The Use of Probiotic Therapy to Modulate the Gut Microbiota and Dendritic Cell Responses in Inflammatory Bowel Diseases
Abstract
:1. Background
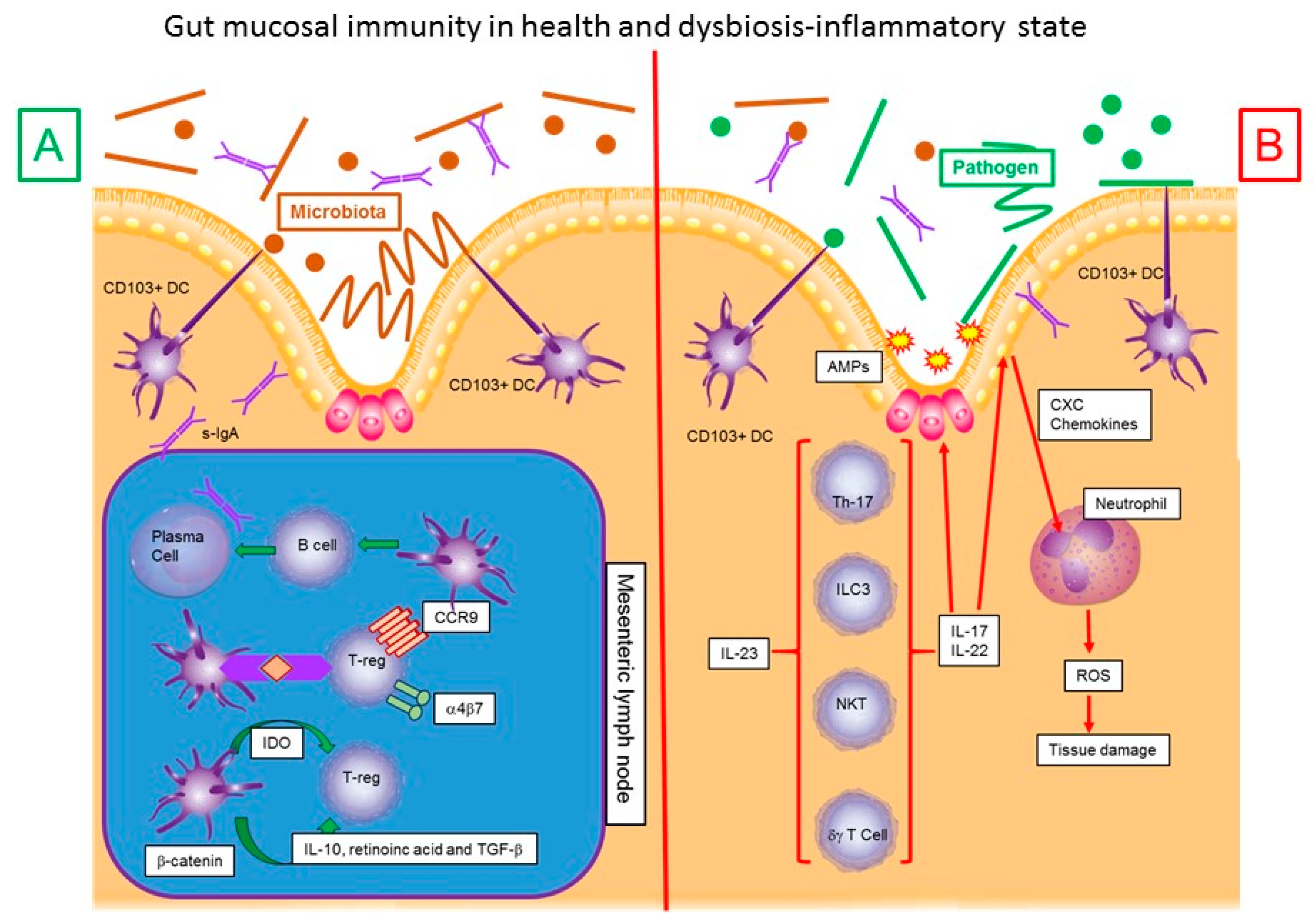
2. Microbiota–Dendritic Cell-Mucosal Immune Response–IBD Interaction
3. Microbiota and Inflammatory Bowel Disease
4. Inflammation in Inflammatory Bowel Disease
5. Probiotics
6. Use of Probiotics in Inflammatory Bowel Diseases
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zain, K.; Belga, S.; Roifman, I.; Hirota, S.; Jijon, H.; Kaplan, G.; Ghosh, S.; Beck, P. Inflammatory Bowel Disease Cause-specific Mortality: A Primer for Clinicians. Inflamm. Bowel. Dis. 2014, 20, 2483–2492. [Google Scholar] [CrossRef]
- Kappelman, M.; Rifas-Shiman, S.; Kleinman, K.; Ollendorf, D.; Bousvaros, A.; Grand, R.; Finklestein, J. The Prevalence and Geographic Distribution of Crohn’s Disease and Ulcerative Colitis in the United States. Clin. Gastroenterol. Hepatol. 2007, 5, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Loftus, C.; Loftus, E.; Harmsen, S.; Zinsmeister, A.; Tremaine, W.; Melton, J.; Sandborn, W. Update on the Incidence and Prevalence of Crohn’s Disease and Ulcerative Colitis in Olmsted County, Minnesota, 1940–2000. Inflamm. Bowel. Dis. 2007, 13, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A comprehensive review and update on Crohns disease. Dis. Mon. 2018, 64, 20–57. [Google Scholar] [CrossRef] [PubMed]
- Victoria, C.R.; Sassak, L.Y.; Nunes, H.R.D.C. Incidence and prevalence rates of inflammatory bowel diseases, in midwestern of São Paulo State, Brazil. Arq. Gastroenterol. 2009, 46, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Mendez, J.; Otoya, G.; Mestanza, A.; Lazo, L.; Acuña, K.; Arenas, J.; Huamán, E.; Julia, F. Caracteristicas epidemiológicas y clínicas de la enfermedad inflamatoria intestinal en un hospital de referencia de Lima-Perú. Rev. Gastroenterol. Perú 2016, 36, 209–218. [Google Scholar] [PubMed]
- Kucharzik, T.; Maaser, C.; Lügering, A.; Kagnoff, M.; Mayer, L.; Targan, S.; Domschke, W. Recent Understanding of IBD Pathogenesis: Implications for Future Therapies. Inflamm. Bowel Dis. 2006, 12, 1068–1083. [Google Scholar] [CrossRef]
- Kelsen, J.; Sullivan, K. Inflammatory Bowel Disease in Primary Immunodeficiencies. Curr. Allergy Asthma Rep. 2017, 17, 57. [Google Scholar] [CrossRef]
- Solano-Gálvez, S.G.; Tovar-Torres, S.M.; Tron-Gómez, M.S.; Weiser-Smeke, A.E.; Álvarez-Hernández, D.A.; Franyuti-Kelly, G.A.; Tapia-Moreno, M.; Ibarra, A.; Gutiérrez-Kobeh, L.; Vázquez-López, R. Human Dendritic Cells: Ontogeny and Their Subsets in Health and Disease. Med. Sci. 2018, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Tang, H.; Manicassamy, S. Programming dendritic cells to induce TH2 and tolerogenic responses. Nat. Immunol. 2010, 11, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol. Rev. 2004, 199, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Denning, T.L.; Norris, B.A.; Medina-Contreras, O.; Manicassamy, S.; Geem, D.; Madan, R.; Karp, C.L.; Pulendran, B. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J. Immunol. 2011, 187, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Banchereau, J. Taking dendritic cells into medicine. Nature 2007, 449, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Tang, H.; Denning, T.L. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr. Opin. Immunol. 2008, 20, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, M.V.; Steinman, R.M.; Krasovsky, J.; Munz, C.; Bhardwaj, N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 2001, 193, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Jonuleit, H.; Schmitt, E.; Schuler, G.; Knop, J.; Enk, A.H. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 2000, 192, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Mahnke, K.; Qian, Y.; Knop, J.; Enk, A.H. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood 2003, 101, 4862–4869. [Google Scholar] [CrossRef]
