Apolipoprotein E Epsilon 4 Genotype, Mild Traumatic Brain Injury, and the Development of Chronic Traumatic Encephalopathy
Abstract
1. Introduction
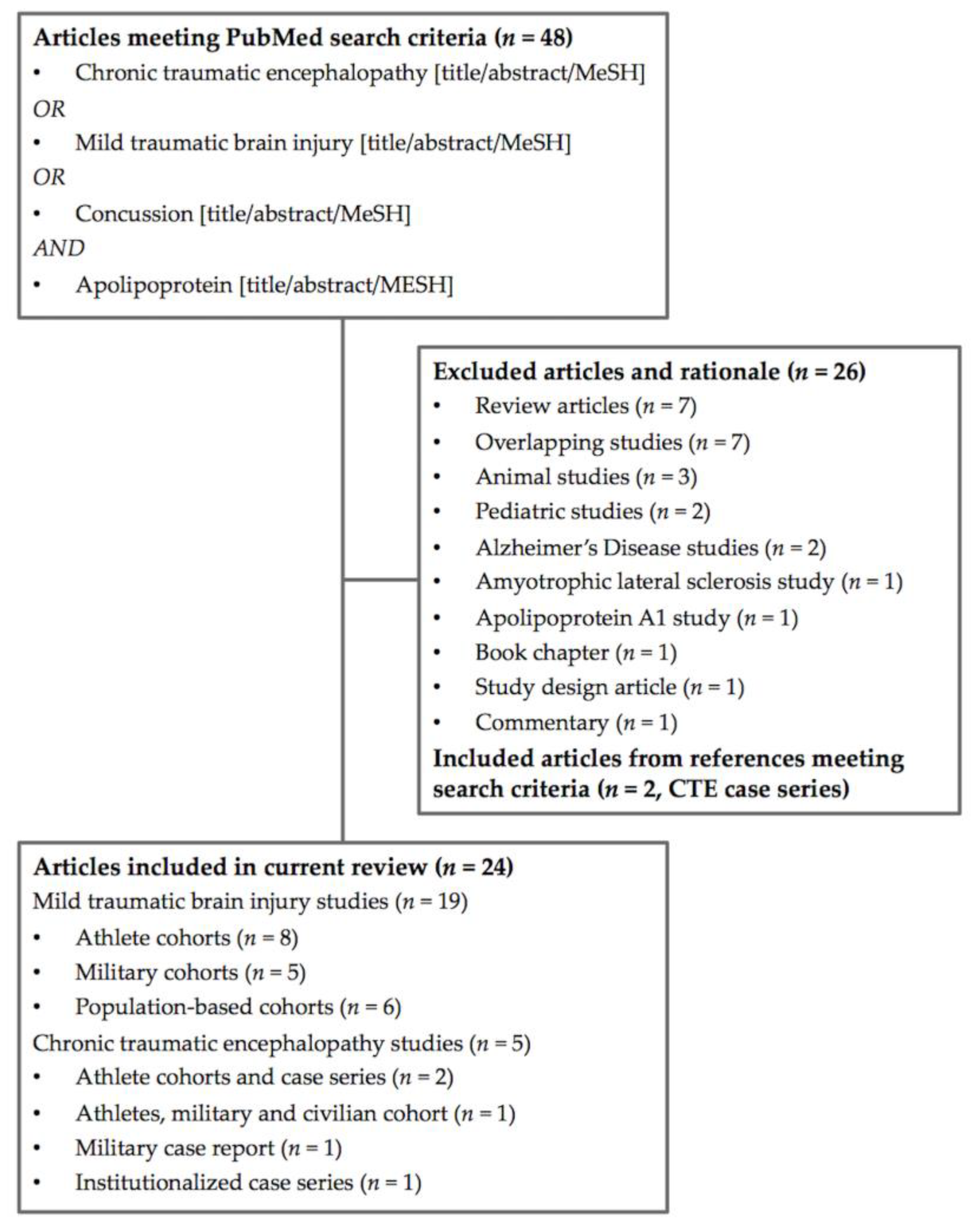
2. Methods
Study Selection
3. Results
3.1. Mild Traumatic Brain Injury in Athletes
3.2. Mild Traumatic Brain Injury in Military Cohorts
3.3. Mild Traumatic Brain Injury in Population—Based Cohorts
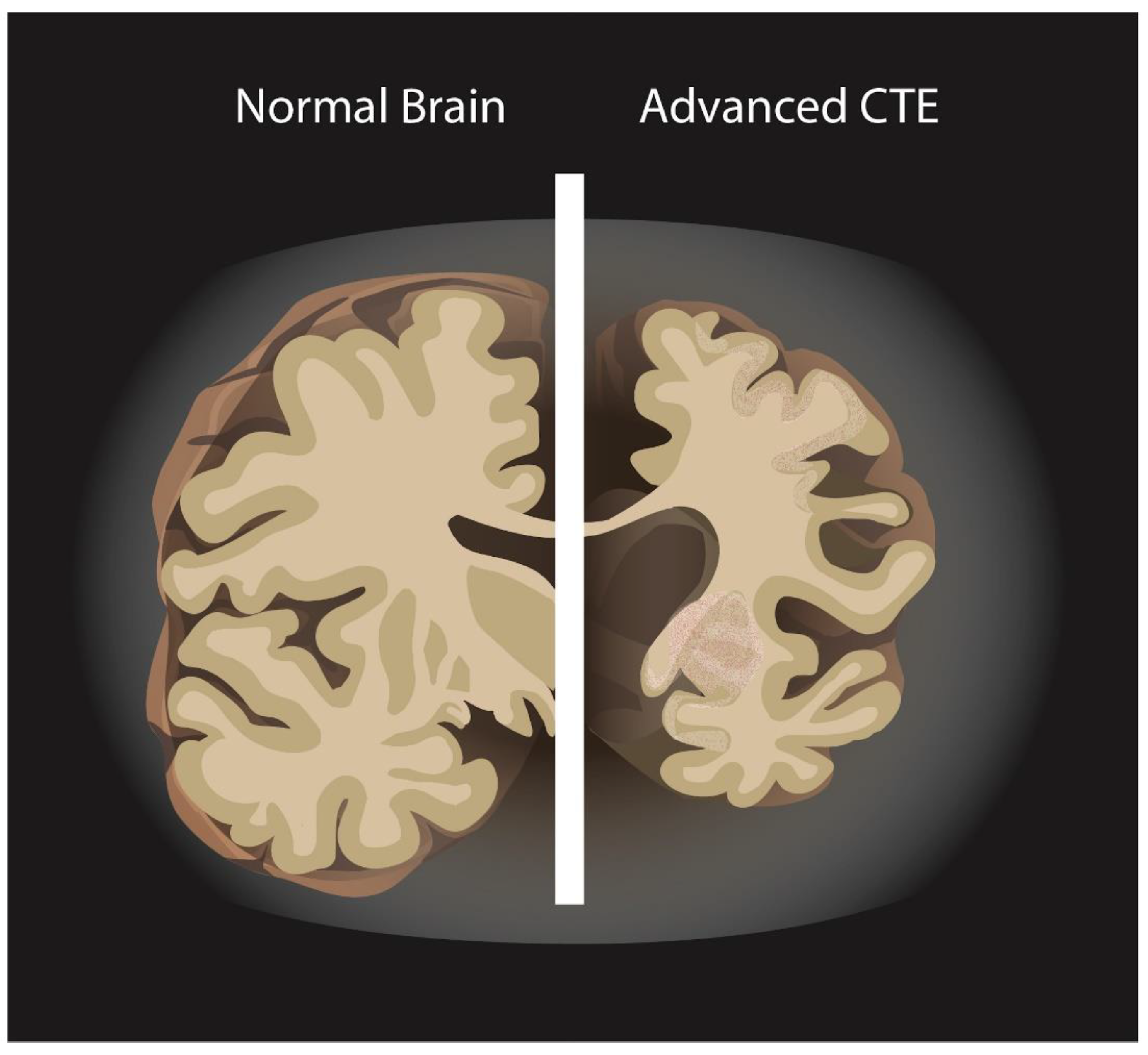
3.4. Chronic Traumatic Encephalopathy in Athletes and Veterans
3.5. Chronic Traumatic Encephalopathy in Leucotomy Patients
4. Discussion
4.1. Role of APOE in the Central Nervous System
4.2. Evidence on APOE and Mild Traumatic Brain Injury
4.3. Evidence on APOE and Chronic Traumatic Encephalopathy
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cassidy, J.D.; Carroll, L.J.; Peloso, P.M.; Borg, J.; von Holst, H.; Holm, L.; Kraus, J.; Coronado, V.G.; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004, 36, 28–60. [Google Scholar] [CrossRef]
- Arciniegas, D.B.; Anderson, C.A.; Topkoff, J.; McAllister, T.W. Mild traumatic brain injury: A neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr. Dis. Treat. 2005, 1, 311–327. [Google Scholar] [PubMed]
- Alexander, M.P. Mild traumatic brain injury: Pathophysiology, natural history, and clinical management. Neurology 1995, 45, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Millspaugh, J.A. Dementia pugilistica. US Nav. Med. Bull. 1937, 35, 297–303. [Google Scholar]
- Martland, H.S. Punch Drunk. J. Am. Med. Assoc. 1928, 91, 1103–1107. [Google Scholar] [CrossRef]
- Critchley, M. Punch-drunk syndromes: The chronic traumatic encephalopathy of boxers. In Hommage a Clovis Vincent; Maloine: Paris, France, 1949. [Google Scholar]
- Corsellis, J.A.N.; Bruton, C.J.; Freeman-Browne, D. The aftermath of boxing. Psychol. Med. 1973, 3, 270–303. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Cairns, N.J.; Dickson, D.W.; Folkerth, R.D.; Dirk Keene, C.; Litvan, I.; Perl, D.P.; Stein, T.D.; Vonsattel, J.P.; Stewart, W.; et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2015, 131, 75–86. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Cantu, R.C.; Nowinski, C.J.; Hedley-Whyte, E.T.; Gavett, B.E.; Budson, A.E.; Santini, V.E.; Lee, H.S.; Kubilus, C.A.; Stern, R.A. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009, 68, 709–735. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Connolly, I.D.; Dangelmajer, S.; Kintzing, J.; Ho, A.L.; Grant, G. Sports-related brain injuries: Connecting pathology to diagnosis. Neurosurg. Focus 2016, 40, E14. [Google Scholar] [CrossRef] [PubMed]
- Bailes, J.E.; Cantu, R.C. Head injury in athletes. Neurosurgery 2001, 48, 26–45, discussion 45–46. [Google Scholar] [PubMed]
- Macciocchi, S.N.; Barth, J.T.; Alves, W.; Rimel, R.W.; Jane, J.A. Neuropsychological functioning and recovery after mild head injury in collegiate athletes. Neurosurgery 1996, 39, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.E.; McKee, A.C. Response to Comment on “Chronic Traumatic Encephalopathy in Blast-Exposed Military Veterans and a Blast Neurotrauma Mouse Model”. Sci. Transl. Med. 2012, 4, 157lr5. [Google Scholar] [CrossRef]
- Omalu, B.; Hammers, J.L.; Bailes, J.; Hamilton, R.L.; Ilyas Kamboh, M.; Webster, G.; Fitzsimmons, R.P. Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurg. Focus 2011, 31, E3. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Stern, R.A.; Nowinski, C.J.; Stein, T.D.; Alvarez, V.E.; Daneshvar, D.H.; Lee, H.-S.; Wojtowicz, S.M.; Hall, G.; Baugh, C.M.; et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013, 136, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, K.F.; Ross, O.A.; Cormier, K.A.; Walton, R.L.; Soto-Ortolaza, A.; Johnston, A.E.; DeSaro, P.; Boylan, K.B.; Graff-Radford, N.R.; Wszolek, Z.K.; et al. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol. 2015, 130, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Samatovicz, R.A. Genetics and brain injury: Apolipoprotein E. J. Head Trauma Rehabil. 2000, 15, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.I.; Horsburgh, K.; Nicoll, J.A.; Teasdale, G.M. Apolipoprotein E and the response of the brain to injury. Acta Neurochir. Suppl. 1999, 73, 89–92. [Google Scholar] [PubMed]
- Chen, Y.; Lomnitski, L.; Michaelson, D.M.; Shohami, E. Motor and cognitive deficits in apolipoprotein E-deficient mice after closed head injury. Neuroscience 1997, 80, 1255–1262. [Google Scholar] [CrossRef]
- Vitek, M.P.; Brown, C.M.; Colton, C.A. APOE genotype-specific differences in the innate immune response. Neurobiol. Aging 2009, 30, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Horsburgh, K.; Kelly, S.; McCulloch, J.; Higgins, G.A.; Roses, A.D.; Nicoll, J.A.R. Increased neuronal damage in apolipoprotein E-deficient mice following global ischaemia. Neuroreport 1999, 10, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Buttini, M.; Orth, M.; Bellosta, S.; Akeefe, H.; Pitas, R.E.; Wyss-Coray, T.; Mucke, L.; Mahley, R.W. Expression of human apolipoprotein E3 or E4 in the brains of Apoe−/− mice: Isoform-specific effects on neurodegeneration. J. Neurosci. 1999, 19, 4867–4880. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Koga, S.; Konno, T.; Nix, J.; Shinohara, M.; Aoki, N.; Das, P.; Parisi, J.E.; Petersen, R.C.; Rosenberry, T.L.; et al. Distinct spatiotemporal accumulation of N-truncated and full-length amyloid-β42 in Alzheimer’s disease. Brain 2017, 140, 3301–3316. [Google Scholar] [CrossRef] [PubMed]
