric parietal primate brain systems for memory, action in space, and navigation. Hippocampus 2020, 30, 332–353. [Google Scholar] [CrossRef]
- Ward, A.M.; Schultz, A.P.; Huijbers, W.; Van Dijk, K.R.; Hedden, T.; Sperling, R.A. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum. Brain Mapp. 2014, 35, 1061–1073. [Google Scholar] [CrossRef]
- Rugg, M.D.; Vilberg, K.L. Brain networks underlying episodic memory retrieval. Curr. Opin. Neurobiol. 2013, 23, 255–260. [Google Scholar] [CrossRef]
- Huijbers, W.; Pennartz, C.M.; Cabeza, R.; Daselaar, S.M. The hippocampus is coupled with the default network during memory retrieval but not during memory encoding. PLoS ONE 2011, 6, e17463. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Cacciola, A.; Bruschetta, D.; Milardi, D.; Quattrini, F.; Sciarrone, F.; la Rosa, G.; Bramanti, P.; Anastasi, G. Neuroanatomy and function of human sexual behavior: A neglected or unknown issue? Brain Behav. 2019, 9, e01389. [Google Scholar] [CrossRef]
- Georgiadis, J.R. Functional neuroanatomy of human cortex cerebri in relation to wanting sex and having it. Clin. Anat. 2015, 28, 314–323. [Google Scholar] [CrossRef]
- Ågmo, A.; Laan, E. Sexual incentive motivation.; sexual behavior.; and general arousal: Do rats and humans tell the same story? Neurosci. Biobehav. Rev. 2022, 135, 104595. [Google Scholar] [CrossRef]
- Stoléru, S.; Fonteille, V.; Cornélis, C.; Joyal, C.; Moulier, V. Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: A review and meta-analysis. Neurosci. Biobehav. Rev. 2012, 36, 1481–1509. [Google Scholar] [CrossRef]
- Georgiadis, J.R.; Kringelbach, M.L. The human sexual response cycle: Brain imaging evidence linking sex to other pleasures. Prog. Neurobiol. 2012, 98, 49–81. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Esposito, R.; Cieri, F.; Chiacchiaretta, P.; Cera, N.; Lauriola, M.; Di Giannantonio, M.; Tartaro, A.; Ferretti, A. Modifications in resting state functional anticorrelation between default mode network and dorsal attention network: Comparison among young adults.; healthy elders and mild cognitive impairment patients. Brain Imaging Behav. 2018, 12, 127–141. [Google Scholar] [CrossRef]
- Rolls, E.T.; Huang, C.C.; Lin, C.P.; Feng, J.; Joliot, M. Automated anatomical labelling atlas 3. Neuroimage 2020, 206, 116189. [Google Scholar] [CrossRef]
- Kim, G.W.; Jeong, G.W. A comparative study of brain activation patterns associated with sexual arousal between males and females using 3.0-T functional magnetic resonance imaging. Sex. Health 2014, 11, 11–16. [Google Scholar] [CrossRef]
- Demos, K.E.; Heatherton, T.F.; Kelley, W.M. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J. Neurosci. 2012, 32, 5549–5552. [Google Scholar] [CrossRef]
- Wehrum-Osinsky, S.; Klucken, T.; Kagerer, S.; Walter, B.; Hermann, A.; Stark, R. At the second glance: Stability of neural responses toward visual sexual stimuli. J. Sex. Med. 2014, 11, 2720–2737. [Google Scholar] [CrossRef]
- Borg, C.; Georgiadis, J.R.; Renken, R.J.; Spoelstra, S.K.; Weijmar Schultz, W.; de Jong, P.J. Brain processing of visual stimuli representing sexual penetration versus core and animal-reminder disgust in women with lifelong vaginismus. PLoS ONE 2014, 9, e84882. [Google Scholar] [CrossRef]
- Oei, N.Y.L.; Both, S.; van Heemst, D.; van der Grond, J. Acute stress-induced cortisol elevations mediate reward system activity during subconscious processing of sexual stimuli. Psychoneuroendocrinology 2014, 39, 111–120. [Google Scholar] [CrossRef]
- Wehrum, S.; Klucken, T.; Kagerer, S.; Walter, B.; Hermann, A.; Vaitl, D.; Stark, R. Gender commonalities and differences in the neural processing of visual sexual stimuli. J. Sex. Med. 2013, 10, 1328–1342. [Google Scholar] [CrossRef]
- Kim, T.H.; Kang, H.K.; Jeong, G.W. Assessment of brain metabolites change during visual sexual stimulation in healthy women using functional MR spectroscopy. J. Sex. Med. 2013, 10, 1001–1011. [Google Scholar] [CrossRef]
- Kagerer, S.; Klucken, T.; Wehrum, S.; Zimmermann, M.; Schienle, A.; Walter, B.; Vaitl, D.; Stark, R. Neural activation toward erotic stimuli in homosexual and heterosexual males. J. Sex. Med. 2011, 8, 3132–3143. [Google Scholar] [CrossRef]