- Hawiger, D.; Inaba, K.; Dorsett, Y.; Guo, M.; Mahnke, K.; Rivera, M.; Ravetch, J.V.; Steiman, R.M.; Nussenzweig, M.C. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001, 194, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Hawiger, D.; Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003, 21, 685–711. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Nussenzweig, M.C. Avoiding horror autotoxicus: The importance of dendritic cells in peripheral T cell tolerance. Proc. Natl. Acad. Sci. USA 2002, 99, 351–358. [Google Scholar] [CrossRef]
- Maldonado-Lopez, R.; De Smedt, T.; Michel, P.; Godfroid, J.; Pajak, B.; Heirman, C.; Thielemans, K.; Leo, O.; Urbain, J.; Moser, M. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999, 189, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Bloom, O.; Ono, S.; Cui, W.; Unternaehrer, J.; Jiang, S.; Whitney, J.A.; Connolly, J.; Banchereau, J.; Mellman, I. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity 2007, 27, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Li, M.O.; Flavell, R.A. TGF-β: A master of all T cell trades. Cell 2008, 134, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Soumelis, V.; Watanabe, N.; Ito, T.; Wang, Y.H.; de Waal Malefyt, R.; Omori, M.; Zhou, B.; Ziegler, S.F. TSLP: An epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 2007, 25, 193–219. [Google Scholar] [CrossRef] [PubMed]
- Manicassamy, S.; Pulendran, B. Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Semin. Immunol. 2009, 21, 22–27. [Google Scholar] [CrossRef]
- Guilliams, K.; Crozat, S.; Henri, S.; Tamoutounour, S.; Grenot, P.; Devilard, E.; de Bovis, B.; Alexopoulou, L.; Dalod, M.; Malessen, B. Skin-draining lymph nodes contain dermis-derived CD103− dendritic cells that constitutively produce retinoic acid and induce Foxp3+ regulatory T cells. Blood 2010, 115, 1958–1968. [Google Scholar] [CrossRef]
- Laffont, S.; Siddiqui, K.R.; Powrie, F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur J. Immunol. 2010, 40, 1877–1883. [Google Scholar] [CrossRef]
- Dillon, S.; Agrawal, S.; Banerjee, K.; Letterio, J.; Denning, T.L.; Oswald-Richter, K.; Kasprowicz, D.J.; Kellar, K.; Pare, J.; van Dyke, T.; et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Investig. 2006, 116, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Manicassamy, S.; Ravindran, R.; Deng, J.; Oluoch, H.; Denning, T.L.; Kasturi, S.P.; Rosentahl, K.M.; Evavold, B.D.; Pulendran, B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat. Med. 2009, 15, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Karumuthil-Melethil, S.; Perez, N.; Li, R.; Vasu, C. Induction of innate immune response through TLR2 and dectin 1 prevents type 1 diabetes. J. Immunol. 2008, 181, 8323–8334. [Google Scholar] [CrossRef] [PubMed]
- Burton, O.T.; Zaccone, P.; Phillips, J.M.; De la Peña, H.; Fehérvári, Z.; Azuma, M.; Gibbs, S.; Stockinger, B.; Cooke, A. Roles for TGF-β and programmed cell death 1 ligand 1 in regulatory T cell expansion and diabetes suppression by zymosan in non-obese diabetic mice. J. Immunol. 2010, 185, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Depaolo, R.W.; Tang, F.; Kim, I.; Han, M.; Levin, N.; Ciletti, N.; Lin, A.; Anderson, D.; Schneewind, O.; Jabri, B. Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe 2008, 4, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Orabona, C.; Puccetti, P.; Vacca, C.; Bicciato, S.; Luchini, A.; Fallarino, F.; Bianchi, R.; Velardi, E.; Peruccio, K.; Velardi, A.; et al. Toward the identification of a tolerogenic signature in IDO-competent dendritic cells. Blood 2006, 107, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Moseman, E.A.; Liang, X.; Dawson, A.J.; Panoskaltsis-Mortari, A.; Krieg, A.M.; Liu, Y.J.; Blazar, B.R.; Chen, W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 2004, 173, 4433–4442. [Google Scholar] [CrossRef] [PubMed]