- Abner, E.L.; Neltner, J.H.; Jicha, G.A.; Patel, E.; Anderson, S.L.; Wilcock, D.M.; Van Eldik, L.J.; Nelson, P.T. Diffuse Amyloid-β Plaques, Neurofibrillary Tangles, and the Impact of APOE in Elderly Persons’ Brains Lacking Neuritic Amyloid Plaques. J. Alzheimers Dis. 2018, 64, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.L.; Chidgey, C.; Peng, P.; Masters, C.L.; Roberts, B.R. The Neurobiology and Age-Related Prevalence of the ε4 Allele of Apolipoprotein E in Alzheimer’s Disease Cohorts. J. Mol. Neurosci. 2016, 60, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, B.; Abud, E.M.; Abud, A.M.; Poon, W.W.; Gylys, K.H. Tau Spread, Apolipoprotein E, Inflammation, and More: Rapidly Evolving Basic Science in Alzheimer Disease. Neurol. Clin. 2017, 35, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Hohman, T.J.; Dumitrescu, L.; Barnes, L.L.; Thambisetty, M.; Beecham, G.; Kunkle, B.; Gifford, K.A.; Bush, W.S.; Chibnik, L.B.; Mukherjee, S.; et al. Alzheimer’s Disease Genetics Consortium and the Alzheimer’s Disease Neuroimaging Initiative Sex-Specific Association of Apolipoprotein E with Cerebrospinal Fluid Levels of Tau. JAMA Neurol. 2018, 75, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Farfel, J.M.; Yu, L.; De Jager, P.L.; Schneider, J.A.; Bennett, D.A. Association of APOE with tau-tangle pathology with and without β-amyloid. Neurobiol. Aging 2016, 37, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Kerr, M.E.; Kim, Y.; Kamboh, M.I.; Beers, S.R.; Conley, Y.P. Apolipoprotein E4 allele presence and functional outcome after severe traumatic brain injury. J. Neurotrauma 2007, 24, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, X.; Gui, L.; Xia, Y.; Tang, W.; Cao, Y.; Gu, Y. Correlation between APOE-491AA promoter in epsilon4 carriers and clinical deterioration in early stage of traumatic brain injury. J. Neurotrauma 2007, 24, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.-F.; Chang, J.G.; Hu, C.J. Association between apolipoprotein E genotype and outcome of traumatic brain injury. Acta Neurochir. 2003, 145, 649–653, discussion 653–654. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.D.; Relkin, N.R.; Ravdin, L.D.; Jacobs, A.R.; Bennett, A.; Gandy, S. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA 1997, 278, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.; Froom, P.; Sazbon, L.; Grinblatt, I.; Shochina, M.; Tsenter, J.; Babaey, S.; Yehuda, B.; Groswasser, Z. Apolipoprotein E-epsilon4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology 1999, 52, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, A.; Marklund, P.; Nilsson, L.G.; Cruts, M.; Adolfsson, R.; Van Broeckhoven, C.; Nyberg, L. APOE influences on neuropsychological function after mild head injury: Within-person comparisons. Neurology 2004, 62, 1963–1966. [Google Scholar] [CrossRef] [PubMed]
- Millar, K.; Nicoll, J.A.R.; Thornhill, S.; Murray, G.D.; Teasdale, G.M. Long term neuropsychological outcome after head injury: Relation to APOE genotype. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Chamelian, L.; Reis, M.; Feinstein, A. Six-month recovery from mild to moderate Traumatic Brain Injury: The role of APOE-epsilon4 allele. Brain 2004, 127, 2621–2628. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, G.M.; Murray, G.D.; Nicoll, J.A.R. The association between APOE epsilon4, age and outcome after head injury: A prospective cohort study. Brain 2005, 128, 2556–2561. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.A.; Daneshvar, D.H.; Baugh, C.M.; Seichepine, D.R.; Montenigro, P.H.; Riley, D.O.; Fritts, N.G.; Stamm, J.M.; Robbins, C.A.; McHale, L.; et al. Clinical presentation of chronic traumatic encephalopathy. Neurology 2013, 81, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Kristman, V.L.; Tator, C.H.; Kreiger, N.; Richards, D.; Mainwaring, L.; Jaglal, S.; Tomlinson, G.; Comper, P. Does the Apolipoprotein ε4 Allele Predispose Varsity Athletes to Concussion? A Prospective Cohort Study. Clin. J. Sport Med. 2008, 18, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Tierney, R.T.; Mansell, J.L.; Higgins, M.; McDevitt, J.K.; Toone, N.; Gaughan, J.P.; Mishra, A.; Krynetskiy, E. Apolipoprotein E genotype and concussion in college athletes. Clin. J. Sport Med. 2010, 20, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Casson, I.R.; Viano, D.C.; Haacke, E.M.; Kou, Z.; LeStrange, D.G. Is There Chronic Brain Damage in Retired NFL Players? Neuroradiology, Neuropsychology, and Neurology Examinations of 45 Retired Players. Sports Health 2014, 6, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, S.; Mc Fie, S.; Patricios, J.; Suter, J.; Posthumus, M.; September, A.V. An association between polymorphisms within the APOE gene and concussion aetiology in rugby union players. J. Sci. Med. Sport 2018, 21, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Merritt, V.C.; Arnett, P.A. Apolipoprotein E (APOE) ε4 Allele Is Associated with Increased Symptom Reporting Following Sports Concussion. J. Int. Neuropsychol. Soc. 2016, 22, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Merritt, V.C.; Rabinowitz, A.R.; Arnett, P.A. The Influence of the Apolipoprotein E (APOE) Gene on Subacute Post-Concussion Neurocognitive Performance in College Athletes. Arch. Clin. Neuropsychol. 