- Bianchi-Demicheli, F.; Cojan, Y.; Waber, L.; Recordon, N.; Vuilleumier, P.; Ortigue, S. Neural bases of hypoactive sexual desire disorder in women: An event-related FMRI study. J. Sex. Med. 2011, 8, 2546–2559. [Google Scholar] [CrossRef]
- Ponseti, J.; Granert, O.; Jansen, O.; Wolff, S.; Mehdorn, H.; Bosinski, H.; Siebner, H. Assessment of sexual orientation using the hemodynamic brain response to visual sexual stimuli. J. Sex. Med. 2009, 6, 1628–1634. [Google Scholar] [CrossRef]
- Schiffer, B.; Paul, T.; Gizewski, E.; Forsting, M.; Leygraf, N.; Schedlowski, M.; Kruger, T.H. Functional brain correlates of heterosexual paedophilia. Neuroimage 2008, 41, 80–91. [Google Scholar] [CrossRef]
- Moulier, V.; Mouras, H.; Pélégrini-Issac, M.; Glutron, D.; Rouxel, R.; Grandjean, B.; Bittoun, J.; Stoléru, S. Neuroanatomical correlates of penile erection evoked by photographic stimuli in human males. Neuroimage 2006, 33, 689–699. [Google Scholar] [CrossRef]
- Stark, R.; Schienle, A.; Girod, C.; Walter, B.; Kirsch, P.; Blecker, C.; Ott, U.; Schäfer, A.; Sammer, G.; Zimmermann, M.; et al. Erotic and disgust-inducing pictures--differences in the hemodynamic responses of the brain. Biol. Psychol. 2005, 70, 19–29. [Google Scholar] [CrossRef]
- Klucken, T.; Schweckendiek, J.; Merz, C.J.; Tabbert, K.; Walter, B.; Kagerer, S.; Vaitl, D.; Stark, R. Neural activations of the acquisition of conditioned sexual arousal: Effects of contingency awareness and sex. J. Sex. Med. 2009, 6, 3071–3085. [Google Scholar] [CrossRef] [PubMed]
- Mouras, H.; Stoléru, S.; Bittoun, J.; Glutron, D.; Pélégrini-Issac, M.; Paradis, A.L.; Burnod, Y. Brain processing of visual sexual stimuli in healthy men: A functional magnetic resonance imaging study. Neuroimage 2003, 20, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Bermpohl, F.; Mouras, H.; Schiltz, K.; Tempelmann, C.; Rotte, M.; Heinze, H.J.; Bogerts, B.; Northoff, G. Distinguishing specific sexual and general emotional effects in fMRI-subcortical and cortical arousal during erotic picture viewing. Neuroimage 2008, 40, 1482–1494. [Google Scholar] [CrossRef] [PubMed]
- Strahler, J.; Kruse, O.; Wehrum-Osinsky, S.; Klucken, T.; Stark, R. Neural correlates of gender differences in distractibility by sexual stimuli. Neuroimage 2018, 176, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Safron, A.; Klimaj, V.; Sylva, D.; Rosenthal, A.M.; Li, M.; Walter, M.; Bailey, J.M. Neural Correlates of Sexual Orientation in Heterosexual, Bisexual, and Homosexual Women. Sci. Rep. 2018, 8, 673. [Google Scholar] [CrossRef] [PubMed]
- Ponseti, J.; Bosinski, H.A.; Wolff, S.; Peller, M.; Jansen, O.; Mehdorn, H.M.; Büchel, C.; Siebner, H.R. A functional endophenotype for sexual orientation in humans. Neuroimage 2006, 33, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Unterhorst, K.; Gerwinn, H.; Pohl, A.; Kärgel, C.; Massau, C.; Ristow, I.; Kneer, J.; Amelung, T.; Walter, H.; Beier, K.; et al. An Exploratory Study on the Central Nervous Correlates of Sexual Excitation and Sexual Inhibition. J. Sex. Res. 2020, 57, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Arnow, B.A.; Desmond, J.E.; Banner, L.L.; Glover, G.H.; Solomon, A.; Polan, M.L.; Lue, T.F.; Atlas, S.W. Brain activation and sexual arousal in healthy.; heterosexual males. Brain 2002, 125, 1014–1023. [Google Scholar] [CrossRef]
- Oh, S.K.; Kim, G.W.; Yang, J.C.; Kim, S.K.; Kang, H.K.; Jeong, G.W. Brain activation in response to visually evoked sexual arousal in male-to-female transsexuals: 3.0 tesla functional magnetic resonance imaging. Korean J. Radiol. 2012, 13, 257–264. [Google Scholar] [CrossRef]
- Wernicke, M.; Hofter, C.; Jordan, K.; Fromberger, P.; Dechent, P.; Müller, J.L. Neural correlates of subliminally presented visual sexual stimuli. Conscious. Cogn. 2017, 49, 35–52. [Google Scholar] [CrossRef]
