Evaluating the Influence of Musical and Monetary Rewards on Decision Making through Computational Modelling
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Stimuli
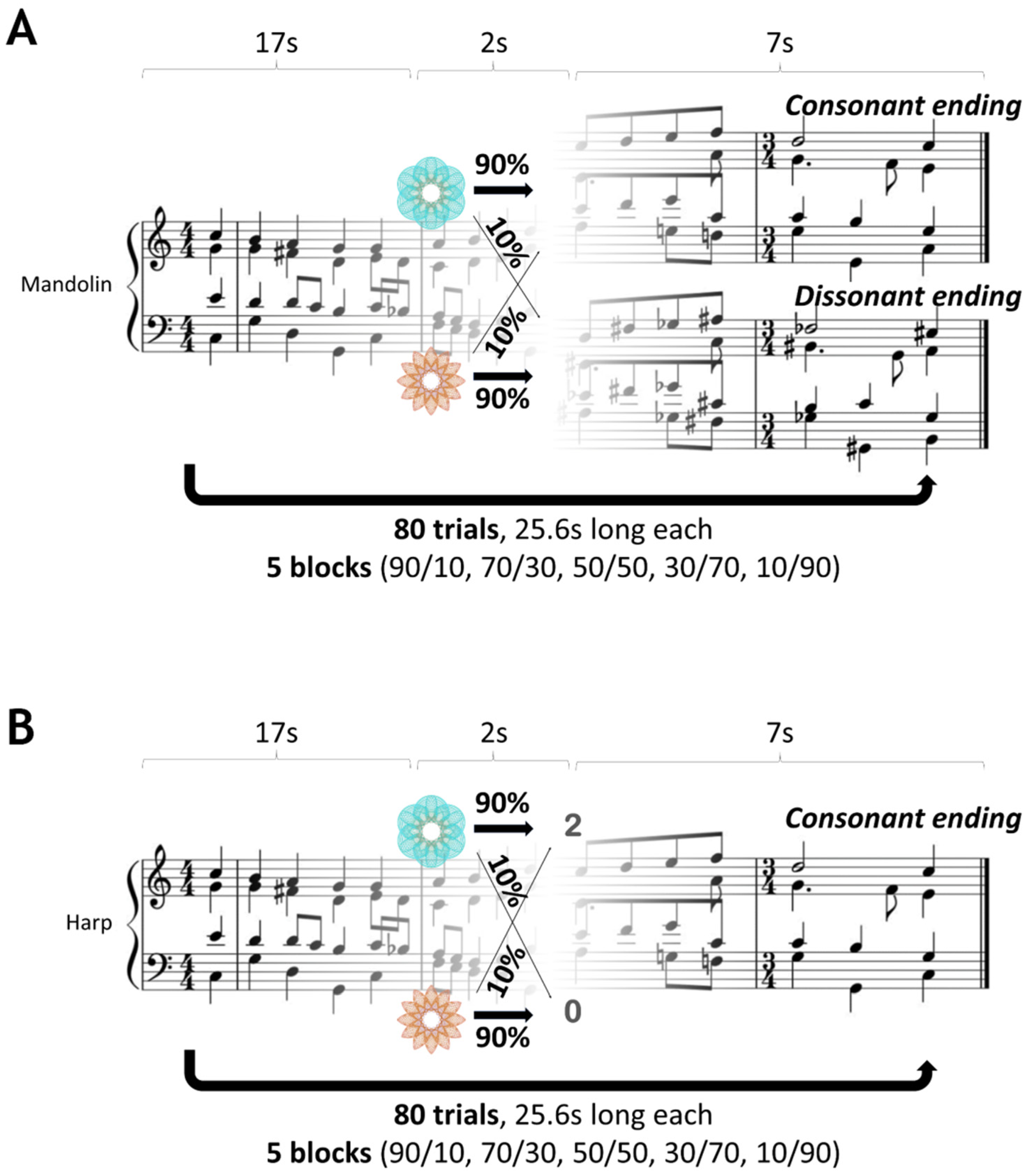
2.3. Experimental Design
2.4. General Decision-Making Performance
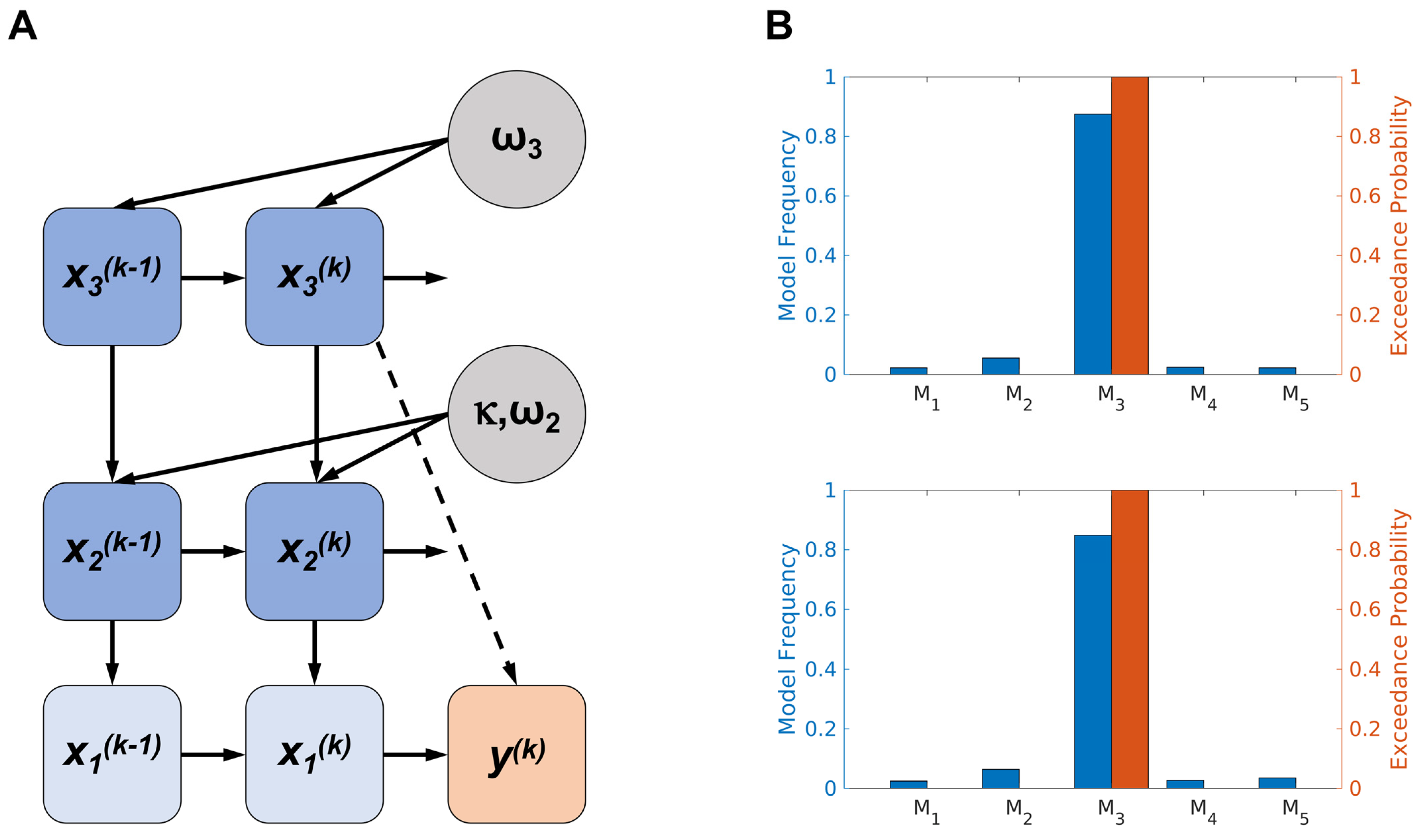
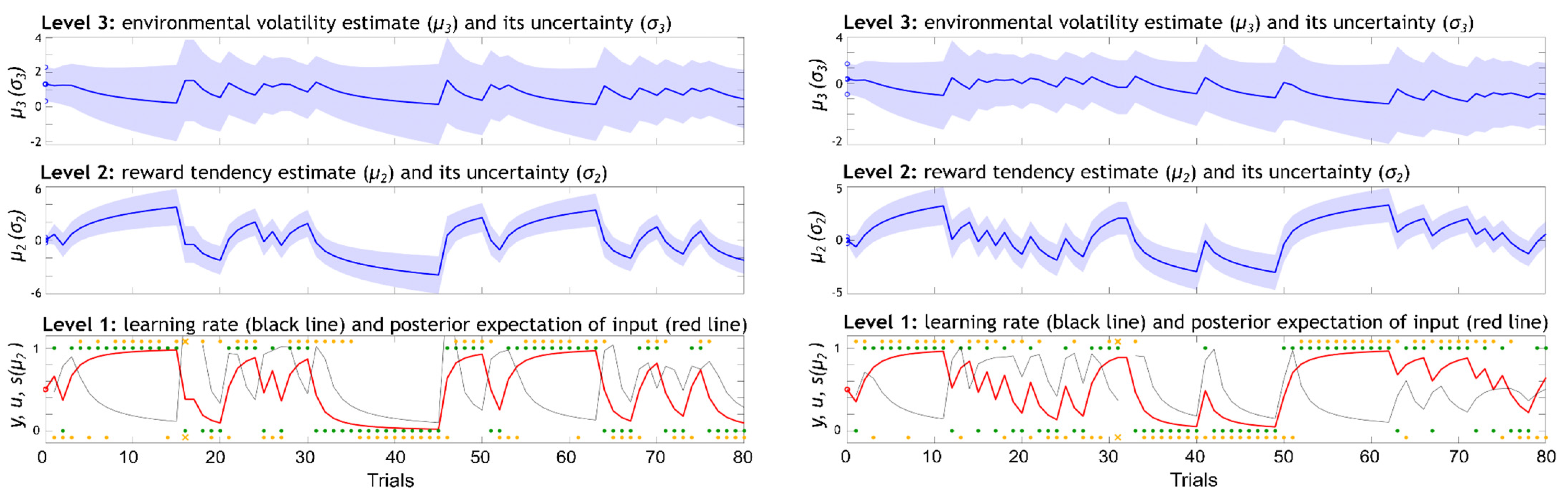
2.5. Modelling Decision-Making Behaviour with the Hierarchical Gaussian Filter (HGF)
2.6. Assessing Learning in the Musical Reward Task as a Function of Musical Preferences
3. Results
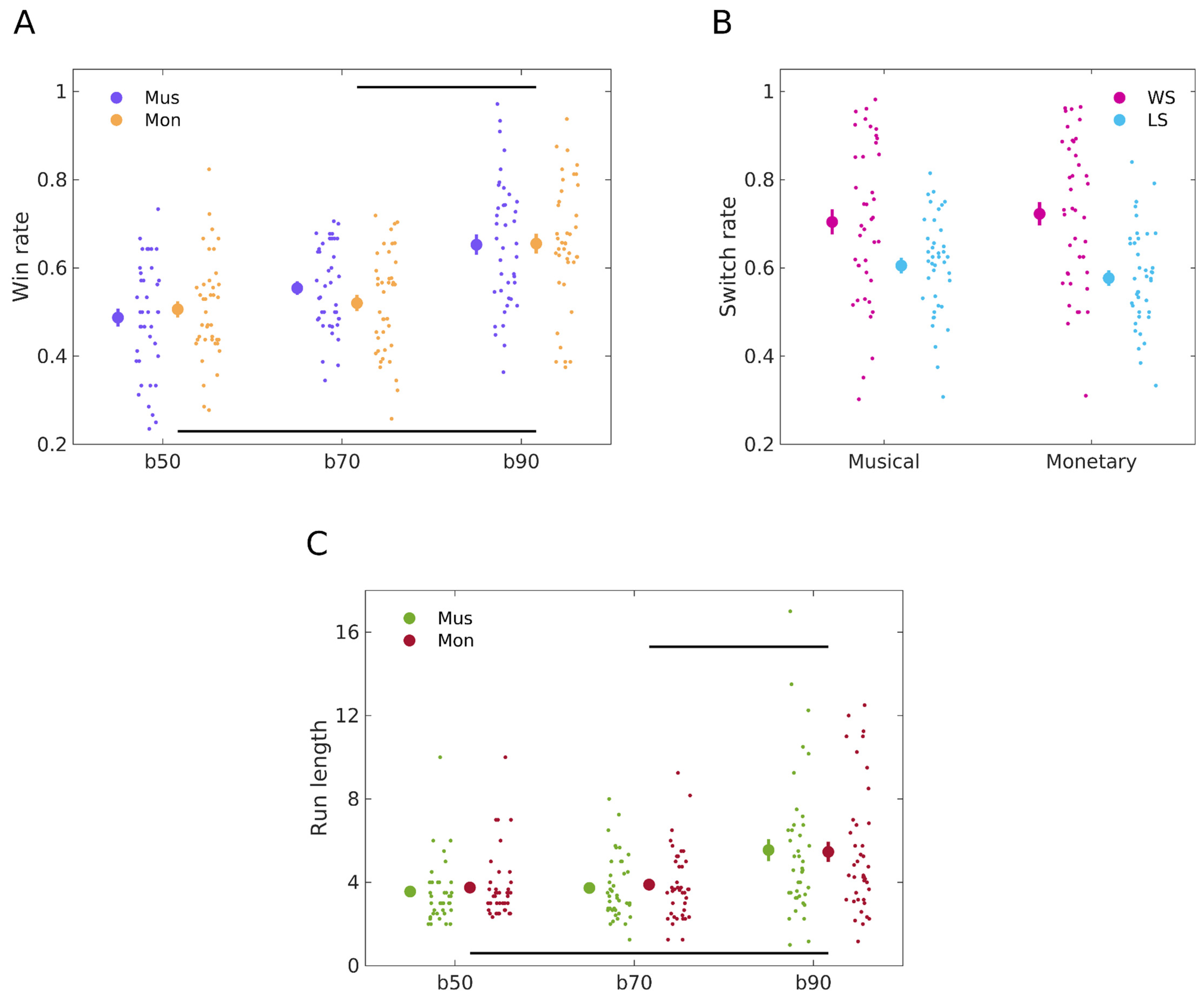
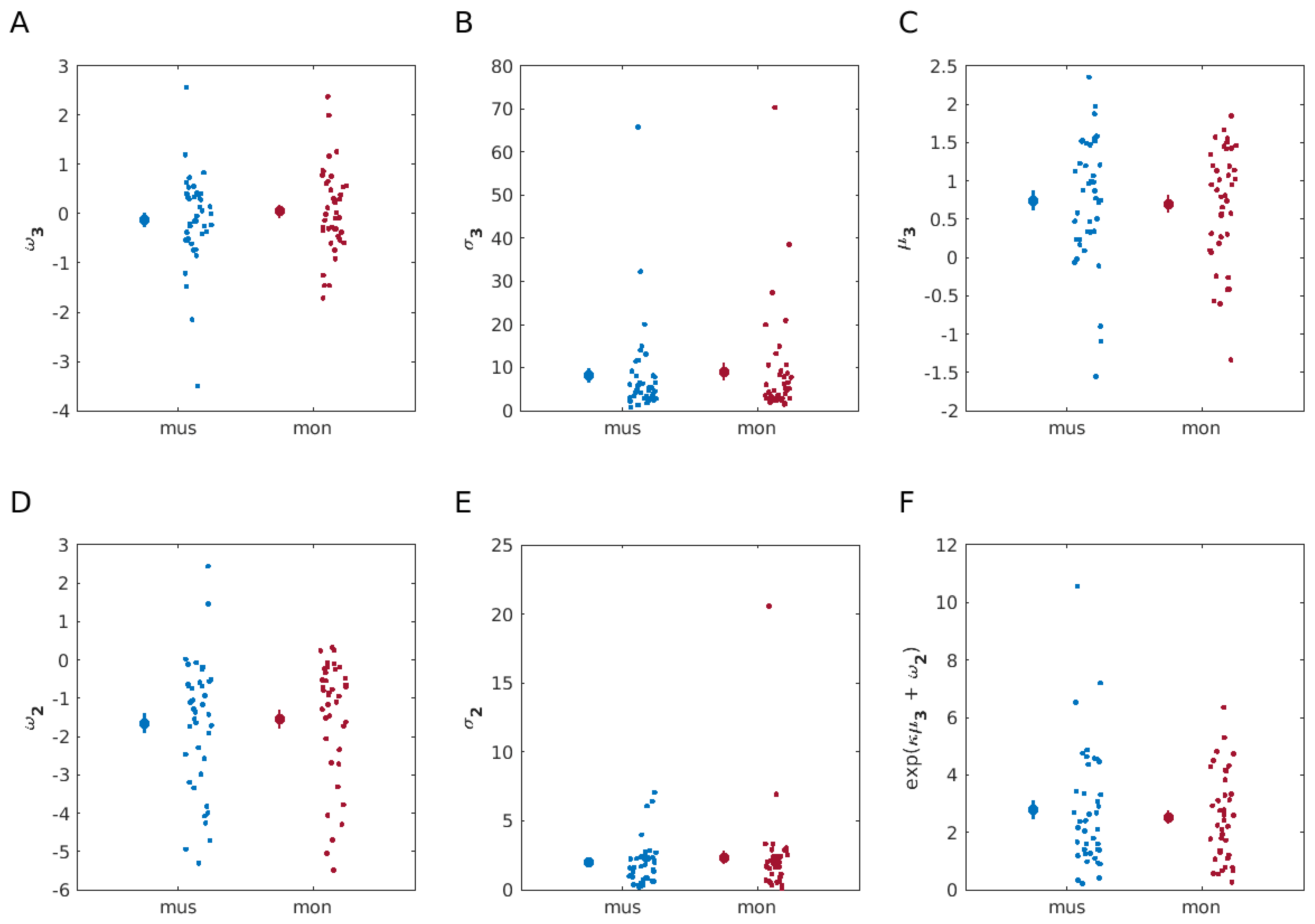
3.1. Similar Decision-Making Behaviour When Learning from Musical and Monetary Reward
3.2. Model-Based Results
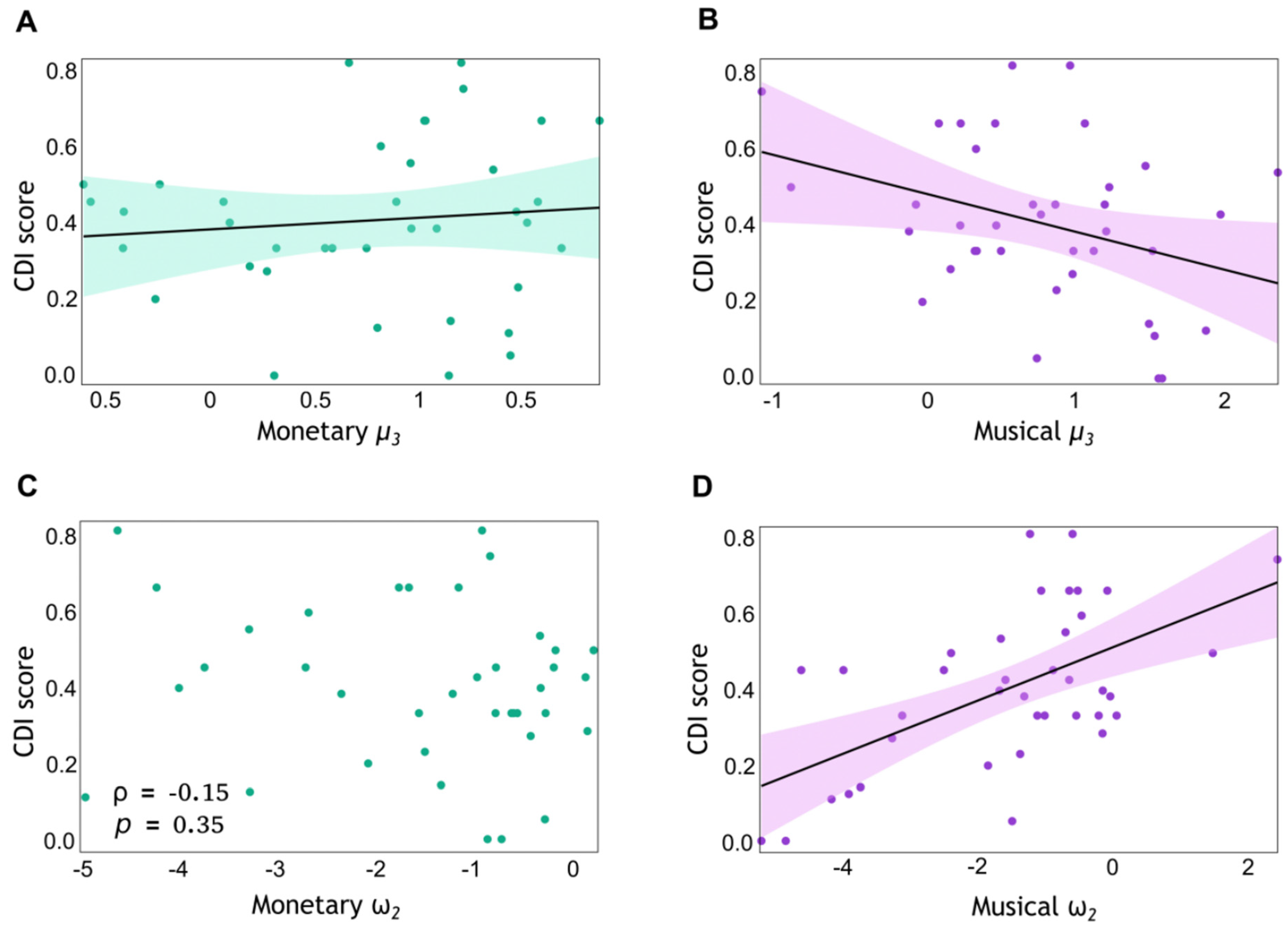
3.3. Musical Preferences Modulate Belief Updates and Stochasticity in the Musical Reward Task
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schultz, W.; Dayan, P.; Montague, P.R. A Neural Substrate of Prediction and Reward. Science 1997, 275, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W.; Dickinson, A. Neuronal Coding of Prediction Errors. Annu. Rev. Neurosci. 2000, 23, 473–500. [Google Scholar] [CrossRef]
- Green, L.; Myerson, J. A Discounting Framework for Choice with Delayed and Probabilistic Rewards. Psychol. Bull. 2004, 130, 769–792. [Google Scholar] [CrossRef] [PubMed]
- Hollerman, J.R.; Schultz, W. Dopamine Neurons Report an Error in the Temporal Prediction of Reward during Learning. Nat. Neurosci. 1998, 1, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Lak, A.; Stauffer, W.R.; Schultz, W. Dopamine Prediction Error Responses Integrate Subjective Value from Different Reward Dimensions. Proc. Natl. Acad. Sci. USA 2014, 111, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Beugré, C. The Neural Basis of Motivation and Rewards. Neurosci. Organ. Behav. 2018, 73–90. [Google Scholar] [CrossRef]
