The Superior Visual Perception Hypothesis: Neuroaesthetics of Cave Art
Abstract
1. Introduction
2. Materials and Methods
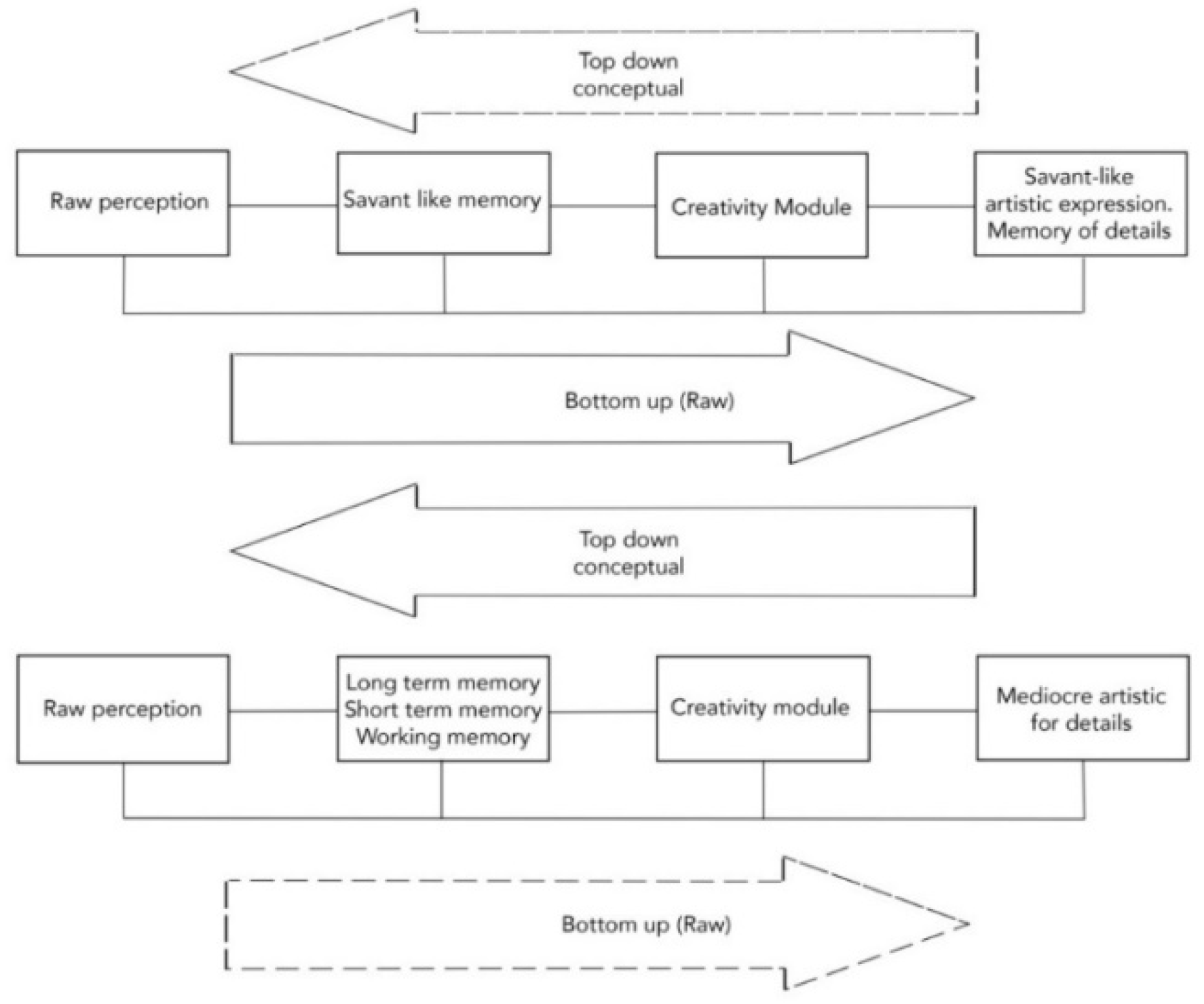
2.1. Savant-Like Representation of Details
2.2. Animals in Motion
2.3. Perception in Modern Autists, Savants and Visual Thinkers
Autism
2.4. Archaic Homo Sapiens and Genetic Links to Raw Perception
Autism and Brain Size—Evolutionary Aspects
2.5. Sudden Boost of Creativity: What about the Neanderthals?
2.6. Volume of Visual Areas: Sapiens Sapiens vs. Sapiens Neanderthalensis
3. Results
4. Discussion: Enhanced Visual Perception
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snyder, A.W.; Mulchhy, E.; Taylor, J.L.; Mitchell, D.J.; Sachdev, P.; Gandevia, S.C. Savant-like skills exposed in normal people by suppressing the left fronto-temporal lobe. J. Integr. Neurosci. 2003, 2, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A. Explaining and inducing savant skills: Privileged access to lower level, less-processed information. Philos. Trans. R. Soc. B 2009, 364, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.S.; Bossomaier, T.; Mitchell, D.J. Concept formation: Object attributes dynamically inhibited from conscious awareness. J. Integr. Neurosci. 2004, 3, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.W.; Thomas, M. Austistic artists give clues to cognition. Perception 1997, 26, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.D.; Kippenhan, J.S.; Eisenberg, D.P.; Kohn, P.D.; Dickinson, D.; Mattay, V.S.; Chen, Q.; Weinberger, D.R.; Saad, Z.S.; Berman, K.F. Neanderthal-Derived Genetic Variation Shapes Modern Human Cranium and Brain. Sci. Rep. 2017, 7, 6308. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.A.; Gadian, D.G.; Johnsrude, I.S.; Good, C.D.; Ashburner, J.; Frackowiak, R.S.J.; Frith, C.D. Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. USA 2000, 97, 4398–4403. [Google Scholar] [CrossRef] [PubMed]
- Malafouris, L. Metaplasticity and the human becoming: Principles of neuroarchaeology. J. Anthropol. Sci. 2010, 88, 49–72. [Google Scholar]
- Roberts, P. “We have never been behaviourally modern”: The implications of material engagement theory and metaplasiticity for understanding the late pleistoscene record of human behaviour. Quat. Int. 2016, 405, 8–20. [Google Scholar] [CrossRef]
- Hood, B. The Domesticated Brain; Pelican Books: London, UK, 2014. [Google Scholar]
- Selfe, L. Nadia: A Case of Extraordinary Drawing Ability in an Autistic Child; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Ramachandran, V.S.; Hirstein, W. The Science of Art: A Neurological Theory of Aesthetic Experience. J. Conscious. Stud. 1999, 6, 15–51. [Google Scholar]
- Humphrey, N. Cave art, autism, and the evolution of the human mind. Camb. Archaeol. J. 1998, 8, 165–191. [Google Scholar] [CrossRef][Green Version]
