Postdiction in Visual Awareness in Schizophrenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
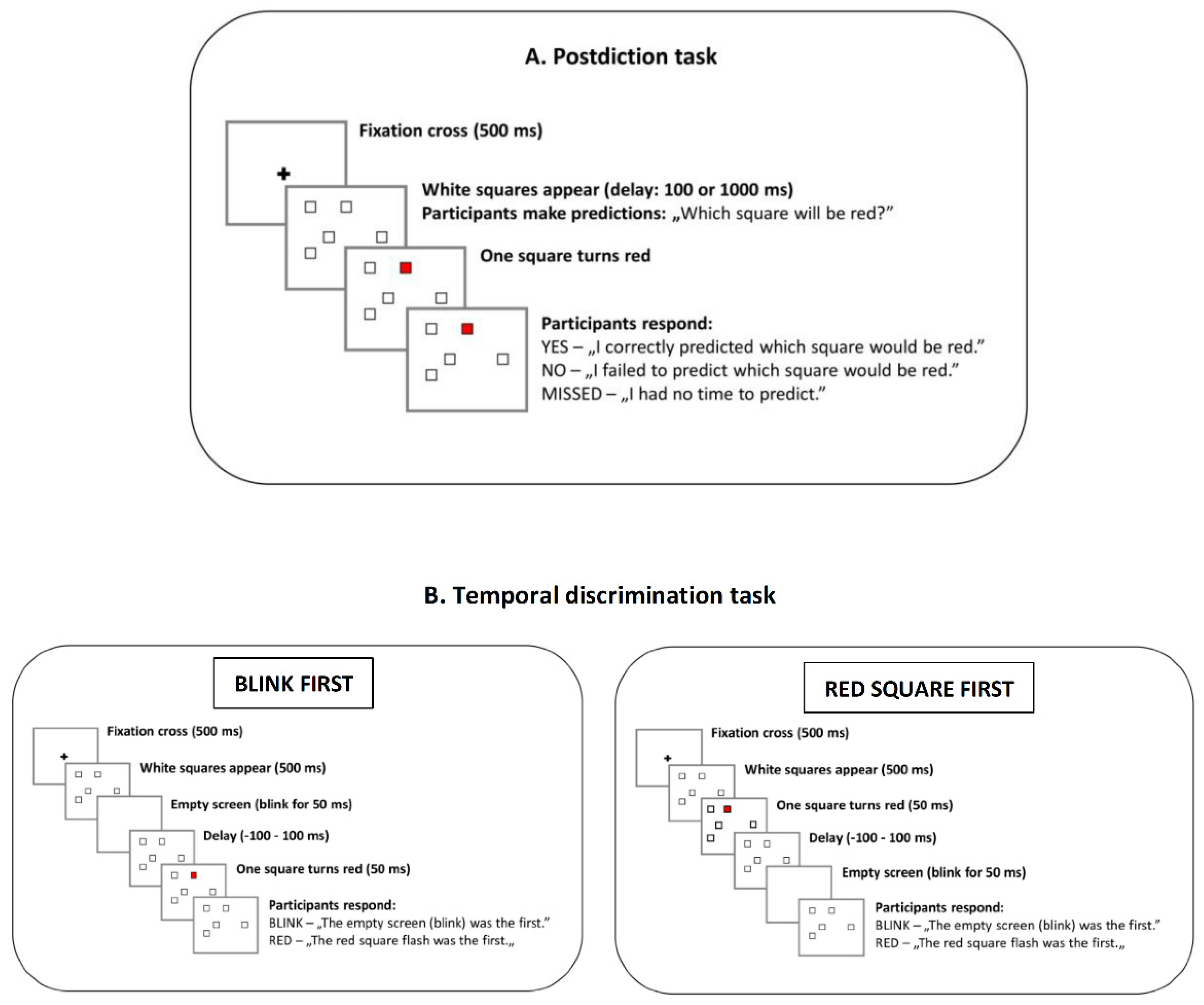
2.3. Postdiction Task
2.4. Temporal Discrimination Task
2.5. Clinical Assessment
2.5.1. Positive and Negative Syndrome Scale (PANSS)
2.5.2. Peters et al. Delusion Inventory (PDI)
2.6. Cognitive-Behavioral Therapy for Psychosis (CBTp)
2.7. Data Analysis
3. Results
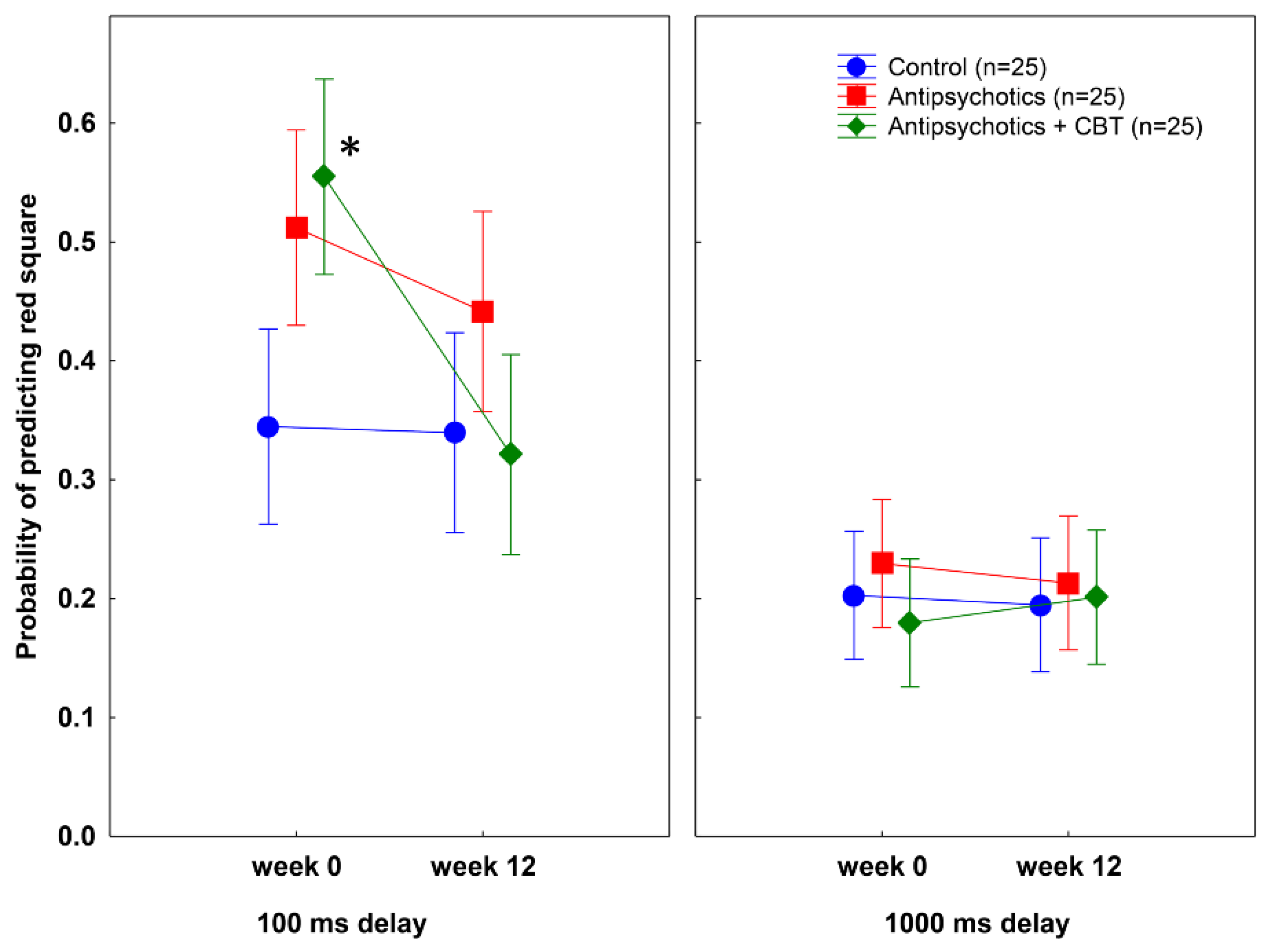
3.1. Postdiction Performance
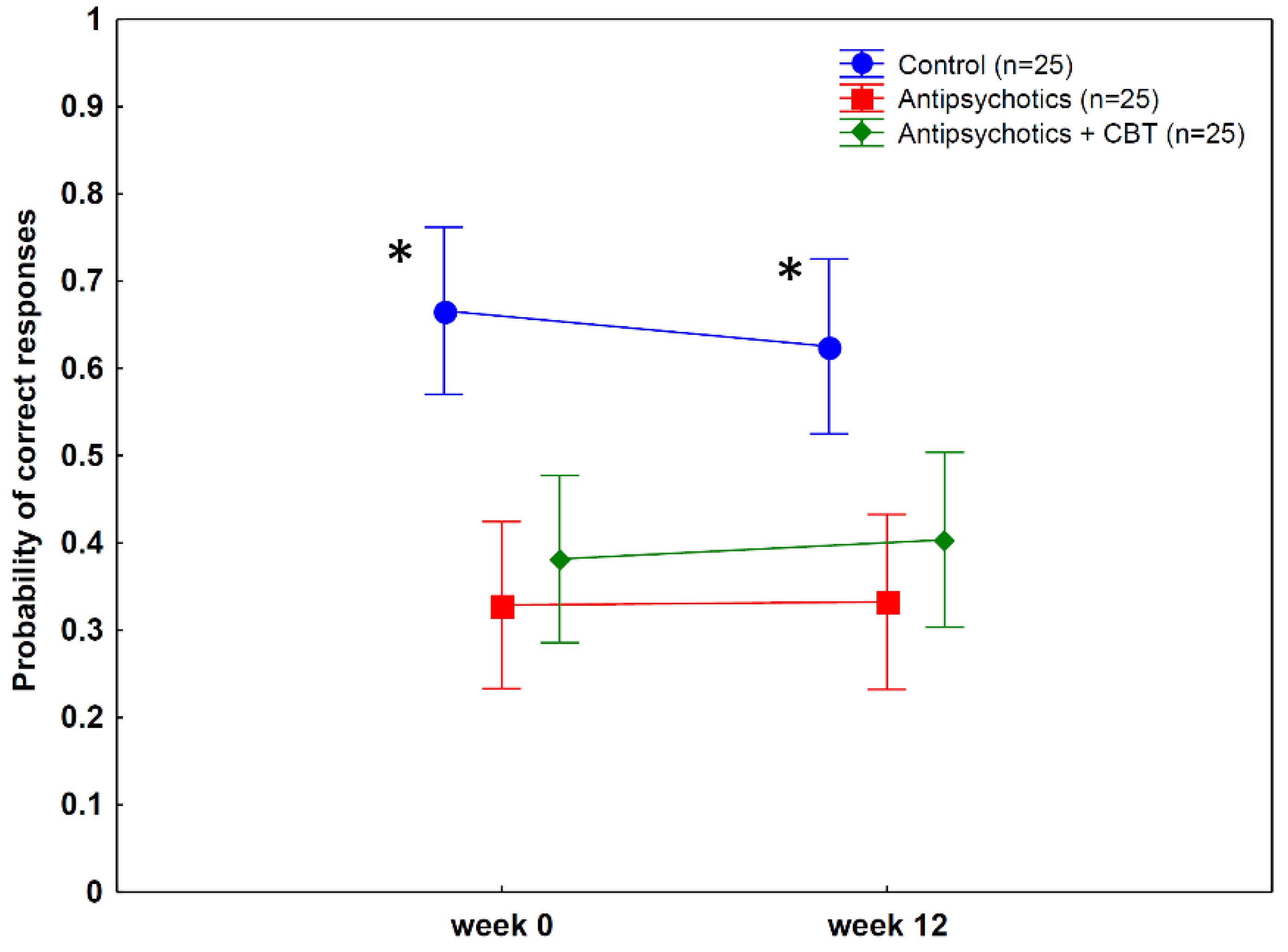
3.2. Probability of Making Predictions in the Postdiction Task
3.3. Temporal Discrimination Performance
3.4. Test-Retest Reliability of Postdiction and Temporal Discrimination
3.5. Clinical Outcomes
3.5.1. PANSS
3.5.2. PDI
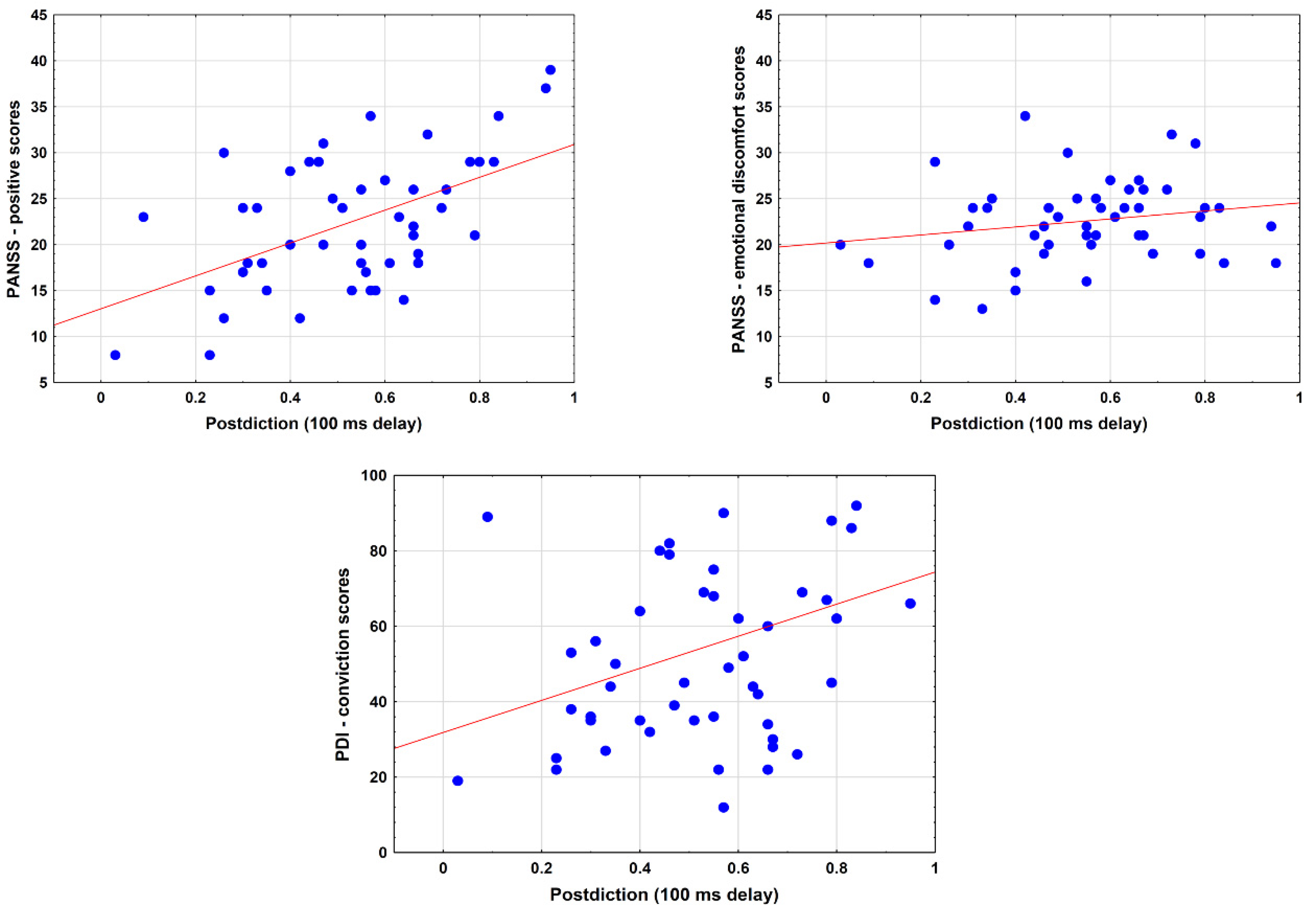
3.6. Relationship between Postdiction and Clinical Measures
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Connors, M.H.; Halligan, P.W. Belief and belief formation: Insights from delusions. In Processes of Believing: The Acquisition, Maintenance, and Change in Creditions; New Approaches to the Scientific Study of Religion; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 153–165. [Google Scholar]
- McKay, R.T.; Ross, R.M. Religion and delusion. Curr. Opin. Psychol. 2021, 40, 160–166. [Google Scholar] [CrossRef] [PubMed]
- McCauley, R.N.; Graham, G. Hearing Voices and Other Matters of the Mind—What Mental Abnormalities Can Teach Us about Religions; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Heinrichs, R.W.; Zakzanis, K.K. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology 1998, 12, 426–445. [Google Scholar] [CrossRef] [PubMed]
- Fett, A.J.; Reichenberg, A.; Velthorst, E. Lifespan evolution of neurocognitive impairment in schizophrenia—A narrative review. Schizophr. Res. Cogn. 2022, 28, 100237. [Google Scholar] [CrossRef]
- Silverstein, S.M.; Fradkin, S.I.; Demmin, D.L. Schizophrenia and the retina: Towards a 2020 perspective. Schizophr. Res. 2020, 219, 84–94. [Google Scholar] [CrossRef]
