Cognitive Function, and Its Relationships with Comorbidities, Physical Activity, and Muscular Strength in Korean Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Variables
2.2.1. Cognitive Function
2.2.2. Comorbidities
2.2.3. Physical Activity and Lower-Body Muscle Strength
2.2.4. Covariates
2.3. Statistics
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alkema, L.; Raftery, A.E.; Gerland, P.; Clark, S.J.; Pelletier, F.; Buettner, T.; Heilig, G.K. Probabilistic projections of the total fertility rate for all countries. Demography 2011, 48, 815–839. [Google Scholar] [CrossRef] [PubMed]
- Statistics Korea. 2017 Statistics on the Aged. Available online: http://kostat.go.kr/portal/korea/kor_nw/3/index.board?bmode=read&bSeq=&aSeq=363363&pageNo=1&rowNum=10&navCount=10&currPg=&sTarget=title&sTxt (accessed on 10 November 2022).
- Lopreite, M.; Mauro, M. The effects of population ageing on health care expenditure: A Bayesian VAR analysis using data from Italy. Health Policy 2017, 121, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Kang, J.M.; Lee, H.; Kim, K.; Kim, S.; Yu, T.Y.; Lee, E.M.; Kim, C.T.; Kim, D.K.; Lewis, M.; et al. Subjective cognitive decline and subsequent dementia: A nationwide cohort study of 579,710 people aged 66 years in South Korea. Alzheimers Res. Ther. 2020, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Bouldin, E.D.; McGuire, L.C. Subjective cognitive decline among adults aged ≥45 Years—United States, 2015–2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 753–757. [Google Scholar] [CrossRef]
- Vance, D.; Larsen, K.I.; Eagerton, G.; Wright, M.A. Comorbidities and cognitive functioning: Implications for nursing research and practice. J. Neurosci. Nurs. 2011, 43, 215–224. [Google Scholar] [CrossRef]
- Shilpa, K.; Norman, G. Prevalence of frailty and its association with lifestyle factors among elderly in rural Bengaluru. J. Fam. Med. Prim. Care 2022, 11, 2083–2089. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Vecchio, L.M.; Meng, Y.; Xhima, K.; Lipsman, N.; Hamani, C.; Aubert, I. The neuroprotective effects of exercise: Maintaining a healthy brain throughout aging. Brain Plast. 2018, 4, 17–52. [Google Scholar] [CrossRef]
- Dougherty, R.J.; Boots, E.A.; Lindheimer, J.B.; Stegner, A.J.; Van Riper, S.; Edwards, D.F.; Gallagher, C.L.; Carlsson, C.M.; Rowley, H.A.; Bendlin, B.B.; et al. Fitness, independent of physical activity is associated with cerebral blood flow in adults at risk for Alzheimer’s disease. Brain Imaging Behav. 2020, 14, 1154–1163. [Google Scholar] [CrossRef]
- Perrey, S. Promoting motor function by exercising the brain. Brain Sci. 2013, 3, 101–122. [Google Scholar] [CrossRef]