- Masataka, N. Autism, its cultural modulation and niche construction in societies. Reply to comments on: “Implications of the idea of neurodiversity for understanding the origins of developmental disorders”. Phys. Life Rev. 2017, 20, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T. The healing balm nature: Understanding and supporting the naturalist intelligence in individuals diagnosed with ASD. Comment on: “Implications of the idea of neurodiversity for understanding the origins of developmental disorders” by N. Masataka. Phys. Life Rev. 2017, 20, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S. Beyond “deficit-based” thinking in autism research. Comment on: “Implications of the idea of neurodiversity for understanding the origins of developmental disorders” by N. Masataka. Phys. Life Rev. 2017, 20, 119–121. [Google Scholar] [CrossRef]
- Spikins, P. Autism, the integrations of “difference” and the origins of modern human behaviour. Camb. Archaeol. J. 2009, 19, 179–201. [Google Scholar] [CrossRef][Green Version]
- Fabricius, T. The savant hypothesis: Is autism a signal-processing problem? Med. Hypotheses 2010, 75, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Koshino, H.; Carpenter, P.A.; Minshew, N.J.; Cherkassy, V.L.; Keller, T.A.; Just, M.A. Functional connectivity in an fMRI working memory task in highfunctioning autism. Neuroimage 2005, 24, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Mottron, L.; Dawson, M.; Soulières, I. Enhanced perception in savant syndrome: Patterns, structure and creativity. Philos. Trans. R. Soc. B 2009, 364, 1385–1391. [Google Scholar] [CrossRef]
- Frith, C. What do imaging studies tell us about the neural basis of autism? In Autism: Neural Basis and Treatment Possibilities. Novartis Foundations Symposium 251; Bock, G., Goode, J., Eds.; Wiley: Hoboken, NJ, USA, 2003; pp. 149–176. [Google Scholar] [CrossRef]
- Pring, L. Memory characteristics in individuals with savant skills. In Memory in Autism: Theory and Evidence; Boucher, J., Bowler, D., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 210–230. [Google Scholar]
- Spikins, P. The Stone Age Origin of Autism. In Recent Advances in Autism Spectrum Disorders Volume II; Fitzgerald, M., Ed.; InTech Open: London, UK, 2013. [Google Scholar] [CrossRef][Green Version]
- Mottron, L.; Dawson, M.; Soulières, I.; Hubert, B. Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. J. Autism Dev. Disord. 2006, 36, 27–43. [Google Scholar] [CrossRef]
- Azéma, M. La représentation du mouvement au Paléolithique supérieur: Apport du comparatisme éthographique à l’interprétation de l’art pariétal. Bull. Société Préhistorique Française 2006, 3, 479–505. [Google Scholar] [CrossRef]
- Azéma, M.; Rivère, F. Animation in Palaeolithic art: A pre-echo of cinema. Antiquity 2012, 86, 316–324. [Google Scholar] [CrossRef]
- Azéma, M. Representation of movement in the Upper Palaeolithic: An ethological approach to the interpretation of parietal art. Anthropozoologica 2008, 43, 117–154. [Google Scholar]
