Abstract
Depression is the most common behavior disorder that leads to many disabilities. The main aim of this study was to evaluate the effects of a Polygonum minus (P. minus) aqueous extract on chronic ultra-mild stress (CUMS)-induced depressive mice model. Chronic ultra-mild stress can disturb the neurotransmitters levels and plasticity of the hippocampus. Balb/c male mice were used in this study, which consisted of six groups (n = 14). Treatment was given for eight weeks, and chronic ultra-mild stress was applied for six weeks. Commercially available P. minus extract (BioKesum®) was used in this study. The behavior and neurochemical parameters were investigated through behavioral Tests and ELISA assays. P. minus administration significantly (p < 0.05) restored CUMS-induced behavior abnormalities, decreased the immobility time, and increased the sucrose preference and increased the spatial memory. P. minus treatment also showed the decreased level of serum corticosterone and increased the level of hippocampal neurotransmitters (Serotonin and Norepinephrine) significantly (p < 0.05). The brain-derived neurotrophic factor (BDNF) level also increased significantly in both the prefrontal cortex and hippocampus (p < 0.05). P. minus treatment exhibited significant (p < 0.05) reduction of Monoamine Oxidase-A (MAO-A) in the hippocampus. These findings indicate that P. minus aqueous extract exhibits antidepressant effects, including decreased immobility time, increased spatial memory, reduced corticosterone, increased BDNF level, and reduced MAO-A enzyme level with increasing the monoamines (serotonin and norepinephrine) in the hippocampus.
1. Introduction
Depression is a common illness around the world. According to a WHO report, around 350 million people of all ages are influenced by this disorder. It is a heterogeneous syndrome and can lead to poorer quality of life. In the worst case, depression can also lead to suicide [1,2]. Several clinical features have been linked to the severity levels of mood disorders including cognitive impairment, anxiety, insomnia, and hopelessness [3,4]. Depressive disorders are expensive and are associated with significant morbidity and mortality as well [5]. There are some hypotheses related to the pathophysiology of depression, such as the neurotrophic hypothesis (reduction of a neurotrophic factor in the brain, imbalance of monoaminergic systems (imbalance of serotonergic, noradrenergic and dopaminergic neurotransmission), and HPA axis dysfunction (hypercortisolemia) hypothesis [6,7]. Clinical evidence also suggested that disorders of serotonin (5-HT) [8], norepinephrine (NE), and dopaminergic neurotransmission in the central nervous system are the main causes of depression. Almost all currently available antidepressants act on one or more of the stated mechanisms, such as the inhibition of 5-HT or NE reuptake, antagonism of inhibitory presynaptic 5-HT or NE receptors, or the inhibition of monoamine oxidase. All these mechanisms lead to an increase in the concentration of 5-HT and NE in the brain [9]. Another target for treating depression is a monoamine oxidase (MAO), an enzyme responsible for breaking down monoamines [10]. Many researchers concluded that flavonoids, alkaloids, coumarins, and their derivatives from natural herbal medicines have a strong MAO-A inhibition ability. These natural drugs became suitable choices for screening MAO-A inhibitors, as they show a synergistic pattern with antidepressant-like effects. A large variety of plant extracts and their isolated components with MAO-A inhibitory activity are now used for treating depression [11,12]. New antidepressant agents are needed that are inexpensive, rapid-acting, effective, and safe [13].
Stress is a common reason for depression. Chronic stress causes major depressive disorders by altering neurotrophic and neuroinflammatory factors in an organism [14]. The chronic ultra-mild Stress (CUMS) is an authentic, validated, and widely used animal model of depression that causes the alteration of animal behavior and nervous system and consists of a different schedule of minor stress protocols [15,16]. Recent studies have reported that the chronic ultra-mild stress (CUMS) procedure—which is the prolonged recurrence of low-intensity stressors, including social isolation, circadian rhythm disturbances, and changes in daily living habits—induces mood disorders in mice [17]. Neurobiological abnormalities in mice treated with CUMS would be similar to those observed in patients with depression, such as decreased BDNF in the hippocampus or the suppression of neurogenesis. The hippocampus is one of the limbic structures associated with emotional responses and is the most studied brain region in depression research. From the point of view of neuronal plasticity, it is generally accepted that hippocampal plasticity plays an important role in the onset and development of depression. Therefore, measuring neurotransmitters in the hippocampus that target the brain is very useful for studying the mechanisms underlying disease processes and treatment goals for chronic ultra-mild depressive stress [17,18].
Polygonum minus Huds, the synonym for Persicaria minor comes from the Polygonaceae family and is commonly known as Kesum or Laksa leaf in Malaysia. It is used as a flavoring in many dishes and is also consumed as ulam (salad) for health care [19]. The P. minus leaf extract contained flavonoids such as quercitrin (quercetin 3-rhamnoside) and quercetin 3. glucuronide has been suggested to have antioxidant, anti-inflammatory, antimicrobial, and cognitive-enhancing properties. An aqueous extract P. minus showed significant enhancing effects on memory and cognitive functions with a 100 mg/kg dose in mic [20] e. Recent research using P. minus extract has shown significant improvements in attention and memory, mood, and quality of life among middle-aged women after six weeks of administration. BioKesum is a Malaysian brand of P. minus leaf extract which consist of standardized flavonoids including quercitrin and quercetin 3. glucuronide. The latest research of six months of BioKesum® administration potentially improved visual memory, and negative mood among older patients but its mechanism was unknown. BioKesum® was developed and registered under the National Pharmaceutical Control Bureau (NPCB) with the registration number of MAL14015033T [21]. The anti-depressant potential of this plant regarding its effects on MAO-A enzyme, BDNF, neurotransmitters (serotonin and norepinephrine), and stress hormones such as corticosterone, were undiscovered, which is why this plant has been selected for further research in this study.
The main aim of this study was to evaluate the effects of Polygonum minus aqueous extract on stress-induced depressive, and anxiety behavior in mice through measurement of various neurochemicals.
The current study investigated the effects of Polygonum minus on chronic ultra-mild stress-induced mice models through various behavioral and experimental assays. Behavioral tests consisted of the sucrose preference test (SPT), Barnes Maze assay, open field test (OFT), and forced swimming test (FST). ELISA assays were performed for the measurement of the brain-derived neurotrophic factor (BDNF), serotonin (5HT), norepinephrine (NE), monoamine oxidase A (MAO-A, and serum corticosterone level.
Male Balb/c mice (n = 14) were used in this study because the latest research about the cognitive effect of this extract was on male mice [20]. The study consisted of six groups. The control group was treated with simple distilled water, and the negative control was a simple CUMS group (the only stress group without treatment, though distilled water was administered); the positive control received amitriptyline at 20 mg with CUMS, and there were three treatment groups based on different doses (P. minus 50 mg,100 mg, and 200 mg with CUMS). Treatment was continued for eight weeks and chronic ultra-mild stress was applied for six weeks. The chronic ultra-mild stress protocol included some interventions like restraining stress, food, and water deprivation, wet bedding, cage tilting, tail pinching, foreign object incorporation, forced swimming, crowded housing, and continuous lighting.