- Green, M.F.; Horan, W.P.; Lee, J. Nonsocial and social cognition in schizophrenia: Current evidence and future directions. World Psychiatry 2019, 18, 146–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, M.F.; Horan, W.P.; Lee, J. Social cognition in schizophrenia. Nat. Rev. Neurosci. 2015, 16, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.M.; Waltz, J.A.; Frank, M.J. Effort cost computation in schizophrenia: A commentary on the recent literature. Biol. Psychiatry 2015, 78, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Connors, M.H.; Halligan, P.W. A cognitive account of belief: A tentative road map. Front. Psychol. 2014, 5, 1588. [Google Scholar] [CrossRef] [Green Version]
- Ashinoff, B.K.; Singletary, N.M.; Baker, S.C.; Horga, G. Rethinking delusions: A selective review of delusion research through a computational lens. Schizophr. Res. 2021. [Google Scholar] [CrossRef]
- Seitz, R.J.; Angel, H.-F.; Paloutzian, R.F. The Processes of Believing, Mental Abnormalities, and Other Matters of the Mind: Where Do They Come from? What Are They Good For? J. Cogn. Sci. Relig. 2021, 7, 54–72. [Google Scholar] [CrossRef]
- Seitz, R.J.; Angel, H.F. Belief formation—A driving force for brain evolution. Brain Cogn. 2020, 140, 105548. [Google Scholar] [CrossRef] [PubMed]
- Shermer, M. The Believing Brain. From Ghosts and Gods to Politics and Conspiracies—How We Construct Beliefs and Reinforce Them as Truths; Times Books: New York, NY, USA, 2011. [Google Scholar]
- Garety, P.A.; Freeman, D. Cognitive approaches to delusions: A critical review of theories and evidence. Br. J. Clin. Psychol. 1999, 38, 113–154. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, P.C.; Frith, C.D. Perceiving is believing: A Bayesian approach to explaining the positive symptoms of schizophrenia. Nat. Rev. Neurosci. 2009, 10, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Fleminger, S. Seeing is believing: The role of ‘preconscious’ perceptual processing in delusional misidentification. Br. J. Psychiatry 1992, 160, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.; Young, A.W. Delusions and Brain Injury: The Philosophy and Psychology of Belief. Mind Lang. 1997, 12, 327–364. [Google Scholar] [CrossRef]