- Sescousse, G.; Caldú, X.; Segura, B.; Dreher, J.C. Processing of Primary and Secondary Rewards: A Quantitative Meta-Analysis and Review of Human Functional Neuroimaging Studies. Neurosci. Biobehav. Rev. 2013, 37, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Arsalidou, M.; Vijayarajah, S.; Sharaev, M. Basal Ganglia Lateralization in Different Types of Reward. Brain Imaging Behav. 2020, 14, 2618–2646. [Google Scholar] [CrossRef]
- Jones, R.M.; Somerville, L.H.; Li, J.; Ruberry, E.J.; Libby, V.; Glover, G.; Voss, H.U.; Ballon, D.J.; Casey, B.J. Behavioral and Neural Properties of Social Reinforcement Learning. J. Neurosci. 2011, 31, 13039–13045. [Google Scholar] [CrossRef]
- Wassiliwizky, E.; Koelsch, S.; Wagner, V.; Jacobsen, T.; Menninghaus, W. The Emotional Power of Poetry: Neural Circuitry, Psychophysiology and Compositional Principles. Soc. Cogn. Affect. Neurosci. 2017, 12, 1229–1240. [Google Scholar] [CrossRef]
- Franklin, R.G.; Adams, R.B. The Reward of a Good Joke: Neural Correlates of Viewing Dynamic Displays of Stand-up Comedy. Cogn. Affect. Behav. Neurosci. 2011, 11, 508–515. [Google Scholar] [CrossRef]
- Blood, A.J.; Zatorre, R.J.; Bermudez, P.; Evans, A.C. Emotional Responses to Pleasant and Unpleasant Music Correlate with Activity in Paralimbic Brain Regions. Nat. Neurosci. 1999, 2, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Koelsch, S. Brain Correlates of Music-Evoked Emotions. Nat. Rev. Neurosci. 2014, 15, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Isoda, M. Socially Relative Reward Valuation in the Primate Brain. Curr. Opin. Neurobiol. 2021, 68, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Veselic, S.; Smid, C.R.; Steinbeis, N. Developmental Changes in Reward Processing Are Reward Specific. Preprint. PsyArXive Preprints 2021. Available online: https://osf.io/preprints/psyarxiv/fzk9t (accessed on 5 September 2022). [CrossRef]
