Effect of Physical Exercise on Executive Functions Using the Emotional Stroop Task in Perimenopausal Women: A Pilot Study
Abstract
1. Introduction
1.1. Cognitive Function in Perimenopausal Women
1.2. Exercise and Cognition
1.3. Emotional Stroop Tasks
2. Materials and Methods
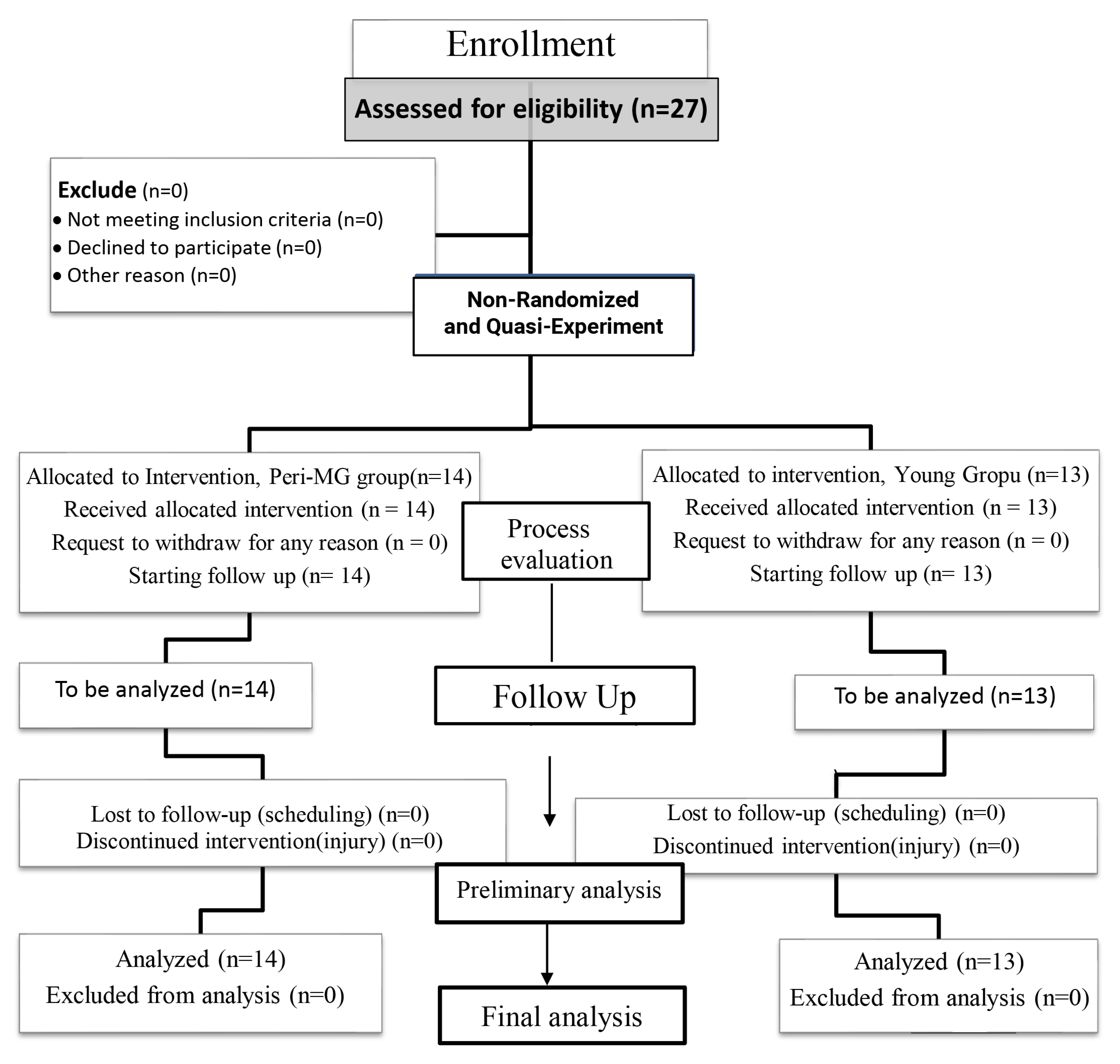
2.1. Study Design and Participants
2.2. Aerobic Exercise
2.3. Pre- and Post-Exercise ESTs
2.4. Sample Size and Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Neuropsychological Assessment
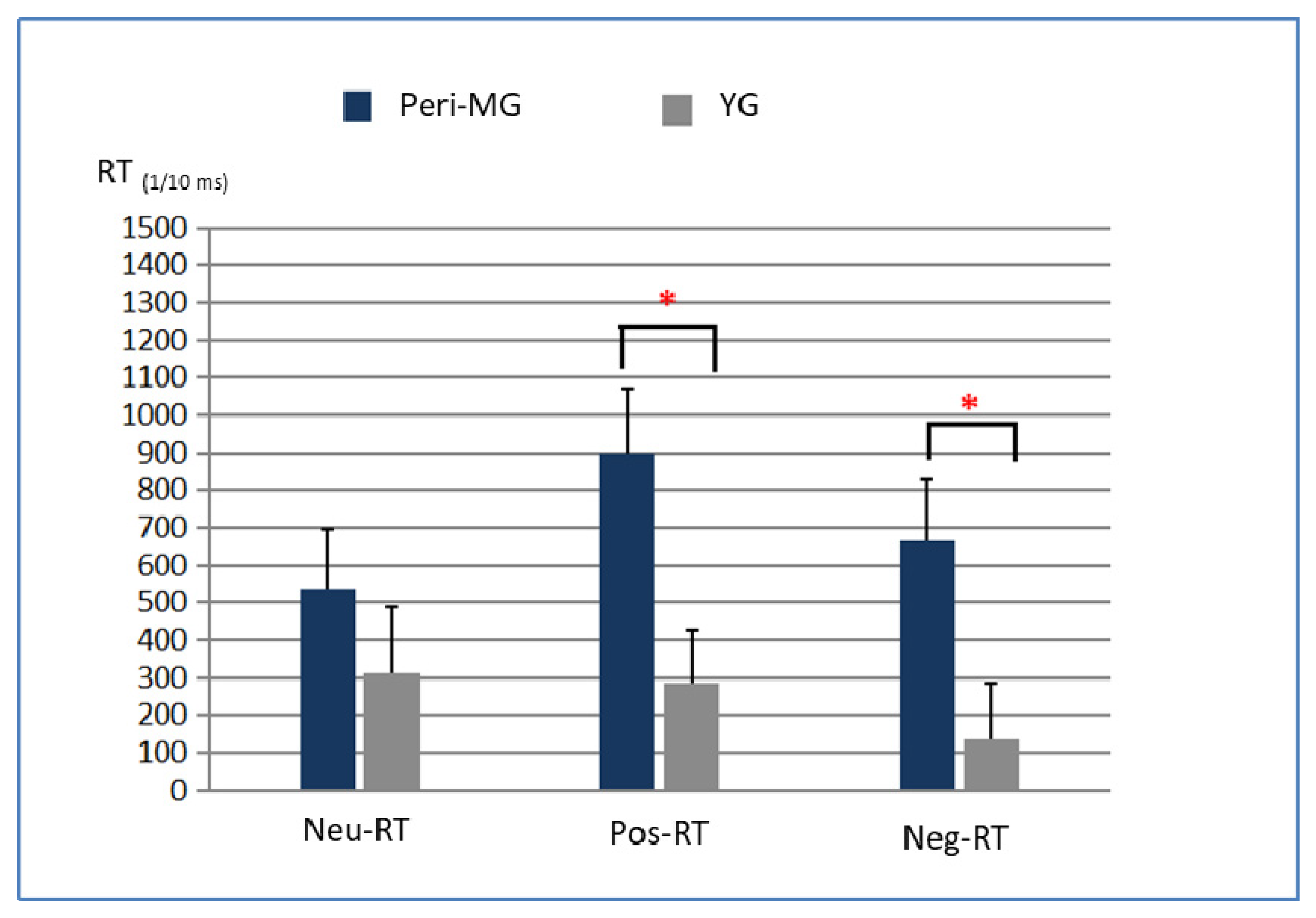
3.3. Differences in RTs and ERs
3.4. Different Effects of Exercise on EST Performance
4. Discussion
4.1. Exercise and Improved RTs during ESTs
4.2. RTs in the Peri-MG
4.3. Exercise and ER Alteration
4.4. Positive and Negative Word RT Improvement
4.5. Strengths
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, M.T.; Maki, P.M.; McDermott, M.P. Cognition and mood in perimenopause: A systematic review and meta-analysis. J. Steroid Biochem. Mol. Biol. 2014, 142, 90–98. [Google Scholar] [CrossRef]
- Zhao, D.; Lv, G.; Zhang, Y.; Xie, Z.; Wang, Q.; Zhou, M.; Li, P. Identifying the different subtypes in menopausal symptoms among middle-aged women in China: A latent class analysis. Menopause 2021, 28, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Suss, H.; Ehlert, U. Psychological resilience during the perimenopause. Maturitas 2020, 131, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Hunter, M.S.; Chen, R.; Crandall, C.J.; Gordon, J.L.; Mishra, G.D.; Rother, V.; Joffe, H.; Hickey, M. Promoting good mental health over the menopause transition. Lancet 2024, 403, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Brinton, R.D.; Yao, J.; Yin, F.; Mack, W.J.; Cadenas, E. Perimenopause as a neurological transition state. Nat. Rev. Endocrinol. 2015, 11, 393–405. [Google Scholar] [CrossRef]
- Burger, H.G.; Dudley, E.C.; Robertson, D.M.; Dennerstein, L. Hormonal changes in the menopause transition. Recent Prog. Horm. Res. 2002, 57, 257–275. [Google Scholar] [CrossRef]
- Freeman, E.W. Associations of depression with the transition to menopause. Menopause 2010, 17, 823–827. [Google Scholar] [CrossRef]
- Gunning-Dixon, F.M.; Brickman, A.M.; Cheng, J.C.; Alexopoulos, G.S. Aging of cerebral white matter: A review of MRI findings. Int. J. Geriatr. Psychiatry 2009, 24, 109–117. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Pitluk Barash, M.; Shuper Engelhard, E.; Elboim-Gabyzon, M. Feasibility and Effectiveness of a Novel Intervention Integrating Physical Therapy Exercise and Dance Movement Therapy on Fall Risk in Community-Dwelling Older Women: A Randomized Pilot Study. Healthcare 2023, 11, 1104. [Google Scholar] [CrossRef]