- Lewis-Williams, J.D. The Mind in the Cave. Consciousness and the Origins of Art; Thames & Hudson: London, UK, 2002. [Google Scholar]
- White, R. Prehistoric Art: The Symbolic Journey of Humankind; H.N. Abrams: New York, NY, USA, 2003. [Google Scholar]
- Horvath, G.; Farkas, E.; Boncz, I.; Blaho, M.; Kriska, G.; Fenton, B. Cavemen were better at depicting quadruped walking than modern artists: Erroneous walking illustrations in the fine arts from prehistory to today. PLoS ONE 2012, 7, e49786. [Google Scholar] [CrossRef]
- Grandin, T. Thinking in Pictures—And Other Reports from my Life with Autism; Vintage Books: New York, NY, USA, 2006. [Google Scholar]
- Rimland, B. Inside the Mind of the Autistic Savant. Psychol. Today 1978, 12, 69–80. [Google Scholar]
- Walenski, M.; Mostofsky, S.H.; Gidley-Larson, J.C.; Ullman, M.T. Brief Report: Enhanced picture naming in autism. J. Autism Dev. Disord. 2008, 38, 1395–1399. [Google Scholar] [CrossRef]
- Frith, U.; Happé, F. Autism: Beyond “theory of mind”. Cognition 1994, 50, 115–132. [Google Scholar] [CrossRef]
- Supekar, K.; Uddin, L.Q.; Khouzam, A.; Phillips, J.; Gaillard, W.D.; Kenworthy, L.E.; Yerys, B.E.; Vaidya, C.J.; Menon, V. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep. 2013, 5, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Schwarzkopf, D.S.; Anderson, E.J.; de Haas, B.; White, S.J.; Rees, G. Larger extrastriate population receptive fields in autism spectrum disorders. J. Neurosci. 2014, 34, 2713–2724. [Google Scholar] [CrossRef]
- El-Ansary, A.; Al-Ayadhi, L. GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J. Neuroinflamm. 2014, 11, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Heaney, C.F.; Kinney, J.W. Role of GABA (B) receptors in learning and memory and neurological disorders. Neurosci. Biobehav. Rev. 2016, 63, 1–28. [Google Scholar] [CrossRef]
- Sung Kim, Y.; Yoon, B.-E. Altered GABAergic signaling in brain disease at various stages of life. Exp. Neurobiol. 2017, 26, 122–131. [Google Scholar] [CrossRef]
- Foss-Feig, J.H.; Tadin, D.; Schauder, K.B.; Cascio, C.J. A Substantial and Unexpected Enhancement of Motion Perception in Autism. J. Neurosci. 2013, 33, 8243–8249. [Google Scholar] [CrossRef]
- Chi, R.P.; Fregni, F.; Snyder, A. Visual memory improved by non-invasive brain stimulation. Brain Res. 2010, 1353, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Mozzi, A.; Forni, D.; Cagliani, R.; Pozzoli, U.; Clerici, M.; Sironi, M. Distinct selective forces and Neanderthal introgression shaped genetic diversity at genes involved in neurodevelopmental disorders. Sci. Rep. 2017, 7, 6116. [Google Scholar] [CrossRef] [PubMed]
- Gobet, F.; Snyder, A.; Bossomaier, T.; Harré, M. Designing a “better” brain: Insights from experts and savants. Front. Psychol. 2014, 5, 470. [Google Scholar] [CrossRef]