- Seok, J.W.; Park, M.S.; Sohn, J.H. Neural pathways in processing of sexual arousal: A dynamic causal modeling study. Int. J. Impot. Res. 2016, 28, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Jeong, B.; Kim, J.W.; Choi, J. Plasma concentration of prolactin.; testosterone might be associated with brain response to visual erotic stimuli in healthy heterosexual males. Psychiatry Investig. 2009, 6, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Costumero, V.; Barrós-Loscertales, A.; Bustamante, J.C.; Ventura-Campos, N.; Fuentes, P.; Rosell-Negre, P.; Ávila, C. Reward sensitivity is associated with brain activity during erotic stimulus processing. PLoS ONE 2013, 8, e66940. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; Wieser, K.; Methfessel, I.; Fromberger, P.; Dechent, P.; Müller, J.L. Sex attracts—Neural correlates of sexual preference under cognitive demand. Brain Imaging Behav. 2018, 12, 109–126. [Google Scholar] [CrossRef]
- Seo, Y.; Jeong, B.; Kim, J.W.; Choi, J. The relationship between age and brain response to visual erotic stimuli in healthy heterosexual males. Int. J. Impot. Res. 2010, 22, 234–239. [Google Scholar] [CrossRef][Green Version]
- Sundaram, T.; Jeong, G.W.; Kim, T.H.; Kim, G.W.; Baek, H.S.; Kang, H.K. Time-course analysis of the neuroanatomical correlates of sexual arousal evoked by erotic video stimuli in healthy males. Korean J. Radiol. 2010, 11, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.; Snagowski, J.; Laier, C.; Maderwald, S. Ventral striatum activity when watching preferred pornographic pictures is correlated with symptoms of Internet pornography addiction. Neuroimage 2016, 129, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.; Schiffer, B.; Zwarg, T.; Krüger, T.H.; Karama, S.; Schedlowski, M.; Forsting, M.; Gizewski, E.R. Brain response to visual sexual stimuli in heterosexual and homosexual males. Hum. Brain Mapp. 2008, 29, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Wang, Q.D.; Xu, Y.; Liao, Z.L.; Xu, L.J.; Liao, Z.L.; Xu, X.J.; Wei, E.Q.; Yan, L.Q.; Hu, J.B.; et al. Haemodynamic brain response to visual sexual stimuli is different between homosexual and heterosexual men. J. Int. Med. Res. 2011, 39, 199–211. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, S.; Xu, L.; Wang, Q.; Xu, X.; Wei, E.; Yan, L.; Hu, J.; Wei, N.; Zhou, W.; et al. Neural circuits of disgust induced by sexual stimuli in homosexual and heterosexual men: An fMRI study. Eur. J. Radiol. 2011, 80, 418–425. [Google Scholar] [CrossRef]
- Safron, A.; Sylva, D.; Klimaj, V.; Rosenthal, A.M.; Li, M.; Walter, M.; Bailey, J.M. Neural Correlates of Sexual Orientation in Heterosexual, Bisexual, and Homosexual Men. Sci. Rep. 2017, 7, 41314. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Wei, N.; Wang, Q.D.; Yan, L.Q.; Wei, E.Q.; Zhang, M.M.; Hu, J.B.; Huang, M.L.; Zhou, W.H.; Xu, Y. Patterns of brain activation during visually evoked sexual arousal differ between homosexual and heterosexual men. AJNR Am. J. Neuroradiol. 2008, 29, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Savic, I.; Lindström, P. PET and MRI show differences in cerebral asymmetry and functional connectivity between homo- and heterosexual subjects. Proc. Natl. Acad. Sci. USA 2008, 105, 9403–9408. [Google Scholar] [CrossRef] [PubMed]
- Gizewski, E.R.; Krause, E.; Schlamann, M.; Happich, F.; Ladd, M.E.; Forsting, M.; Senf, W. Specific cerebral activation due to visual erotic stimuli in male-to-female transsexuals compared with male and female controls: An fMRI study. J. Sex. Med. 2009, 6, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.W.; Kim, S.K.; Jeong, G.W. Neural activation-based sexual orientation and its correlation with free testosterone level in postoperative female-to-male transsexuals: Preliminary study with 3.0-T fMRI. Surg. Radiol. Anat. 2016, 38, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Karama, S.; Lecours, A.R.; Leroux, J.M.; Bourgouin, P.; Beaudoin, G.; Joubert, S.; Beauregard, M. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum. Brain Mapp. 2002, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sylva, D.; Safron, A.; Rosenthal, A.M.; Reber, P.J.; Parrish, T.B.; Bailey, J.M. Neural correlates of sexual arousal in heterosexual and homosexual women and men. Horm. Behav. 2013, 64, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Metzger, C.D.; Eckert, U.; Steiner, J.; Sartorius, A.; Buchmann, J.E.; Stadler, J.; Tempelmann, C.; Speck, O.; Bogerts, B.; Abler, B.; et al. High field FMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. Front. Neuroanat. 2010, 4, 138. [Google Scholar] [CrossRef] [PubMed]