- Makizako, H.; Tsutsumimoto, K.; Doi, T.; Hotta, R.; Nakakubo, S.; Liu-Ambrose, T.; Shimada, H. Effects of exercise and horticultural intervention on the brain and mental health in older adults with depressive symptoms and memory problems: Study protocol for a randomized controlled trial [UMIN000018547]. Trials 2015, 16, 499. [Google Scholar] [CrossRef] [PubMed]
- Basso, J.C.; Suzuki, W.A. The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review. Brain Plast 2017, 2, 127–152. [Google Scholar] [CrossRef] [PubMed]
- Perimenopausal risk factors and future health. Hum. Reprod. Update 2011, 17, 706–717. [CrossRef] [PubMed]
- Rothon, C.; Edwards, P.; Bhui, K.; Viner, R.M.; Taylor, S.; Stansfeld, S.A. Physical activity and depressive symptoms in adolescents: A prospective study. BMC Med. 2010, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Matsukawa, K.; Liang, N.; Nakatsuka, C.; Tsuchimochi, H.; Okamura, H.; Hamaoka, T. Dynamic exercise improves cognitive function in association with increased prefrontal oxygenation. J. Physiol. Sci. JPS 2013, 63, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Berchicci, M.; Lucci, G.; Di Russo, F. Benefits of physical exercise on the aging brain: The role of the prefrontal cortex. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Churchill, J.D.; Galvez, R.; Colcombe, S.; Swain, R.A.; Kramer, A.F.; Greenough, W.T. Exercise, experience and the aging brain. Neurobiol. Aging 2002, 23, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Ingold, M.; Tulliani, N.; Chan, C.C.H.; Liu, K.P.Y. Cognitive function of older adults engaging in physical activity. BMC Geriatr. 2020, 20, 229. [Google Scholar] [CrossRef]
- Guitar, N.A.; Connelly, D.M.; Nagamatsu, L.S.; Orange, J.B.; Muir-Hunter, S.W. The effects of physical exercise on executive function in community-dwelling older adults living with Alzheimer’s-type dementia: A systematic review. Ageing Res. Rev. 2018, 47, 159–167. [Google Scholar] [CrossRef]
- Berryman, N.; Bherer, L.; Nadeau, S.; Lauziere, S.; Lehr, L.; Bobeuf, F.; Kergoat, M.J.; Vu, T.T.; Bosquet, L. Executive functions, physical fitness and mobility in well-functioning older adults. Exp. Gerontol. 2013, 48, 1402–1409. [Google Scholar] [CrossRef]