- Daimiel, L.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Schröder, H.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Wärnberg, J.; Lopez-Miranda, J.; et al. Physical fitness and physical activity association with cognitive function and quality of life: Baseline cross-sectional analysis of the PREDIMED-Plus trial. Sci. Rep. 2020, 10, 3472. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Hwang, G.; Cho, Y.H.; Kim, E.J.; Woang, J.W.; Hong, C.H.; Son, S.J.; Roh, H.W. Relationships of physical activity, depression, and sleep with cognitive function in community-dwelling older adults. Int J. Environ. Res. Public Health 2022, 19, 15655. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Srivastava, S.; Muhammad, T. Relationship between physical activity and cognitive functioning among older Indian adults. Sci. Rep. 2022, 12, 2725. [Google Scholar] [CrossRef] [PubMed]
- Dupré, C.; Helmer, C.; Bongue, B.; Dartigues, J.F.; Roche, F.; Berr, C.; Carrière, I. Associations between physical activity types and multi-domain cognitive decline in older adults from the three-city cohort. PLoS ONE 2021, 16, e0252500. [Google Scholar] [CrossRef] [PubMed]
- Frith, E.; Loprinzi, P.D. The Association between lower extremity muscular strength and cognitive function in a national sample of older adults. J. Lifestyle Med. 2018, 8, 99–104. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, H.; Du, C. Association of physical fitness with cognitive function in the community-dwelling older adults. BMC Geriatr. 2022, 22, 868. [Google Scholar] [CrossRef]
- Kim, T.H.; Jhoo, J.H.; Park, J.H.; Kim, J.L.; Ryu, S.H.; Moon, S.W.; Choo, I.H.; Lee, D.W.; Yoon, J.C.; Do, Y.J.; et al. Korean version of mini mental status examination for dementia screening and its’ short form. Psychiatry Investig. 2010, 7, 102–108. [Google Scholar] [CrossRef]
- Park, B.; Ock, M.; Lee, H.A.; Lee, S.; Han, H.; Jo, M.W.; Park, H. Multimorbidity and health-related quality of life in Koreans aged 50 or older using KNHANES 2013–2014. Health Qual. Life Outcomes 2018, 16, 186. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010; Available online: https://www.who.int/publications/i/item/9789241599979 (accessed on 5 December 2022).
- Buatois, S.; Miljkovic, D.; Manckoundia, P.; Gueguen, R.; Miget, P.; Vancon, G.; Perrin, P.; Benetos, A. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J. Am. Geriatr. Soc. 2008, 56, 1575–1577. [Google Scholar] [CrossRef]
- Nam, S.M.; Kim, S.G. Effects of a five times sit to stand test on the daily life independence of Korean elderly and cut-off analysis. J. Korean Soc. Phys. Med. 2019, 14, 29–35. [Google Scholar] [CrossRef]