2. Materials and Methods
2.1. Chemicals and Drugs
BioKesum®, a water-soluble Polygonum minus aqueous extract containing flavonoids extract from its leaf part, as commercially available, was obtained from Biotropics Malaysia Berhad. Amitriptyline (Apotex Inc, Toronto, ON, Canada), serotonin, norepinephrine, monoamine oxidase A, serum corticosterone, and brain-derived neurotrophic factor (BDNF) Elisa kits were purchased from (Qayee Bio-Technology Co., Ltd. Unit 201–229 Shanghai, China). 70% Ethanol, Sucrose, and Phosphate buffer (Sigma-Aldrich, Inc, Taufkirchen, Germany).
2.2. Animals and Experimental Protocol
Adult male balb/c mice (22–26 g) were purchased from the Animal Research and Service Centre, Kelantan Campus, Universiti Sains Malaysia. All experimental procedures were carried out after getting the approval from USM Institutional Animal Care and Use Committee (USM IACUC) of Universiti Sains Malaysia (No of Animal Ethical Approvals: USM/IACUC/2020/(123)(1075) & USM/IACUC/2021/ (127) (1129).
The animals were housed under standard environmental conditions (24 ± 1 °C) in the Animal Transit Room at the School of Pharmaceutical Sciences, Universiti Sains, Malaysia. They were maintained on a 12-h light/dark cycle. The animals were randomly separated into six experimental groups. A chronic ultra-mild stress method was adopted to induce stress in the animals. The normal control group was without any chronic ultra- mild stress (CUMS), and the remaining groups were influenced by chronic ultra-mild stress (CUMS). Chronic ultra-mild stress remained for six weeks. Treatment remained for eight weeks means until the end of behavioral tests and sampling.
Group 1: Control group (Without CUMS) treated with distilled water only (n = 14). Group 2: (CUMS group, a negative Control) Chronic ultra- mild stress (CUMS) treated with 6 weeks of CUMS and distilled water for 8 weeks (n = 14). Group 3: CUMS + Amitriptyline 20 mg, a Positive Control (n = 14). Group 4: CUMS + P. minus treated group with 50 mg/kg (n = 14). Group 5: CUMS + P. minus treated group with 100 mg/kg (n = 14). Group 6: CUMS + P. minus treated group with 200 mg/kg (n = 14). Treatment was given through the oral route by using Oral gavage for mice. A complete design of the study is expressed in Figure 1.
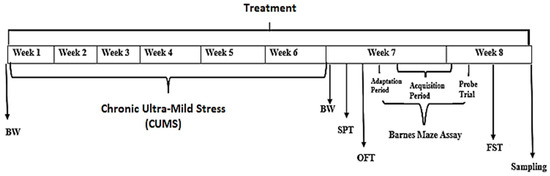
Figure 1.
Schematic experimental design. BW means measurement of body weight, SPT means Sucrose preference test, OFT means Open field test and FST stands for Forced swimming test.
2.3. Chronic Ultra Mild Stress Protocol
Chronic Ultra mild stress was given for 6 weeks. A weekly stress plan was made as shown in Table 1. Two different stressors were given on daily basis, first stress was applied in the morning and the second stress in the evening as followed by the latest research [16,22].

Table 1.
Chronic ultra-mild stress weekly protocol.
2.4. Body Weight Test
The body weight of each animal was measured at 0 day and after 6 weeks.
2.5. Behavioral Tests
Sucrose Preference Test
Mice were exposed to both the test solution (1% sucrose) and tap water for a 24 h period before 2 days from the experimental trial. Mice were food and water-deprived for 14 h before the start of the experiment. During the test, sucrose preference was evaluated for 6 h by using two bottles of 1% sucrose and tap water and the position of the bottles was switched after 3 h. The sucrose preference was calculated by the ratio of the consumed sucrose solution to the total amount of liquid consumed [23].
2.6. Open Field Test
The open-field test is a classic and well-validated test for detecting the anxiety and locomotion activity of animals. Many researchers explained that an anxious object shows less movement in the central zone as compared to normal, and they spend more time in the periphery and not in the central zone. Thigmotaxis theory and rearing can also provide an idea as an exploratory measure for anxiety [24,25]. An automated open-field apparatus (Pan Lab, Spain) was used to detect the parameters as distance travelled in the central zone, total distance travelled, time spent in the central zone, and the number of rearing. The apparatus had a Perspex cage (height: 40 cm, length: 90 cm; width: 90 cm), with the five zones divided into the bottom. The detection unit consisted of a 45 × 45 cm frame containing a total of 16 × 16 infrared beams at an interval of 2.5 cm located on the sides, and there were 32 cells in one frame. The mice’s movements were stored and analyzed. For testing, each animal was placed in the center of the open field and spontaneous activity (travelling and rearing) was recorded for 10 min (n = 14/group) [26].
2.7. Barnes Maze Assay
The Barnes Maze procedure was first developed by Carol Barnes. It is a behavioral test based on dry-land. It was developed to study spatial memory in rodents [27]. The Barnes Maze consisted of a wooden circular platform with 12 holes placed 2.5 cm from the edge, and all holes were equally distributed around the surface. Its surface platform was 92 cm in diameter and 92 cm above the ground. The Barnes Maze takes advantage of the animal’s natural reluctance to open up lit places. Therefore, subjects are motivated by a bright light to locate an escape hole that leads to a dark box. The Barnes Maze test consisted of three phases: an adaptation period, an acquisition period, and a probe trial. Before starting the test on the first day, a preliminary test (adaptation period) was performed. In this study, the acquisition trial consisted of 5 days and then a probe trial was performed twenty-four hours after the final acquisition trial. The behavior of the experimental subject was captured by a video camera and was recorded on the computer. Four parameters were observed in this assay: the total path length, number of errors (number of wrong holes explored during acquisition trail), latency (the time taken to escape from target hole during acquisition trial days), and the percentage of time spent during the probe trial [23]. Kinovea® (0.8.15) a video tracking software was used to track the path length.
2.7.1. Adaptation Period
During this period, the subject was placed in the center area on the maze surface. A chamber was placed on mice for 10 s after it was removed, and the subject was allowed to explore the maze for 30 s, then was gently guided towards the escape hole. If the subject did not enter the escape hole, it was placed inside the black box. The hole was then covered and the mice remained there for 3 min. After this, the subject was returned to its original cage and the surface of the maze was cleaned with 70% ethanol.
2.7.2. Acquisition Period
In this period, the subject could explore the maze for 8 min and then be gently guided to the escape hole. If the subject did not enter the escape hole, it was placed inside. The hole was then covered and the subject remained there for 1 min. The subject was returned to its home cage and the platform was cleaned with 70% ethanol. All movements of mice were recorded by the camera every day.