- Bear, A.; Bloom, P. A Simple Task Uncovers a Postdictive Illusion of Choice. Psychol. Sci. 2016, 27, 914–922. [Google Scholar] [CrossRef]
- Bear, A.; Fortgang, R.G.; Bronstein, M.V.; Cannon, T.D. Mistiming of thought and perception predicts delusionality. Proc. Natl. Acad. Sci. USA 2017, 114, 10791–10796. [Google Scholar] [CrossRef] [Green Version]
- Eagleman, D.M.; Sejnowski, T.J. Motion integration and postdiction in visual awareness. Science 2000, 287, 2036–2038. [Google Scholar] [CrossRef] [Green Version]
- Shimojo, S. Postdiction: Its implications on visual awareness, hindsight, and sense of agency. Front. Psychol. 2014, 5, 196. [Google Scholar] [CrossRef] [Green Version]
- Cleary, A.M.; Huebert, A.M.; McNeely-White, K.L.; Spahr, K.S. A postdictive bias associated with déjà vu. Psychon. Bull. Rev. 2019, 26, 1433–1439. [Google Scholar] [CrossRef]
- Frith, C.D.; Blakemore, S.J.; Wolpert, D.M. Abnormalities in the awareness and control of action. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1771–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daprati, E.; Franck, N.; Georgieff, N.; Proust, J.; Pacherie, E.; Dalery, J.; Jeannerod, M. Looking for the agent: An investigation into consciousness of action and self-consciousness in schizophrenic patients. Cognition 1997, 65, 71–86. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.; Haggard, P. Awareness of action: Inference and prediction. Conscious. Cogn. 2008, 17, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Plinio, S.; Arnò, S.; Perrucci, M.G.; Ebisch, S.J.H. The evolving sense of agency: Context recency and quality modulate the interaction between prospective and retrospective processes. Conscious. Cogn. 2020, 80, 102903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jauhar, S.; McKenna, P.J.; Radua, J.; Fung, E.; Salvador, R.; Laws, K.R. Cognitive-behavioural