2018, 33, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Esopenko, C.; Chow, T.W.; Tartaglia, M.C.; Bacopulos, A.; Kumar, P.; Binns, M.A.; Kennedy, J.L.; Müller, D.J.; Levine, B. Cognitive and psychosocial function in retired professional hockey players. J. Neurol. Neurosurg. Psychiatry 2017, 88, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, G.D.; Sundman, M.H.; Hall, E.E.; Kostek, M.C.; Patel, K.; Barnes, K.P.; Ketcham, C.J. Genetics Influence Neurocognitive Performance at Baseline but Not Concussion History in Collegiate Student-Athletes. Clin. J. Sport Med. 2017, 28, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Dretsch, M.N.; Silverberg, N.; Gardner, A.J.; Panenka, W.J.; Emmerich, T.; Crynen, G.; Ait-Ghezala, G.; Chaytow, H.; Mathura, V.; Crawford, F.C.; et al. Genetics and Other Risk Factors for Past Concussions in Active-Duty Soldiers. J. Neurotrauma 2017, 34, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.P.; Logue, M.W.; Sadeh, N.; Spielberg, J.M.; Verfaellie, M.; Hayes, S.M.; Reagan, A.; Salat, D.H.; Wolf, E.J.; McGlinchey, R.E.; et al. Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain 2017, 140, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Han, S.D.; Duke Han, S.; Suzuki, H.; Drake, A.I.; Jak, A.J.; Houston, W.S.; Bondi, M.W. Clinical, Cognitive, and Genetic Predictors of Change in Job Status Following Traumatic Brain Injury in a Military Population. J. Head Trauma Rehabil. 2009, 24, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Emmerich, T.; Abdullah, L.; Crynen, G.; Dretsch, M.; Evans, J.; Ait-Ghezala, G.; Reed, J.; Montague, H.; Chaytow, H.; Mathura, V.; et al. Plasma Lipidomic Profiling in a Military Population of Mild Traumatic Brain Injury and Post-Traumatic Stress Disorder with Apolipoprotein E ε4–Dependent Effect. J. Neurotrauma 2016, 33, 1331–1348. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.A.; Spellicy, C.J.; Harding, M.J.; Graham, D.P. Apolipoprotein E DNA methylation and posttraumatic stress disorder are associated with plasma ApoE level: A preliminary study. Behav. Brain Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Liberman, J.N.; Stewart, W.F.; Wesnes, K.; Troncoso, J. Apolipoprotein E epsilon 4 and short-term recovery from predominantly mild brain injury. Neurology 2002, 58, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Sundström, A.; Nilsson, L.G.; Cruts, M.; Adolfsson, R.; Van Broeckhoven, C.; Nyberg, L. Fatigue before and after mild traumatic brain injury: Pre–post-injury comparisons in relation to Apolipoprotein E. Brain Inj. 2007, 21, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Ingebrigtsen, T.; Wilsgaard, T.; Wikran, G.; Fagerheim, T.; Romner, B.; Waterloo, K. Prediction of time trends in recovery of cognitive function after mild head injury. Neurosurgery 2009, 64, 698–704, discussion 704. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.K.; Robinson, C.K.; Burke, J.F.; Winkler, E.A.; Deng, H.; Cnossen, M.C.; Lingsma, H.F.; Ferguson, A.R.; McAllister, T.W.; Rosand, J.; et al. The TRACK-TBI Investigators Apolipoprotein E epsilon 4 (APOE-ε4) genotype is associated with decreased 6-month verbal memory performance after mild traumatic brain injury. Brain Behav. 2017, 7, e00791. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Hsiao, I.T.; Hsieh, C.J.; Chiang, Y.H.; Yen, T.C.; Chiu, W.T.; Lin, K.J.; Hu, C.J. Accumulation of amyloid in cognitive impairment after mild traumatic brain injury. J. Neurol. Sci. 2015, 349, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Yeh, C.T.; Ou, J.C.; Ma, H.P.; Chen, K.Y.; Chang, C.F.; Lai, J.H.; Liao, K.H.; Lin, C.M.; Lin, S.Y.; et al. The Association of Apolipoprotein E Allele 4 Polymorphism with the Recovery of Sleep Disturbance after Mild Traumatic Brain Injury. Acta Neurol. Taiwan 2017, 26, 13–19. [Google Scholar] [PubMed]
- Omalu, B.; Bailes, J.; Hamilton, R.L.; Kamboh, M.I.; Hammers, J.; Case, M.; Fitzsimmons, R. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery 2011, 69, 173–183, discussion 183. [Google Scholar] [CrossRef] [PubMed]
- Shively, S.B.; Edgerton, S.L.; Iacono, D.; Purohit, D.P.; Qu, B.-X.; Haroutunian, V.; Davis, K.L.; Diaz-Arrastia, R.; Perl, D.P. Localized cortical chronic traumatic encephalopathy pathology after single, severe axonal injury in human brain. Acta Neuropathol. 2017, 133, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Buzas, D.; Jacobson, N.A.; Morawa, L.G. Concussions from 9 Youth Organized Sports: Results from NEISS Hospitals Over an 11-Year Time Frame, 2002–2012. Orthop. J. Sports Med. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.J.; Patel, D.R. Sports related mild traumatic brain injury in adolescents. Indian J. Pediatr. 2000, 67, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.; Lovell, M.R.; Collins, M.W.; Pardini, J. Neurocognitive and symptom predictors of recovery in high school athletes. Clin. J. Sport Med. 2009, 19, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W.; Weisgraber, K.H.; Huang, Y. Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2006, 103, 5644–5651. [Google Scholar] [CrossRef] [PubMed]