- Lyu, J.; Lee, S.H. Alcohol consumption and cognitive impairment among Korean older adults: Does gender matter? Int. Psychogeriatr. 2014, 26, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, E.; An, M. The cognitive impact of chronic diseases on functional capacity in community-dwelling adults. J. Nurs. Res. 2019, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sujitha, P.; Gopalakrishnan, S.; Swetha, N.B.; Grace, G.A. Cognitive impairment and its correlation with comorbidities among elderly residing in old age homes in Southern India. Natl. J. Community Med. 2022, 13, 235–241. [Google Scholar] [CrossRef]
- Winning, L.; Naseer, A.; De Looze, C.; Knight, S.P.; Kenny, R.A.; O’Connell, B. Tooth loss and cognitive decline in community-dwelling older Irish adults: A cross-sectional cohort study. J. Dent. 2022, 119, 104077. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.E.; Jiang, D.; Schweizer, T.A. Determining the association of medical co-morbidity with subjective and objective cognitive performance in an inner city memory disorders clinic: A retrospective chart review. BMC Geriatr. 2010, 10, 89. [Google Scholar] [CrossRef]
- Whitelock, V.; Rutters, F.; Rijnhart, J.J.M.; Nouwen, A.; Higgs, S. The mediating role of comorbid conditions in the association between type 2 diabetes and cognition: A cross-sectional observational study using the UK Biobank cohort. Psychoneuroendocrinology 2021, 123, 104902. [Google Scholar] [CrossRef]
- She, R.; Yan, Z.; Hao, Y.; Zhang, Z.; Du, Y.; Liang, Y.; Vetrano, D.L.; Dekker, J.; Bai, B.; Lau, J.T.F.; et al. Comorbidity in patients with first-ever ischemic stroke: Disease patterns and their associations with cognitive and physical function. Front. Aging Neurosci. 2022, 14, 887032. [Google Scholar] [CrossRef]
- Martínez-Horta, S.; Bejr-Kasem, H.; Horta-Barba, A.; Pascual-Sedano, B.; Santos-García, D.; de Deus-Fonticoba, T.; Jesús, S.; Aguilar, M.; Planellas, L.; García-Caldentey, J.; et al. Identifying comorbidities and lifestyle factors contributing to the cognitive profile of early Parkinson’s disease. BMC Neurol. 2021, 21, 477. [Google Scholar] [CrossRef]
- Kao, S.L.; Wang, J.H.; Chen, S.C.; Li, Y.Y.; Yang, Y.L.; Lo, R.Y. Impact of comorbidity burden on cognitive decline: A prospective cohort study of older adults with dementia. Dement. Geriatr. Cogn. Disord. 2021, 50, 43–50. [Google Scholar] [CrossRef]
