Interpreting fMRI Studies in Populations with Cerebrovascular Risk: The Use of a Subject-Specific Hemodynamic Response Function
Abstract
1. Introduction
1.1. Vascular Changes in Older Adults and Brain Function
1.2. Estimating Task-Evoked Brain Activity in fMRI
1.3. The Present Study
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.2.1. Overall Study
2.2.2. Health Questionnaire
2.2.3. Health Metrics
2.2.4. fMRI Checkerboard Task
2.2.5. fMRI Memory Task
2.3. Data Analyses
2.3.1. Vascular Risk Score Calculation
2.3.2. fMRI Acquisition and Preprocessing
2.3.3. Statistical Analyses
3. Results
3.1. Participant Demographics
3.2. Factor Analysis of Vascular Risk Factors
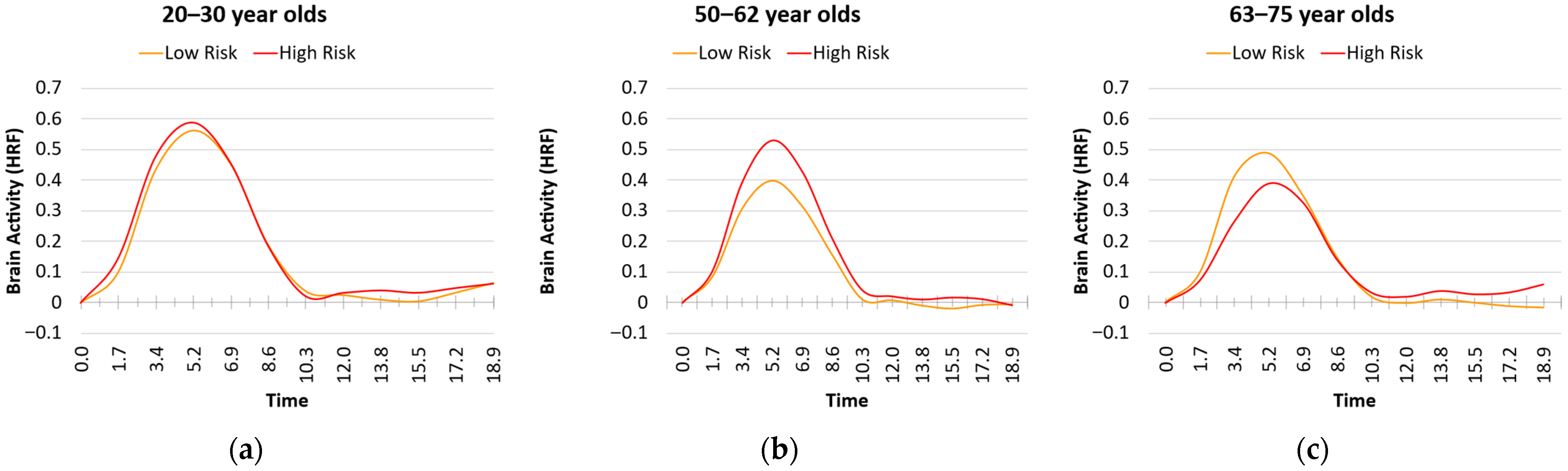
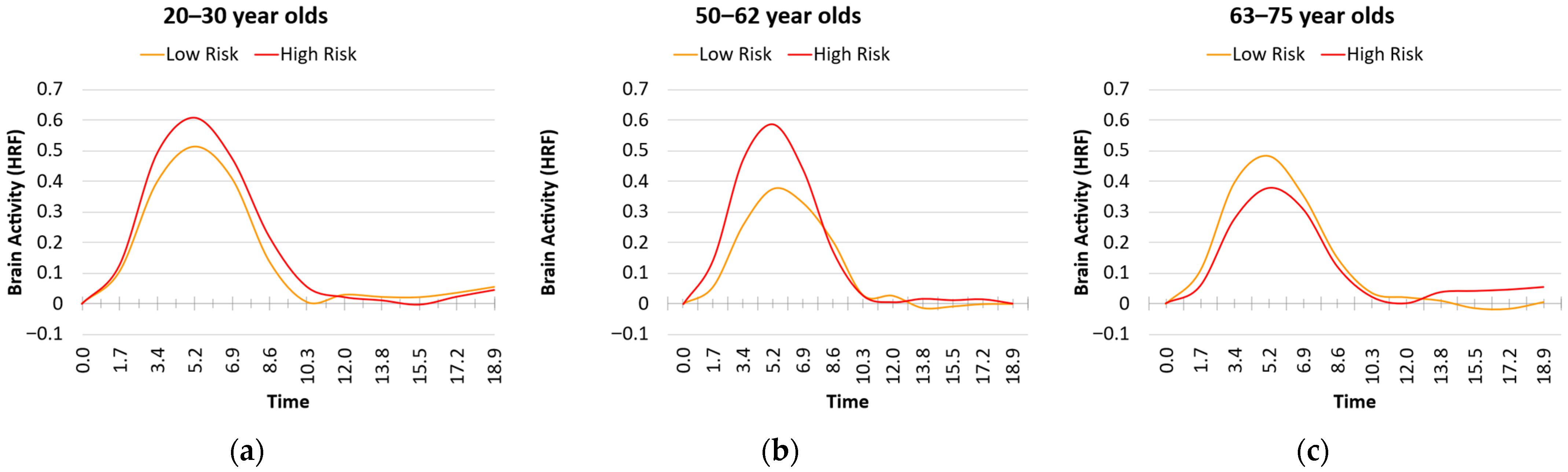
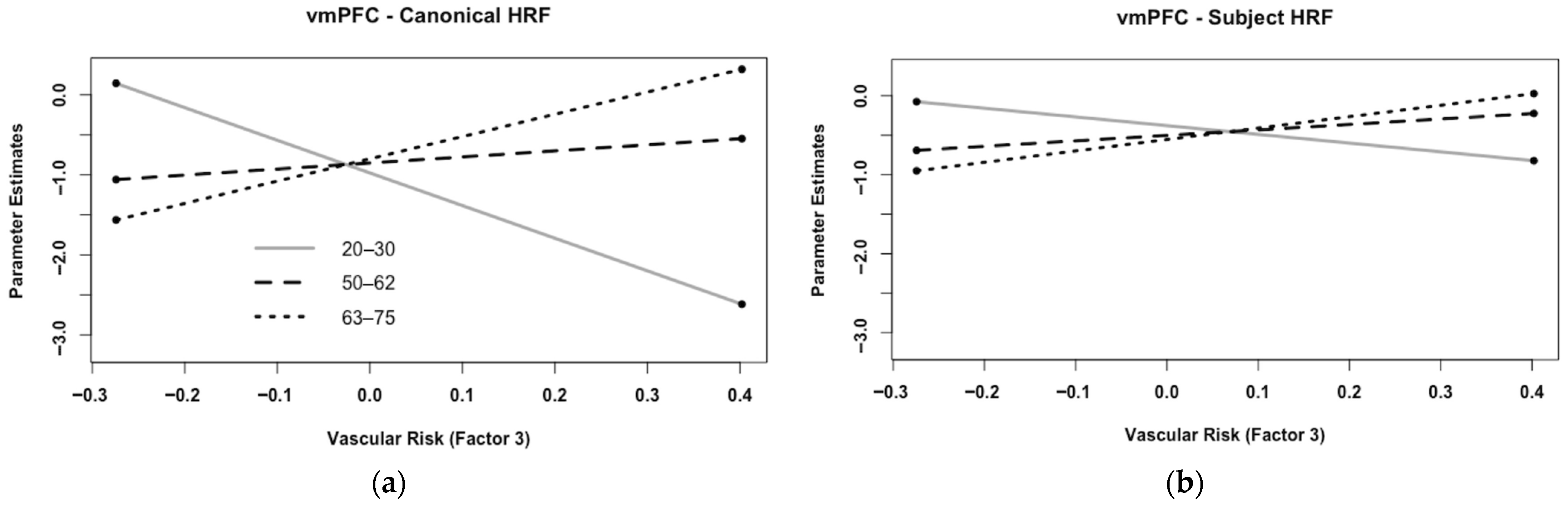
3.3. Age and Risk Effects on HRF Metrics
3.4. Age, Risk, and Analysis Type Effects on the Estimates of Brain Activity in the ROIs
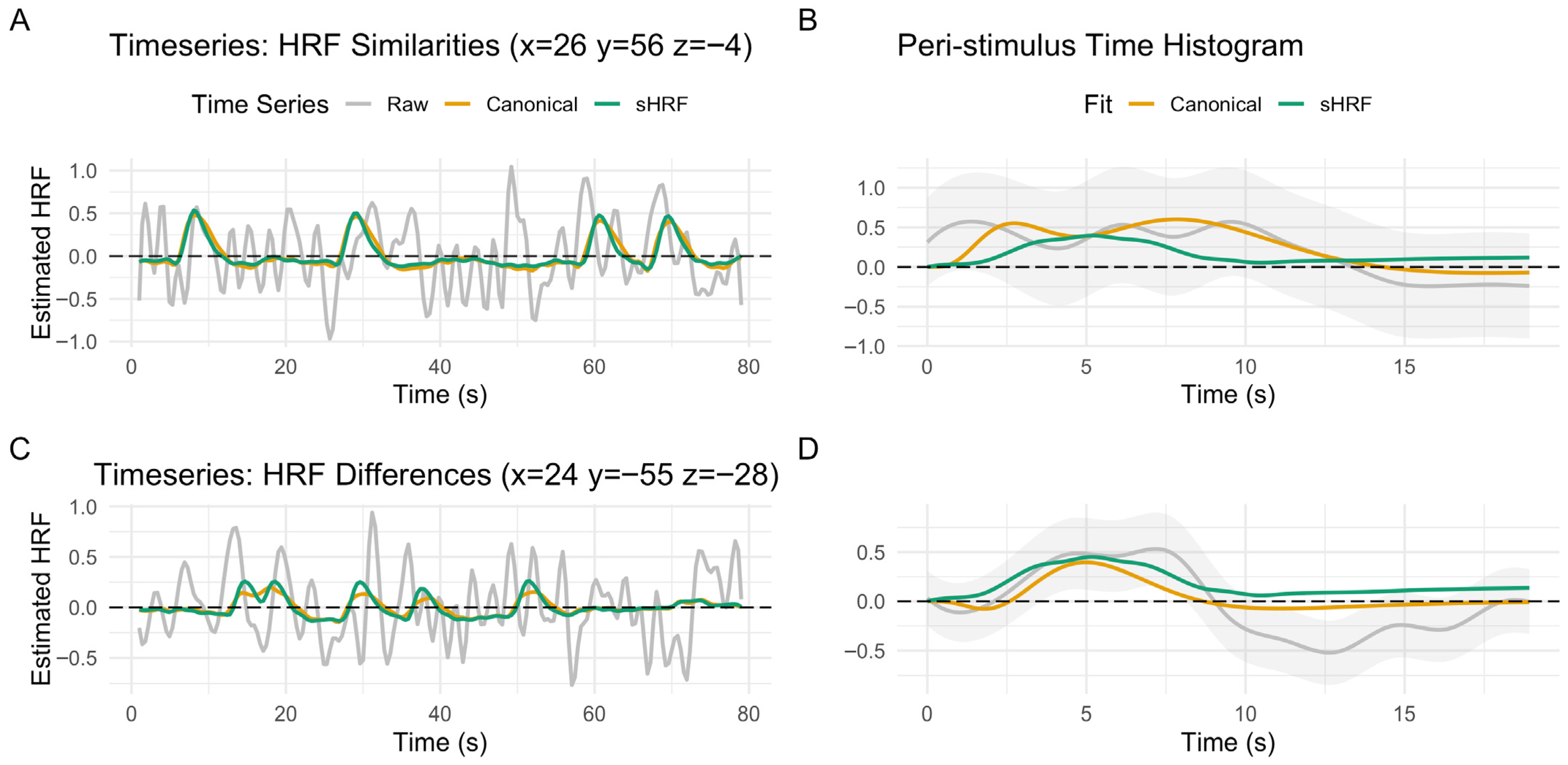
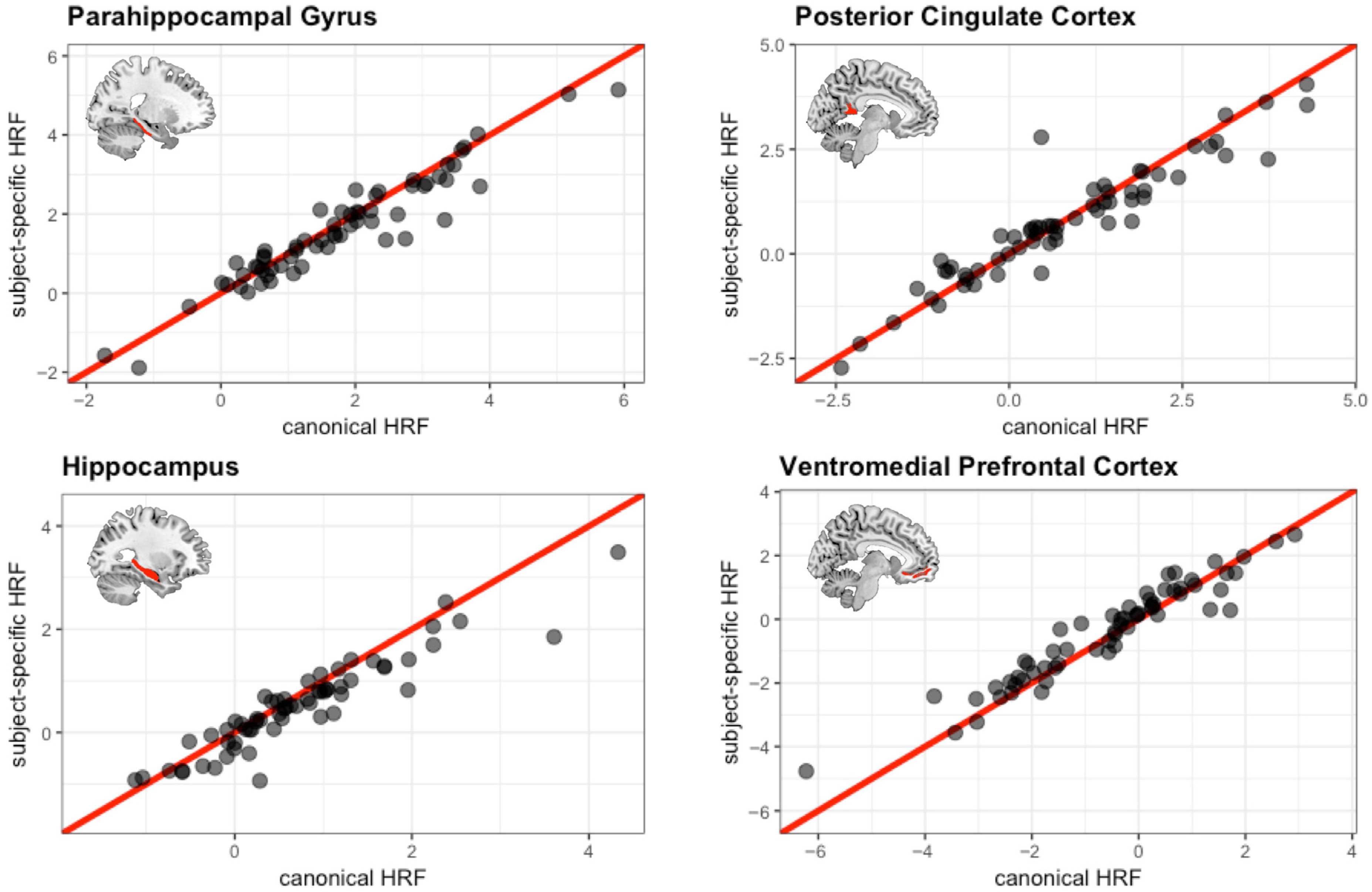
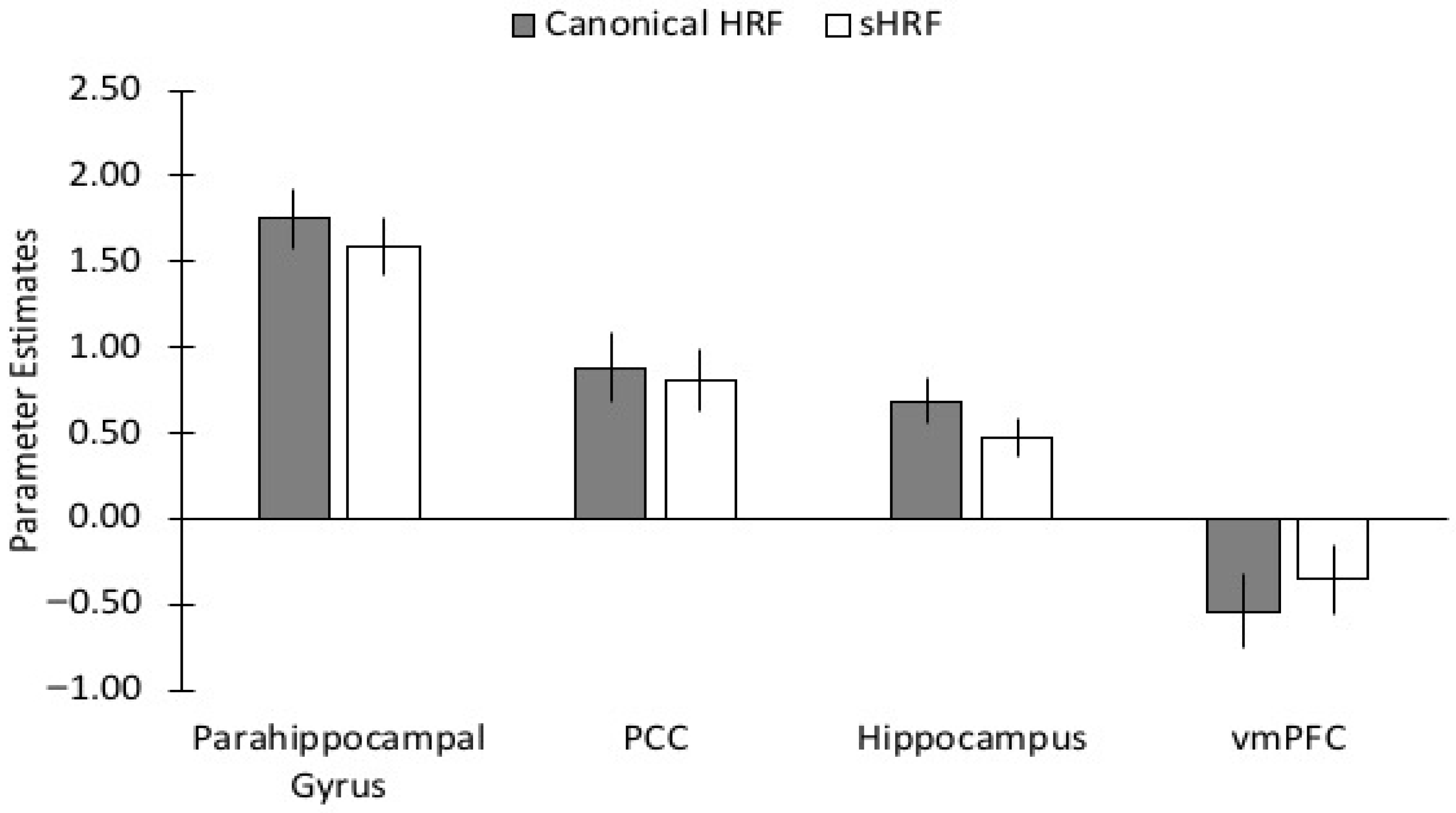
3.5. Comparison of Canonical HRF vs. Subject-Specific HRF During Encoding
3.6. Age and Risk Effects Using Canonical vs. Subject-Specific HRF: Whole Brain Analysis
3.7. Brain–Behavior Correlations
4. Discussion
4.1. Alternative Interpretations
4.2. Implications for Studies on Aging, Health, and Disease
4.3. Alternative Methods to Calibrate the BOLD Signal
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BOLD | blood oxygen level dependent |
| CMRO2 | cerebral metabolic rate of oxygen consumption |
| DMN | default mode network |
| fMRI | functional magnetic resonance imaging |
| FWHM | full-width half-max |
| GLM | general linear model |
| HRF | hemodynamic response function |
| MTL | medial temporal lobe |
| PCC | posterior cingulate cortex |
| ROI | region of interest |
| RSFA | Resting state fluctuation analyses |
| sHRF | subject-specific HRF |
| SLUMS | St. Louis University Mental Status |
| vmPFC | ventromedial prefrontal cortex |
References
- Aguirre, G. K., Zarahn, E., & D’esposito, M. (1998). The variability of human, BOLD hemodynamic responses. Neuroimage, 8, 360–369. [Google Scholar] [CrossRef]
- Anderson, K. M., Odell, P. M., Wilson, P. W., & Kannel, W. B. (1991). Cardiovascular disease risk profiles. American Heart Journal, 121, 293–298. [Google Scholar] [CrossRef]
- Bandettini, P. A., & Wong, E. C. (1997). A hypercapnia-based normalization method for improved spatial localization of human brain activation with fMRI. NMR in Biomedicine, 10, 197–203. [Google Scholar] [CrossRef]
- Bangen, K. J., Nation, D. A., Clark, L. R., Harmell, A. L., Wierenga, C. E., Dev, S. I., Delano-Wood, L., Zlatar, Z. Z., Salmon, D. P., Liu, T. T., & Bondi, M. W. (2014). Interactive effects of vascular risk burden and advanced age on cerebral blood flow. Frontiers in Aging Neuroscience, 6, 159. [Google Scholar] [CrossRef]
- Bangen, K. J., Restom, K., Liu, T. T., Jak, A. J., Wierenga, C. E., Salmon, D. P., & Bondi, M. W. (2009). Differential age effects on cerebral blood flow and BOLD response to encoding: Associations with cognition and stroke risk. Neurobiology of Aging, 30, 1276–1287. [Google Scholar] [CrossRef]