2.7.3. Probe Trial
In the probe trial, the subject was placed in the center zone of the maze surrounded by a chamber for 10 s. The chamber was removed and the subject was fully allowed to explore the area for sharp 90 s after the subject was returned and the surface was cleaned with 70% alcohol [28].
2.7.4. Forced Swimming Test
Mice were introduced into a glass cylinder (50 cm deep, 30 cm in diameter) filled with water up to a height of 40 cm from the base and forced to swim for 6-min. The swim session was recorded by a video recorder. After each swimming session, the mice were removed from the cylinder, dried with towels, and placed in a temporary resting cage for 20 min, and then mice were returned to their home cage. Water in the cylinder was renewed after every 6 subjects. The time of swimming and immobility was recorded using a stopwatch. The duration of each behavior was calculated [29].
2.8. Experimental Assays
Blood Sample Collection
The blood samples were collected by cardiac puncture and were kept at room temperature for 30 min, then centrifuged at 3000 rpm for 20 min for serum collection, and stored at −80 °C for the corticosterone assays [11].
2.9. Brain Tissue Sample Collection and Homogenization
The mice were sacrificed, and the brains were quickly removed on ice. The prefrontal cortex and hippocampus were isolated and stored at −80 °C. Further at the time of assays, the prefrontal cortex and hippocampus were weighed and homogenized by 1:4 w/v with phosphate buffer saline (PBS- Sigma 7.2–7.6). The supernatant was collected for further assays after centrifuging it at 12,000 rpm [11].
2.10. Serum Corticosterone Assay
Serum corticosterone level was measured by using an enzyme-linked immunosorbent assay (ELISA) kit (Qayee Bio-Technology Co., Ltd. Unit 201–229 Shanghai, China) by following the manufacturer’s protocol at 450 nm absorbance in a microplate reader.
2.11. Measurement of Brain-Derived Neurotrophic Factor (BDNF) Level
The brain-derived neurotrophic factor BDNF level was measured from both hippocampus and prefrontal cortex separately by using an enzyme-linked immunosorbent assay kit (ELISA) kit (Qayee Bio-Technology Co., Ltd. Unit 201–229 Shanghai, China) by following the manufacturer’s protocol at 450 nm absorbance in a microplate reader.
2.12. Measurement of Serotonin (5HT) and Norepinephrine (NE), Monoamine Oxidase A (MAO-A) from the Hippocampus
The concentrations of serotonin, norepinephrine, and monoamine oxidase A (MAO-A) were measured from the hippocampus only by Elisa Kits (Qayee Bio-Technology Co., Ltd. Unit 201–229 Shanghai, China) at 450 nm by using a microplate reader and all protocol was followed absolutely as provided by the manufacturer.
2.13. Statistical Analysis
Statistical analysis was performed using SPSS version 26 statistical software (IBM, New York, NY, USA). Most data were analyzed by one-way ANOVA followed by post hoc comparisons between all groups by using Tukey HSD, except for the Barnes Maze assay. For the Barnes Maze assay, data of three parameters (travelled distance, latency, and the number of errors) were analyzed by repeated measure ANOVA. Differences in mean values with p < 0.05 were considered as a level of significance in all tests. The means were calculated with standard error means (SEM).
3. Results
3.1. Effects of P. minus Aqueous Extract on Body Weight
Figure 2 shows the relationship between the body weight of mice at zero-day and after 6 weeks. The body weight results showed an increasing trend in all treatment groups significantly in comparison to their zero-day measurements, except for the CUMS group. The CUMS group showed a non-significant difference in body weight as compared to its zero-day value.
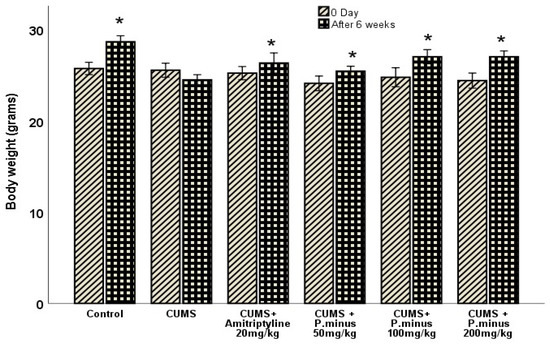
Figure 2.
Effects of P. minus aqueous extract on body weight of CUMS induced stressed mice. * (p < 0.05), vs. zero-day of each group, (n = 14).
3.2. Behavioral Tests Results
Effects of P. Minus Aqueous Extract on Sucrose Preference Test.
As depicted in Figure 3, the CUMS group showed a significant (p < 0.05) decrease in sucrose preference in comparison to the control group, which was improved in all treatment groups significantly compared to CUMS group. CUMS+ Amitriptyline 20 mg showed larger, and CUMS + P. minus 50 mg showed minimum sucrose preference among all treated groups.
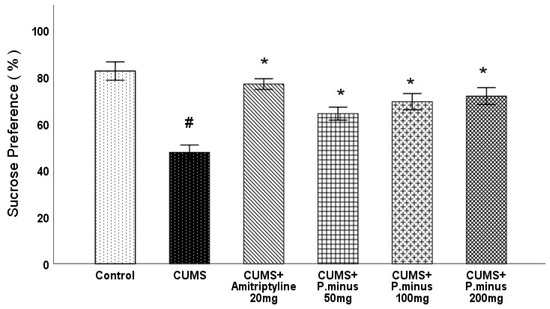
Figure 3.
Effects of P. minus aqueous extract on sucrose preference test in CUMS induced depressive mice. # (p < 0.05), vs. Control group; * (p < 0.05), vs. CUMS group, (n = 14).
3.3. Effects of P. minus Aqueous Extract on Open Field Test
Open field test results have been expressed in four parts: total distance travelled by mice (Figure 4A), percentage of time spent in the central zone (Figure 4B), distance travelled in the central zone (Figure 4C), and no of rearing (Figure 4D). In the first part (A), the CUMS group showed a less travelled distance with a p < 0.05 level of significance as compared to the control, which was only reversed by CUMS + Amitriptyline 20 mg group by p < 0.05 significance. The remaining CUMS+ P. minus treated groups showed a non-significant increase in travelled distance as compared to CUMS group. For the percentage of time spent and covered distance in the central zone, results have been shown in Figure 4B,C respectively, which expressed that the CUMS group had shown a significantly (p < 0.05) small residence time and shorter distance in the central zone in comparison to the control group. Only CUMS + Amitriptyline 20 mg treatment improved timings and travelled distance in the central zone significantly (p < 0.05), whereas the CUMS + P. minus 50 mg,100 mg and 200 mg groups showed non-significance improvement in both timings and distance as compared to CUMS group. Results for rearing behavior are presented in Figure 4(D), which indicates that there was no significant difference between the control and CUMS groups, as well as all remaining treated groups had non-significant differences in comparison to the CUMS group.