- Calderón-Larrañaga, A.; Vetrano, D.L.; Ferrucci, L.; Mercer, S.W.; Marengoni, A.; Onder, G.; Eriksdotter, M.; Fratiglioni, L. Multimorbidity and functional impairment-bidirectional interplay, synergistic effects and common pathways. J. Intern. Med. 2019, 285, 255–271. [Google Scholar] [CrossRef]
- Taylor, C.A.; Bouldin, E.D.; Greenlund, K.J.; McGuire, L.C. Comorbid chronic conditions among older adults with subjective cognitive decline, United States, 2015–2017. Innov. Aging 2020, 4, igz045. [Google Scholar] [CrossRef] [PubMed]
- Lutski, M.; Weinstein, G.; Goldbourt, U.; Tanne, D. Cardiovascular health and cognitive decline 2 decades later in men with preexisting coronary artery disease. Am. J. Cardiol. 2018, 121, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Patel, R.; Figley, C.R.; Kornelsen, J.; Bolton, J.M.; Graff, L.A.; Mazerolle, E.L.; Helmick, C.; Uddin, M.N.; Figley, T.D.; et al. Effects of vascular comorbidity on cognition in multiple sclerosis are partially mediated by changes in brain structure. Front. Neurol. 2022, 13, 910014. [Google Scholar] [CrossRef] [PubMed]
- Yigit, B.; Oner, C.; Cetin, H.; Simsek, E.E. Association between sarcopenia and cognitive functions in older Individuals: A Cross-Sectional Study. Ann. Geriatr. Med. Res. 2022, 26, 134–139. [Google Scholar] [CrossRef]
- Dhikav, V.; Kumar, P.; Anand, P.K. Cardiovascular comorbidities and cognitive impairment. OBM Geriatr. 2022, 6, 215. [Google Scholar] [CrossRef]
- Kobayashi, H.; Arai, H. Donepezil may reduce the risk of comorbidities in patients with Alzheimer’s disease: A large-scale matched case-control analysis in Japan. Alzheimers Dement. 2018, 4, 130–136. [Google Scholar] [CrossRef]
- Song, H.; Park, J.H. Effects of changes in physical activity with cognitive decline in Korean home-dwelling older adults. J. Multidiscip. Healthc. 2022, 15, 333–341. [Google Scholar] [CrossRef]
- Krell-Roesch, J.; Syrjanen, J.A.; Bezold, J.; Trautwein, S.; Barisch-Fritz, B.; Boes, K.; Woll, A.; Forzani, E.; Kremers, W.K.; Machulda, M.M.; et al. Physical activity and trajectory of cognitive change in older persons: Mayo Clinic Study of Aging. J. Alzheimers Dis. 2021, 79, 377–388. [Google Scholar] [CrossRef]
- Lee, J.M.J.; Ryan, E. The Relationship between muscular strength and depression in older adults with chronic disease comorbidity. Int J. Environ. Res. Public Health 2020, 17, 6830. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, J. Prospective association of handgrip strength with risk of new-onset cognitive dysfunction in Korean adults: A 6-year national cohort study. Tohoku J. Exp. Med. 2018, 244, 83–91. [Google Scholar] [CrossRef]