- Arnow, B.A.; Millheiser, L.; Garrett, A.; Lake Polan, M.; Glover, G.H.; Hill, K.R.; Lightbody, A.; Watson, C.; Banner, L.; Smart, T.; et al. Women with hypoactive sexual desire disorder compared to normal females: A functional magnetic resonance imaging study. Neuroscience 2009, 158, 484–502. [Google Scholar] [CrossRef]
- Safron, A.; Barch, B.; Bailey, J.M.; Gitelman, D.R.; Parrish, T.B.; Reber, P.J. Neural correlates of sexual arousal in homosexual and heterosexual men. Behav. Neurosci. 2007, 121, 237–248. [Google Scholar] [CrossRef][Green Version]
- Brunetti, M.; Babiloni, C.; Ferretti, A.; Del Gratta, C.; Merla, A.; Olivetti Belardinelli, M.; Romani, G.L. Hypothalamus.; sexual arousal and psychosexual identity in human males: A functional magnetic resonance imaging study. Eur. J. Neurosci. 2008, 27, 2922–2927. [Google Scholar] [CrossRef]
- Ferretti, A.; Caulo, M.; Del Gratta, C.; Di Matteo, R.; Merla, A.; Montorsi, F.; Pizzella, V.; Pompa, P.; Rigatti, P.; Rossini, P.M.; et al. Dynamics of male sexual arousal: Distinct components of brain activation revealed by fMRI. Neuroimage 2005, 26, 1086–1096. [Google Scholar] [CrossRef]
- Sabatinelli, D.; Bradley, M.M.; Lang, P.J.; Costa, V.D.; Versace, F. Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. J. Neurophysiol. 2007, 98, 1374–1379. [Google Scholar] [CrossRef]
- Kim, G.W.; Jeong, G.W. Menopause-related brain activation patterns during visual sexual arousal in menopausal women: An fMRI pilot study using time-course analysis. Neuroscience 2017, 343, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.S.; Kim, G.W.; Sundaram, T.; Park, K.; Jeong, G.W. Brain Morphological Changes With Functional Deficit Associated With Sexual Arousal in Postmenopausal Women. Sex. Med. 2019, 7, 480–488. [Google Scholar] [CrossRef]
- Woodard, T.L.; Nowak, N.T.; Balon, R.; Tancer, M.; Diamond, M.P. Brain activation patterns in women with acquired hypoactive sexual desire disorder and women with normal sexual function: A cross-sectional pilot study. Fertil. Steril. 2013, 100, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.W.; Jeong, G.W. Neural mechanisms underlying sexual arousal in connection with sexual hormone levels: A comparative study of the postoperative male-to-female transsexuals and premenopausal and menopausal women. Neuroreport 2014, 25, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Kennis, M.; Dewitte, M.; T’Sjoen, G.; Stinkens, K.; Sack, A.T.; Duecker, F. The behavioral component of sexual inhibition and its relation with testosterone levels: An fMRI study in transgender and cisgender individuals. Psychoneuroendocrinology 2024, 163, 106963. [Google Scholar] [CrossRef]
- Poeppl, T.B.; Nitschke, J.; Dombert, B.; Santtila, P.; Greenlee, M.W.; Osterheider, M.; Mokros, A. Functional cortical and subcortical abnormalities in pedophilia: A combined study using a choice reaction time task and fMRI. J. Sex. Med. 2011, 8, 1660–1674. [Google Scholar] [CrossRef]
- Ristow, I.; Foell, J.; Kärgel, C.; Borchardt, V.; Li, S.; Denzel, D.; Witzel, J.; Drumkova, K.; Beier, K.; Kruger, T.H.C.; et al. Expectation of sexual images of adults and children elicits differential dorsal anterior cingulate cortex activation in pedophilic sexual offenders and healthy controls. Neuroimage Clin. 2019, 23, 101863. [Google Scholar] [CrossRef]
- Cera, N.; Di Pierro, E.D.; Ferretti, A.; Tartaro, A.; Romani, G.L.; Perrucci, M.G. Brain networks during free viewing of complex erotic movie: New insights on psychogenic erectile dysfunction. PLoS ONE 2014, 9, e105336. [Google Scholar] [CrossRef]