- Beason-Held, L. L., Thambisetty, M., Deib, G., Sojkova, J., Landman, B. A., Zonderman, A. B., Ferrucci, L., Kraut, M. A., & Resnick, S. M. (2012). Baseline cardiovascular risk predicts subsequent changes in resting brain function. Stroke, 43, 1542–1547. [Google Scholar] [CrossRef]
- Beauchet, O., Celle, S., Roche, F., Bartha, R., Montero-Odasso, M., Allali, G., & Annweiler, C. (2013). Blood pressure levels and brain volume reduction: A systematic review and meta-analysis. Journal of Hypertension, 31, 1502–1516. [Google Scholar] [CrossRef] [PubMed]
- Bonakdarpour, B., Parrish, T. B., & Thompson, C. K. (2007). Hemodynamic response function in patients with stroke-induced aphasia: Implications for fMRI data analysis. Neuroimage, 36, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Boraxbekk, C. J., Stomby, A., Ryberg, M., Lindahl, B., Larsson, C., Nyberg, L., & Olsson, T. (2015). Diet-induced weight loss alters functional brain responses during an episodic memory task. Obesity Facts, 8, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Braskie, M. N., Small, G. W., & Bookheimer, S. Y. (2010). Vascular health risks and fMRI activation during a memory task in older adults. Neurobiology of Aging, 31, 1532–1542. [Google Scholar] [CrossRef]
- Brown, W. R., & Thore, C. R. (2011). Cerebral microvascular pathology in ageing and neurodegeneration. Neuropathology & Applied Neurobiology, 37, 56–74. [Google Scholar]
- Buxton, R. B., Uludağ, K., Dubowitz, D. J., & Liu, T. T. (2004). Modeling the hemodynamic response to brain activation. Neuroimage, 23, S220–S233. [Google Scholar] [CrossRef]
- Celone, K. A., Calhoun, V. D., Dickerson, B. C., Atri, A., Chua, E. F., Miller, S. L., DePeau, K., Rentz, D. M., Selkoe, D. J., Blacker, D., & Albert, M. S. (2006). Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: An independent component analysis. Journal of Neuroscience, 26, 10222–10231. [Google Scholar] [CrossRef] [PubMed]
- Chambless, L. E., Heiss, G., Shahar, E., Earp, M. J., & Toole, J. (2004). Prediction of ischemic stroke risk in the atherosclerosis risk in communities study. American Journal of Epidemiology, 160, 259–269. [Google Scholar] [CrossRef]
- Chan, M. Y., Na, J., Agres, P. F., Savalia, N. K., Park, D. C., & Wig, G. S. (2018). Socioeconomic status moderates age-related differences in the brain’s functional network organization and anatomy across the adult lifespan. Proceedings of the National Academy of Sciences, 115, E5144–E5153. [Google Scholar] [CrossRef] [PubMed]
- Chen, J., Duan, X., Shu, H., Wang, Z., Long, Z., Liu, D., Liao, W., Shi, Y., Chen, H., & Zhang, Z. (2016). Differential contributions of subregions of medial temporal lobe to memory system in amnestic mild cognitive impairment: Insights from fMRI study. Scientific Reports, 6, 26148. [Google Scholar] [CrossRef]
- Claus, J. J., Breteler, M. M. B., Hasan, D., Krenning, E. P., Bots, M. L., Grobbee, D. E., Van Swieten, J. C., Van Harskamp, F., & Hofman, A. (1998). Regional cerebral blood flow and cerebrovascular risk factors in the elderly population. Neurobiology of Aging, 19, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S. J., Kramer, A. F., Erickson, K. I., Scalf, P., McAuley, E., Cohen, N. J., Webb, A., Jerome, G. J., Marquez, D. X., & Elavsky, S. (2004). Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences, 101, 3316–3321. [Google Scholar] [CrossRef]