therapy for the symptoms of schizophrenia: Systematic review and meta-analysis with examination of potential bias. Br. J. Psychiatry 2014, 204, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wykes, T.; Steel, C.; Everitt, B.; Tarrier, N. Cognitive behavior therapy for schizophrenia: Effect sizes, clinical models, and methodological rigor. Schizophr. Bull. 2008, 34, 523–537. [Google Scholar] [CrossRef]
- Mehl, S.; Werner, D.; Lincoln, T.M. Does Cognitive Behavior Therapy for psychosis (CBTp) show a sustainable effect on delusions? A meta-analysis. Front. Psychol. 2015, 6, 1450. [Google Scholar] [CrossRef] [Green Version]
- Morrison, A.P.; Law, H.; Carter, L.; Sellers, R.; Emsley, R.; Pyle, M.; French, P.; Shiers, D.; Yung, A.R.; Murphy, E.K.; et al. Antipsychotic drugs versus cognitive behavioural therapy versus a combination of both in people with psychosis: A randomised controlled pilot and feasibility study. Lancet Psychiatry 2018, 5, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Morrison, A.P.; Turkington, D.; Pyle, M.; Spencer, H.; Brabban, A.; Dunn, G.; Christodoulides, T.; Dudley, R.; Chapman, N.; Callcott, P.; et al. Cognitive therapy for people with schizophrenia spectrum disorders not taking antipsychotic drugs: A single-blind randomised controlled trial. Lancet 2014, 383, 1395–1403. [Google Scholar] [CrossRef] [Green Version]
- Morrison, A.P.; Pyle, M.; Maughan, D.; Johns, L.; Freeman, D.; Broome, M.R.; Husain, N.; Fowler, D.; Hudson, J.; MacLennan, G.; et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): A multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry 2020, 7, 788–800. [Google Scholar] [CrossRef]
- Kapur, S. How antipsychotics become anti-“psychotic”—From dopamine to salience to psychosis. Trends Pharmacol. Sci. 2004, 25, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A.; Beck, A.T. Cognitive theory and therapy of anxiety and depression: Convergence with neurobiological findings. Trends Cogn. Sci. 2010, 14, 418–424. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Williams, J.B.W.; Karg, R.S.; Spitzer, R.L. Structured Clinical Interview for DSM-5® Disorders—Clinician Version (SCID-5-CV); American Psychiatric Publishing: Washington, DC, USA, 2016. [Google Scholar]