- Smits, H.; Engering, A.; van der Kleij, D.; de Jong, E.; Schipper, K.; van Capel, T.; Zaat, B.; Yazdanbakhsh, M.; Wierenga, E.; van Kooyk, Y.; et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 2005, 115, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.P.; Engering, A.; Smits, H.H.; Savan Vliet, S.J.; Bodegraven, A.A.; Wirth, H.P.; Kapsenberg, M.L.; Vandenbroucke-Grauls, C.; van Kooyk, Y.; Appelmelk, B.J. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 2004, 200, 979–990. [Google Scholar] [CrossRef]
- Konstantinov, S.R.; Smidt, H.; de Vos, W.M.; Brujins, S.C.; Singh, S.K.; Valence, F.; Molle, D.; Lortal, S.; Altermann, E.; Klaenhammer, T.R.; et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl. Acad. Sci. USA 2008, 105, 19474–19479. [Google Scholar] [CrossRef]
- Zhou, Y.; Kawasaki, H.; Hsu, S.C.; Lee, R.T.; Yao, X.; Plunkett, B.; Fu, J.; Yang, K.; Lee, Y.C.; Huang, S.K. Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1. Nat. Med. 2010, 16, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Garin, M.I.; Chu, C.C.; Golshayan, D.; Cernuda-Morollon, E.; Wait, R.; Lechler, R.I. Galectin-1, a key effector of regulation mediated by CD4+CD25+ T cells. Blood 2007, 109, 2058–2065. [Google Scholar] [CrossRef]
- Kubach, J.; Lutter, P.; Bopp, T.; Stoll, S.; Becker, C.; Huter, E.; Richter, C.; Weingarten, P.; Warger, T.; Knop, J.; et al. Human CD4+CD25+ regulatory T cells: Proteome analysis identifies galectin-10 as a novel marker essential for their anergy and suppressive function. Blood 2007, 110, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Smythies, L.E.; Sellers, M.; Clements, R.H.; Mosteller-Barnum, M.; Meng, G.; Benjamin, W.H.; Orenstein, J.M.; Smith, P.D. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Investig. 2005, 115, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tang, H.; Guo, Z.; An, H.; Zhu, X.; Song, W.; Guo, J.; Huang, X.; Chen, T.; Wang, J.; et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat. Immunol. 2004, 5, 1124–1133. [Google Scholar] [CrossRef]
- Svensson, M.; Maroof, A.; Ato, M.; Kaye, P.M. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity 2004, 21, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M.; Di Sabatino, A. Dendritic cells in intestinal homeostasis and disease. J. Clin. Investig. 2009, 119, 2441–2450. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Artis, D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu. Rev. Immunol. 2010, 28, 623–667. [Google Scholar] [CrossRef]
- Grainger, J.R.; Hall, J.A.; Bouladoux, N.; Oldenhove, G.; Belkaid, Y. Microbe-dendritic cell dialog controls regulatory T-cell fate. Immunol. Rev. 2010, 234, 305–316. [Google Scholar] [CrossRef]
- Zeuthen, L.H.; Fink, L.N.; Frokiaer, H. Epithelial cells prime the immune response to an array of gut- derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology 2008, 123, 197–208. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; Tanoue, T.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef]