- Finch, C.E.; Morgan, T.E. Systemic inflammation, infection, ApoE alleles, and Alzheimer disease: A position paper. Curr. Alzheimer Res. 2007, 4, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; LaDu, M.J.; Van Eldik, L.J. A dual role for apolipoprotein e in neuroinflammation: Anti- and pro-inflammatory activity. J. Mol. Neurosci. 2004, 23, 205–212. [Google Scholar] [CrossRef]
- Zlatar, Z.Z.; Bischoff-Grethe, A.; Hays, C.C.; Liu, T.T.; Meloy, M.J.; Rissman, R.A.; Bondi, M.W.; Wierenga, C.E. Higher Brain Perfusion May Not Support Memory Functions in Cognitively Normal Carriers of the ApoE ε4 Allele Compared to Non-Carriers. Front. Aging Neurosci. 2016, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.E.; Kraus, M.; Marion, D.; Kamboh, I. Evaluation of Apolipoprotein E Genotypes on Cerebral Blood Flow and Metabolism Following Traumatic Brain Injury. In Advances in Experimental Medicine and Biology; Springer Nature International Publishing: New York, NY, USA, 1999; pp. 117–124. [Google Scholar]
- Methia, N.; André, P.; Hafezi-Moghadam, A.; Economopoulos, M.; Thomas, K.L.; Wagner, D.D. ApoE deficiency compromises the blood brain barrier especially after injury. Mol. Med. 2001, 7, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Bigler, E.D.; Johnson, S.C.; Anderson, C.V.; Blatter, D.D. Traumatic brain injury and memory: The role of hippocampal atrophy. Neuropsychology 1996, 10, 333–342. [Google Scholar] [CrossRef]
- Bigler, E.D.; Anderson, C.V.; Blatter, D.D.; Andersob, C.V. Temporal lobe morphology in normal aging and traumatic brain injury. AJNR Am. J. Neuroradiol. 2002, 23, 255–266. [Google Scholar] [PubMed]
- Teasdale, G.M.; Nicoll, J.A.; Murray, G.; Fiddes, M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet 1997, 350, 1069–1071. [Google Scholar] [CrossRef]
- Crawford, F.C.; Vanderploeg, R.D.; Freeman, M.J.; Singh, S.; Waisman, M.; Michaels, L.; Abdullah, L.; Warden, D.; Lipsky, R.; Salazar, A.; et al. APOE genotype influences acquisition and recall following traumatic brain injury. Neurology 2002, 58, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Mayeux, R.; Ottman, R.; Maestre, G.; Ngai, C.; Tang, M.X.; Ginsberg, H.; Chun, M.; Tycko, B.; Shelanski, M. Synergistic Effects of Traumatic Head Injury and Apolipoprotein-epsilon4 in Patients with Alzheimer’s Disease. Neurology 1995, 45, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Omalu, B.I.; DeKosky, S.T.; Minster, R.L.; Ilyas Kamboh, M.; Hamilton, R.L.; Wecht, C.H. Chronic Traumatic Encephalopathy in a National Football League Player. Neurosurgery 2006, 59, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Gavett, B.E.; Stern, R.A.; McKee, A.C. Chronic traumatic encephalopathy: A potential late effect of sport-related concussive and subconcussive head trauma. Clin. Sports Med. 2011, 30, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.R.; Marshall, S.W.; Mueller, F.O.; Yang, J.; Weaver, N.L.; Kalsbeek, W.D.; Bowling, J.M. Incidence and risk factors for concussion in high school athletes, North Carolina, 1996–1999. Am. J. Epidemiol. 2004, 160, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Yuh, E.L.; Mukherjee, P.; Lingsma, H.F.; Yue, J.K.; Ferguson, A.R.; Gordon, W.A.; Valadka, A.B.; Schnyer, D.M.; Okonkwo, D.O.; Maas, A.I.R.; et al. TRACK-TBI Investigators Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann. Neurol. 2013, 73, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Büki, A.; Povlishock, J.T. All roads lead to disconnection?—Traumatic axonal injury revisited. Acta Neurochir. 2006, 148, 181–193, discussion 193–194. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.; Pueyo, R.; Matarín Mdel, M.; Junqué, C.; Mataró, M.; Clemente, I.; Moral, P.; Poca, M.A.; Garnacho, A.; Sahuquillo, J. Influence of APOE polymorphism on cognitive and behavioural outcome in moderate and severe traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1191–1193. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.; Giacino, J.; Harrison-Felix, C.; Manley, G.; Valadka, A.; Wilde, E.A. Progress in developing common data elements for traumatic brain injury research: Version two—The end of the beginning. J. Neurotrauma 2013, 30, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Wilde, E.A.; Whiteneck, G.G.; Bogner, J.; Bushnik, T.; Cifu, D.X.; Dikmen, S.; French, L.; Giacino, J.T.; Hart, T.; Malec, J.F.; et al. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch. Phys. Med. Rehabil. 2010, 91, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Broglio, S.P.; Kontos, A.P.; Levin, H.; Schneider, K.; Wilde, E.A.; Cantu, R.C.; Feddermann-Demont, N.; Fuller, G.W.; Gagnon, I.; Gioia, G.A.; et al. National Institute of Neurological Disorders and Stroke and Department of Defense Sport-Related Concussion Common Data Elements Version 1.0 Recommendations. J. Neurotrauma 2018. [Google Scholar] [CrossRef] [PubMed]
- Geddes, J.F.; Vowles, G.H.; Nicoll, J.A.; Révész, T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol. 1999, 98, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Gavett, B.E.; Stern, R.A.; Cantu, R.C.; Nowinski, C.J.; McKee, A.C. Mild traumatic brain injury: A risk factor for neurodegeneration. Alzheimers Res. Ther. 2010, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.M.; Brecht, W.J.; Xu, Q.; Mahley, R.W.; Huang, Y. Increased tau phosphorylation in apolipoprotein E4 transgenic mice is associated with activation of extracellular signal-regulated kinase: Modulation by zinc. J. Biol. Chem. 2004, 279, 44795–44801. [Google Scholar] [CrossRef] [PubMed]