- Daulatzai, M. A. (2012). Quintessential risk factors: Their role in promoting cognitive dysfunction and Alzheimer’s disease. Neurochemical Research, 37, 2627–2658. [Google Scholar] [CrossRef]
- Deckers, K., Van Boxtel, M. P., Schiepers, O. J., De Vugt, M., Munoz Sanchez, J. L., Anstey, K. J., Brayne, C., Dartigues, J. F., Engedal, K., Kivipelto, M., & Ritchie, K. (2015). Target risk factors for dementia prevention: A systematic review and Delphi consensus study on the evidence from observational studies. International Journal of Geriatric Psychiatry, 30, 234–246. [Google Scholar] [CrossRef]
- de La Torre, J. C. (2012). Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovascular Psychiatry and Neurology, 2012, 367516. [Google Scholar] [CrossRef]
- Del Zoppo, G. J. (2012). Aging and the neurovascular unit. Annals of the New York Academy of Sciences, 1268, 127–133. [Google Scholar] [CrossRef]
- D’Esposito, M., Deouell, L. Y., & Gazzaley, A. (2003). Alterations in the BOLD fMRI signal with ageing and disease: A challenge for neuroimaging. Nature Reviews Neuroscience, 4, 863–872. [Google Scholar] [CrossRef]
- Di Carlo, A., Baldereschi, M., Amaducci, L., Maggi, S., Grigoletto, F., Scarlato, G., & Inzitari, D. (2000). Cognitive impairment without dementia in older people: Prevalence, vascular risk factors, impact on disability. The Italian longitudinal study on aging. Journal of the American Geriatrics Society, 48, 775–782. [Google Scholar] [CrossRef]
- Fitzpatrick, A. L., Kuller, L. H., Lopez, O. L., Diehr, P., O’Meara, E. S., Longstreth, W. T., & Luchsinger, J. A. (2009). Midlife and late-life obesity and the risk of dementia: Cardiovascular health study. Archives of Neurology, 66, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Grady, C. L., Springer, M. V., Hongwanishkul, D., McIntosh, A. R., & Winocur, G. (2006). Age-related changes in brain activity across the adult lifespan. Journal of Cognitive Neuroscience, 18, 227–241. [Google Scholar] [CrossRef]
- Haight, T. J., Bryan, R. N., Erus, G., Davatzikos, C., Jacobs, D. R., D’esposito, M., Lewis, C. E., & Launer, L. J. (2015). Vascular risk factors, cerebrovascular reactivity, and the default-mode brain network. Neuroimage, 115, 7–16. [Google Scholar] [CrossRef]
- Han, S. D., Bangen, K. J., & Bondi, M. W. (2009). Functional magnetic resonance imaging of compensatory neural recruitment in aging and risk for Alzheimer’s disease: Review and recommendations. Dementia and Geriatric Cognitive Disorders, 27, 1–10. [Google Scholar] [CrossRef]
- Handwerker, D. A., Ollinger, J. M., & D’Esposito, M. (2004). Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage, 21, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Harb, R., Whiteus, C., Freitas, C., & Grutzendler, J. (2013). In vivo imaging of cerebral microvascular plasticity from birth to death. Journal of Cerebral Blood Flow & Metabolism, 33(1), 146–156. [Google Scholar]
- Huang, S., & Wikswo, J. (2006). Dimensions of systems biology. Reviews of Physiology, Biochemistry and Pharmacology, 157, 81–104. [Google Scholar]