- Van der Gaag, M.; Hoffman, T.; Remijsen, M.; Hijman, R.; de Haan, L.; van Meijel, B.; van Harten, P.N.; Valmaggia, L.; de Hert, M.; Cuijpers, A.; et al. The five-factor model of the Positive and Negative Syndrome Scale II: A ten-fold cross-validation of a revised model. Schizophr. Res. 2006, 85, 280–287. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Peters, E.; Joseph, S.; Day, S.; Garety, P. Measuring delusional ideation: The 21-item Peters et al. Delusions Inventory (PDI). Schizophr. Bull. 2004, 30, 1005–1022. [Google Scholar] [CrossRef]
- Landa, Y. Cognitive behavioral therapy for psychosis (CBTp): An introductory manual for clinicians. Ment. Illn. Res. Educ. Clin. Cent. 2017. Available online: https://www.mirecc.va.gov/visn2/docs/CBTp_Manual_VA_Yulia_Landa_2017.pdf (accessed on 19 February 2019).
- Blackburn, I.-M.; James, I.A.; Milne, D.L.; Baker, C.; Standart, S.; Garland, A.; Reichelt, F.K. The revised cognitive therapy scale (CTS-R): Psychometric properties. Behav. Cogn. Psychother. 2001, 29, 431–446. [Google Scholar] [CrossRef]
- Kapur, S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 2003, 160, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Katthagen, T.; Kaminski, J.; Heinz, A.; Buchert, R.; Schlagenhauf, F. Striatal Dopamine and Reward Prediction Error Signaling in Unmedicated Schizophrenia Patients. Schizophr. Bull. 2020, 46, 1535–1546. [Google Scholar] [CrossRef] [Green Version]
- Maia, T.V.; Frank, M.J. An integrative perspective on the role of dopamine in schizophrenia. Biol. Psychiatry 2017, 81, 52–66. [Google Scholar] [CrossRef] [Green Version]
- Millard, S.J.; Bearden, C.E.; Karlsgodt, K.H.; Sharpe, M.J. The prediction-error hypothesis of schizophrenia: New data point to circuit-specific changes in dopamine activity. Neuropsychopharmacology 2022, 47, 628–640. [Google Scholar] [CrossRef]