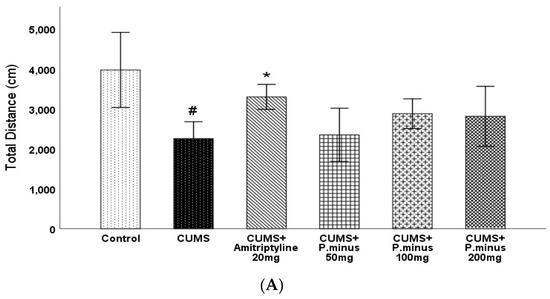
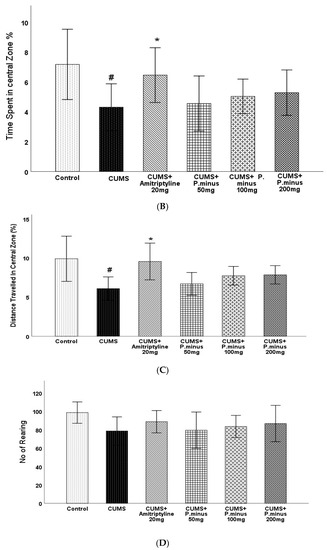
Figure 4.
Effects of P. minus aqueous extract on Open Field Test in CUMS induced depressive mice. (A) Total distance travelled (cm), (B) Time spent in central zone (%), (C) Distance travelled in central zone. (D). No of rearing # (p < 0.05), vs. Control group; * (p < 0.05), vs. CUMS group. (n = 14).
3.4. Effects of P. minus Aqueous Extract on Barnes Maze Assay
The results of the Barnes maze are expressed in Figure 5. As shown in (A) CUMS group had travelled a larger distance as compared to the Control group significantly, which showed that the control group had completed its task by covering less distance in comparison to CUMS group. On day 1, there was no significant difference between CUMS group and treatment groups, but on Day 2, significant differences appeared. From Day 2 to Day 4 all treatment groups (CUMS + Amitryptyline20 mg, CUMS + P. minus 100 mg, CUMS+ P. minus 200 mg) except CUMS + P. minus 50 mg showed a significant less travelled distance as compare to CUMS. On the last day of the acquisition trial (Day 5), only CUMS + Amitriptyline 20 mg and CUMS+ P. minus 200 mg showed a significant decrease in the travelled distance in comparison to CUMS group. Part B of Figure 5 presented the results for latency time (the taken by subjects to escape from the hole). According to the results, CUMS group showed a significantly higher latency period as compared to the control in the last two days (Day 4 and Day 5), whereas, on Day 2, this difference was also significant. Day 1 results showed no significant difference between any groups, from day 2 to day 5 CUMS + Amitriptyline 20 mg group showed significantly less latency time in comparison to the simple CUMS group. It was observed that among P. minus treatment groups CUMS + P. minus 100 mg and CUMS + P. minus 200 mg showed significant less latency in comparison to CUMS group. The same pattern had been observed in CUMS+ P. minus 200 mg for the last 2 days (Day 4 and Day 5), whereas CUMS + P. minus 100 mg expressed this result only on the last day (Day 5). Figure 5 presents results for the number of errors during the acquisition trial in part C. Results related to this part showed that CUMS group had made larger numbers of errors on all days during the acquisition trial, but a significant difference was reported on Day3 and Day 4 by p < 0.05, whereas Day 5 was also significant. In the case of comparison between the treatment group and CUMS group, there was no significant difference in these groups from Day 1 to Day 3, at Day 4 CUMS + Amitriptyline showed a significantly small number of errors by p < 0.05, and on Day 5 this group also showed significance variance with decreased number of errors. Among P. minus treated groups, CUMS + P. minus 100 mg showed a significantly small number of errors on Day 5 with p < 0.05, and CUMS + P. minus 200 mg group also showed a significance in the decreased number of errors on Day 5. The results of the probe trial in form of percentage time spent by subjects to achieve their task in fixed provided time have been shown in the D part of Figure 5, these results expressed that CUMS group had spent significant (p < 0.05) time to reach their destination in comparison of Control group. CUMS + Amitriptyline 20 mg, CUMS + P. minus 100 mg, and CUMS + P. minus 200 mg groups have been showing a significantly shorter time to escape from a hole than the CUMS group.
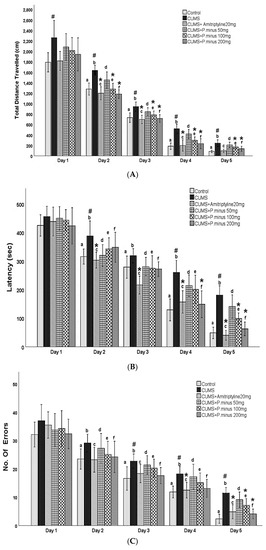
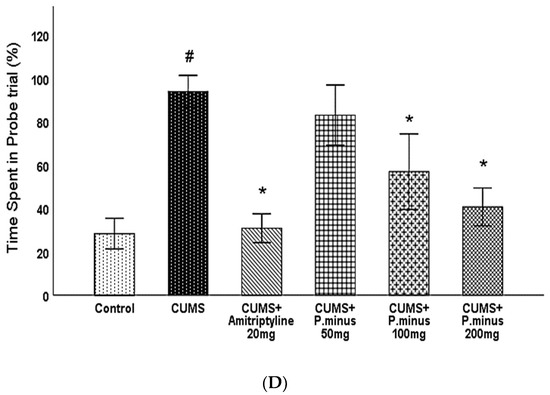
Figure 5.
Effects of P. minus aqueous extract on Barnes Maze Assay in CUMS induced depressive mice. (A)Total distance travelled (cm), (B) Latency (sec), (C) No of Errors (D) Time spent during prob trial # (p < 0.05), vs. Control group; * (p < 0.05), vs. CUMS group with respect to each day. CUMS group. a showed significant variance between the Control group (Day 1) and control groups from other days, b showed significant variance between CUMS group (Day 1) and other CUMS groups. c showed significant variance between CUMS + Amitriptyline20 mg group (Day 1) with other CUMS + Amitriptyline 20 mg groups on different days. d showed significant variance in between P. minus 50 mg group (Day 1) with other P. minus 50 mg groups on different days. e showed significant variance between P. minus 100 mg group (Day 1) with other P. minus 100 mg groups on different days, whereas f showed significant variance between P. minus 200 mg group (Day 1) with other P. minus 200 mg groups on different days. Whereas n = 14.
As a whole, the results of Barnes maze analysis showed that all groups learnt about the task day by day, but CUMS group and CUMS + P. minus 50 mg group were significantly slower than other groups, whereas CUMS + Amitriptyline, CUMS + P. minus 200 mg and CUMS + P. minus 100 mg showed significantly better power of learning and efficient memory characteristics than CUMS group.