- Grainger, J.; Smith, K.; Hewitson, J.; McSorley, H.; Harcus, Y.; Filbey, K.; Finney, C.; Greenwood, E.; Knox, D.; Wilson, M.; et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J. Exp. Med. 2010, 207, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; Amand, A.L.S.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Microbiology Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Salas-Jara, J.; Ilabaca, A.; Vega, M.; García, A. Biofilm Forming Lactobacillus: New Challenges for the Development of Probiotics. Microorganisms 2016, 4, 35. [Google Scholar] [CrossRef]
- Satokari, R. Contentious host—microbiota relationship in inflammatory bowel disease—can foes become friends again? Scand. J. Gastroenterol. 2015, 50, 34–42. [Google Scholar] [CrossRef]
- Ogura, Y.; Bonen, D.K.; Inohara, N.; Nicolae, D.; Chen, F.; Ramos, R.; Britton, H.; Moran, T.; Karaliuskas, R.; Duerr, R.; et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001, 411, 603–606. [Google Scholar] [CrossRef]
- Swidsinski, A.; Ladhoff, A.; Pernthaler, A.; Swidsinski, S.; Loening-Baucke, V.; Ortner, M.; Weber, J.; Hoffma, U.; Schreiber, S.; Dietel, M.; et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002, 122, 44–54. [Google Scholar] [CrossRef]
- Noguchi, E.; Homma, Y.; Kang, X.; Netea, M.; Ma, X. A Crohn’s disease–associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1. Nat. Immunol. 2009, 10, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Denman, S.E.; Morrison, M.; Yu, Z.; Dore, J.; Leclerc, M.; McSweeny, C. Dysbiosis of fecal microbiota in Crohn’s disease patients as revealed by a custom phylogenetic microarray. Inflamm. Bowel Dis. 2010, 16, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Lepage, P.; Seksik, P.; Doré, J.; Marteau, P. Temperature gradient gel electrophoresis of fecal 16S rRNA reveals active Escherichia coli in the microbiota of patients with ulcerative colitis. J. Clin. Microbiol. 2006, 44, 3172–3177. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.; Gallini, C.; Michaud, M.; Clancy, T.; Chung, D.; Locchead, P.; Hold, G. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Tysk, C.; Lindberg, E.; Jarnerot, G.; Flodérus-Myrhed, B. Ulcerative colitis and Crohn’s disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking. Gut 1988, 29, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Spehlmann, M.E.; Begun, A.Z.; Burghardt, J.; Lepage, P.; Radler, A.; Schreiber, S. Epidemiology of inflammatory bowel disease in a German twin cohort: Results of a nationwide study. Inflamm. Bowel Dis. 2008, 14, 968–976. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Duncan, S.; García-Gil, L.; Martínez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef]
- Sjöberg, F.; Barkman, C.; Nookaew, I.; Östman, S.; Adlerberth, I.; Saalman, R.; Wold, A. Low-complexity microbiota in the duodenum of children with newly diagnosed ulcerative colitis. PLoS ONE 2017, 12, e0186178. [Google Scholar] [CrossRef]
- Sokol, H.; Lay, C.; Seksik, P.; Tannock, G. Analysis of bacterial bowel communities of IBD patients: What has it revealed? Inflamm. Bowel Dis. 2008, 14, 858–867. [Google Scholar] [CrossRef]
- Lepage, P.; Hasler, R.; Spehlmann, M.E.; Rehman, A.; Zvibliene, A.; Begun, A.; Ott, S.; Kupcinskas, L.; Doré, J.; Raedler, A.; et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 2011, 141, 227–236. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Imerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitziidefines dysbiosis in patients with ulcerative colitis. Gut 2013, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; Shanahan, F.; Guarner, F.; de Vos, W. Phylogenetic Analysis of Dysbiosis in Ulcerative Colitis During Remission. Inflamm. Bowel Dis. 2013, 19, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Garcia Vilela, E.; De Lourdes De Abreu Ferrari, M.; Oswaldo Da Gama Torres, H.; Guerra Pinto, A.; Carolina Carneiro Aguirre, A.; Paiva Martins, F.; Marcos Andrade Goulart, E.; Sales Da Cunha, A. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn’s disease in remission. Scand. J. Gastroenterol. 2008, 43, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Orel, R.; Tina, K. Intestinal microbiota, probiotics and prebiotics in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 11505. [Google Scholar] [CrossRef] [PubMed]