- Bilalić, M.; McLeod, P.; Gobet, F. Inflexibility of Experts-Reality or Myth? Quantifying the Einstellung Effect in Chess Masters. Cogn. Psychol. 2008, 56, 73–102. [Google Scholar] [CrossRef]
- Bilalić, M.; McLeod, P.; Gobet, F. The mechanism of the Einstellung (Set) effect: A pervasive sourse of cognitive bias. Curr. Dir. Psychol. Sci. 2010, 19, 111–115. [Google Scholar] [CrossRef]
- Casanova, M.F.; Buxhoeveden, D.; Gomez, J. Disruption in the inhibitory architecture of the cell minicolumn: Implications for autism. Prog. Clin. Neurosci. 2003, 9, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Persico, A.M.; Bourgeron, T. Searching for ways out of the autism maze: Genetic, epigenetics and environmental clues. Trends Neurosci. 2006, 29, 349–358. [Google Scholar] [CrossRef]
- Rubenstein, J.L.R.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef]
- Rho, H.-J.; Kim, J.-H.; Lee, S.-H. Function of Selective Neuromodulatory Projections in the Mammalian Cerebral Cortex: Comparison Between Cholinergic and Noradrenergic Systems. Front. Neural Circuits 2018, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Matzusawa, T. Working memory of numerals in chimpanzees. Curr. Biol. 2007, 17, R1004–R1005. [Google Scholar] [CrossRef]
- Green, R.E.; Krause, J.; Briggs, A.W.; Maricic, T.; Stenzel, U.; Kircher, M.; Patterson, N.; Li, H.; Zhai, W.; Fritz, M.H.-Y.; et al. A Draft Sequence of the Neandertal Genome. Science 2010, 328, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Gabriele, S.; Persico, A.M. Head circumference and brain size in autism spectrum disorder: A systematic review and meta-analysis. Psychiatric Res. Neuroimaging 2015, 234, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Mouton, P.R.; Calhoun, M.E.; Semendeferi, K.; Ahrens-Barbeau, C.; Hallet, M.J.; Carter Barnes, M.J.; Pierce, K. Neuron Number and Size in Prefrontal Cortex of Children with Autism. JAMA 2011, 306, 2001–2010. [Google Scholar] [CrossRef]
- Amaral, D.G.; Mills Schumann, C.; Wu Nordahl, C. Neuroanatomy of autism. Trends Neurosci. 2008, 31, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Redcay, E.; Courchesne, E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol. Psychiatry 2005, 58, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hutsler, J.J.; Casanova, M.F. Review: Cortical construction in autism spectrum disorder: Columns, connectivity and the subplate. Neuropathol. Appl. Neurobiol. 2015, 14, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Pierce, K. Brain overgrowth in autism during critical time in development: Implications for frontal pyramidal neuron and interneuron development and connectivity. Int. J. Dev. Neurosci. 2005, 23, 153–170. [Google Scholar] [CrossRef]
- McAuliffe, K. If Modern Humans Are So Smart, Why Are Our Brains Shrinking? Discover. September 2010. Available online: https://www.discovermagazine.com/the-sciences/if-modern-humans-are-so-smart-why-are-our-brains-shrinking (accessed on 25 May 2021).