- Jensen, A.R.; Rohwer, W.D., Jr. The Stroop color-word test: A review. Acta Psychol. 1966, 25, 36–93. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Mathews, A.; MacLeod, C. The emotional Stroop task and psychopathology. Psychol. Bull. 1996, 120, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Chajut, E.; Schupak, A.; Algom, D. Emotional dilution of the Stroop effect: A new tool for assessing attention under emotion. Emotion 2010, 10, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Xu, Q.; Wang, X.; Xu, F.; Yang, Y.; Lu, Z. The automatic activation of emotion words measured using the emotional face-word Stroop task in late Chinese-English bilinguals. Cogn. Emot. 2018, 32, 315–324. [Google Scholar] [CrossRef]
- Kappes, C.; Bermeitinger, C. The Emotional Stroop as an Emotion Regulation Task. Exp. Aging Res. 2016, 42, 161–194. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.T.; Rubin, L.H.; Maki, P.M. Cognition in perimenopause: The effect of transition stage. Menopause 2013, 20, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Tomporowski, P.D. Effects of acute bouts of exercise on cognition. Acta Psychol. 2003, 112, 297–324. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.D.; Muller, S.M.; Kim, C.H.; Ryan, E.J.; Gunstad, J.; Glickman, E.L. Mood and selective attention in the cold: The effect of interval versus continuous exercise. Eur. J. Appl. Physiol. 2011, 111, 1321–1328. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Mitsui, S.; Fukuyama, R.; Yamaya, N.; Fujita, T.; Shimoda, K.; Tozato, F. An acute bout of housework activities has beneficial effects on executive function. Neuropsychiatr. Dis. Treat. 2018, 14, 61–72. [Google Scholar] [CrossRef]
- Biondi, M.; Picardi, A. Psychological stress and neuroendocrine function in humans: The last two decades of research. Psychother. Psychosom. 1999, 68, 114–150. [Google Scholar] [CrossRef]
- Joyal, M.; Wensing, T.; Levasseur-Moreau, J.; Leblond, J.; Sack, A.T.; Fecteau, S. Characterizing emotional Stroop interference in posttraumatic stress disorder, major depression and anxiety disorders: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0214998. [Google Scholar] [CrossRef] [PubMed]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; de Villiers, T.J.; Group, S.C. Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. Menopause 2012, 19, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Weissman, M.M.; Sholomskas, D.; Pottenger, M.; Prusoff, B.A.; Locke, B.Z. Assessing depressive symptoms in five psychiatric populations: A validation study. Am. J. Epidemiol. 1977, 106, 203–214. [Google Scholar] [CrossRef]
- Chan, K.K.; Mak, W.W. Attentional Bias Associated with Habitual Self-Stigma in People with Mental Illness. PLoS ONE 2015, 10, e0125545. [Google Scholar] [CrossRef][Green Version]