- Lautner, R.; Insel, P.S.; Skillbäck, T.; Olsson, B.; Landén, M.; Frisoni, G.B.; Herukka, S.-K.; Hampel, H.; Wallin, A.; Minthon, L.; et al. Preclinical effects of APOE ε4 on cerebrospinal fluid Aβ42 concentrations. Alzheimers Res. Ther. 2017, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Sutphen, C.L.; Jasielec, M.S.; Shah, A.R.; Macy, E.M.; Xiong, C.; Vlassenko, A.G.; Benzinger, T.L.S.; Stoops, E.E.J.; Vanderstichele, H.M.J.; Brix, B.; et al. Longitudinal Cerebrospinal Fluid Biomarker Changes in Preclinical Alzheimer Disease During Middle Age. JAMA Neurol. 2015, 72, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; van Duijn, C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997, 278, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
| Search Criteria [Title/Abstract/MeSH Terms]: (Chronic Traumatic Encephalopathy or Mild Traumatic Brain Injury or Concussion) and Apolipoprotein E | ||||||
|---|---|---|---|---|---|---|
| MTBI in Athlete Cohorts (8 Studies) | ||||||
| Author and Year | Study Type | N | Sex; Age | Description | Outcome Measures | Results |
| Cochrane et al., 2018 | MTBI, prospective, athletes | 250 collegiate athletes (95 football, 58 baseball/softball, 67 soccer, 18 basketball, 2 cross-country/track and field) | 184 male, 66 female; 19.0 ± 1.3 years old | APOE-ε2 carriers (n = 28), APOE-ε3 carriers (n = 128), APOE-ε4 carriers (n = 68), 24% self-reported a history of prior concussion. | Self-reported history of concussions and neurocognitive performance from Immediate Post-concussion Assessment and Cognitive Testing (ImPACT). | Apo-ε4 carriers had significantly slower reaction time (p = 0.002) as measured by the ImPACT. |
| Kristman et al., 2008 | MTBI, prospective, athletes | 318 collegiate athletes (43 football, 26 field hockey, 32 basketball, 23 ice hockey, 45 lacrosse, 49 rugby, 53 soccer, 47 volleyball) | 164 male, 154 female, mean age 20.5 ± 2.4 years (range 17–31) | 79 (24.8%) APOE-ε4(+) athletes | Concussions in athletes were diagnosed by the sport-medicine team and verified by a sport-medicine physician. | In the proportional hazards model, presence of the APOE-ε4 was not significantly associated with concussion (p = 0.68). |
| Merritt et al., 2016 | MTBI, prospective, athletes | 42 collegiate athletes (sports not defined) | 35 male, 7 female; subjects with mild concussions (14.3% LOC); ε4 carriers aged 19.9 ± 1.4 years, noncarriers aged 20.0 ± 1.6 years | 15 (35.7%) APOE-ε4(+) athletes, and 27 (64.3%) APOE-ε4(−) athletes | Team physicians determined TBI and concussed athletes were referred for neuropsychological testing post-injury. The Post-Concussion Symptom Scale was used to evaluate self-report symptoms. Physical and cognitive symptom clusters were each dichotomized into “symptoms present” versus “symptoms absent” groups | ε4-carriers associated with “symptoms present” group; ε4(+) athletes more likely to endorse physical symptoms than ε4(−) athletes (odds ratio (OR) = 5.25 (1.33–20.76); p = 0.015)). For cognitive symptoms, ε4-carriers associated with “symptoms present” group; ε4(+) athletes more likely to endorse cognitive symptoms than ε4(−) athletes, (OR = 4.75 (1.23–18.41), p = 0.020) |
| Merritt et al., 2018 | MTBI, prospective, athletes | 57 collegiate athletes (16 football, 11 basketball, 8 lacrosse, 7 rugby, 7 hockey, 4 soccer, 2 wresting, 2 other). | 44 males, 13 females; ε4 carriers 20.3 ± 1.4 years old, ε4 noncarriers 20.3 ± 1.4 years old | 20 athletes (35.1%) APOE-ε4(+); 37 athletes (64.9%) APOE-ε4(−) | Neurocognitive test battery including learning and memory, attention, processing speed, and executive functions: Brief Visuospatial Memory Test, Hopkins Verbal Learning Test; Symbol-Digit Modalities Test; Vigil Continuous Performance Test; Digit Span Test from the WAIS-III; Trail-Making Test; Penn State University Cancellation Test; Stroop Color-Word Test; ImPACT. | No significant differences were seen between athletes with and without the APOE-ε4 allele when examining mean neurocognitive scores (all p > 0.05). However, ε4(+) subjects were more likely to show a greater number of impaired neurocognitive scores post-injury compared to ε4(−) athletes (p < 0.05). More ε4+ athletes (40.0%; 8 of 20) fell in the impaired group compared with ε4- athletes (16.2%; 6 of 37; p = 0.046). APOE-ε4(+) athletes demonstrated greater neurocognitive variability than athletes without APOE-ε4 (p < 0.05). |
| Abrahams et al., 2017 | MTBI retrospective, athletes (rugby) | 288 rugby athletes (121 high school, 116 club, 51 professional) | Males; controls younger than concussed group (19.2 ± 3.5 years vs. 20.5 ± 4.4 years; p = 0.008) | The concussed group (N = 160) reported 1.9 ± 1.0 prior concussions (45%: 1; 34.4%: 2; 8.8%: 3; 11.9%: 4 concussions) | Self-reported duration of symptoms (<1 week versus ≥1 week) | APOE-ε4 isoforms did not differ significantly between concussion and control groups. APOE-ε4 isoform frequency distribution did not differ significantly between duration of symptoms groups |