- Renfrew, C. Archaeology and Language: The Puzzle of Indo-European Origins; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Gould, S.J.; Lewontin, R.C. The Spandrels of San Marco and the Panglossian paradigm: A critique of the adaptationist program. Proc. R. Soc. London B Biol. Sci. 1979, 205, 581–598. [Google Scholar]
- Pigliucci, M.; Kaplan, J. The fall and rise of Dr Pangloss: Adaptationism and the Spandrels paper 20 years later. TREE 2010, 15, 66–70. [Google Scholar] [CrossRef]
- Falk, D. Brain evolution in Homo: The “radiator” theory. Behav. Brain Sci. 1990, 13, 333–381. [Google Scholar] [CrossRef]
- Hofman, M.A. Evolution of the human brain: When bigger is better. Front. Neuroanat. 2014, 8, 15. [Google Scholar] [CrossRef]
- Kruska, D.C.T. The effects of domestication on brain size. Evol. Nerv. Syst. 2007, 3, 143–153. [Google Scholar]
- Baron-Cohen, S. Mind Blindness: An Essay on Autism and Theory of Mind; MIT press: Cambridge, MA, USA, 1997. [Google Scholar]
- von dem Hagen, E.A.H.; Stoyanova, R.S.; Rowe, J.B.; Baron-Cohen, S.; Calder, A.J. Direct gaze elicits atypical activation of the Theory-of-Mind network in Autism Spectrum Conditions. Cereb. Cortex 2013, 24, 1485–1492. [Google Scholar] [CrossRef]
- Gallese, V.; Eagle, M.N.; Migone, P. Intentional Attunement: Mirror Neurons and the Neural Underpinnings of Interpersonal Relations. J. Am. Psychoanal. Assoc. 2007, 55, 131–175. [Google Scholar] [CrossRef]
- Gallese, V.; Goldman, A. Mirror neurons and the simulation theory of mind-reading. Trends Cogn. Sci. 1998, 2, 493–501. [Google Scholar] [CrossRef]
- Gallese, V.; Mastrogiorgio, A.; Petracca, E.; Viale, R. Embodied Bounded Rationality. Chapter 23. In Routledge Handbook on Bounded Rationality; Viale, R., Ed.; Routledge: London, UK, 2021; ISBN 9781315658353. [Google Scholar]
- Gallese, V. Intentional attunement: A neurophysiological perspective on social cognition and its disruption in autism. Brain Res. 2006, 1079, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Fabbri-Destro, M. Mirror neurons: From discovery to autism. Exp. Brain Res. 2010, 200, 223–237. [Google Scholar] [CrossRef]
- Changeux, J.-P. Climbing brain levels of organisation from genes to consciousness. Trends Cogn. Sci. 2017, 21, 168–181. [Google Scholar] [CrossRef]
- Crespy, B. Developmental heterochrony and the evolution of autistic perception, cognition and behavior. BMC Med. 2013, 11, 119. [Google Scholar] [CrossRef]
- Bednarik, R.G. The Domestication of Humans. Anthropologie 2008, 46, 1–18. [Google Scholar]
- Hardy, B.L.; Moncel, M.H.; Kerfant, C.; Lebon, M.; Bellot-Gurlet, L.; Mélard, N. Direct evidence of Neanderthal fibre technology and its cognitive and behavioral implications. Sci. Rep. 2020, 10, 4889. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.L.; Skove, S.L.; Rouanet, J.P.; Pilaz, L.-J.; Bepler, T.; Gordân, R.; Wray, G.A.; Silver, D.L. Human-Chimpanzee Differences in a FZD8 Enhancer Alter Cell-Cycle Dynamics in the Developing Neocortex. Curr. Biol. 2015, 25, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Stowe, L.; Haverkort, M.; Zwarts, F. Rethinking the neurological basis of language. Lingua 2005, 115, 997–1042. [Google Scholar] [CrossRef]