- Sheu, C.F.; Lee, Y.S.; Shih, P.Y. Analyzing recognition performance with sparse data. Behav. Res. Methods 2008, 40, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, K.T. Attentional Processing of Unpleasant Stimuli in Alexithymia. Psychol. Rep. 2022. [Google Scholar] [CrossRef] [PubMed]
- Jhou, Y.-U.; Hung, D.-L. Using Emotional Stroop Tasks Examine the Emotion-Cognition Interactions across the Sex Offenders and the Normal Controls. Unpublished Master’s Thesis, National Yang-Ming University, Taipei, Taiwan, 2008. [Google Scholar]
- Ben-Haim, M.S.; Williams, P.; Howard, Z.; Mama, Y.; Eidels, A.; Algom, D. The Emotional Stroop Task: Assessing Cognitive Performance under Exposure to Emotional Content. J. Vis. Exp. JoVE 2016, 29, 53720. [Google Scholar] [CrossRef]
- Parris, B.A.; Hasshim, N.; Wadsley, M.; Augustinova, M.; Ferrand, L. The loci of Stroop effects: A critical review of methods and evidence for levels of processing contributing to color-word Stroop effects and the implications for the loci of attentional selection. Psychol. Res. 2022, 86, 1029–1053. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.J.; Ainslie, P.N.; Murrell, C.J.; Thomas, K.N.; Franz, E.A.; Cotter, J.D. Effect of age on exercise-induced alterations in cognitive executive function: Relationship to cerebral perfusion. Exp. Gerontol. 2012, 47, 541–551. [Google Scholar] [CrossRef]
- Abe, T.; Fujii, K.; Hyodo, K.; Kitano, N.; Okura, T. Effects of acute exercise in the sitting position on executive function evaluated by the Stroop task in healthy older adults. J. Phys. Ther. Sci. 2018, 30, 609–613. [Google Scholar] [CrossRef]
- Davidson, D.J.; Zacks, R.T.; Williams, C.C. Stroop interference, practice, and aging. Neuropsychol. Dev. Cogn. Sect. B Aging Neuropsychol. Cogn. 2003, 10, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, H.; Dan, I.; Tsuzuki, D.; Kato, M.; Okamoto, M.; Kyutoku, Y.; Soya, H. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. NeuroImage 2010, 50, 1702–1710. [Google Scholar] [CrossRef]
- McMorris, T.; Sproule, J.; Draper, S.; Child, R. Performance of a psychomotor skill following rest, exercise at the plasma epinephrine threshold and maximal intensity exercise. Percept. Mot. Ski. 2000, 91, 553–562. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T.; Sproule, J.; Turner, A.; Hale, B.J. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: A meta-analytical comparison of effects. Physiol. Behav. 2011, 102, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.; Shukla, D. A Single Bout of Aerobic Exercise Provides an Immediate “Boost” to Cognitive Flexibility. Front. Psychol. 2020, 11, 1106. [Google Scholar] [CrossRef] [PubMed]