- Yang, X.; Liu, X.; Zeng, Y.; Wu, R.; Zhao, W.; Xin, F.; Yao, S.; Kendrick, K.M.; Ebstein, R.P.; Becker, B. Secondary Rewards Acquire Enhanced Incentive Motivation via Increasing Anticipatory Activity of the Lateral Orbitofrontal Cortex. Brain Struct. Funct. 2021, 226, 2339–2355. [Google Scholar] [CrossRef] [PubMed]
- Mathar, D.; Wiebe, A.; Tuzsus, D.; Knauth, K.; Peters, J. Erotic Cue Exposure Increases Physiological Arousal, Biases Choices toward Immediate Rewards, and Attenuates Model-based Reinforcement Learning. Psychophysiology 2023, 60, e14381. [Google Scholar] [CrossRef] [PubMed]
- Mason, L.; Eldar, E.; Rutledge, R.B. Mood Instability and Reward Dysregulation—A Neurocomputational Model of Bipolar Disorder. JAMA Psychiatry 2017, 74, 1275. [Google Scholar] [CrossRef] [PubMed]
- Arensburg, B.; Tillier, A.M.; Vandermeersch, B.; Duday, H.; Schepartz, L.A.; Rak, Y. A Middle Palaeolithic Human Hyoid Bone. Nature 1989, 338, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Conard, N.J.; Malina, M.; Münzel, S.C. New Flutes Document the Earliest Musical Tradition in Southwestern Germany. Nature 2009, 460, 737–740. [Google Scholar] [CrossRef]
- Higham, T.; Basell, L.; Jacobi, R.; Wood, R.; Ramsey, C.B.; Conard, N.J. Τesting Models for the Beginnings of the Aurignacian and the Advent of Figurative Art and Music: The Radiocarbon Chronology of Geißenklösterle. J. Hum. Evol. 2012, 62, 664–676. [Google Scholar] [CrossRef]
- Fishman, Y.I.; Volkov, I.O.; Noh, M.D.; Garell, P.C.; Bakken, H.; Arezzo, J.C.; Howard, M.A.; Steinschneider, M. Consonance and Dissonance of Musical Chords: Neural Correlates in Auditory Cortex of Monkeys and Humans. J. Neurophysiol. 2001, 86, 2761–2788. [Google Scholar] [CrossRef] [PubMed]