| Casson et al., 2014 | Chronic brain damage, retrospective cross-sectional, athletes | 45 retired National Football League (NFL) players | 45 males; 45.6 ± 8.9 years old (range, 30–60 years) | Athletes reported 6.9 ± 6.2 concussions (maximum 25) throughout their careers | MMSE, dysarthria, pyramidal system dysfunction, extrapyramidal system dysfunction, cerebellar dysfunction, depression, PHQ9, ImPACT. susceptibility weighted imaging (SWI) and diffusion tensor imaging (DTI) evaluation for brain injuries | APOE-ε4 allele present in 38% of players, a larger number than expected in the general male population (23–26%). No association of the APOE-ε4 genotype with anatomical magnetic resonance imaging (MRI), clinical neurologic, depression, or neuropsychological test results |
| Esopenko et al., 2017 | MTBI, retrospective, alumni athletes | 33 retired National Hockey League (NHL) players | 53 males; alumni athletes 54.3 ± 10.4 years old, controls 53.4 ± 10.2 years old, | Concussions in athletes were 4.8 ± 2.7; in controls were 0.6 ± 0.8; 9 alumni and 4 controls were ε4(+); 1 ε4/ε4, 2 ε2/ε4, rest were ε2/ε3 or ε3/ε3 | Self-reported history of concussion, cognitive function and questionnaires on psychosocial/psychiatric function | ε4-carriers associated with increased psychiatric complaints (p = 0.009) but not objective cognitive performance. Executive function associated with number of concussions after accounting for confounders (β = −0.55 and −0.39, p = 0.005 and p = 0.039 for concussion and age, respectively) |
| Tierney et al., 2010 | MTBI, retrospective, athletes | 196 collegiate athletes (163 football; 33 soccer) | 163 male football and 33 female soccer athletes, age was 19.7 ± 1.5 years old | 48 (24.5%) with 1 concussion, 9/48 (4.6%) with >1 concussions. ε2 and ε4 present in 35 (17.7%) and 62 (31.9%) athletes. | Self-reported history of concussions. | No association between ε4 and ε2 carriers and history of concussion. Significant association (OR = 9.8; p = 0.05) between carrying APOE rare alleles and prior concussions. |
| MTBI in Military Cohorts (5 Studies) | ||||||
| Author | Study Type | N | Sex; Age | Description | Outcome Measures | Results |
| Dretsch et al., 2017 | MTBI, retrospective, active-duty military | 458 military members from two brigade combat teams preparing to deploy to Iraq and Afghanistan | 430 male, 28 female; age was 26.0 ± 7.0 years | History of concussion in 36.5%, with 10.7% having 3+ concussions. 38% (162/430) of men with 1+ concussions, vs. 18% (5/28) of women | Self-reported history of concussions | APOE not associated with risk for concussions. Of ε3/ε4 or ε4/ε4 soldiers, 44.1% (41/93) had a history of one or more prior concussions compared with 34.5% (126/365) of the comparison group (OR = 1.50; p = 0.087) |
| Emmerich et al., 2016 | MTBI, retrospective, active-duty military | 120 demographically matched soldiers with prior deployment to Iraq or Afghanistan (21 with TBI, 34 with posttraumatic stress disorder (PTSD); 13 with TBI + PTSD; 52 controls) | 120 males; TBI subjects’ age was 25.9 ± 1.4 years, PTSD subjects’ age was 26.4 ± 1.3 years, TBI + PTSD subjects’ age was 29.9 ± 1.6 years old; control subjects’ age was 26.7 ± 1.0 years | 85 APOE-ε4(−) (control n = 37, TBI n = 16, PTSD n = 24, TBI + PTSD n = 8); APOE-ε4(+) n = 35 (control n = 15, TBI = 5, PTSD = 10, TBI + PTSD n = 5) | Self-reported history of TBI, including military-associated injury and all other traumas; alcohol use; medical history; prior deployments; current medications; PTSD symptoms; and depression. Blood samples for lipidomic analysis. | ε4(+) subjects exhibited higher plasma phospholipids levels than their ε4(−) counterparts within diagnostic groups. ε4 noncarriers showed decreased saturated fatty acids (SFA)- and monounsaturated fatty acids (MUFA)-containing phosphatidylethanolamine (PE) species for TBI, PTSD, and TBI+PTSD groups, compared with controls. ε4 carriers showed no significant differences between TBI and PTSD groups for SFA- and MUFA. Interaction between ε4-carriers and diagnosis of TBI+PTSD on MUFA-containing lysophosphatidylcholine (LPC) and lysophosphatidylethanolamide (LPE) species. |
| Han et al., 2009 | Mild to moderate TBI, retrospective, active-duty military | 53 military personnel | 42 male, 4 female; Mean age of APOE-ε4(+) was 22.6 ± 3.8 years, mean age of APOE-ε4(−) was 25.2 ± 6.1 years | 16 APOE-ε4(+), 30 APOE-ε4(−) | Job change (reduction in duties for any reason e.g., medical hold, rehabilitation or assignment to light/limited duties (n = 24), reduction due to TBI (n = 3), referral to Medical Board (n = 3), or administrative separation (n = 1)), using neuropsychological assessments | In ε4-carriers, job status was determined by a long-delay free recall on the CVLT-II. If the percent change between long-delay free recall and short-delay free recall (defined as ((long-delay free recall raw score) minus (short-delay free recall raw score))/(short-delay free recall raw score)) >3.55%, subjects correctly predicted as no change in work status (85.7% accuracy). If the percentage change was <3.55%, subjects were correctly predicted to have a change in their job status with 88.9% accuracy |