- Alonso Debreczeni, F.; Bailey, P.E. A Systematic Review and Meta-Analysis of Subjective Age and the Association with Cognition, Subjective Wellbeing, and Depression. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2021, 76, 471–482. [Google Scholar] [CrossRef]
- Lyoo, Y.; Yoon, S. Brain Network Correlates of Emotional Aging. Sci. Rep. 2017, 7, 15576. [Google Scholar] [CrossRef] [PubMed]
- Drag, L.L.; Bieliauskas, L.A. Contemporary review 2009: Cognitive aging. J. Geriatr. Psychiatry Neurol. 2010, 23, 75–93. [Google Scholar] [CrossRef]
- Gilbert, J.R.; Moran, R.J. Inputs to prefrontal cortex support visual recognition in the aging brain. Sci. Rep. 2016, 6, 31943. [Google Scholar] [CrossRef]
- Pudas, S.; Persson, J.; Josefsson, M.; de Luna, X.; Nilsson, L.G.; Nyberg, L. Brain characteristics of individuals resisting age-related cognitive decline over two decades. J. Neurosci. 2013, 33, 8668–8677. [Google Scholar] [CrossRef]
- Ruffman, T.; Henry, J.D.; Livingstone, V.; Phillips, L.H. A meta-analytic review of emotion recognition and aging: Implications for neuropsychological models of aging. Neurosci. Biobehav. Rev. 2008, 32, 863–881. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.A.; Janowich, J.R.; Gazzaley, A. Differential Impact of Interference on Internally- and Externally-Directed Attention. Sci. Rep. 2018, 8, 2498. [Google Scholar] [CrossRef] [PubMed]
- Dresler, T.; Meriau, K.; Heekeren, H.R.; van der Meer, E. Emotional Stroop task: Effect of word arousal and subject anxiety on emotional interference. Psychol. Res. 2009, 73, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Kalanthroff, E.; Henik, A.; Derakshan, N.; Usher, M. Anxiety, emotional distraction, and attentional control in the Stroop task. Emotion 2016, 16, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Portin, R.; Polo-Kantola, P.; Polo, O.; Koskinen, T.; Revonsuo, A.; Irjala, K.; Erkkola, R. Serum estrogen level, attention, memory and other cognitive functions in middle-aged women. Climacteric 1999, 2, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Riemersma-van der Lek, R.F.; Patxot, M.; Li, P.; Shea, S.A.; Scheer, F.A.; Van Someren, E.J. Progression of Dementia Assessed by Temporal Correlations of Physical Activity: Results From a 3.5-Year, Longitudinal Randomized Controlled Trial. Sci. Rep. 2016, 6, 27742. [Google Scholar] [CrossRef] [PubMed]
