Shall We Focus on the Eosinophil to Guide Treatment with Systemic Corticosteroids during Acute Exacerbations of COPD?: PRO
Abstract
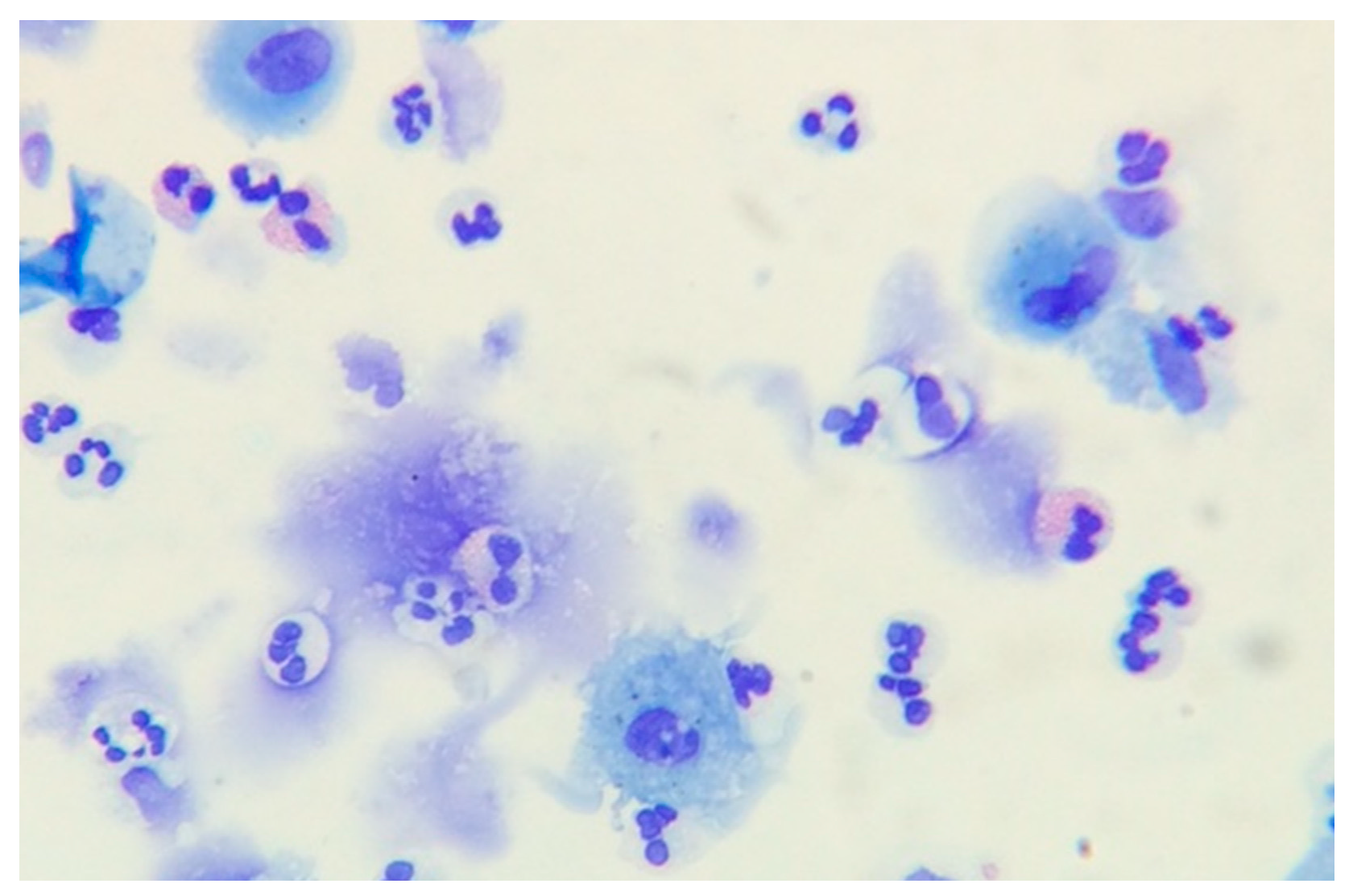
1. Eosinophil Cell Biology
2. Eosinophils in Chronic Obstructive Pulmonary Disease
3. Eosinophils in Exacerbations of COPD
4. Systemic Corticosteroids at the Onset of an Exacerbation: A Poorly Effective Treatment?
5. Eosinophils at the Onset of an Exacerbation to Direct Prednisolone Treatment: Time to Move Towards Precision
Funding
Conflicts of Interest
References
- Kay, A.B. The early history of the eosinophil. Clin. Exp. Allergy 2015, 45, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Redwan, M. Eosinophils, Cytokines, and Allergic Inflammation. Ann. N. Y. Acad. Sci. 1994, 725, 223–233. [Google Scholar]
- Young, B. Wheater’s Functional Histology: A Text and Colour Atlas; Elsevier Health Sciences: New York, NY, USA, 2006. [Google Scholar]
- Sanderson, C.J. Interleukin-5, eosinophils, and disease. Blood 1992, 79, 3101–3109. [Google Scholar] [PubMed]
- Luijk, B.; Lindemans, C.A.; Kanters, D.; van der Heijde, R.; Bertics, P.; Lammers, J.W.; Bates, M.E.; Koenderman, L. Gradual increase in priming of human eosinophils during extravasation from peripheral blood to the airways in response to allergen challenge. J. Allergy Clin. Immunol. 2005, 115, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.N.; Friend, D.S.; Zimmermann, N.; Sarafi, M.N.; Luster, A.D.; Pearlman, E.; Wert, S.E.; Rothenberg, M.E. Eotaxin is required for the baseline level of tissue eosinophils. Proc. Natl. Acad. Sci. USA 1998, 95, 6273–6278. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.M.; Naik, C.; Holguin, F.; Ray, A.; Ray, P.; Trudeau, J.B.; Wenzel, S.E. Epithelial eotaxin-2 and eotaxin-3 expression: Relation to asthma severity, luminal eosinophilia and age at onset. Thorax 2012, 67, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Ramos, E.; Avalos, A.F.; Perez-Fernandez, L.; Cuevas-Schacht, F.; Valencia-Maqueda, E.; Teran, L.M. Role of the chemokines RANTES, monocyte chemotactic proteins-3 and -4, and eotaxins-1 and -2 in childhood asthma. Eur. Res. J. 2003, 22, 310–316. [Google Scholar] [CrossRef]