| Hayes et al., 2017 | MTBI, retrospective, veterans | 160 veterans of OEF/OIF and/or New Dawn | 149 male, 11 female; non-MTBI aged 32.9 ± 8.9 years, MTBI aged 30.6 ± 8.1 years | 55 with no MTBI, 105 with MTBI. ε4(+): 10/55 with no MTBI, 27/105 with MTBI | Linear models examined the main effect of APOE (ε4-carriers, n = 37; non-ε4 carriers, n = 123) and MTBI × APOE interaction factor on cortical thickness. | No main effect of APOE on cortical thickness; no MTBI/APOE interaction. |
| Nielsen et al., 2018 | MTBI, retrospective, veterans | 87 veterans with or without MTBI | Demographics not published | 47 veterans with MTBI and 40 controls | Hierarchical linear regression to evaluate the association between DNA methylation, MTBI, and APOE genotype with plasma APOE, controlling for age, sex, population structure, depression and PTSD | Plasma APOE associated with PTSD severity (p = 0.013). Higher APOE levels in ε3/ε3 compared to ε4 carriers (p = 0.031). Plasma APOE was associated with DNA methylation at CpG sites −877 (p = 0.021), and −775 (p = 0.014). The interaction factor ε4 × PTSD was associated with DNA methylation at CpG −675 (p = 0.009) |
| MTBI in Population-Based Cohorts (6 Studies) | ||||||
| Author | Study Type | N | Sex; Age | Description | Outcome Measures | Results |
| Lee et al., 2017 | MTBI, prospective, population-based | 189 patients from emergency departments (ED) of three hospitals | 76 male, 113 female; Mean age of ε4(+) was 42.2 ± 14.7 years and ε4(−) was 40.1 ± 15.2 years | 35 ε4(+), 154 ε4(−) | 1st week post-mTBI and 6th week post-mTBI) sleep assessments, using the Pittsburgh Sleep Quality Index (PSQI) | No difference in PSQI at baseline and week 6 between ε4-carriers and noncarriers. Both ε4 carriers and noncarriers exhibited improvement in overall PSQI scores between baseline and week 6 follow-up (carrier: baseline 8.3 ± 3.9, 6th week: 7.4 ± 4.9, p = 0.05; noncarrier: baseline 8.5 ± 4.4, 6th week: 8.1 ± 3.8, p = 0.03) |
| Liberman et al., 2002 | MTBI, prospective, population-based | 87 adult patients presenting with mild or moderate TBI to a shock trauma center | 48 males, 32 females; <30 years of age (n = 25), 30–49 years old (n = 28) and ≥50 years old (n = 27) | 18 ε4(+), 62 ε4(−) | 13 neuropsychological tests administered twice at 3 and 6 weeks post-injury | 90% with MTBI; 18 (22.5%) ε4-carriers, who had lower scores on 12 of 13 neuropsychological outcomes at visit 1 compared to noncarriers, 2 were significant (grooved pegboard test, p = 0.005; paced auditory serial addition task 2.8-s trial, p = 0.004). At visit 2, ε4(+) had lower adjusted mean scores on 11/13 neuropsychological outcomes, though none were statistically significant. |
| Muller et al., 2009 | MTBI, prospective, population-based | 59 patients with MTBI | 47 male, 12 female; Mean age 35.1 years (range 18–74) | 13 APOE-ε4(+), 46 APOE-ε4(−) | GCS in ED, head computed tomography (CT) and MRI, neurophysiological assessments at baseline and 6-months. Serum S100B was measured. | GCS < 15, TBI on CT/MRI, and serum S-100B > 0.14 μg/L predicted impaired cognitive performance at baseline and 6-months while APOE did not. APOE-ε4 genotype was the only independent factor significantly predicting less improvement from baseline to 6-months. |
| Sundstrom et al., 2007 | MTBI, prospective, population-based | 31 MTBI patients and 62 matched controls | 18 male, 13 female; Mean age 55.2 ± 13.6 years | APOE-ε4 present in 38.7% of MTBI subjects and controls | Self-reported pre and postinjury fatigue, anxiety, depression and sleep disturbance was compared within-group and between groups | In MTBI, fatigue was more commonly reported among ε4 carriers (58%) than noncarriers (32%). MTBI ε4-carriers were more often fatigued than controls with ε4 (58% vs. 17%, p = 0.02). No significant between-group differences between MTBI and controls without ε4 |
| Yang et al., 2015 | MTBI, prospective, population-based | 21 MTBI patients without dementia, 6 MTBI patients with dementia, and 10 controls without MTBI | 15 male, 22 female; (controls: 2 M, 8 F, aged 50.6 ± 6.8 years; MTBI without dementia: 9 M, 12 F, aged 53.7 ± 7.9 years; MTBI with dementia: 4 M, 2 F; aged 60.0 ± 7.5 years) | ε4 carriers: 5 of 21 MTBI without dementia, 4 of 6 MTBI with dementia, 1 of 10 controls | MMSE, amyloid-PET | ε4 frequency high in MTBI patients with dementia (p = 0.049). Linear regression between APOE-ε4 and average amyloid standardized uptake value ratio (SUVR) showed significant correlation for all subjects (p < 0.05) |
| Yue et al., 2017 | MTBI, prospective, population-based | 114 MTBI patients | 76 male, 38 female; aged 49.6 ± 13.6 for ε4(+); aged 39.7 ± 16.5 years for noncarriers | 79 ε4(−), 35 ε4(+) | 6-month verbal memory using the CVLT-II, including Short-Delay Free Recall (SDFR), Short-Delayed Cued Recall (SDCR), Long-Delay Free Recall (LDFR), and Long-Delay Cued Recall (LDCR). | ε4-carriers associated with long-delay verbal memory deficits (LDFR: B = −1.17 points, 95% CI (−2.33, −0.01), p = 0.049; LDCR: B = −1.58 (−2.63, −0.52), p = 0.004), and a marginal decrease on SDCR (B = −1.02 (−2.05, 0.00), p = 0.050). CT pathology was the strongest predictor of decreased verbal memory. |
| CTE in Athlete and Military Cohorts (4 Studies) | ||||||
| Author | Study Type | N | Sex; Age | Description | Outcome Measures | Results |