- Huettel, S. A., Singerman, J. D., & McCarthy, G. (2001). The effects of aging upon the hemodynamic response measured by functional MRI. Neuroimage, 13, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, W., Pennartz, C. M., Beldzik, E., Domagalik, A., Vinck, M., Hofman, W. F., Cabeza, R., & Daselaar, S. M. (2014). Respiration phase-locks to fast stimulus presentations: Implications for the interpretation of posterior midline “deactivations”. Human Brain Mapping, 35, 4932–4943. [Google Scholar] [CrossRef] [PubMed]
- Irie, F., Fitzpatrick, A. L., Lopez, O. L., Kuller, L. H., Peila, R., Newman, A. B., & Launer, L. J. (2008). Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE ε4: The cardiovascular health study cognition study. Archives of Neurology, 65, 89–93. [Google Scholar] [CrossRef]
- Kalcher, K., Boubela, R. N., Huf, W., Biswal, B. B., Baldinger, P., Sailer, U., Filzmoser, P., Kasper, S., Lamm, C., Lanzenberger, R., & Moser, E. (2013). RESCALE: Voxel-specific task-fMRI scaling using resting state fluctuation amplitude. Neuroimage, 70, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Kannurpatti, S. S., Motes, M. A., Biswal, B. B., & Rypma, B. (2014). Assessment of unconstrained cerebrovascular reactivity marker for large age-range FMRI studies. PLoS ONE, 9(2), e88751. [Google Scholar] [CrossRef]
- Kim, H. (2011). Neural activity that predicts subsequent memory and forgetting: A meta-analysis of 74 fMRI studies. Neuroimage, 54, 2446–2461. [Google Scholar] [CrossRef]
- Kivipelto, M., Ngandu, T., Laatikainen, T., Winblad, B., Soininen, H., & Tuomilehto, J. (2006). Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. The Lancet Neurology, 5, 735–741. [Google Scholar] [CrossRef]
- Konkle, T., Brady, T. F., Alvarez, G. A., & Oliva, A. (2010a). Conceptual distinctiveness supports detailed visual long-term memory for real-world objects. Journal of Experimental Psychology: General, 139, 558–578. [Google Scholar] [CrossRef]
- Konkle, T., Brady, T. F., Alvarez, G. A., & Oliva, A. (2010b). Scene memory is more detailed than you think: The role of categories in visual long-term memory. Psychological Science, 21, 1551–1556. [Google Scholar] [CrossRef]
- Kovacic, J. C., Moreno, P., Nabel, E. G., Hachinski, V., & Fuster, V. (2011). Cellular senescence, vascular disease, and aging: Part 2 of a 2-part review: Clinical vascular disease in the elderly. Circulation, 123(17), 1900–1910. [Google Scholar] [CrossRef]
- Leenders, K. L., Perani, D., Lammertsma, A. A., Heather, J. D., Buckingham, P., Jones, T., Healy, M. J. R., Gibbs, J. M., Wise, R. J. S., Hatazawa, J., & Herold, S. (1990). Cerebral blood flow, blood volume and oxygen utilization: Normal values and effect of age. Brain, 113, 27–47. [Google Scholar] [CrossRef]
- Li, J., Wang, Y. J., Zhang, M., Xu, Z. Q., Gao, C. Y., Fang, C. Q., Yan, J. C., & Zhou, H. D. (2011). Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology, 76, 1485–1491. [Google Scholar] [CrossRef]
- Li, L., Men, W. W., Chang, Y. K., Fan, M. X., Ji, L., & Wei, G. X. (2014). Acute aerobic exercise increases cortical activity during working memory: A functional MRI study in female college students. PLoS ONE, 9(6), e99222. [Google Scholar] [CrossRef]
- Liu, P., Hebrank, A. C., Rodrigue, K. M., Kennedy, K. M., Park, D. C., & Lu, H. (2013a). A comparison of physiologic modulators of fMRI signals. Human Brain Mapping, 34, 2078–2088. [Google Scholar] [CrossRef]