- Yuan, S.; Wu, H.; Wu, Y.; Xu, H.; Yu, J.; Zhong, Y.; Zhang, N.; Li, J.; Xu, Q.; Wang, C. Neural Effects of Cognitive Behavioral Therapy in Psychiatric Disorders: A Systematic Review and Activation Likelihood Estimation Meta-Analysis. Front. Psychol. 2022, 13, 853804. [Google Scholar] [CrossRef] [PubMed]
- Fatouros-Bergman, H.; Cervenka, S.; Flyckt, L.; Edman, G.; Farde, L. Meta-analysis of cognitive performance in drug-naïve patients with schizophrenia. Schizophr. Res. 2014, 158, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddi, S.; Petretto, D.R.; Preti, A. Neuropsychological correlates of schizotypy: A systematic review and meta-analysis of cross-sectional studies. Cogn. Neuropsychiatry 2017, 22, 186–212. [Google Scholar] [CrossRef] [PubMed]
- Thoenes, S.; Oberfeld, D. Meta-analysis of time perception and temporal processing in schizophrenia: Differential effects on precision and accuracy. Clin. Psychol. Rev. 2017, 54, 44–64. [Google Scholar] [CrossRef] [PubMed]

| Controls (n = 25) | Antipsychotics (n = 25) | Antipsychotics Plus CBT (n = 25) | |
|---|---|---|---|
| Male/female | 15/10 | 17/8 | 18/7 |
| Age (years) | 38.8 (12.9) | 39.8 (10.9) | 35.9 (9.2) |
| Education (years) | 11.4 (2.3) | 11.6 (2.7) | 11.5 (2.5) |
| Duration of illness (years) | - | 8.7 (2.6) | 9.3 (2.8) |
| Type of antipsychotics | - | Olanzapine (n = 11) Amisulpride (n = 7) Clozapine (n = 6) | Olanzapine (n = 13) Amisulpride (n = 5) Clozapine (n = 6) |
| df | F | p | η2 | |
|---|---|---|---|---|
| Main effect of group (antipsychotics, antipsychotics + CBT, non-clinical controls) | 2, 72 | 3.47 | 0.04 | 0.09 |
| Main effect of treatment (week 0 vs. 12) | 1, 72 | 12.91 | 0.001 | 0.15 |
| Main effect of delay (100 vs. 1000 ms) | 1, 72 | 80.25 | <0.0001 | 0.53 |
| Group by treatment interaction | 2, 72 | 4.02 | 0.02 | 0.10 |
| Group by delay interaction | 2, 72 | 2.25 | 0.11 | 0.06 |
| Treatment by delay interaction | 1, 72 | 9.12 | 0.004 | 0.11 |