- Johansson-Lindbom, B.; Svensson, M.; Wurbel, M.A.; Malissen, B.; Márquez, G.; Agace, W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): Requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 2003, 198, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Bono, M.R.; Manjunath, N.; Weninger, W.; Cavanagh, L.; Rosemblatt, M.; Von Andrian, U. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature 2003, 424, 88–93. [Google Scholar] [CrossRef]
- Stagg, A.J.; Kamm, M.A.; Knight, S.C. Intestinal dendritic cells increase T cell expression of α4β7 integrin. Eur. J. Immunol. 2002, 32, 1445–1454. [Google Scholar] [CrossRef]
- Ahiro, S.; Ohtani, H.; Suzuki, M.; Murata, M.; Ejima, C.; Oki, M.; Kinouchi, Y.; Fukushima, K.; Sasaki, I.; Nakamura, S.; et al. Differential expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in ulcerative colitis and Crohn’s disease. Pathol. Int. 2002, 52, 367–374. [Google Scholar] [CrossRef]
- Briskin, M.; Winsor-Hines, D.; Shyjan, A.; Cochran, N.; Bloom, S.; Wilson, J.; McEvoy, L.; Butcher, E.; Kassam, N.; Mackay, C.; et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am. J. Pathol. 1997, 151, 97–110. [Google Scholar]
- Hart, A.L.; Kamm, M.A.; Knight, S.C.; Stagg, A.J. Prospective evaluation of intestinal homing memory T cells in ulcerative colitis. Inflamm. Bowel Dis. 2004, 10, 496–503. [Google Scholar] [CrossRef]
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.P.; Raffatellu, M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 2016, 16, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.R.; Bernardo, D.; Ng, S.C.; Rigby, R.J.; Al-Hassi, H.O.; Landy, J.; Peake, S.T.; Spranger, H.; English, N.R.; Thomas, L.V.; et al. Human gut dendritic cells drive aberrant gut-specific t-cell responses in ulcerative colitis, characterized by increased IL-4 production and loss of IL-22 and IFNγ. Inflamm. Bowel Dis. 2014, 20, 2299–2307. [Google Scholar] [CrossRef]
- Harbour, S.; Maynard, C.; Zindl, C.; Schoeb, T.; Weaver, C. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc. Natl. Acad. Sci. USA 2015, 112, 7061–7066. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.; Elson, C.; Fouser, L.; Kolls, J. The Th17 Pathway and Inflammatory Diseases of the Intestines, Lungs and Skin. Annu. Rev. Pathol. 2013, 8, 477–512. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Feidi, C.; Zhanju, L.; Yingzi, C. Microbiota-specific Th17 cells: Yin and Yang in regulation of inflammatory bowel disease. Inflamm. Bowel Dis. 2016, 22, 1473–1482. [Google Scholar] [CrossRef]
- Atreya, R.; Neurath, M. IBD Pathogenesis in 2014, Molecular pathways controlling barrier function in IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Steenholdt, C.; Coskun, M.; Buhl, S.; Bendtzen, K.; Ainsworth, M.; Brynskov, J.; Nielsen, O. Circulating Cytokines and Cytokine Receptors in Infliximab Treatment Failure Due to TNF-α Independent Crohn Disease. Medicine 2016, 95, e3417. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.; Targan, S. Immunopathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 390–400. [Google Scholar] [CrossRef]
- Wen, Z.; Fiocchi, C. Inflammatory Bowel Disease: Autoimmune or Immune-mediated Pathogenesis? Clin. Dev. Immunol. 2004, 11, 195–204. [Google Scholar] [CrossRef]