- Cera, N.; Di Pierro, E.D.; Sepede, G.; Gambi, F.; Perrucci, M.G.; Merla, A.; Tartaro, A.; Del Gratta, C.; Galatioto Paradiso, G.; Vicentini, C.; et al. The role of left superior parietal lobe in male sexual behavior: Dynamics of distinct components revealed by FMRI. J. Sex. Med. 2012, 9, 1602–1612. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, G.W.; Kim, S.K.; Jeong, G.W. Brain activation-based sexual orientation in female-to-male transsexuals. Int. J. Impot. Res. 2016, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Bühler, M.; Vollstädt-Klein, S.; Klemen, J.; Smolka, M.N. Does erotic stimulus presentation design affect brain activation patterns? Event-related vs. blocked fMRI designs. Behav. Brain Funct. 2008, 4, 30. [Google Scholar] [CrossRef]
- Gizewski, E.R.; Krause, E.; Karama, S.; Baars, A.; Senf, W.; Forsting, M. There are differences in cerebral activation between females in distinct menstrual phases during viewing of erotic stimuli: A fMRI study. Exp. Brain Res. 2006, 174, 101–108. [Google Scholar] [CrossRef]
- Karama, S.; Armony, J.; Beauregard, M. Film excerpts shown to specifically elicit various affects lead to overlapping activation foci in a large set of symmetrical brain regions in males. PLoS ONE 2011, 6, e22343. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, A.; Walter, M.; Schneider, F.; Rotte, M.; Matthiae, C.; Tempelmann, C.; Heinze, H.J.; Bogerts, B.; Northoff, G. Self-related processing in the sexual domain: A parametric event-related fMRI study reveals neural activity in ventral cortical midline structures. Soc. Neurosci. 2006, 1, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Abler, B.; Seeringer, A.; Hartmann, A.; Grön, G.; Metzger, C.; Walter, M.; Stingl, J. Neural correlates of antidepressant-related sexual dysfunction: A placebo-controlled fMRI study on healthy males under subchronic paroxetine and bupropion. Neuropsychopharmacology 2011, 36, 1837–1847. [Google Scholar] [CrossRef]
- Bosch, O.G.; Havranek, M.M.; Baumberger, A.; Preller, K.H.; von Rotz, R.; Herdener, M.; Kraehenmann, R.; Staempfli, P.; Scheidegger, M.; Klucken, T.; et al. Neural underpinnings of prosexual effects induced by gamma-hydroxybutyrate in healthy male humans. Eur. Neuropsychopharmacol. 2017, 27, 372–382. [Google Scholar] [CrossRef]
- Yang, J.C. Functional neuroanatomy in depressed patients with sexual dysfunction: Blood oxygenation level dependent functional MR imaging. Korean J. Radiol. 2004, 5, 87–95. [Google Scholar] [CrossRef]
- Habermeyer, B.; Esposito, F.; Händel, N.; Lemoine, P.; Klarhöfer, M.; Mager, R.; Dittmann, V.; Seifritz, E.; Graf, M. Immediate processing of erotic stimuli in paedophilia and controls: A case control study. BMC Psychiatry 2013, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, X.; Parkinson, C.; Cai, C.; Gao, S.; Hu, P. Brain activation evoked by erotic films varies with different menstrual phases: An fMRI study. Behav. Brain Res. 2010, 206, 279–285. [Google Scholar] [CrossRef]
- Bartels, A.; Zeki, S. The neural basis of romantic love. Neuroreport 2000, 11, 3829–3834. [Google Scholar] [CrossRef] [PubMed]
- Parada, M.; Gérard, M.; Larcher, K.; Dagher, A.; Binik, Y.M. Neural Representation of Subjective Sexual Arousal in Men and Women. J. Sex. Med. 2016, 13, 1508–1522. [Google Scholar] [CrossRef]
- Parada, M.; Gérard, M.; Larcher, K.; Dagher, A.; Binik, Y.M. How Hot Are They? Neural Correlates of Genital Arousal: An Infrared Thermographic and Functional Magnetic Resonance Imaging Study of Sexual Arousal in Men and Women. J. Sex. Med. 2018, 15, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.; Park, K.; Hwang, I.S.; Jung, S.I.; Kim, H.J.; Chung, T.W.; Jeong, G.W. Brain activation areas of sexual arousal with olfactory stimulation in men: A preliminary study using functional MRI. J. Sex. Med. 2008, 5, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Ertl, N.; Mills, E.G.; Wall, M.B.; Thurston, L.; Yang, L.; Suladze, S.; Hunjan, T.; Phylactou, M.; Patel, B.; Bassett, P.A.; et al. Women and men with distressing low sexual desire exhibit sexually dimorphic brain processing. Sci. Rep. 2024, 14, 11051. [Google Scholar] [CrossRef] [PubMed]