- Liu, P., Hebrank, A. C., Rodrigue, K. M., Kennedy, K. M., Section, J., Park, D. C., & Lu, H. (2013b). Age-related differences in memory-encoding fMRI responses after accounting for decline in vascular reactivity. Neuroimage, 78, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, N. K. (2008). What we can do and what we cannot do with fMRI. Nature, 453, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Lustig, C., Snyder, A. Z., Bhakta, M., O’Brien, K. C., McAvoy, M., Raichle, M. E., Morris, J. C., & Buckner, R. L. (2003). Functional deactivations: Change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Sciences USA, 100, 14504–14509. [Google Scholar] [CrossRef]
- Ma, D. S., Correll, J., & Wittenbrink, B. (2015). The Chicago face database: A free stimulus set of faces and norming data. Behavior Research Methods, 4, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Marder, T. J., Flores, V. L., Bolo, N. R., Hoogenboom, W. S., Simonson, D. C., Jacobson, A. M., Foote, S. E., Shenton, M. E., Sperling, R. A., & Musen, G. (2014). Task-induced brain activity patterns in type 2 diabetes: A potential biomarker for cognitive decline. Diabetes, 63, 3112–3119. [Google Scholar] [CrossRef]
- Mazaika, P. K., Whitfield, S., & Cooper, J. C. (2005). Detection and repair of transient artifacts in fMRI data. Neuroimage, 26(Suppl. S1), S36. [Google Scholar]
- McDonough, I. M., & Allen, R. S. (2019). Biological markers of aging and mental health: A seed and soil model of neurocognitive disorders. Aging & Mental Health, 23(7), 793–799. [Google Scholar]
- McDonough, I. M., Festini, S. B., & Wood, M. M. (2020). Risk for Alzheimer’s disease: A review of long-term episodic memory encoding and retrieval fMRI studies. Ageing Research Reviews, 62, 101133. [Google Scholar] [CrossRef] [PubMed]
- McDonough, I. M., Letang, S. K., & Stinson, E. A. (2019). Dementia risk elevates brain activity during memory retrieval: A functional MRI analysis of middle aged and older adults. Journal of Alzheimer’s Disease, 70(4), 1005–1023. [Google Scholar] [CrossRef]
- Meusel, L. A. C., Kansal, N., Tchistiakova, E., Yuen, W., MacIntosh, B. J., Greenwood, C. E., & Anderson, N. D. (2014). A systematic review of type 2 diabetes mellitus and hypertension in imaging studies of cognitive aging: Time to establish new norms. Frontiers in Aging Neuroscience, 6, 148. [Google Scholar] [CrossRef]
- Miezin, F. M., Maccotta, L., Ollinger, J. M., Petersen, S. E., & Buckner, R. L. (2000). Characterizing the hemodynamic response: Effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage, 11, 735–759. [Google Scholar] [CrossRef]
- Miller, K. L., Alfaro-Almagro, F., Bangerter, N. K., Thomas, D. L., Yacoub, E., Xu, J., Bartsch, A. J., Jbabdi, S., Sotiropoulos, S. N., Andersson, J. L., & Griffanti, L. (2016). Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nature Neuroscience, 19, 1523–1536. [Google Scholar] [CrossRef]
- Miller, S. L., Celone, K., DePeau, K., Diamond, E., Dickerson, B. C., Rentz, D., Pihlajamäki, M., & Sperling, R. A. (2008). Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Sciences, 105, 2181–2186. [Google Scholar] [CrossRef]