- Chiandetti, C.; Vallortigara, G. Chicks like Consonant Music. Psychol. Sci. 2011, 22, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Jentschke, S.; Friederici, A.D.; Koelsch, S. Neural Correlates of Music-Syntactic Processing in Two-Year Old Children. Dev. Cogn. Neurosci. 2014, 9, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Lahdelma, I.; Eerola, T. Cultural Familiarity and Musical Expertise Impact the Pleasantness of Consonance/Dissonance but Not Its Perceived Tension. Sci. Rep. 2020, 10, 8693. [Google Scholar] [CrossRef] [PubMed]
- Midya, V.; Valla, J.; Balasubramanian, H.; Mathur, A.; Singh, N.C. Cultural Differences in the Use of Acoustic Cues for Musical Emotion Experience. PLoS ONE 2019, 14, e0222380. [Google Scholar] [CrossRef] [PubMed]
- Lahdelma, I.; Eerola, T.; Armitage, J. Is Harmonicity a Misnomer for Cultural Familiarity in Consonance Preferences? Front. Psychol. 2022, 13, 802385. [Google Scholar] [CrossRef] [PubMed]
- Virtala, P.; Tervaniemi, M. Neurocognition of Major-Minor and Consonance-Dissonance. Music. Percept. 2017, 34, 387–404. [Google Scholar] [CrossRef]
- Greenberg, D.M.; Wride, S.J.; Snowden, D.A.; Spathis, D.; Potter, J.; Rentfrow, P.J. Universals and Variations in Musical Preferences: A Study of Preferential Reactions to Western Music in 53 Countries. J. Pers. Soc. Psychol. 2022, 122, 286–309. [Google Scholar] [CrossRef]
- Gold, B.P.; Mas-Herrero, E.; Zeighami, Y.; Benovoy, M.; Dagher, A.; Zatorre, R.J. Musical Reward Prediction Errors Engage the Nucleus Accumbens and Motivate Learning. Proc. Natl. Acad. Sci. USA 2019, 116, 3310–3315. [Google Scholar] [CrossRef]
- Beron, C.C.; Neufeld, S.Q.; Linderman, S.W.; Sabatini, B.L. Mice Exhibit Stochastic and Efficient Action Switching during Probabilistic Decision Making. Proc. Natl. Acad. Sci. USA 2022, 119, e2113961119. [Google Scholar] [CrossRef]