- Safron, A.; Sylva, D.; Klimaj, V.; Rosenthal, A.M.; Bailey, J.M. Neural Responses to Sexual Stimuli in Heterosexual and Homosexual Men and Women: Men’s Responses Are More Specific. Arch. Sex. Behav. 2020, 49, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, J.R.; Farrell, M.J.; Boessen, R.; Denton, D.A.; Gavrilescu, M.; Kortekaas, R.; Renken, R.J.; Hoogduin, J.M.; Egan, G.F. Dynamic subcortical blood flow during male sexual activity with ecological validity: A perfusion fMRI study. Neuroimage 2010, 50, 208–216. [Google Scholar] [CrossRef]
- Wise, N.J.; Frangos, E.; Komisaruk, B.R. Brain Activity Unique to Orgasm in Women: An fMRI Analysis. J. Sex. Med. 2017, 14, 1380–1391. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, E.; Cho, H.; Park, D.W.; Choi, J.; Jang, S.H. Brain activation in response to visual sexual stimuli in male patients with right middle cerebral artery infarction: The first case-control functional magnetic resonance imaging study. Medicine 2021, 100, e25823. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nieto, G.; Sack, A.T.; Dewitte, M.; Emmerling, F.; Schuhmann, T. The Modulatory Role of Cortisol in the Regulation of Sexual Behavior in Young Males. Front. Behav. Neurosci. 2020, 14, 552567. [Google Scholar] [CrossRef] [PubMed]
- Versace, F.; Engelmann, J.M.; Jackson, E.F.; Slapin, A.; Cortese, K.M.; Bevers, T.B.; Schover, L.R. Brain responses to erotic and other emotional stimuli in breast cancer survivors with and without distress about low sexual desire: A preliminary fMRI study. Brain Imaging Behav. 2013, 7, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Gillath, O.; Canterberry, M. Neural correlates of exposure to subliminal and supraliminal sexual cues. Soc. Cogn. Affect. Neurosci. 2012, 7, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.M.; Heo, S.H.; Yoon, W.; Baek, B.H.; Shin, S.S.; Kim, S.K.; Lee, Y.Y. Altered Sexual Response-Related Functional Connectivity and Morphometric Changes Influenced by Sex Hormones across Menopausal Status. J. Clin. Med. 2024, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Flannigan, R.; Heier, L.; Voss, H.; Chazen, J.L.; Paduch, D.A. Functional Magnetic Resonance Imaging Detects Between-Group Differences in Neural Activation Among Men with Delayed Orgasm Compared with Normal Controls: Preliminary Report. J. Sex. Med. 2019, 16, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.; Spiegelberg, J.; Jacob, G.; van Zutphen, L.; Zeeck, A.; Hartmann, A.; Tüscher, O.; Holovics, L.; van Elst, L.T.; Joos, A. Neural correlates of intimate picture stimuli in females. Psychiatry Res. Neuroimaging 2018, 273, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Abler, B.; Kumpfmüller, D.; Grön, G.; Walter, M.; Stingl, J.; Seeringer, A. Neural correlates of erotic stimulation under different levels of female sexual hormones. PLoS ONE 2013, 8, e54447. [Google Scholar] [CrossRef] [PubMed]
- Montorsi, F.; Perani, D.; Anchisi, D.; Salonia, A.; Scifo, P.; Rigiroli, P.; Zanoni, M.; Heaton, J.P.; Rigatti, P.; Fazio, F. Apomorphine-induced brain modulation during sexual stimulation: A new look at central phenomena related to erectile dysfunction. Int. J. Impot. Res. 2003, 15, 203–209. [Google Scholar] [CrossRef]
- Comninos, A.N.; Wall, M.B.; Demetriou, L.; Shah, A.J.; Clarke, S.A.; Narayanaswamy, S.; Nesbitt, A.; Izzi-Engbeaya, C.; Prague, J.K.; Abbara, A.; et al. Kisspeptin modulates sexual and emotional brain processing in humans. J. Clin. Investig. 2017, 127, 709–719. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Eickhoff, S.B.; Laird, A.R.; Fox, M.; Wiener, M.; Fox, P. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum. Brain Mapp. 2012, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Bzdok, D.; Laird, A.R.; Kurth, F.; Fox, P.T. Activation likelihood estimation meta-analysis revisited. Neuroimage 2012, 59, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.L.; Cykowski, M.D.; McKay, D.R.; Kochunov, P.V.; Fox, P.T.; Rogers, W.; Toga, A.W.; Zilles, K.; Amunts, K.; Mazziotta, J. Anatomical global spatial normalization. Neuroinformatics 2010, 8, 171–182. [Google Scholar] [CrossRef]