- Mohtasib, R. S., Lumley, G., Goodwin, J. A., Emsley, H. C., Sluming, V., & Parkes, L. M. (2012). Calibrated fMRI during a cognitive Stroop task reveals reduced metabolic response with increasing age. Neuroimage, 59, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Obisesan, T. O., Obisesan, O. A., Martins, S., Alamgir, L., Bond, V., Maxwell, C., & Gillum, R. F. (2008). High blood pressure, hypertension, and high pulse pressure are associated with poorer cognitive function in persons aged 60 and older: The third national health and nutrition examination survey. Journal of the American Geriatrics Society, 56, 501–509. [Google Scholar] [CrossRef]
- Park, H., Kennedy, K. M., Rodrigue, K. M., Hebrank, A., & Park, D. C. (2013). An fMRI study of episodic encoding across the lifespan: Changes in subsequent memory effects are evident by middle-age. Neuropsychologia, 51, 448–456. [Google Scholar] [CrossRef]
- Persson, J., Lustig, C., Nelson, J. K., & Reuter-Lorenz, P. A. (2007). Age differences in deactivation: A link to cognitive control? Journal of Cognitive Neuroscience, 19, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamäki, M., DePeau, K. M., Blacker, D., & Sperling, R. A. (2008). Impaired medial temporal repetition suppression is related to failure of parietal deactivation in Alzheimer disease. The American Journal of Geriatric Psychiatry, 16, 283–292. [Google Scholar] [CrossRef]
- Prvulovic, D., Van de Ven, V., Sack, A. T., Maurer, K., & Linden, D. E. (2005). Functional activation imaging in aging and dementia. Psychiatry Research: Neuroimaging, 140, 97–113. [Google Scholar] [CrossRef]
- Riecker, A., Grodd, W., Klose, U., Schulz, J. B., Gröschel, K., Erb, M., Ackermann, H., & Kastrup, A. (2003). Relation between regional functional MRI activation and vascular reactivity to carbon dioxide during normal aging. Journal of Cerebral Blood Flow & Metabolism, 23, 565–573. [Google Scholar] [CrossRef]
- Rosendorff, C., Beeri, M. S., & Silverman, J. M. (2007). Cardiovascular risk factors for Alzheimer’s disease. The American Journal of Geriatric Cardiology, 16, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Sestieri, C., Tosoni, A., Mignogna, V., McAvoy, M. P., Shulman, G. L., Corbetta, M., & Romani, G. L. (2014). Memory accumulation mechanisms in human cortex are independent of motor intentions. Journal of Neuroscience, 34, 6993–7006. [Google Scholar] [CrossRef]
- Spaniol, J., Davidson, P. S., Kim, A. S., Han, H., Moscovitch, M., & Grady, C. L. (2009). Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia, 47, 1765–1779. [Google Scholar] [CrossRef]
- Sperling, R. A., LaViolette, P. S., O’Keefe, K., O’Brien, J., Rentz, D. M., Pihlajamaki, M., Marshall, G., Hyman, B. T., Selkoe, D. J., Hedden, T., & Buckner, R. L. (2009). Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron, 63, 178–188. [Google Scholar] [CrossRef]
- Spreng, R. N., Wojtowicz, M., & Grady, C. L. (2010). Reliable differences in brain activity between young and old adults: A quantitative meta-analysis across multiple cognitive domains. Neuroscience & Biobehavioral Reviews, 34, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S., Perera, S., Patel, K., Rosano, C., Faulkner, K., Inzitari, M., Brach, J., Chandler, J., Cawthon, P., Connor, E. B., & Nevitt, M. (2011). Gait speed and survival in older adults. JAMA, 305(1), 50–58. [Google Scholar] [CrossRef]