- Noonan, M.P.; Chau, B.K.H.; Rushworth, M.F.S.; Fellows, L.K. Contrasting Effects of Medial and Lateral Orbitofrontal Cortex Lesions on Credit Assignment and Decision-Making in Humans. J. Neurosci. 2017, 37, 7023–7035. [Google Scholar] [CrossRef] [PubMed]
- Barto, A.G.; Sutton, R.S. Introduction to Reinforcement Learning; MIT Press: Cambridge, UK, 1998; Volume 125. [Google Scholar]
- Mathys, C.D.; Lomakina, E.I.; Daunizeau, J.; Iglesias, S.; Brodersen, K.H.; Friston, K.J.; Stephan, K.E. Uncertainty in Perception and the Hierarchical Gaussian Filter. Front. Hum. Neurosci. 2014, 8, 825. [Google Scholar] [CrossRef] [PubMed]
- Gläscher, J.; Daw, N.; Dayan, P.; O’Doherty, J.P. States versus Rewards: Dissociable Neural Prediction Error Signals Underlying Model-Based and Model-Free Reinforcement Learning. Neuron 2010, 66, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Diaconescu, A.O.; Mathys, C.; Weber, L.A.E.; Kasper, L.; Mauer, J.; Stephan, K.E. Hierarchical Prediction Errors in Midbrain and Septum during Social Learning. Soc. Cogn. Affect. Neurosci. 2017, 12, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Hein, T.P.; de Fockert, J.; Ruiz, M.H. State Anxiety Biases Estimates of Uncertainty and Impairs Reward Learning in Volatile Environments. Neuroimage 2021, 224, 117424. [Google Scholar] [CrossRef] [PubMed]
- Mathys, C.; Daunizeau, J.; Friston, K.J.; Stephan, K.E. A Bayesian Foundation for Individual Learning under Uncertainty. Front. Hum. Neurosci. 2011, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Wetzels, R.; Wagenmakers, E.J. A Default Bayesian Hypothesis Test for Correlations and Partial Correlations. Psychon. Bull. Rev. 2012, 19, 1057–1064. [Google Scholar] [CrossRef]
- Peirce, J.; Gray, J.R.; Simpson, S.; MacAskill, M.; Höchenberger, R.; Sogo, H.; Kastman, E.; Lindeløv, J.K. PsychoPy2: Experiments in Behavior Made Easy. Behav. Res. Methods 2019, 51, 195–203. [Google Scholar] [CrossRef]