- Gable, P.A.; Harmon-Jones, E. Reducing attentional capture of emotion by broadening attention: Increased global attention reduces early electrophysiological responses to negative stimuli. Biol. Psychol. 2012, 90, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Padmala, S.; Pessoa, L. Reward learning and negative emotion during rapid attentional competition. Front. Psychol. 2015, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Chu, C.H.; Wang, C.C.; Wang, Y.C.; Song, T.F.; Tsai, C.L.; Etnier, J.L. Dose-response relation between exercise duration and cognition. Med. Sci. Sports Exerc. 2015, 47, 159–165. [Google Scholar] [CrossRef]
- Garg, M.; Lata, H.; Walia, L.; Goyal, O. Effect of aerobic exercise on auditory and visual reaction times: A prospective study. Indian J. Physiol. Pharmacol. 2013, 57, 138–145. [Google Scholar]
- Stigger, F.; Marcolino, M.A.Z.; Plentz, R.D.M. Commentary: Exercise-dependent BDNF as a Modulatory Factor for the Executive Processing of Individuals in Course of Cognitive Decline. A Systematic Review. Front. Psychol. 2017, 8, 1858. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.J.; Chen, L.F.; Yeh, T.C.; Tu, P.C.; Tu, C.H.; Hsieh, J.C. The resting frontal alpha asymmetry across the menstrual cycle: A magnetoencephalographic study. Horm. Behav. 2008, 54, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Pfabigan, D.M.; Lamplmayr-Kragl, E.; Pintzinger, N.M.; Sailer, U.; Tran, U.S. Sex differences in event-related potentials and attentional biases to emotional facial stimuli. Front. Psychol. 2014, 5, 1477. [Google Scholar] [CrossRef] [PubMed][Green Version]
| (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Perimenopause Group (Peri-MG) Mean Differences (MDs) | Young Group (YG) Mean Differences (MDs) | |||||||
| Baseline Mean (SD) | Post-Exercise Mean (SD) | MDs | t (df = 13) | Baseline Mean (SD) | Post-Exercise Mean (SD) | MDs | t (df = 12) | |
| Neutral-RTs | 8337 (980) | 7799 (929) | 537 | 3.364 ** | 6604 (752) | 6292 (526) | 312 | 1.787 * |
| Positive-RTs | 8586 (1108) | 7687 (1000) | 899 | 5.229 *** | 6672 (760) | 6388 (613) | 284 | 1.976 * |
| Negative-RTs | 8377 (1119) | 7715 (1011) | 662 | 4.021 *** | 6580 (664) | 6444 (614) | 136 | 0.914 |
| Neutral-ERs | 0.143 (0.363) | 0.071 (0.267) | 0.071 | 0.563 | 0.077 (0.277) | 0.154 (0.376) | −0.077 | −0.562 |
| Positive-ERs | 0.357 (0.277) | 0.214 (0.426) | 0.143 | 0.693 | 0.231 (0.439) | 0.077 (0.277) | 0.154 | 1.000 |
| Negative-ERs | 0.571 (0.938) | 0.143 (0.363) | 0.429 | 1.578 | 0.077 (0.277) | 0.077 (0.277) | 0.000 | 0.000 |
| (b) | ||||||||