- Stuerenburg, H. J., Ganzer, S., Arlt, S., & Müller-Thomsen, T. (2005). The influence of smoking on plasma folate and lipoproteins in Alzheimer disease, mild cognitive impairment and depression. Neuro Endocrinology Letters, 26, 261–263. [Google Scholar]
- Sun, X., He, G., Qing, H., Zhou, W., Dobie, F., Cai, F., Staufenbiel, M., Huang, L. E., & Song, W. (2006). Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proceedings of the National Academy of Sciences, 103, 18727–18732. [Google Scholar] [CrossRef] [PubMed]
- Takada, H., Nagata, K., Hirata, Y., Satoh, Y., Watahiki, Y., Sugawara, J., Yokoyama, E., Kondoh, Y., Shishido, F., Inugami, A., & Fujita, H. (1992). Age-related decline of cerebral oxygen metabolism in normal population detected with positron emission tomography. Neurological Research, 14, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S. H., Tumosa, N., Chibnall, J. T., Perry, M. H., III, & Morley, J. E. (2006). Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—A pilot study. The American Journal of Geriatric Psychiatry, 14, 900–910. [Google Scholar] [CrossRef]
- Taylor, H. A. (2005). The Jackson heart study. Ethnicity & Disease, 15, 1–3. [Google Scholar]
- Thomason, M. E., Foland, L. C., & Glover, G. H. (2007). Calibration of BOLD fMRI using breath holding reduces group variance during a cognitive task. Human Brain Mapping, 28, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Tsvetanov, K. A., Henson, R. N., Tyler, L. K., Davis, S. W., Shafto, M. A., Taylor, J. R., Williams, N., & Rowe, J. B. (2015). The effect of ageing on f MRI: Correction for the confounding effects of vascular reactivity evaluated by joint f MRI and MEG in 335 adults. Human Brain Mapping, 36, 2248–2269. [Google Scholar] [CrossRef]
- Tyson, J. J., Chen, K., & Novak, B. (2001). Network dynamics and cell physiology. Nature Reviews Molecular Cell Biology, 2, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Valle, S., Li, W., & Qin, S. J. (1999). Selection of the number of principal components: The variance of the reconstruction error criterion with a comparison to other methods. Industrial & Engineering Chemistry Research, 38, 4389–4401. [Google Scholar] [CrossRef]
- Van Kan, G. A., Rolland, Y., Andrieu, S., Bauer, J., Beauchet, O., Bonnefoy, M., Cesari, M., Donini, L. M., Gillette-Guyonnet, S., Inzitari, M., & Nourhashemi, F. (2009). Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. The Journal of Nutrition, Health & Aging, 13(10), 881–889. [Google Scholar]
- Vannini, P., Hedden, T., Becker, J. A., Sullivan, C., Putcha, D., Rentz, D., Johnson, K. A., & Sperling, R. A. (2012). Age and amyloid-related alterations in default network habituation to stimulus repetition. Neurobiology of Aging, 33, 1237–1252. [Google Scholar] [CrossRef]
- Veldsman, M., Cumming, T., & Brodtmann, A. (2015). Beyond BOLD: Optimizing functional imaging in stroke populations. Human Brain Mapping, 36, 1620–1636. [Google Scholar] [CrossRef]
- Wood, A. G., Chen, J., Moran, C., Phan, T., Beare, R., Cooper, K., Litras, S., & Srikanth, V. (2016). Brain activation during memory encoding in type 2 diabetes mellitus: A discordant twin pair study. Journal of Diabetes Research, 3978428. [Google Scholar] [CrossRef] [PubMed]