- Lee, J.; Suh, Y.; Park, J.; Kim, G.U.; Lee, S. Combined effects of handgrip strength and sensory impairment on the prevalence of cognitive impairment among older adults in Korea. Sci. Rep. 2022, 12, 6713. [Google Scholar] [CrossRef] [PubMed]
- Strandkvist, V.; Larsson, A.; Pauelsen, M.; Nyberg, L.; Vikman, I.; Lindberg, A.; Gustafsson, T.; Röijezon, U. Hand grip strength is strongly associated with lower limb strength but only weakly with postural control in community-dwelling older adults. Arch. Gerontol. Geriatr. 2021, 94, 104345. [Google Scholar] [CrossRef]
- Tatangelo, T.; Muollo, V.; Ghiotto, L.; Schena, F.; Rossi, A.P. Exploring the association between handgrip, lower limb muscle strength, and physical function in older adults: A narrative review. Exp. Gerontol. 2022, 167, 111902. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Meher, T. Association of late-life depression with cognitive impairment: Evidence from a cross-sectional study among older adults in India. BMC Geriatr. 2021, 21, 364. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.Y.; Hong, H.; Kang, H. Relationship between physical activity and depressive symptoms in older Korean adults: Moderation analysis of muscular strength. BMC Geriatr. 2022, 22, 884. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Martin, B.; Maudsley, S. Anti-inflammatory effects of physical activity in relationship to improved cognitive status in humans and mouse models of Alzheimer’s disease. Curr. Alzheimer Res. 2012, 9, 86–92. [Google Scholar] [CrossRef]
- Shakouri, E.; Azarbayjani, M.A.; Jameie, S.B.; Peeri, M.; Farhadi, M. Effect of physical activity on cognitive function and neurogenesis: Roles of BDNF and oxidative stress. Thrita 2020, 9, e109723. [Google Scholar] [CrossRef]
- Yorston, L.C.; Kolt, G.S.; Rosenkranz, R.R. Physical activity and physical function in older adults: The 45 and up study. J. Am. Geriatr. Soc. 2012, 60, 719–725. [Google Scholar] [CrossRef]
- Ezzati, A.; Zammit, A.R.; Katz, M.J.; Derby, C.A.; Zimmerman, M.E.; Lipton, R.B. Health-related quality of life, cognitive performance, and incident dementia in a community-based elderly cohort. Alzheimer Dis. Assoc. Disord. 2019, 33, 240–245. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.W.; McLaughlin, S.J. Psychological well-being and cognitive function among older adults in China: A population-based longitudinal study. J. Aging Health 2022, 34, 173–183. [Google Scholar] [CrossRef]
- Ogonowska-Slodownik, A.; Morgulec-Adamowicz, N.; Geigle, P.R.; Kalbarczyk, M.; Kosmol, A. Objective and self-reported assessment of physical activity of women over 60 years old. Ageing Int. 2022, 47, 307–320. [Google Scholar] [CrossRef]