- Aronson, R.A.; Cook, S.L.; Roche, J.K. Sensitization to epithelial antigens in chronic mucosal inflammatory disease. I. Purification, characterization, and immune reactivity of murine epithelial cell-associated components (ECAC). J. Immunol. 1983, 131, 2796. [Google Scholar]
- Mitsuyama, K.; Niwa, M.; Takedatsu, H.; Yamasaki, H.; Kuwaki, K.; Yoshioka, S.; Yamauchi, R.; Fukunaga, S.; Torimura, T. Antibody markers in the diagnosis of inflammatory bowel disease. World J. Gastroenterol. 2015, 22, 1304–1310. [Google Scholar] [CrossRef]
- Figueroa-González, I.; Quijano, G.; Ramírez, G.; Cruz-Guerrero, A. Probiotics and prebiotics—perspectives and challenges. J. Sci. Food Agric. 2011, 91, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Metchnikoff, E. Optimistic Studies of the Prolongation of Life; Putman’s Sons: New York, NY, USA, 1998; pp. 161–183. [Google Scholar]
- FAO/WHO. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, UK, 2002. [Google Scholar]
- Costerton, J.W.; Stewwart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Post, J.C.; Stoodley, P.; Hall-Stoodley, L.; Ehrlich, G.D. The role of biofilms in otolaryngologic infections. Curr. Opin. Otolaryngol. Head Neck Surg. 2004, 12, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Terraf, M.C.; Juarez, M.S.; Nader-Macias, M.E.; Silva, C. Screening of biofilm formation by beneficial vaginal lactobacilli and influence of culture media components. J. Appl. Microbiol. 2012, 113, 1517–1529. [Google Scholar] [CrossRef] [PubMed]
- Leccese, M.; Juárez, M.; Rault, L.; Le Loir, Y.; Even, S.; Nader-Macías, M. Biofilms of vaginal Lactobacillus rhamnosus CRL 1332, Kinetics of formation and matrix characterization. Arch. Microbiol. 2016, 198, 689–700. [Google Scholar] [CrossRef]
- Sarxelin, M.; Tynkkynen, S.; Mattila-sandholm, T.; Vos, W.M. Probiotic and other functional microbes: From markets to mechanisms. Curr. Opin. Microbiol. 2005, 16, 204–211. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Haberer, P.; Geisen, R.; Björkroth, J.; Schillinger, U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am. J. Clin. Nutr. 2001, 73, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Rajagopalan, S.; Mohan, R. Management of liver hydatid cysts—Current perspectives. Med. J. Armed Forces India 2012, 68, 304–309. [Google Scholar] [CrossRef] [PubMed]
- De Vos, P.; Fass, M.; Spasojevic, M.; Sikkema, J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- Klayraung, S.; Viernstein, H.; Okonogi, S. Development of tablet containing probiotics: Effects of formulation and processing parameters on bacterial viability. Int. J. Pharm. 2008, 370, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–493. [Google Scholar] [CrossRef]
- Kiew, T.; Cheow, W.; Hadinoto, K. Importance of biofilm age and growth medium on the viability of probiotics capsules containing Lactobacillus rhamnosus GG biofilm. Food Sci. Technol. 2014, 59, 956–963. [Google Scholar] [CrossRef]
- McCarthy, J.; O’Mahony, L.; O’Callaghan, L.; Sheil, B.; Vaughan, E.E.; Fitzsimons, N.; Fitzgibbon, J.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut 2002, 52, 975–980. [Google Scholar] [CrossRef]
- Lammers, K.; Brigidi, P.; Vitali, B.; Gionchetti, P.; Rizello, F.; Caramelli, E.; Matteuzzi, D.; Campieri, M. Immunomodulatory effects of probiotic bacteria DNA: IL-1 and IL-10 response in human peripheral blood mononuclear cells. FEMS Immunol. Med. Microbiol. 2003, 38, 165–172. [Google Scholar] [CrossRef]
- Zaylaa, M.; Al Kassaa, I.; Alard, J.; Peucelle, V.; Boutillier, D.; Desramaut, J.; Dabboussi, F.; Pot, B.; Grangette, C. Probiotics in IBD: Combining in vitro and in vivo models for selecting strains with both anti-inflammatory potential as well as a capacity to restore the gut epithelial barrier. J. Funct. Foods 2018, 47, 304–315. [Google Scholar] [CrossRef]