3.5. Effects of P. minus Aqueous Extract on Forced Swimming Test (FST)
As sketched in Figure 6A, CUMS group showed an increased immobility time which indicates that CUMS had induced depression significantly which was reversed in all treatment groups. CUMS + Amitriptyline 20 mg and CUMS + P. minus 200 mg reduced immobility by p < 0.05 level of significance, whereas CUMS + P. minus 100 mg and 50 mg treated groups also reduced immobility significantly. The results of swimming behavior during the forced swimming test are presented in (B) part of Figure 6, which expressed that swimming time was significantly less in CUMS group as compared to the Control. This reduced swimming time was significantly enhanced in CUMS + Amitriptyline 20 mg, CUMS + P. minus 200 mg and P. minus 100 mg. The significance level of improved swimming behavior in CUMS + P. minus 50 mg treated group was p < 0.05. The results demonstrate the anti-depressant effect of Amitriptyline and P. minus treated groups with CUMS induced depression model of mice.
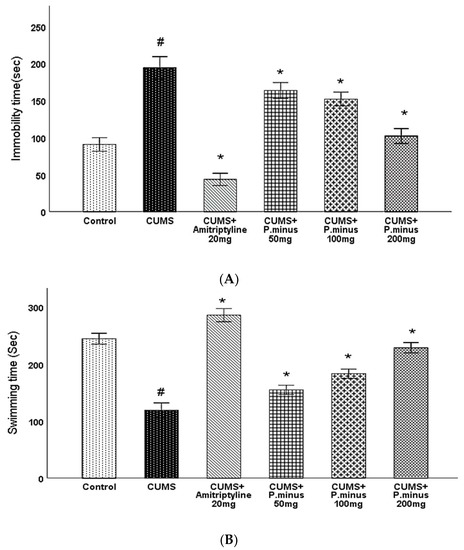
Figure 6.
Effects of P. minus aqueous extract on Forced Swimming Test (FST) in CUMS induced depressive mice. (A) Immobility time(sec) (B) Swimming time(sec). # (p < 0.05), vs. Control group; * (p < 0.05), vs. CUMS group. (n = 14).
3.6. The Results of Experimental Assays
3.6.1. Effects of P. minus Aqueous Extract on Serum Corticosterone Level
As described in Figure 7, CUMS treated group has significantly enhanced the serum corticosterone level with a comparison of Control. An enhanced corticosterone level was significantly decreased in all treatment groups. Reduction of corticosterone level in CUMS + Amitriptyline 20 mg was more than P. minus treated groups, whereas CUMS + P. minus 50 mg treated group showed a minimum decrease as compared to other groups. The reduction pattern in CUMS+ P. minus 100 mg and CUMS + P. minus 200 mg was quite similar.
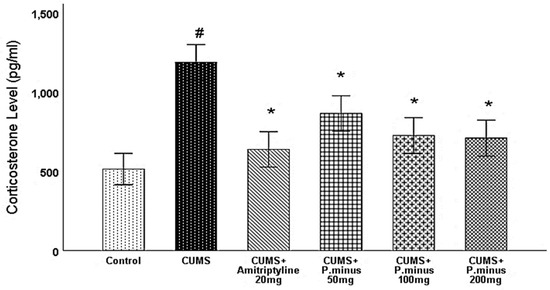
Figure 7.
Effects of P. minus aqueous extract on serum corticosterone level (pg/mL) in CUMS induced depressive mice. # (p < 0.05), vs. Control group; * (p < 0.05), vs. CUMS group, (n = 6).
3.6.2. Effects of P. minus Aqueous Extract on Brain-Derived Neurotrophic Factor (BDNF) Level
Figure 8A, B presents the results of BDNF levels influenced by treatment and CUMS in the hippocampus and prefrontal cortex. Variations of BDNF levels in the hippocampus are expressed, which demonstrates that CUMS group had significantly reduced the BDNF level in the hippocampus. This reduction was inverted by CUMS + Amitriptyline+ 20 mg, P. minus 200 mg and100 mg treated groups significantly, but CUMS + P. minus 50 mg treated group did not show any significant variations in hippocampal BDNF level. In Figure 8B BDNF level variations in the prefrontal cortex are shown, according to these results CUMS also decreased the BDNF level in the prefrontal cortex but this effect was reversed by all treatment groups significantly. The CUMS + Amitriptyline 20 mg showed more improvement in BDNF levels in the hippocampus and prefrontal cortex as compared to P. minus treated groups
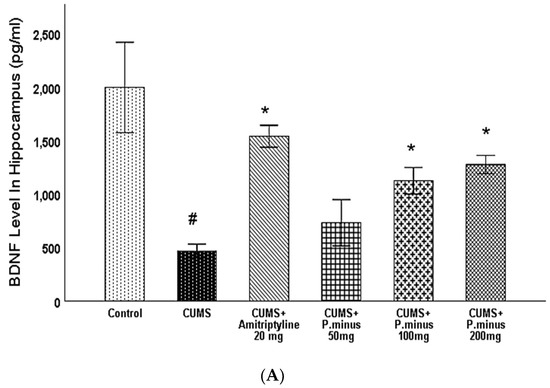
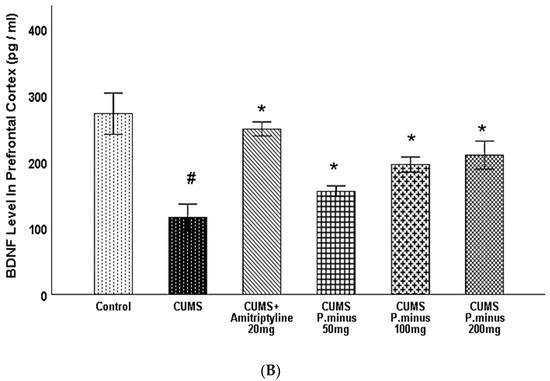
Figure 8.
Effects of P. minus aqueous extract on BDNF level in CUMS induced depressive mice. (A) BDNF level in Hippocampus(pg/mL) (B) BDNF level in Prefrontal cortex (pg/mL). # (p < 0.05), vs. Control group; * (p < 0.05), vs. CUMS group, (n = 6).
3.6.3. Effects of P. minus Aqueous Extract on Monoamine Oxidase -A (MAO-A)
MAO-A level was measured from the hippocampus of the brain. Figure 9 shows a significantly increased level of MAO-A in CUMS group as compared to the control group which was significantly reversed in all treatment groups whereas more MAO-A inhibition was observed with administration of CUMS + P. minus 200 mg than in other treatment groups.
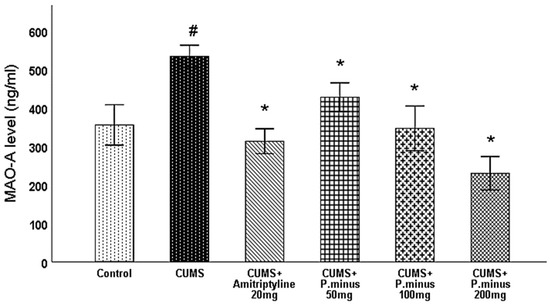
Figure 9.