| Variables | Number of Comorbidities | p for Linear Trends | ||
|---|---|---|---|---|
| 0 (n = 1686/16.7%) | 1 (n = 2956/29.3%) | 2 (n = 5445/54.0%) | ||
| Age in years, mean (95% CI) | 70.9 (70.6~71.2) | 72.6 (72.4~72.8) | 74.9 (74.7~75.1) | <0.001 |
| BMI in kg/m2, mean (95% CI) | 23.3 (23.2~23.4) | 23.5 (23.4~23.6) | 23.7 (23.6~23.8) | <0.001 |
| Gender, n (%) | <0.001 | |||
| Male | 831 (49.3) | 1326 (44.9) | 1878 (34.4) | |
| Female | 855 (50.7) | 1630 (55.1) | 3577 (65.6) | |
| Marriage status | <0.001 | |||
| Never married | 13 (0.8) | 5 (0.2) | 25 (0.5) | |
| Married with a spouse | 1179 (69.9) | 1891 (64.0) | 2861 (52.4) | |
| Married without a spouse | 494 (29.3) | 1060 (35.9) | 2569 (47.1) | |
| Educational background, n (%) | <0.001 | |||
| Elementary or less | 527 (31.3) | 1133 (38.3) | 2888 (52.9) | |
| Middle/high school | 1044 (61.9) | 1644 (55.6) | 2349 (43.1) | |
| College or higher | 115 (6.8) | 179 (6.1) | 218 (4.0) | |
| Smoking status, n (%) | <0.001 | |||
| Current/past smokers | 234 (13.9) | 363 (12.3) | 507 (9.3) | |
| Alcohol intake (times/week) | 0.006 | |||
| 0 | - | - | - | |
| 1–6 | 619 (81.1) | 1058 (89.0) | 1483 (87.2) | |
| ≥7 | 144 (18.9) | 131 (11.0) | 218 (12.8) | |
| Physical-activity status, n (%) | <0.001 | |||
| Sufficient | 957 (56.8) | 1540 (52.1) | 2745 (50.3) | |
| Insufficient | 729 (43.2) | 1416 (47.9) | 2710 (49.7) | |
| Lower-body muscle strength, n (%) | <0.001 | |||
| Normal | 1469 (91.5) | 2336 (83.0) | 3519 (67.8) | |
| Weak | 136 (8.5) | 479 (17.0) | 1672 (32.2) | |
| Cognitive function, mean (95% CI) | 25.3 (25.1~25.6) | 24.9 (24.8~25.1) | 23.7 (23.5~23.8) | <0.001 |
| # Cognitive impairment, n (%) | 493 (29.4) | 915 (31.4) | 1905 (36.0) | <0.001 |
| Variables | Unstandardized Beta | Standardized Beta | 95% CI | p-Value |
|---|---|---|---|---|
| Age | −0.281 | −0.346 | −0.296~−0.266 | <0.001 |
| Gender | 1.306 | 0.121 | 1.094~1.519 | <0.001 |
| Marriage | 1.185 | 0.168 | 1.605~2.205 | <0.001 |
| Body mass index | 0.100 | 0.049 | 0.060~0.140 | <0.001 |
| Education | 0.447 | 0.337 | 0.423~0.472 | <0.001 |
| Smoking | −1.496 | −0.088 | −1.831~−1.162 | <0.001 |
| Alcohol intake | 0.221 | 0.091 | 0.143~0.299 | <0.001 |
| Comorbidities | −0.535 | −0.148 | −0.996~−0.705 | <0.001 |
| PA | −1.482 | −0.139 | −1.690~−1.275 | <0.001 |
| LBMS | −4.215 | −0.338 | −4.452~−3.977 | <0.001 |
| Predictors | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| PA | ||||
| Sufficient | 1 (reference) | 1 (reference) | ||
| Insufficient | 1.325 (1.219~1.441) | <0.001 | 1.340 (1.160~1.547) | <0.001 |
| LBMS | ||||
| Normal | 1 (reference) | 1 (reference) | ||