- Dunbar, R.I.M. Co-evolution of neocortical size, group size and language in humans. Behav. Brain Sci. 1993, 16, 681–735. [Google Scholar] [CrossRef]
- Deacon, T.W. Confounded correlations, again. Behav. Brain Sci. 1993, 16, 698–699. [Google Scholar] [CrossRef]
- Pearce, E.; Stringer, C.; Dunbar, R.I.M. New insights into differences in brain organization between Neanderthals and anatomically modern humans. Proc. R. Soc. B 2013, 280, 20130168. [Google Scholar] [CrossRef]
- Kochiyama, T.; Ogihara, N.; Tanabe, H.C.; Kondo, O.; Amano, H.; Hasegawa, K.; Suzuki, H.; Ponce de León, M.S.; Zollikofer, C.P.E.; Bastir, M.; et al. Reconstructing the Neanderthal brain using computational anatomy. Sci. Rep. 2018, 8, 6296. [Google Scholar] [CrossRef]
- Ogihara, N.; Amano, H.; Kikuchi, T.; Morita, Y.; Suzuki, H.; Kondo, O. Digital Reconstruction of Neanderthal and Early Homo sapiens Endocasts. In Digital Endocasts; Bruner, E., Ogihara, N., Tanabe, H., Eds.; Replacement of Neanderthals by Modern Humans Series; Springer: Tokyo, Japan, 2018. [Google Scholar] [CrossRef]
- Bruner, E. Evolving Human Brains: Paleoneurology and the Fate of Middle Pleistocene. J. Archaeol. Method Theory 2021, 28, 76–94. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Leech, R.; Hellyer, P.J.; Shanahan, M.; Feilding, A.; Tagliazucchi, E.; Chialvo, D.R.; Nutt, D. The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Front. Human Neurosci. 2014, 8, 20. [Google Scholar] [CrossRef]
- Bednarik, R.G. The Late Pleistocene Cultural Shift in Europe. Anthropos 2007, 102, 347–370. [Google Scholar] [CrossRef]
- Marean, C.W. When the sea saved humanity. Sci. Am. 2010, 303, 54–61. Available online: https://www.jstor.org/stable/26002131 (accessed on 25 May 2021). [CrossRef] [PubMed]
- Marean, C.W. Pinnacle Point Cave 13B (Western Cape Province, South Africa) in context: The Cape Floral kingdom, shellfish, and modern human origins. J. Hum. Evol. 2010, 59, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E. The Acquatic Ape Hypothesis; Souvenir Press: London, UK, 2017; ISBN 978-0-285-64361-1. [Google Scholar]
- Aubert, M.; Lebe, R.; Oktaviana, A.A.; Tang, M.; Burhan, B.; Hamrullah; Jusdi, A.; Abdullah; Hakim, B.; Zhao, J.; et al. Earliest hunting scene in prehistoric art. Nature 2019, 576, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Finch, D.; Gleadow, A.; Hergt, J.; Heaney, P.; Green, H.; Myers, C.; Veth, P.; Harper, S.; Ouzman, S.; Levchenko, V.A. Ages for Australia’s oldest rock paintings. Nat. Hum. Behav. 2021, 5, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C. A View from Language; Traveaux de L’Institut de Linguistique de Lund; Lund University Press: Lund, Sweden, 1997; Volume XXXIV. [Google Scholar]