Effects of P. minus aqueous extract on MAO-A level (ng/mL) in CUMS induced depressive mice. # (p < 0.05), vs. Control group; * (p < 0.05), vs. CUMS group, (n = 6).
3.6.4. Effects of P. minus Aqueous Extract on Serotonin (5-HT) Level
In this section serotonin results measured from the hippocampus are discussed, as per Figure 10 serotonin level was significantly reduced in CUMS group as compared to the control. CUMS+ amitriptyline 20 mg, CUMS +P. minus 100 mg and P. minus, 200 mg groups showed a significant increase in serotonin (5-HT) level, whereas CUMS + P. minus 50 mg did not show a significant difference in serotonin level in comparison to CUMS group. These results indicate that administration with higher doses of P. minus can enhance the level of serotonin in the hippocampus.
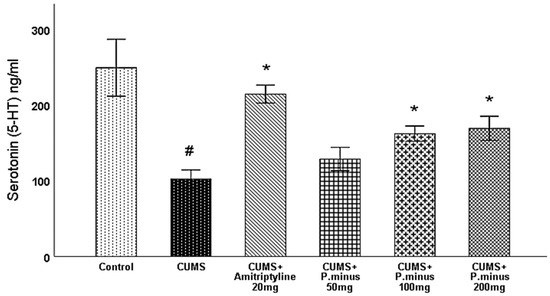
Figure 10.
Effects of P. minus aqueous extract on Serotonin (5-HT) level(ng/mL) in CUMS induced depressive mice. # (p < 0.05), vs. Control group; * (p < 0.05), vs. CUMS group, (n = 6).
3.6.5. Effects of P. minus Aqueous Extract on Norepinephrine (NE) Level
Norepinephrine level was also observed in the hippocampus part of the brain, According to Figure 11, CUMS + Amitriptyline 20 mg, P. minus 100 and 200 mg treated groups showed a significant increase in norepinephrine level in comparison to CUMS group. While CUMS group decreases the norepinephrine level significantly as compared to the control group. In addition, CUMS + P. minus 50 mg has not shown any significant difference in comparison to CUMS group
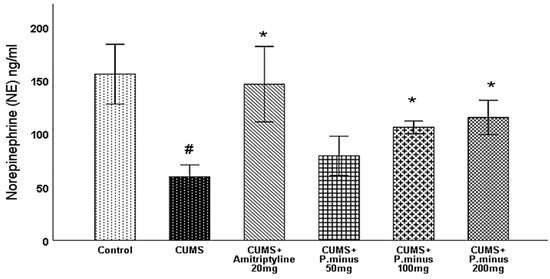
Figure 11.
Effects of P. minus aqueous extract on Norepinephrine (NE) level(ng/mL) in CUMS induced depressive mice. # (p < 0.05), vs. Control group; * (p < 0.05), vs. CUMS group, (n = 6).
4. Discussion
BioKesum® extract is registered under the National Pharmaceutical Control Bureau (NPCB) (Reg# MAL14015033T). BioKesum® contains bioactive compounds including quercetin-3-glucuronide (not less than 0.45%), and quercitrin (not less than 0.15%), These are the most abundant compounds in the total phenolic extract of P. minus aqueous extract of leaf part [21].
Our primary finding of this research work showed that administration with P. minus aqueous extract (BioKesum®) has a significant role in improvement on CUMS induced cognition deficit and depression with maximum doses (P. minus 100 mg, P. minus 200 mg). A previously done work on the effects of P. minus extract on scopolamine-induced cognition deficit supports the results (Figure 5) of this current study. But the cognition deficit induction method was different in both studies. That work also suggests that this property of P. minus aqueous extract is due to the presence of quercitrin and quercetin 3 glucuronide (Q3G) like flavonoids. Q3G is an abundant compound in this extract, which is a derivative of quercetin [20]. According to previously done studies, it is proved that quercetin 3 glucuronide and quercitrin cross the blood-brain barrier and can be absorbed from the intestine and further distributed to the central nervous system [30,31]. Recent research explained that P. minus extract can enhance mood and BDNF levels in a human trial [21], through results (Figure 8) of the current study we support their findings as shown which indicates that P. minus extract administration significantly enhanced the BDNF level in CUMS induced depressive mice.
Despite all these findings and studies, there was a gap in research regarding its antidepressant effects because no proper mechanisms were found in all previously done work regarding its beneficial activities. So, the current study demonstrates some potential regarding anti-depressant and cognitive enhancing properties of P. minus aqueous extract.
This research showed that P. minus has the potential to reverse the CUMS induced behavioral disturbances significantly by using the method of sucrose preference test, Barnes maze assay and forced swimming test with higher doses of P. minus 100 mg and P. minus 200 mg mostly. Our findings regarding antidepressant effect are strongly supported by previously done work on individual flavonoids which are present in P. minus extract, Quercitrin (quercetin 3-rhamnoside) alleviated depression-like behaviors with a maximum dose (10 mg/kg) among depressive mice in a previous study [32]. Whereas for Q3 G, a researcher suggested that it has a potential role in the antidepressant effect of Hypericum perforatum (St. John’s wort) through forced swimming test in mice, it can reduce the immobility time significantly which is similar to our result (Figure 6) of immobility time [33]. In the case of anxiety behavior, P.minus treated groups showed a decrease in the anxiety level through open field test in comparison to CUMS but it was not significantly (Figure 4), The same pattern was also observed in another study in which quercetin 3 glucuronide showed non-significance improvement on anxiety as a part of Hypericum perforatum (St. John’s wort) with 0.6 mg/kg dose through open field test [34], but it has already reversed the immobility time in forced swimming test by the same researcher in another study [33]. It is suggested that might be significant anxiolytic effect can be achieved by increasing the duration of time. P. minus aqueous extract also significantly inhibit the MAO-A level in CUMS induced depressive mice which clearly expressed that P. minus extract may be proven as an MAO-A inhibitor by its anti-depressant mechanism of action as shown in Figure 9. As MAO-A inhibition is directly involved to increase the level of the monoamine because it is involved in the degradation of serotonin and norepinephrine, its inhibition is directly proportional to enhancing the level of the monoamine, in our experiment P. minus aqueous extract aqueous has significantly increased the serotonin and norepinephrine with maximum doses (P. minus 100 mg and P. minus 200 mg) only (Figure 10 and Figure 11). These results are supported by some previously done studies, in which quercetin 3 glucuronide (Q3G) was a part of Apocynum venetum leaves extract, that extract increased the level of serotonin and norepinephrine level in the depression model [35]. If we discuss furthermore another hypothesis of depression model HPA axis, we can observe that Corticosterone level is the main factor in this theory which indicates that increased cortisol or corticosterone level is the major cause of depression, our results showed that P. minus aqueous extract has reduced the corticosterone level significantly as compare to chronic ultra-mild stress (CUMS) induced depressive mice group (Figure 7). It is recommended that a future study should be done on each constituent of this extract especially on Q3G because it has very less reported literature regarding anti-depressant effects as an individual constituent, it needs a separate full comprehensive research study which will describe its doses and will demonstrate its various beneficial and side effects along with the dose-response relationship. Based on all current findings, it is suggested that quercetin 3-glucuronide and quercitrin (quercetin 3-rhamnoside) are may be responsible for the anti-depressant-like effects of P. minus aqueous extract, quercetin-3-glucuronide is the most abundant constituent (0.45%) in P. minus aqueous extract and other studies showed that 0.6 mg/kg dose of this constituent had significant antidepressant potential which was near to concentration used in current research even P. minus 200 mg/kg (maximum dose group) had more concentration of Q3G as compare to that extract which was used in previous studies [33], whereas quercitrin showed a significant increase in monoamine level and antidepressant potential with 10 mg/kg only in previous studies, even nothing was significant with 5 mg/kg dose in one of a previous study [32,36]. Based on previous evidence, the concentration of quercitrin in the present extract (P. minus aqueous extract) was not sufficient for significant anti-depressant potential.