| Stern et al., 2013 | CTE, retrospective, athletes | 36 athletes (29 football (22 pro, 4 college, 3 high school), 3 hockey, 1 wrestling, 3 boxing (1 pro, 2 amateur)) with neuropathologically confirmed CTE | All male; aged 56.8 ± 21.9 years (range 17–98) | APOE-ε4 genotype distribution was 3% ε2/ε3, 63% ε3/ε3, 26% ε3/ε4, and 9% ε4/ε4 | Next-of-kin interviewed for neuropsychiatric, social/occupational histories, dementia, depression, changes in cognition, behavior, mood, motor function, and ADLs | Proportions of APOE genotypes (i.e., ε4/ε4, ε4 carriers, and ε4 noncarriers) in this CTE sample were significantly different from those found in an age-matched normative sample (p < 0.05). Primary difference between this CTE sample and population norms was a greater proportion of ε4/ε4 in this sample (p < 0.05). More ε4/ε4 in the cognition group than expected (p < 0.05). Relative proportions of APOE in the 10 subjects with dementia did not differ significantly from those seen in Alzheimer’s (p > 0.05) |
| Mckee et al., 2013 | CTE, retrospective, athletes, military veterans, and civilians | 80 athletes (22 veterans), 3 military veterans, 1 civilian with history of falls, 1 civilian with history of self-injurious head banging behavior; 18 age- and sex-matched controls | 84 males, 1 female; Mean age of 54.2 ± 23.3 years (age range 14–98) | Of all subjects, including controls, 21 were carriers of Apo-ε4, 5 were homozygous for Apo-ε4 | Post-mortem brains of subjects with histories of repetitive MTBI were analyzed for evidence of CTE. Hyperphosphorylated tau pathology ranged in severity from focal pathology in the frontal lobe to a more global tauopathy, allowing for a progressive staging of pathology in these subjects. APOE genotyping was performed | In the 68 subjects diagnosed with CTE, the proportion of carrying at least one APOE-ε4 allele was not significantly different than that observed in the general population (p = 0.334) |
| Omalu et al., 2011 | CTE, case series, athlete | 14 pro athletes (8 football, 4 wrestling, 1 boxing, 1 mixed martial arts, 3 high school football) | Subjects male; age range 16–52 years | ε3/ε3 in 6 athletes (60%), ε3/ε4 in 2 athletes (20%), ε2/ε3 in 1 athlete (10%), ε2/ε4 (10%) in 1 athlete. 9 of the pro athletes (90%) with at least 1 ε3 allele. 7 of 10 pro athletes with known APOE genotype had CTE (70%). For the 3 deceased high school football players, the APOE genotype in 1 case could not be determined (blood samples were not available), and the genotypes in the other 2 were ε3/ε3 and ε3/ε3 | Histochemical and immunohistochemical brain tissue analysis for CTE changes and apolipoprotein E genotyping | Three pro athletes carried APOE-ε4, two of which (ε3/ε4 genotype) were positive for CTE, and the remaining (ε2/ε4 genotype) negative for CTE |
| Omalu et al., 2011 | CTE, case report, military | Case report of a military individual | 27-year-old male | The APOE genotype was ε3/ε4 | Histochemical and immunohistochemical brain tissue analysis for CTE changes | Autopsy, as well as gross and histomorphological examination of this brain revealed CTE changes similar to those observed in USA athletes |
| CTE in an Institutionalized Cohort (1 Study) | ||||||
| Author | Study Type | N | Sex; Age | Description | Outcome Measures | Results |
| Shively et al., 2017 | Leucotomy, case series, institutionalized | 5 institutionalized patients with schizophrenia and history of surgical leucotomy, with post-diagnosis survival of >40 years | 2 male, 3 female; Ages were 67, 70, 77, 87, and 89 years | Three of 5 are APOE-ε4 carriers, with the other 2 having ε3/ε3 genotype | Immunohistochemistry for abnormally hyperphosphorylated tau, beta-amyloid, antigen CD68; H&E stains on tissue sections for general morphology/structure | The three ε4-carriers showed scattered β-amyloid plaques in the overlying gray matter, which were not seen in the two ε3/ε3 patients |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Ordaz, A.; Upadhyayula, P.S.; Gillis-Buck, E.M.; Suen, C.G.; Melhado, C.G.; Mohammed, N.; Lam, T.; Yue, J.K. Apolipoprotein E Epsilon 4 Genotype, Mild Traumatic Brain Injury, and the Development of Chronic Traumatic Encephalopathy. Med. Sci. 2018, 6, 78. https://doi.org/10.3390/medsci6030078
Deng H, Ordaz A, Upadhyayula PS, Gillis-Buck EM, Suen CG, Melhado CG, Mohammed N, Lam T, Yue JK. Apolipoprotein E Epsilon 4 Genotype, Mild Traumatic Brain Injury, and the Development of Chronic Traumatic Encephalopathy. Medical Sciences. 2018; 6(3):78. https://doi.org/10.3390/medsci6030078
Chicago/Turabian StyleDeng, Hansen, Angel Ordaz, Pavan S. Upadhyayula, Eva M. Gillis-Buck, Catherine G. Suen, Caroline G. Melhado, Nebil Mohammed, Troy Lam, and John K. Yue. 2018. "Apolipoprotein E Epsilon 4 Genotype, Mild Traumatic Brain Injury, and the Development of Chronic Traumatic Encephalopathy" Medical Sciences 6, no. 3: 78. https://doi.org/10.3390/medsci6030078
APA StyleDeng, H., Ordaz, A., Upadhyayula, P. S., Gillis-Buck, E. M., Suen, C. G., Melhado, C. G., Mohammed, N., Lam, T., & Yue, J. K. (2018). Apolipoprotein E Epsilon 4 Genotype, Mild Traumatic Brain Injury, and the Development of Chronic Traumatic Encephalopathy. Medical Sciences, 6(3), 78. https://doi.org/10.3390/medsci6030078