- Grogan, J.P.; Tsivos, D.; Smith, L.; Knight, B.E.; Bogacz, R.; Whone, A.; Coulthard, E.J. Effects of Dopamine on Reinforcement Learning and Consolidation in Parkinson’s Disease. eLife 2017, 6, 26801. [Google Scholar] [CrossRef]
- Lehner, R.; Balsters, J.H.; Herger, A.; Hare, T.A.; Wenderoth, N. Monetary, Food, and Social Rewards Induce Similar Pavlovian-to-Instrumental Transfer Effects. Front. Behav. Neurosci. 2017, 10, 237682. [Google Scholar] [CrossRef]
- Rouder, J.N.; Morey, R.D.; Speckman, P.L.; Province, J.M. Default Bayes Factors for ANOVA Designs. J. Math. Psychol. 2012, 56, 356–374. [Google Scholar] [CrossRef]
- Jeffreys, H. Theory of Probability, 3rd ed.; Oxford University Press: New York, NY, USA, 1961. [Google Scholar]
- Bayarri, M.J.; García-Donato, G. Extending Conventional Priors for Testing General Hypotheses in Linear Models. Biometrika 2007, 94, 135–152. [Google Scholar] [CrossRef]
- Iglesias, S.; Mathys, C.; Brodersen, K.H.; Kasper, L.; Piccirelli, M.; den Ouden, H.E.M.; Stephan, K.E. Hierarchical Prediction Errors in Midbrain and Basal Forebrain during Sensory Learning. Neuron 2013, 80, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Diaconescu, A.O.; Mathys, C.; Weber, L.A.E.; Daunizeau, J.; Kasper, L.; Lomakina, E.I.; Fehr, E.; Stephan, K.E. Inferring on the Intentions of Others by Hierarchical Bayesian Learning. PLoS Comput. Biol. 2014, 10, e1003810. [Google Scholar] [CrossRef] [PubMed]
- De Berker, A.O.; Rutledge, R.B.; Mathys, C.; Marshall, L.; Cross, G.F.; Dolan, R.J.; Bestmann, S. Computations of Uncertainty Mediate Acute Stress Responses in Humans. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hein, T.P.; Herrojo Ruiz, M. State Anxiety Alters the Neural Oscillatory Correlates of Predictions and Prediction Errors during Reward-Based Learning. Neuroimage 2022, 249, 118895. [Google Scholar] [CrossRef] [PubMed]