- Valdovinos, M.A.; Montijo, E.; Abreu, A.T.; Heller, S.; González-Garay, A.; Bacarreza, D.; Bielsa-Fernández, M.; Bojórquez-Ramos, M.C.; Bosques-Padilla, F.; Burguete-García, A.I.; et al. The Mexican consensus on probiotics in gastroenterology. Rev. Gastroenterol. Mex. 2017, 82, 156–178. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2017, 149, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Guslandi, M. Role of Probiotics in Crohn’s Disease and in Pouchitis. J. Clin. Gastroenterol. 2015, 49, S46–S49. [Google Scholar] [CrossRef]
- Guslandi, M.; Mezzi, G.; Sorghi, M.; Testoni, P. Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Digest. Dis. Sci. 2000, 45, 1462–1464. [Google Scholar] [CrossRef]
- Plein, K.; Hotz, J. Therapeutic effects of Saccharomyces boulardii on mild residual symptoms in a stable phase of Crohn’s disease with special respect to chronic diarrhea—a pilot study. Z Gastroenterol. 1993, 31, 129–134. [Google Scholar]
- Bourreille, A.; Cadiot, G.; Le Dreau, G.; Laharie, D.; Beaugerie, L.; Dupas, J.; Marteau, P.; Rampai, P.; Moyse, D.; Saleh, A.; et al. Saccharomyces boulardii Does Not Prevent Relapse of Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2013, 11, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Steed, H.; Macfarlane, G.; Blackett, K.; Bahrami, B.; Reynolds, N.; Walsh, S.; Cummings, J.; Macfarlane, S. Clinical trial: The microbiological and immunological effects of synbiotic consumption—A randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment Pharmacol. Ther. 2010, 32, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, S.; Tatsuguchi, A.; Gudis, K.; Kishida, T.; Mitsui, K.; Ehara, A.; Kobayashi, T.; Sekita, Y.; Seo, T.; Sakamoto, C. High dose probiotic and prebiotic cotherapy for remission induction of active Crohn’s disease. J. Gastroenterol. Hepatol. 2007, 22, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Fedorak, R.; Feagan, B.; Hotte, N.; Leddin, D.; Dieleman, L.; Petrunia, D.; Enns, R.; Bitton, A.; Chiba, N.; Paré, P.; et al. The Probiotic VSL#3 Has Anti-inflammatory Effects and Could Reduce Endoscopic Recurrence After Surgery for Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2015, 13, 928–935. [Google Scholar] [CrossRef]
- Shen, Z.; Zhu, C.; Quan, Y.; Yang, Z.; Wu, S.; Luo, W.; Tan, B.; Wang, X. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef]
- Ghouri, Y.A.; Richards, D.M.; Rahimi, E.F.; Krill, J.T.; Jelinek, K.A.; DuPont, A.W. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin. Exp. Gastroenterol. 2014, 7, 473–487. [Google Scholar] [CrossRef]
- Kruis, W.; Fric, P.; Pokrotnieks, J.; Lukás, M.; Fixa, B.; Kascák, M.; Kamm, M.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef]
- Tsuda, Y.; Yoshimatsu, Y.; Aoki, H.; Nakamura, K.; Irie, M.; Fukuda, K.; Hosoe, N.; Takada, N.; Shirai, K.; Suzuki, Y. Clinical effectiveness of probiotics therapy (BIO-THREE) in patients with ulcerative colitis refractory to conventional therapy. Scand J. Gastroenterol. 2007, 42, 1306–1311. [Google Scholar] [CrossRef]
- Hudson, L.; Anderson, S.; Corbett, A.; Lamb, T. Gleaning Insights from Fecal Microbiota Transplantation and Probiotic Studies for the Rational Design of Combination Microbial Therapies. Clin. Microbiol. Rev. 2016, 30, 191–231. [Google Scholar] [CrossRef]