In the case of P. minus aqueous extract (BioKesum), it is strongly recommended to develop its planned and prescribed use for improving the memory and depression signs based on its beneficial mechanisms of action, observed in current research work.
5. Conclusions
It is concluded that P. minus aqueous extract has an anti-depressant-like effect on behavior, it shows an improved spatial memory, reduced immobility, decreased corticosterone level, elevated serotonin, norepinephrine and BDNF level along with a significant reduction of MAO-A level compared to CUMS induced depressive mice model.
Author Contributions
Conceptualization, N.H.K.A.A., M.I.B. and D.A.M.N.; Data curation, M.I.B.; Formal analysis, D.A.M.N. and M.I.B.; Investigation, M.I.B.; Methodology, M.I.B. and N.H.K.A.A.; Project administration, M.I.B., N.H.K.A.A. and D.A.M.N.; Writing—original draft, M.I.B.; Writing—review & editing, N.H.K.A.A. and D.A.M.N. All authors have read and agreed to the published version of the manuscript.
Funding
There was no funding support for this project.
Institutional Review Board Statement
All experimental procedures were carried out after getting the approval from USM Institutional Animal Care and Use Committee (USM IACUC) of Universiti Sains Malaysia (No of Animal Ethical Approvals: USM/IACUC/2020/(123)(1075) & USM/IACUC/2021/ (127) (1129).
Informed Consent Statement
Not Applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The author acknowledges the USM fellowship for Muhammad Irfan Bashir by Universiti Sains Malaysia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Remes, O.; Lafortune, L.; Wainwright, N.; Surtees, P.; Khaw, K.T.; Brayne, C. Association between Area Deprivation and Major Depressive Disorder in British Men and Women: A Cohort Study. BMJ Open 2019, 9, e027530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulugeta, A.; Zhou, A.; King, C.; Hyppönen, E. Association between Major Depressive Disorder and Multiple Disease Outcomes: A Phenome-Wide Mendelian Randomisation Study in the UK Biobank. Mol. Psychiatry 2019, 25, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, C.; Bosi, M.F.; Volontè, M.; Giordano, F.; Dell’Osso, B.; Viganò, C.A. Duration of Untreated Illness and Depression Severity Are Associated with Cognitive Impairment in Mood Disorders. Int. J. Psychiatry Clin. Pract. 2020, 24, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Palagini, L.; Miniati, M.; Marazziti, D.; Sharma, V.; Riemann, D. Association among Early Life Stress, Mood Features, Hopelessness and Suicidal Risk in Bipolar Disorder: The Potential Contribution of Insomnia Symptoms. J. Psychiatr. Res. 2021, 135, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Stringaris, A.; Wolpert, M. Treatment Outcomes for Depression: Challenges and Opportunities. Lancet Psychiatry 2020, 7, 925–927. [Google Scholar] [CrossRef] [Green Version]
- Govindarajulu, M.; Shankar, T.; Patel, S.; Fabbrini, M.; Manohar, A.; Ramesh, S.; Boralingaiah, P.; Sharma, S.; Clark, R.C.; Deruiter, J.; et al. Reserpine-Induced Depression and Other Neurotoxicity: A Monoaminergic Hypothesis. In Medicinal Herbs and Fungi; Springer: Singapore, 2021; pp. 293–313. [Google Scholar] [CrossRef]
- Chakraborty, S.; Tripathi, S.J.; Raju, T.R.; Rao, S. Mechanisms Underlying Remediation of Depression-Associated Anxiety by Chronic N-Acetyl Cysteine Treatment. Psychopharmacology 2020, 237, 2967–2981. [Google Scholar] [CrossRef]
- Pourhamzeh, M.; Moravej, F.G.; Arabi, M.; Shahriari, E.; Mehrabi, S.; Ward, R.; Ahadi, R.; Joghataei, M.T. The Roles of Serotonin in Neuropsychiatric Disorders. Cell. Mol. Neurobiol. 2021, 1–22. [Google Scholar] [CrossRef]
- Duarte, P.; Cuadrado, A.; León, R. Monoamine Oxidase Inhibitors: From Classic to New Clinical Approaches. In Handbook of Experimental Pharmacology; Springer Press: New York, NY, USA, 2020; Volume 264, pp. 229–259. [Google Scholar] [CrossRef]
- Ege, T.; Şelimen, H.D. Monoamine Oxidase Inhibitory Effects of Medicinal Plants in Management of Alzheimer’s Disease. J. Turk. Chem. Soc. Sect. A Chem. 2021, 8, 239–248. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, C.; Xin, C.; Wang, Z. The Antidepressant-like Effect of Flavonoids from Trigonella Foenum-Graecum Seeds in Chronic Restraint Stress Mice via Modulation of Monoamine Regulatory Pathways. Molecules 2019, 24, 1105. [Google Scholar] [CrossRef] [Green Version]
- Esfahani, A.N.; Mirzaei, M. Flavonoid Derivatives for Monoamine Oxidase–A Inhibition. Adv. J. Chem.-Sect. B Nat. Prod. Med. Chem. 2019, 1, 17–22. [Google Scholar] [CrossRef]
- Cole, E.J.; Stimpson, K.H.; Bentzley, B.S.; Gulser, M.; Cherian, K.; Tischler, C.; Nejad, R.; Pankow, H.; Choi, E.; Aaron, H.; et al. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. Am. J. Psychiatry 2020, 177, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Kim, H.B.; Lee, S.; Kim, M.J.; Kim, K.J.; Han, G.; Han, S.Y.; Lee, E.A.; Yoon, J.H.; Kim, D.O.; et al. Antidepressant-like Effects of β-Caryophyllene on Restraint plus Stress-Induced Depression. Behav. Brain Res. 2020, 380, 112439. [Google Scholar] [CrossRef] [PubMed]