| Group by treatment by delay interaction | 2, 72 | 5.36 | 0.007 | 0.13 |
| df | F | p | η2 | |
|---|---|---|---|---|
| Main effect of group (antipsychotics, antipsychotics + CBT, non-clinical controls) | 2, 72 | 0.79 | 0.46 | 0.02 |
| Main effect of treatment (week 0 vs. 12) | 1, 72 | 0.14 | 0.71 | 0.001 |
| Main effect of delay (100 vs. 1000 ms) | 1, 72 | 181.44 | <0.0001 | 0.72 |
| Group by treatment interaction | 2, 72 | 0.04 | 0.96 | 0.001 |
| Group by delay interaction | 2, 72 | 1.13 | 0.33 | 0.03 |
| Treatment by delay interaction | 1, 72 | 0.01 | 0.93 | 0.0001 |
| Group by treatment by delay interaction | 2, 72 | 0.27 | 0.76 | 0.008 |
| df | F | p | η2 | |
|---|---|---|---|---|
| Main effect of group (antipsychotics, antipsychotics + CBT, non-clinical controls) | 2, 2 | 12.03 | 0.00003 | 0.25 |
| Main effect of treatment (week 0 vs. 12) | 1, 72 | 0.19 | 0.67 | 0.003 |
| Group by treatment interaction | 2, 72 | 2.54 | 0.09 | 0.07 |
| df | F | p | η2 | |
|---|---|---|---|---|
| PANSS—Positive | ||||
| Group | 1, 48 | 0.004 | 0.95 | 0 |
| Treatment | 1, 48 | 89.80 | <0.0001 | 0.65 |
| Group by treatment | 1, 48 | 18.48 | 0.0001 | 0.28 |
| PANSS—Negative | ||||
| Group | 1, 48 | 1.43 | 0.24 | 0.03 |
| Treatment | 1, 48 | 27.05 | <0.0001 | 0.36 |
| Group by treatment | 1, 48 | 0.72 | 0.40 | 0.02 |
| PANSS—Disorganized | ||||
| Group | 1, 48 | 1.21 | 0.28 | 0.02 |
| Treatment | 1, 48 | 65.34 | <0.0001 | 0.58 |
| Group by treatment | 1, 48 | 1.68 | 0.20 | 0.03 |
| PANSS—Excitement | ||||
| Group | 1, 48 | 0.24 | 0.62 | 0.01 |
| Treatment | 1, 48 | 85.65 | <0.0001 | 0.64 |
| Group by treatment | 1, 48 | 1.91 | 0.17 | 0.04 |
| PANSS—Emotional discomfort | ||||
| Group | 1, 48 | 0.003 | 0.96 | 0 |
| Treatment | 1, 48 | 105.91 | <0.0001 | 0.69 |
| Group by treatment | 1, 48 | 9.35 | 0.004 | 0.16 |
| PDI—Conviction | ||||
| Group | 1, 48 | 0.05 | 0.83 | 0.001 |
| Treatment | 1, 48 | 21.38 | <0.0001 | 0.31 |
| Group by treatment | 1, 48 | 4.87 | 0.03 | 0.09 |
| PDI—Preoccupation | ||||
| Group | 1, 48 | 0.23 | 0.63 | 0.005 |