- Daunizeau, J.; den Ouden, H.E.M.; Pessiglione, M.; Kiebel, S.J.; Stephan, K.E.; Friston, K.J. Observing the Observer (I): Meta-Bayesian Models of Learning and Decision-Making. PLoS ONE 2010, 5, e15554. [Google Scholar] [CrossRef]
- Recorla, R.A.; Wagner, A.R. A Theory of Pavlovian Conditioning: Variations in the Effectiveness of Reinforcement and Non-Reinforcement. Class. Cond. Curr. Res. Theory 1972, 2, 64–69. [Google Scholar]
- Sutton, R.S. Gain Adaptation Beats Least Squares? In Proceedings of the Seventh Yale Workshop on Adaptive and Learning Systems, Waltham, MA, USA, 20–22 May 1992; pp. 161–166. [Google Scholar]
- Soch, J.; Allefeld, C. MACS—A New SPM Toolbox for Model Assessment, Comparison and Selection. J. Neurosci. Methods 2018, 306, 19–31. [Google Scholar] [CrossRef]
- Stephan, K.E.; Penny, W.D.; Daunizeau, J.; Moran, R.J.; Friston, K.J. Bayesian Model Selection for Group Studies. Neuroimage 2009, 46, 1004–1017. [Google Scholar] [CrossRef]
- Hein, T.P.; Gong, Z.; Ivanova, M.; Fedele, T.; Nikulin, V.; Herrojo Ruiz, M. Anterior Cingulate and Medial Prefrontal Cortex Oscillations Underlie Learning Alterations in Trait Anxiety in Humans. Commun. Biol. 2023, 6, 271. [Google Scholar] [CrossRef]
- Ly, A.; Verhagen, J.; Wagenmakers, E.-J. Harold Jeffreys’s Default Bayes Factor Hypothesis Tests: Explanation, Extension, and Application in Psychology. J. Math. Psychol. 2016, 72, 19–32. [Google Scholar] [CrossRef]
- Balleine, B.W.; Delgado, M.R.; Hikosaka, O. The Role of the Dorsal Striatum in Reward and Decision-Making. J. Neurosci. 2007, 27, 8161–8165. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C.; Kringelbach, M.L. Affective Neuroscience of Pleasure: Reward in Humans and Animals. Psychopharmacology 2008, 199, 457–480. [Google Scholar] [CrossRef] [PubMed]