- Henshilwood, C.S.; d’Errico, F.; Yates, R.; Jacobs, Z.; Tribolo, C.; Duller, G.A.; Mercier, N.; Sealy, J.C.; Valladas, H.; Watts, I.; et al. Emergence of modern human behavior. Middle Stone Age engravings from South Africa. Science 2002, 295, 1278–1280. [Google Scholar] [CrossRef]
- Zeberg, H.; Pääbo, S. The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature 2020, 587, 610–612. [Google Scholar] [CrossRef]
- Zeberg, H.; Pääbo, S. A genomic region associated with protection against severe COVID-19 is inherited from Neandertals. Proc. Natl. Acad. Sci. USA 2021, 118, e2026309118. [Google Scholar] [CrossRef]
- Conty, L.; N’Diaye, K.; Tijus, C.; George, N. When eye creates the contact! ERP evidence for early dissociation between direct and averted gaze motion processing. Neuropsychologia 2007, 45, 3024–3037. [Google Scholar] [CrossRef]
- Gregory, M.D.; Kippenhan, J.S.; Kohn, P.; Eisenberg, D.P.; Callicott, J.H.; Kolachana, B.; Berman, K.F. Neanderthal-Derived Genetic Variation is Associated with Functional Connectivity in the Brains of Living Humans. Brain Connect. 2021, 11, 38–44. [Google Scholar] [CrossRef]
- Wammes, J.D.; Meade, M.E.; Fernandes, M.A. The drawing effect: Evidence for reliable and robust memory benefits in free recall. Q. J. Exp. Psychol. 2016, 69, 1752–1776. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.F. Planting misinformation in the human mind: A 30-year investigation of the malleability of memory. Learn. Mem. 2005, 12, 361–366. [Google Scholar] [CrossRef]
- Morrot, G.; Brochet, F.; Dubourdieu, D. The color of odors. Brain Lang. 2001, 79, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Oksenberg, N.; Stevison, L.; Wall, J.D.; Ahituv, N. Function and regulation of AUTS2, a gene implicated in Autism and Human Evolution. PLoS Genet. 2013, 9, e1003221. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.L.; Standish, C.D.; García-Diez, M.; Pettitt, P.B.; Milton, J.A.; Zilhão, J.; Alcolea-González, J.J.; Cantalejo-Duarte, P.; Collado, H.; de Balbín, R.; et al. U-Th dating of carbonate crusts reveals Neandertal origin of Iberian cave art. Science 2018, 359, 912–915. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folgerø, P.O.; Johansson, C.; Stokkedal, L.H. The Superior Visual Perception Hypothesis: Neuroaesthetics of Cave Art. Behav. Sci. 2021, 11, 81. https://doi.org/10.3390/bs11060081
Folgerø PO, Johansson C, Stokkedal LH. The Superior Visual Perception Hypothesis: Neuroaesthetics of Cave Art. Behavioral Sciences. 2021; 11(6):81. https://doi.org/10.3390/bs11060081
Chicago/Turabian StyleFolgerø, Per Olav, Christer Johansson, and Linn Heidi Stokkedal. 2021. "The Superior Visual Perception Hypothesis: Neuroaesthetics of Cave Art" Behavioral Sciences 11, no. 6: 81. https://doi.org/10.3390/bs11060081
APA StyleFolgerø, P. O., Johansson, C., & Stokkedal, L. H. (2021). The Superior Visual Perception Hypothesis: Neuroaesthetics of Cave Art. Behavioral Sciences, 11(6), 81. https://doi.org/10.3390/bs11060081