- Brandman, T.; Malach, R.; Simony, E. The surprising role of the default mode network in naturalistic perception. Commun. Biol. 2021, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, A.; Marlatte, H. Neurobiology of Schemas and Schema-Mediated Memory. Trends Cogn. Sci. 2017, 21, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.L.; Cyranowski, J.M.; Espindle, D. Men’s sexual self-schema. J. Pers. Soc. Psychol. 1999, 76, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Reissing, E.D.; Laliberté, G.M.; Davis, H.J. Young women’s sexual adjustment: The role of sexual self-schema, sexual self-efficacy, sexual aversion and body attitudes. Can. J. Hum. Sex. 2005, 14, 77. [Google Scholar]
- Wiederman, M.W.; Hurst, S.R. Physical attractiveness, body image, and women’s sexual self-schema. Psychol. Women Q. 1997, 21, 567–580. [Google Scholar] [CrossRef]
- Baldassano, C.; Hasson, U.; Norman, K.A. Representation of Real-World Event Schemas during Narrative Perception. J. Neurosci. 2018, 38, 9689–9699. [Google Scholar] [CrossRef]
- Chen, J.; Leong, Y.C.; Honey, C.J.; Yong, C.H.; Norman, K.A.; Hasson, U. Shared memories reveal shared structure in neural activity across individuals. Nat. Neurosci. 2017, 20, 115–125. [Google Scholar] [CrossRef]
- Honey, C.J.; Thompson, C.R.; Lerner, Y.; Hasson, U. Not lost in translation: Neural responses shared across languages. J. Neurosci. 2012, 32, 15277–15283. [Google Scholar] [CrossRef] [PubMed]
- Dohmatob, E.; Dumas, G.; Bzdok, D. Dark control: The default mode network as a reinforcement learning agent. Hum. Brain Mapp. 2020, 41, 3318–3341. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Honey, C.J.; Simony, E.; Arcaro, M.J.; Norman, K.A.; Hasson, U. Accessing Real-Life Episodic Information from Minutes versus Hours Earlier Modulates Hippocampal and High-Order Cortical Dynamics. Cereb. Cortex. 2016, 26, 3428–3441. [Google Scholar] [CrossRef] [PubMed]
- van ‘t Hof, S.R.; Cera, N. Specific factors and methodological decisions influencing brain responses to sexual stimuli in women. Neurosci. Biobehav. Rev. 2021, 131, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Koukounas, E.; Over, R. Male sexual arousal elicited by film and fantasy matched in content. Aust. J. Psychol. 1997, 49, 1–5. [Google Scholar] [CrossRef]
- Harter, C.J.L.; Kavanagh, G.S.; Smith, J.T. The role of kisspeptin neurons in reproduction and metabolism. J. Endocrinol. 2018, 238, R173–R183. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.G.; Ertl, N.; Wall, M.B.; Thurston, L.; Yang, L.; Suladze, S.; Hunjan, T.; Phylactou, M.; Patel, B.; Muzi, B.; et al. Effects of Kisspeptin on Sexual Brain Processing and Penile Tumescence in Men With Hypoactive Sexual Desire Disorder: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2254313. [Google Scholar] [CrossRef] [PubMed]
- Thurston, L.; Hunjan, T.; Ertl, N.; Wall, M.B.; Mills, E.G.; Suladze, S.; Patel, B.; Alexander, E.C.; Muzi, B.; Bassett, P.A.; et al. Effects of Kisspeptin Administration in Women With Hypoactive Sexual Desire Disorder: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2236131. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.I.; Chamberlain, L.; Elshourbagy, N.A.; Michalovich, D.; Moore, D.J.; Calamari, A.; Szekeres, P.G.; Sarau, H.M.; Chambers, J.K.; Murdock, P.; et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J. Biol. Chem. 2001, 276, 28969–28975. [Google Scholar] [CrossRef]
- Duggan, S.J.; McCreary, D.R. Body image, eating disorders, and the drive for muscularity in gay and heterosexual men: The influence of media images. J. Homosex. 2004, 47, 45–58. [Google Scholar] [CrossRef]
- Lau, J.T.; Kim, J.H.; Tsui, H.Y. Prevalence and factors of sexual problems in Chinese males and females having sex with the same-sex partner in Hong Kong: A population-based study. Int. J. Impot. Res. 2006, 18, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.T.; Kim, J.H.; Tsui, H.Y. Prevalence and sociocultural predictors of sexual dysfunction among Chinese men who have sex with men in Hong Kong. J. Sex. Med. 2008, 5, 2766–2779. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, M.M.; Nobre, P. Cognitive schemas activated in sexual context: A comparative study with homosexual and heterosexual men and women, with and without sexual problems. Cogn. Ther. Res. 2015, 39, 390–402. [Google Scholar] [CrossRef]