| Treatment | 1, 48 | 106.87 | <0.0001 | 0.69 |
| Group by treatment | 1, 48 | 21.66 | <0.0001 | 0.31 |
| PDI—Distress | ||||
| Group | 1, 48 | 0.46 | 0.50 | 0.009 |
| Treatment | 1, 48 | 65.0 | <0.0001 | 0.58 |
| Group by treatment | 1, 48 | 4.39 | 0.04 | 0.08 |
| 0 Week | 12 Weeks | 0 vs. 12 Weeks | ||||
|---|---|---|---|---|---|---|
| Antipsychotics (n = 25) | Antipsychotics + CBT (n = 25) | Antipsychotics (n = 25) | Antipsychotics + CBT (n = 25) | Antipsychotics | Antipsychotics + CBT | |
| PANSS—Positive | 21.5 (7.5) | 23.6 (6.8) | 19.2 (7.7) | 17.3 (6.1) | d = 0.30 p = 0.004 | d = 0.98 p < 0.001 |
| PANSS—Negative | 15.5 (4.1) | 16.4 (4.3) | 13.2 (3.9) | 14.8 (3.3) | d = 0.58 p = 0.001 | d = 0.45 p < 0.05 |
| PANSS—Disorganized | 13.0 (2.0) | 12.7 (2.1) | 11.6 (1.8) | 10.8 (2.1) | d = 0.73 p < 0.001 | d = 0.90 p < 0.001 |
| PANSS—Excitement | 15.8 (3.1) | 16.0 (3.6) | 12.7 (2.6) | 11.8 (2.9) | d = 1.09 p < 0.001 | d = 1.29 p < 0.001 |
| PANSS—Emotional discomfort | 21.8 (4.1) | 23.2 (4.6) | 18.4 (3.8) | 17.0 (4.4) | d = 0.86 p < 0.001 | d = 1.38 p < 0.001 |
| PDI—Conviction | 50.5 (26.8) | 58.5 (26.3) | 43.0 (19.7) | 37.3 (18.7) | d = 0.31 p < 0.05 | d = 0.94 p < 0.001 |
| PDI—Preoccupation | 43.7 (20.0) | 47.8 (19.9) | 35.9 (15.5) | 27.1 (16.3) | d = 0.44 p = 0.001 | d = 1.14 p < 0.001 |
| PDI—Distress | 43.5 (28.7) | 42.3 (26.1) | 34.0 (23.2) | 22.6 (17.8) | d = 0.37 p = 0.001 | d = 0.90 p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kéri, S. Postdiction in Visual Awareness in Schizophrenia. Behav. Sci. 2022, 12, 198. https://doi.org/10.3390/bs12060198
Kéri S. Postdiction in Visual Awareness in Schizophrenia. Behavioral Sciences. 2022; 12(6):198. https://doi.org/10.3390/bs12060198
Chicago/Turabian StyleKéri, Szabolcs. 2022. "Postdiction in Visual Awareness in Schizophrenia" Behavioral Sciences 12, no. 6: 198. https://doi.org/10.3390/bs12060198
APA StyleKéri, S. (2022). Postdiction in Visual Awareness in Schizophrenia. Behavioral Sciences, 12(6), 198. https://doi.org/10.3390/bs12060198