| Within-Group Mean Differences | Baseline Mean Differences | Between-Group Mean Differences | Second (Post-Exercise) Mean Differences | |||||
| MDs | F (df = 1.25) | MDs | t (df = 25) | MDs | F (df = 1.25) | MDs | t (df = 25) | |
| Neutral-RTs | 424.691 | 12.930 *** | 1733 | 5.126 *** | 1620.47 | 2440.099 *** | −1507.80 | −5.13 *** |
| Positive-RTs | 591.683 | 27.417 *** | 1914 | 5.193 *** | 1606.440 | 2008.113 *** | −1298.70 | −4.03 *** |
| Negative-RTs | 399.233 | 12.781 *** | 1797 | 5.022 *** | 1534.252 | 2029.724 *** | −1271.31 | −3.91 *** |
| Neutral-ERs | −0.003 | 0.001 | 0.066 | 0.527 | −0.008 | 7.155 * | 0.082 | 0.515 |
| Positive-ERs | 0.148 | 1.301 | 0.126 | 0.598 | 0.132 | 12.771 ** | −0.137 | 0.328 |
| Negative-ERs | 0.214 | 2.006 | 0.494 | 1.827 * | 0.280 | 8.729 ** | −0.066 | 0.603 |
| Catagory | Group | Mean (SD) | t (df = 25) | Sig. | Eta Squared |
|---|---|---|---|---|---|
| Neutral-RTs | Perimenopause | 537 (598) | 0.954 | 0.349 | 0.035 |
| Young | 312 (630) | ||||
| Postive-RTs | Perimenopause | 899 (644) | 2.723 | 0.012* | 0.229 |
| Young | 284 (518) | ||||
| Negative-RTs | Menopause | 662 (616) | 2.355 | 0.027* | 0.182 |
| Young | 136 (537) | ||||
| Neutral-ERs | Perimenopause | 0.071 (0.47) | 0.796 | 0.434 | 0.025 |
| Young | −0.076 (0.494) | ||||
| Postive-ERs | Perimenopause | 0.143 (0.770) | −0.042 | 0.967 | 0.000 |
| Young | 0.153 (0.555) | ||||
| Negative-ERs | Perimenopause | 0.429 (1.02) | 1.416 | 0.169 | 0.074 |
| Young | 0 (0.408) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.-Y.; Hsu, H.-C.; Ni, L.-F.; Yan, Y.-J.; Hwang, R.-J. Effect of Physical Exercise on Executive Functions Using the Emotional Stroop Task in Perimenopausal Women: A Pilot Study. Behav. Sci. 2024, 14, 338. https://doi.org/10.3390/bs14040338
Wu L-Y, Hsu H-C, Ni L-F, Yan Y-J, Hwang R-J. Effect of Physical Exercise on Executive Functions Using the Emotional Stroop Task in Perimenopausal Women: A Pilot Study. Behavioral Sciences. 2024; 14(4):338. https://doi.org/10.3390/bs14040338
Chicago/Turabian StyleWu, Li-Yu, Hsiu-Chin Hsu, Lee-Fen Ni, Yu-Jia Yan, and Ren-Jen Hwang. 2024. "Effect of Physical Exercise on Executive Functions Using the Emotional Stroop Task in Perimenopausal Women: A Pilot Study" Behavioral Sciences 14, no. 4: 338. https://doi.org/10.3390/bs14040338
APA StyleWu, L.-Y., Hsu, H.-C., Ni, L.-F., Yan, Y.-J., & Hwang, R.-J. (2024). Effect of Physical Exercise on Executive Functions Using the Emotional Stroop Task in Perimenopausal Women: A Pilot Study. Behavioral Sciences, 14(4), 338. https://doi.org/10.3390/bs14040338