- Everard, A.; Matamoros, S.; Geurts, L.; Delzenne, N.M.; Cani, P.D. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. mBio 2014, 5, e01011-14. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. (Landmark Ed.) 2009, 14, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Finamore, A.; Nuccitelli, S.; Carnevali, P.; Brigidi, P.; Vitali, B.; Nobili, F.; Rami, R.; Garaguso, I.; Mengheri, E. Prevention of TNBS-induced colitis by different Lactobacillus and Bifidobacterium strains is associ- ated with an expansion of gamma delta T and regulatory T cells of intestinal intraepithelial lymphocytes. Inflamm. Bowel. Dis. 2009, 15, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- van der Waal, M.; Flach, J.; Browne, P.; Besseling-van der Vaart, I.; Claassen, E.; van de Burgwal, L. Probiotics for improving quality of life in ulcerative colitis: Exploring the patient perspective. Pharmanutrition 2019, 7, 100139. [Google Scholar] [CrossRef]
- Ghadimi, D.; Helwig, U.; Schrezenmeir, J.; Heller, K.J.; de Vrese, M. Epigenetic imprinting by commensal probiotics inhibits the IL-23/IL-17 axis in an in vitro model of the intestinal mucosal immune system. J. Leukoc Biol. 2012, 92, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, E.; Ogita, T.; Miyamoto, J.; Kawamoto, S.; Morita, H.; Ohno, H.; Suzuki, T.; Tanabe, S. Bifidobacterium longum alleviates dextran sulfate sodium-induced colitis by suppressing IL-17A response: Involvement of intestinal epithelial costimulatory molecules. PLoS ONE 2013, 8, e79735. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Kinuta, Y.; Saito, Y. Bifidobacterium infantis suppresses proinflammatory interleukin-17 production in murine splenocytes and dextran sodium sulfate-induced intestinal inflammation. Int. J. Mol. Med. 2008, 22, 181–185. [Google Scholar] [CrossRef]
- Ogita, T.; Tanii, Y.; Morita, H.; Suzuki, T.; Tanabe, S. Suppression of Th17 response by Streptococcus thermophilus ST28 through induction of IFN-γ. Int. J. Mol. Med. 2011, 28, 817–822. [Google Scholar] [CrossRef]
| Cytokine | CD | UC | Cytokine | CD | UC |
| IL-1b | I | I | IL-5 | N | I |
| IL-6 | I | I | IL-13 | N | I |
| IL-8 | I | I | IL-17 | I | I |
| IL-12 | I | N | IL-21 | I | N |
| IL-18 | I | I | IFN-γ | I | I |
| IL-23 | I | N | IL-4 | N | I |
| IL-27 | I | N | IL-22 | I | I |
| TNF-α | I | I |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alagón Fernández del Campo, P.; De Orta Pando, A.; Straface, J.I.; López Vega, J.R.; Toledo Plata, D.; Niezen Lugo, S.F.; Alvarez Hernández, D.; Barrientos Fortes, T.; Gutiérrez-Kobeh, L.; Solano-Gálvez, S.G.; et al. The Use of Probiotic Therapy to Modulate the Gut Microbiota and Dendritic Cell Responses in Inflammatory Bowel Diseases. Med. Sci. 2019, 7, 33. https://doi.org/10.3390/medsci7020033
Alagón Fernández del Campo P, De Orta Pando A, Straface JI, López Vega JR, Toledo Plata D, Niezen Lugo SF, Alvarez Hernández D, Barrientos Fortes T, Gutiérrez-Kobeh L, Solano-Gálvez SG, et al. The Use of Probiotic Therapy to Modulate the Gut Microbiota and Dendritic Cell Responses in Inflammatory Bowel Diseases. Medical Sciences. 2019; 7(2):33. https://doi.org/10.3390/medsci7020033
Chicago/Turabian StyleAlagón Fernández del Campo, Pablo, Alejandro De Orta Pando, Juan Ignacio Straface, José Ricardo López Vega, Diego Toledo Plata, Sebastian Felipe Niezen Lugo, Diego Alvarez Hernández, Tomás Barrientos Fortes, Laila Gutiérrez-Kobeh, Sandra Georgina Solano-Gálvez, and et al. 2019. "The Use of Probiotic Therapy to Modulate the Gut Microbiota and Dendritic Cell Responses in Inflammatory Bowel Diseases" Medical Sciences 7, no. 2: 33. https://doi.org/10.3390/medsci7020033
APA StyleAlagón Fernández del Campo, P., De Orta Pando, A., Straface, J. I., López Vega, J. R., Toledo Plata, D., Niezen Lugo, S. F., Alvarez Hernández, D., Barrientos Fortes, T., Gutiérrez-Kobeh, L., Solano-Gálvez, S. G., & Vázquez-López, R. (2019). The Use of Probiotic Therapy to Modulate the Gut Microbiota and Dendritic Cell Responses in Inflammatory Bowel Diseases. Medical Sciences, 7(2), 33. https://doi.org/10.3390/medsci7020033