- Di Plinio, S.; Ebisch, S.J.H. Brain network profiling defines functionally specialized cortical networks. Hum. Brain Mapp. 2018, 39, 4689–4706. [Google Scholar] [CrossRef] [PubMed]
- Nummenmaa, L.; Lahnakoski, J.M.; Glerean, E. Sharing the social world via intersubject neural synchronisation. Curr. Opin. Psychol. 2018, 4, 7–14. [Google Scholar] [CrossRef]
- Chai, W.; Zhang, P.; Zhang, X.; Wu, J.; Chen, C.; Li, F.; Xie, X.; Shi, G.; Liang, J.; Zhu, C.; et al. Feasibility study of functional near-infrared spectroscopy in the ventral visual pathway for real-life applications. Neurophotonics 2024, 11, 015002. [Google Scholar] [CrossRef]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Design | |
| Experimental studies | Other designs Systematic reviews/meta-analyses |
| Population | |
| Men Women | Animals Children |
| Intervention | |
| fMRI Sexual stimulation VSS (videos, pictures) | EEG MEG fNIRS |
| Topic | |
| Sexual behavior Brain activity Default Mode Network Sexual dysfunctions Paraphilia Sexual offenders Sexual orientation Transsexualism | Other brain networks, different from DMN No sex-related studies |
| Cluster | BA | Hemisphere | x | y | z | ALE | p | Z |
|---|---|---|---|---|---|---|---|---|
| Anterior cingulate cortex | 24 | L | 0 | 34 | 14 | 0.04148 | 0.00000 | 9.389 |
| Middle temporal gyrus | 21 | R | 50 | −60 | 0 | 0.02803 | 0.00000 | 7.215 |
| Posterior cingulate cortex | 23 | L | −2 | −54 | 22 | 0.01799 | 0.00000 | 5.306 |
| Parahippocampal gyrus | -- | L | −30 | −8 | −22 | 0.01898 | 0.00000 | 5.510 |
| Parahippocampal gyrus | -- | L | −22 | −36 | −4 | 0.01617 | 0.00000 | 4.917 |
| Posterior cingulate cortex | 31 | R | 6 | −56 | 26 | 0.01382 | 0.00001 | 4.390 |
| Cluster | BA | Hemisphere | x | y | z | ALE |
|---|---|---|---|---|---|---|
| Videos vs. Pictures (FDR pN < 0.01) | ||||||
| Parahippocampal gyrus | 28 | R | 22 | −14 | −20 | 0.01400 |
| Parahippocampal gyrus | 34 | L | −17 | −2 | −22 | 0.01210 |
| Men vs. Women (p < 0.001). | ||||||
| Anterior cingulate cortex | 32 | L | −4 | 42 | 14 | 0.00202 |
| Medial frontal gyrus | 9 | L | −4 | 50 | 8 | 0.00192 |
| Parahippocampal gyrus | 28 | L | −18 | −4 | −20 | 0.00335 |
| Parahippocampal gyrus | 28 | R | 34 | −18 | −28 | 0.00190 |
| Precuneus | L | −2 | −60 | 50 | 0.00184 | |
| Heterosexuals vs. Homosexuals (p < 0.001). | ||||||
| Anterior cingulate cortex | 32 | L | −12 | 38 | 10 | 0.00230 |
| Anterior cingulate cortex | 32 | L | −12 | 34 | 12 | 0.00228 |
| Inferior parietal lobule | 40 | L | −32 | −52 | 52 | 0.00229 |
| Parahippocampal gyrus | 34 | L | −18 | 0 | −22 | 0.00325 |
| Anterior cingulate cortex | 32 | R | 4 | 42 | −2 | 0.00192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, J.; Comprido, C.; Moreira, V.; Maccarone, M.T.; Cogoni, C.; Faustino, R.; Pignatelli, D.; Cera, N. The Complex Role Played by the Default Mode Network during Sexual Stimulation: A Cluster-Based fMRI Meta-Analysis. Behav. Sci. 2024, 14, 570. https://doi.org/10.3390/bs14070570
Pinto J, Comprido C, Moreira V, Maccarone MT, Cogoni C, Faustino R, Pignatelli D, Cera N. The Complex Role Played by the Default Mode Network during Sexual Stimulation: A Cluster-Based fMRI Meta-Analysis. Behavioral Sciences. 2024; 14(7):570. https://doi.org/10.3390/bs14070570
Chicago/Turabian StylePinto, Joana, Camila Comprido, Vanessa Moreira, Marica Tina Maccarone, Carlotta Cogoni, Ricardo Faustino, Duarte Pignatelli, and Nicoletta Cera. 2024. "The Complex Role Played by the Default Mode Network during Sexual Stimulation: A Cluster-Based fMRI Meta-Analysis" Behavioral Sciences 14, no. 7: 570. https://doi.org/10.3390/bs14070570
APA StylePinto, J., Comprido, C., Moreira, V., Maccarone, M. T., Cogoni, C., Faustino, R., Pignatelli, D., & Cera, N. (2024). The Complex Role Played by the Default Mode Network during Sexual Stimulation: A Cluster-Based fMRI Meta-Analysis. Behavioral Sciences, 14(7), 570. https://doi.org/10.3390/bs14070570