- Xu, W., Qiu, C., Gatz, M., Pedersen, N. L., Johansson, B., & Fratiglioni, L. (2009). Mid-and late-life diabetes in relation to the risk of dementia: A population-based twin study. Diabetes, 58, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T., Kanno, I., Uemura, K., Shishido, F., Inugami, A., Ogawa, T., Murakami, M., & Suzuki, K. (1986). Reduction in regional cerebral metabolic rate of oxygen during human aging. Stroke, 17, 1220–1228. [Google Scholar] [CrossRef] [PubMed]



| Factor | Young (20–30) | Middle-Age (50–62) | Old (63–74) |
|---|---|---|---|
| N | 19 | 24 | 20 |
| Sex (F/M) | 10/9 | 17/7 | 13/7 |
| Race | |||
| Non-Hispanic White (N/%) | 12/63.16% | 16/66.67% | 17/85% |
| African American (N/%) | 1/5.26% | 6/25% | 3/15% |
| Other (N/%) | 6/31.58% | 2/8.33% | 0/0% |
| Education (M/SD) | 15.16/2.14 | 14.67/2.26 | 14.10/2.94 |
| SLUMS (M/SD) | NA | 27.17/2.96 | 26.53/2.29 |
| fMRI Memory Accuracy % (M/SD) | 56.79/16.96 | 38.96/14.03 | 30.90/6.86 |
| Arterial Stiffness (M/SD) | 37.05/12.27 | 46.86/10.11 | 53.50/12.83 |
| Body Mass Index (M/SD) | 25.47/5.61 | 29.98/7.79 | 27.76/4.84 |
| Visceral Fat (M/SD) | 6.16/4.43 | 9.92/4.61 | 11.32/5.63 |
| Abdominal Circumference in cm (M/SD) | 90.84/14.38 | 103.10/22.08 | 103.42/19.82 |
| Body Fat % (M/SD) | 30.19/9.84 | 39.57/11.44 | 35.19/10.00 |
| Presence of Diabetes (N/%) | 1/5.26% | 3/12.5% | 6/30% |
| Family History of Diabetes (N/%) * | 2/10.53% | 14/58.33% | 12/60% |
| High Cholesterol (N/%) * | 0/0% | 9/37.5% | 13/65% |
| History of Heart Attack (N/%) | 0/0% | 3/12.5% | 5/25% |
| Hypertension (N/%) * | 0/0% | 8/33.33% | 12/60% |
| Smoking Status (N/%) | |||
| Never Smoked | 15/78.95% | 14/58.33% | 12/60% |
| Quit | 2/10.52% | 8/33.33% | 6/30% |
| Current | 2/10.52% | 2/8.33% | 2/10% |
| Gait Speed in ms (M/SD) | 2580.95/347.53 | 2837.29/521.80 | 3030.26/776.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDonough, I.M.; Bender, A.R.; Patihis, L.; Stinson, E.A.; Letang, S.K.; Miller, W.S., Jr. Interpreting fMRI Studies in Populations with Cerebrovascular Risk: The Use of a Subject-Specific Hemodynamic Response Function. Behav. Sci. 2025, 15, 1457. https://doi.org/10.3390/bs15111457
McDonough IM, Bender AR, Patihis L, Stinson EA, Letang SK, Miller WS Jr. Interpreting fMRI Studies in Populations with Cerebrovascular Risk: The Use of a Subject-Specific Hemodynamic Response Function. Behavioral Sciences. 2025; 15(11):1457. https://doi.org/10.3390/bs15111457
Chicago/Turabian StyleMcDonough, Ian M., Andrew R. Bender, Lawrence Patihis, Elizabeth A. Stinson, Sarah K. Letang, and William S. Miller, Jr. 2025. "Interpreting fMRI Studies in Populations with Cerebrovascular Risk: The Use of a Subject-Specific Hemodynamic Response Function" Behavioral Sciences 15, no. 11: 1457. https://doi.org/10.3390/bs15111457
APA StyleMcDonough, I. M., Bender, A. R., Patihis, L., Stinson, E. A., Letang, S. K., & Miller, W. S., Jr. (2025). Interpreting fMRI Studies in Populations with Cerebrovascular Risk: The Use of a Subject-Specific Hemodynamic Response Function. Behavioral Sciences, 15(11), 1457. https://doi.org/10.3390/bs15111457