- Willner, P.; Gruca, P.; Lason, M.; Tota-Glowczyk, K.; Litwa, E.; Niemczyk, M.; Papp, M. Validation of Chronic Mild Stress in the Wistar-Kyoto Rat as an Animal Model of Treatment-Resistant Depression. Behav. Pharmacol. 2019, 30, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Du, X.; Yang, Y.; Botchway, B.O.A.; Fang, M. Progesterone and Fluoxetine Treatments of Postpartum Depressive-like Behavior in Rat Model. Cell Biol. Int. 2019, 43, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Geiger, L.; Wiborg, O.; Czéh, B. Stress-Induced Morphological, Cellular and Molecular Changes in the Brain—Lessons Learned from the Chronic Mild Stress Model of Depression. Cells 2020, 9, 1026. [Google Scholar] [CrossRef] [Green Version]
- Boulle, F.; Massart, R.; Stragier, E.; Païzanis, E.; Zaidan, L.; Marday, S.; Gabriel, C.; Mocaer, E.; Mongeau, R.; Lanfumey, L. Hippocampal and Behavioral Dysfunctions in a Mouse Model of Environmental Stress: Normalization by Agomelatine. Transl. Psychiatry 2014, 4, e485. [Google Scholar] [CrossRef] [Green Version]
- Christapher, P.; Parasuraman, S.; Christina, J.; Asmawi, M.Z.; Vikneswaran, M. Review on Polygonum Minus. Huds, a Commonly Used Food Additive in Southeast Asia. Pharmacogn. Res. 2015, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- George, A.; Ng, C.P.; O’Callaghan, M.; Jensen, G.S.; Wong, H.J. In Vitro and Ex-Vivo Cellular Antioxidant Protection and Cognitive Enhancing Effects of an Extract of Polygonum Minus Huds (LineminusTM) Demonstrated in a Barnes Maze Animal Model for Memory and Learning. BMC Complement. Altern. Med. 2014, 14, 166. [Google Scholar] [CrossRef] [Green Version]
- Lau, H.; Shahar, S.; Mohamad, M.; Rajab, N.F.; Yahya, H.M.; Din, N.C.; Hamid, H.A. The Effects of Six Months Persicaria Minor Extract Supplement among Older Adults with Mild Cognitive Impairment: A Double-Blinded, Randomized, and Placebo-Controlled Trial. BMC Complement. Med. Ther. 2020, 20, 315. [Google Scholar] [CrossRef]
- Sakai, Y.; Li, H.; Inaba, H.; Funayama, Y.; Iscience, E.; Kawatake-Kuno, A.; Yamagata, H.; Seki, T.; Hobara, T.; Nakagawa, S.; et al. Gene-Environment Interactions Mediate Stress Susceptibility and Resilience through the CaMKIIβ/TARPγ-8/AMPAR Pathway. iScience 2021, 24, 102504. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, R.; Chen, C.; Du, X.; Ruan, L.; Sun, J.; Li, J.; Zhang, L.; O’Donnell, J.M.; Pan, J.; et al. Antidepressant-like Effect of Trans-Resveratrol in Chronic Stress Model: Behavioral and Neurochemical Evidences. J. Psychiatr. Res. 2013, 47, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Sturman, O.; Germain, P.L.; Bohacek, J. Exploratory Rearing: A Context- and Stress-Sensitive Behavior Recorded in the Open-Field Test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Choukairi, Z.; Hazzaz, T.; Lkhider, M.; Ferrandez, J.M.; Fechtali, T. Effect of Salvia Officinalis L. and Rosmarinus Officinalis L. Leaves Extracts on Anxiety and Neural Activity. Bioinformation 2019, 15, 172–178. [Google Scholar] [CrossRef]
- Pitts, M.W. Barnes Maze Procedure for Spatial Learning and Memory in Mice. Bio-Protocol 2018, 8, e2744. [Google Scholar] [CrossRef] [Green Version]
- Lesuis, S.L.; Weggen, S.; Baches, S.; Lucassen, P.J.; Krugers, H.J. Targeting Glucocorticoid Receptors Prevents the Effects of Early Life Stress on Amyloid Pathology and Cognitive Performance in APP/PS1 Mice. Transl. Psychiatry 2018, 8, 53. [Google Scholar] [CrossRef]
- Chiba, S.; Numakawa, T.; Ninomiya, M.; Richards, M.C.; Wakabayashi, C.; Kunugi, H. Chronic Restraint Stress Causes Anxiety-and Depression-like Behaviors, Downregulates Glucocorticoid Receptor Expression, and Attenuates Glutamate Release Induced by Brain-Derived Neurotrophic Factor in the Prefrontal Cortex. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 39, 112–119. [Google Scholar] [CrossRef]
- Juergenliemk, G.; Boje, K.; Huewel, S.; Lohmann, C.; Galla, H.J.; Nahrstedt, A. In Vitro Studies Indicate That Miquelianin (Quercetin 3-O-β-D- Glucuronopyranoside) Is Able to Reach the CNS from the Small Intestine. Planta Med. 2003, 69, 1013–1017. [Google Scholar] [CrossRef]
- Ferri, P.; Angelino, D.; Gennari, L.; Benedetti, S.; Ambrogini, P.; del Grande, P.; Ninfali, P. Enhancement of Flavonoid Ability to Cross the Blood–Brain Barrier of Rats by Co-Administration with α-Tocopherol. Food Funct. 2015, 6, 394–400. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Wu, Z.; Yu, X.; Yin, Y.; Qian, S.; Wang, Z.; Huang, J.; Wang, W.; Liu, T.; et al. Quercitrin Rapidly Alleviated Depression-like Behaviors in Lipopolysaccharide-Treated Mice: The Involvement of PI3K/AKT/NF-ΚB Signaling Suppression and CREB/BDNF Signaling Restoration in the Hippocampus. ACS Chem. Neurosci. 2021, 12, 3387–3396. [Google Scholar] [CrossRef]
- Butterweck, V.; Jürgenliemk, G.; Nahrstedt, A.; Winterhoff, H. Flavonoids from Hypericum Perforatum Show Antidepressant Activity in the Forced Swimming Test. Planta Med. 2000, 66, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, O.; Kelber, O.; Butterweck, V. Effects of St. John’s Wort Extract and Single Constituents on Stress-Induced Hyperthermia in Mice. Planta Med. 2006, 72, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Fan, Y.; Shi, D.; Liu, C. Antidepressant-like Effect of Flavonoids Extracted from Apocynum Venetum Leaves on Brain Monoamine Levels and Dopaminergic System. J. Ethnopharmacol. 2013, 147, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Q.T.; Chen, Y.; Liu, J.; Shi, J.L.; Liu, Y.; Guo, J.Y. Involvement of 5-HT1A Receptors in the Anxiolytic-like Effects of Quercitrin and Evidence of the Involvement of the Monoaminergic System. Evid.-Based Complement. Altern. Med. 2016, 2016, 6530364. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).