| Weak | 3.240 (2.607~4.027) | <0.001 | 1.719 (1.380~2.143) | <0.001 |
| Number of comorbidities | ||||
| 0 | 1 (reference) | 1 (reference) | ||
| 1 | 1.099 (0.964~1.253) | 0.159 | 1.063 (0.864~1.307) | 0.564 |
| ≥2 | 1.321 (1.167~1.496) | <0.001 | 1.415 (1.154~1.736) | <0.001 |
| Predictors | Coefficients | SE | t | p | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Model 1 (R2 = 0.1277, F = 275.7519, p < 0.001) | ||||||
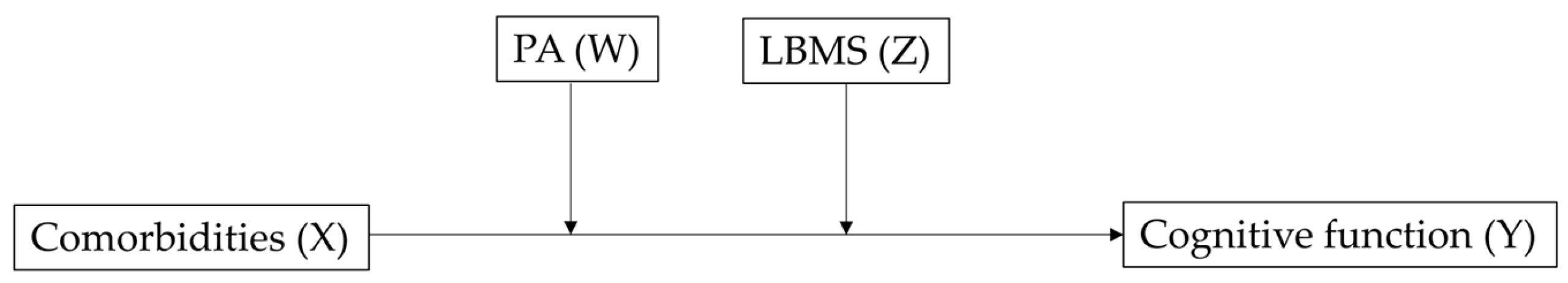
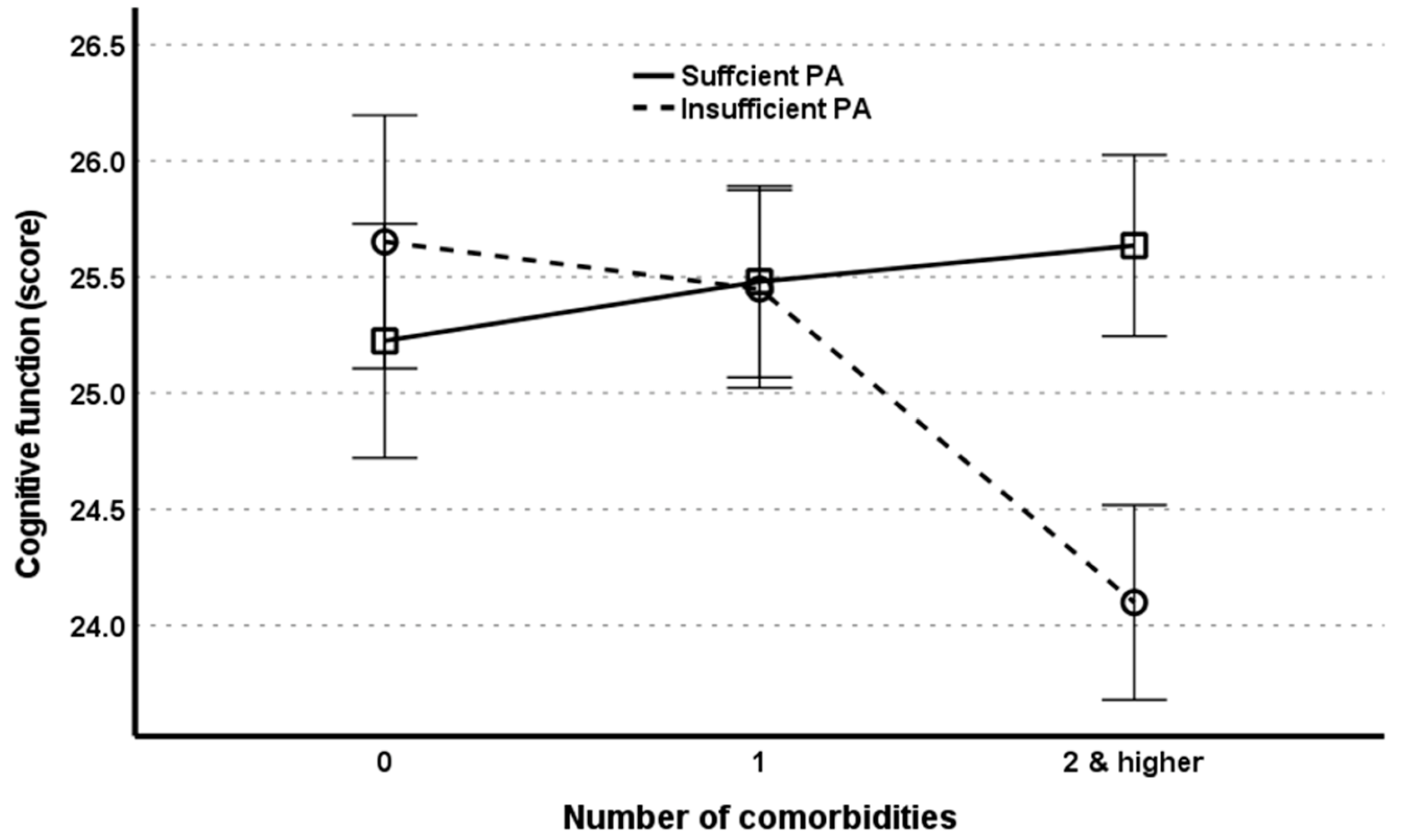
| Comorbidities (X) | 0.2443 | 0.1079 | 2.2631 | 0.0237 | 0.0327 | 0.4559 |
| Physical activity (W) | −0.2432 | 0.1655 | −1.4700 | 0.1416 | −0.5675 | 0.0811 |
| Interaction 1 (X × W) | −0.3753 | 0.0720 | −5.2092 | <0.001 | −0.5165 | −0.2341 |
| LBMS (Z) | −4.2866 | 0.2163 | −19.8182 | <0.001 | −4.7106 | −3.8626 |
| Interaction 2 (X × Z) | 0.2353 | 0.0805 | 2.9210 | 0.035 | 0.0774 | 0.3932 |
| R2 change due to X × W = 0.0025 (F = 27.1362, p < 0.001) R2 change due to X × Z = 0.0008 (F = 6.5323, p = 0.0035) R2 change due to both = 0.0028 (F = 15.0746, p < 0.001) | ||||||
| Model 2 (R2 = 0.1941, F = 226.4264, p < 0.001) | ||||||
| Comorbidities (X) | 0.3852 | 0.1042 | 3.6962 | 0.002 | 0.1809 | 0.5895 |
| Physical activity (W) | −0.0019 | 0.1596 | −0.0119 | 0.991 | −0.3148 | 0.3110 |
| Interaction 1 (X × W) | −0.3383 | 0.0694 | −4.8758 | <0.001 | −0.4743 | −0.2023 |
| LBMS (Z) | −2.5078 | 0.2199 | −11.4042 | <0.001 | −2.9388 | −2.0767 |
| Interaction 2 (X × Z) | 0.0647 | 0.0778 | 0.8309 | 0.406 | −0.0879 | 0.2172 |
| R2 change due to X × W = 0.0020 (F = 23.7736, p < 0.001) R2 change due to X × Z = 0.0001 (F = 0.6904, p = 0.406) R2 change due to both = 0.0020 (F = 11.9443, p < 0.001) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S. Cognitive Function, and Its Relationships with Comorbidities, Physical Activity, and Muscular Strength in Korean Older Adults. Behav. Sci. 2023, 13, 212. https://doi.org/10.3390/bs13030212
Kim S. Cognitive Function, and Its Relationships with Comorbidities, Physical Activity, and Muscular Strength in Korean Older Adults. Behavioral Sciences. 2023; 13(3):212. https://doi.org/10.3390/bs13030212
Chicago/Turabian StyleKim, Shinuk. 2023. "Cognitive Function, and Its Relationships with Comorbidities, Physical Activity, and Muscular Strength in Korean Older Adults" Behavioral Sciences 13, no. 3: 212. https://doi.org/10.3390/bs13030212
APA StyleKim, S. (2023). Cognitive Function, and Its Relationships with Comorbidities, Physical Activity, and Muscular Strength in Korean Older Adults. Behavioral Sciences, 13(3), 212. https://doi.org/10.3390/bs13030212