- Hampton, A.N.; Bossaerts, P.; O’Doherty, J.P. The Role of the Ventromedial Prefrontal Cortex in Abstract State-Based Inference during Decision Making in Humans. J. Neurosci. 2006, 26, 8360–8367. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.W.; Reynolds, J.R.; Power, J.D.; Repovs, G.; Anticevic, A.; Braver, T.S. Multi-Task Connectivity Reveals Flexible Hubs for Adaptive Task Control. Nat. Neurosci. 2013, 16, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.K.; Dunlop, K.; Downar, J. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front. Syst. Neurosci. 2016, 10, 242701. [Google Scholar] [CrossRef] [PubMed]
| Prior | Mean | Variance |
|---|---|---|
| κ | log(1) | 0 |
| ω2 | −2.3 | 4 |
| ω3 | 0.7 | 4 |
| 0 | 0 | |
| log(0.1) | 0 | |
| log(1) | 1 | |
| log(1) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopytin, G.; Ivanova, M.; Herrojo Ruiz, M.; Shestakova, A. Evaluating the Influence of Musical and Monetary Rewards on Decision Making through Computational Modelling. Behav. Sci. 2024, 14, 124. https://doi.org/10.3390/bs14020124
Kopytin G, Ivanova M, Herrojo Ruiz M, Shestakova A. Evaluating the Influence of Musical and Monetary Rewards on Decision Making through Computational Modelling. Behavioral Sciences. 2024; 14(2):124. https://doi.org/10.3390/bs14020124
Chicago/Turabian StyleKopytin, Grigory, Marina Ivanova, Maria Herrojo Ruiz, and Anna Shestakova. 2024. "Evaluating the Influence of Musical and Monetary Rewards on Decision Making through Computational Modelling" Behavioral Sciences 14, no. 2: 124. https://doi.org/10.3390/bs14020124
APA StyleKopytin, G., Ivanova, M., Herrojo Ruiz, M., & Shestakova, A. (2024). Evaluating the Influence of Musical and Monetary Rewards on Decision Making through Computational Modelling. Behavioral Sciences, 14(2), 124. https://doi.org/10.3390/bs14020124