- Lee, J.J.; Jacobsen, E.A.; McGarry, M.P.; Schleimer, R.P.; Lee, N.A. Eosinophils in health and disease: The LIAR hypothesis. Clin. Exp. Allergy 2010, 40, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Gleich, G.J.; Frigas, E.; Loegering, D.A.; Wassom, D.L.; Steinmuller, D. Cytotoxic properties of the eosinophil major basic protein. J. Immunol. 1979, 123, 2925–2927. [Google Scholar] [PubMed]
- Hansel, T.T.; Braunstein, J.B.; Walker, C.; Blaser, K.; Bruijinzeel, P.L.; Virchow, J.C., Jr.; Virchow, C., Sr. Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin. Exp. Immunol. 1991, 86, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Green, R.H.; Brightling, C.E.; McKenna, S.; Hargadon, B.; Parker, D.; Bradding, P.; Wardlaw, A.J.; Pavord, I.D. Asthma exacerbations and sputum eosinophil counts: A randomised controlled trial. Lancet 2002, 360, 1715–1721. [Google Scholar] [CrossRef]
- Haldar, P.; Brightling, C.E.; Hargadon, B.; Gupta, S.; Monteiro, W.; Sousa, A.; Marshall, R.P.; Bradding, P.; Green, R.H.; Wardlaw, A.J.; et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N. Engl. J. Med. 2009, 360, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keen, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Castro, M.; Wenzel, S.E.; Bleecker, E.R.; Pizzichini, E.; Kuna, P.; Busse, W.W.; Gossage, D.L.; Ward, C.K.; Wu, Y.; Wang, B.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: A phase 2b randomised dose-ranging study. Lancet Respir. Med. 2014, 2, 879–890. [Google Scholar] [CrossRef]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Bafadhel, M.; Pavord, I.D.; Russell, R.E.K. Eosinophils in COPD: Just another biomarker? Lancet Respir. Med. 2017, 5, 747–759. [Google Scholar] [CrossRef]
- Saha, S.; Brightling, C.E. Eosinophilic airway inflammation in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2006, 1, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; Monterio, W.; Green, R.H.; Parker, D.; Morgan, M.D.; Wardlaw, A.J.; Pavord, D. Induced sputum and other outcome measures in chronic obstructive pulmonary disease: Safety and repeatability. Respir. Med. 2001, 95, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Pizzichini, E.; Pizzichini, M.M.; Efthimiadis, A.; Evans, S.; Morris, M.M.; Squillace, D.; Gleich, G.J.; Dolovich, J.; Hargreave, F.E. Indices of airway inflammation in induced sputum: Reproducibility and validity of cell and fluid-phase measurements. Am. J. Respir. Crit. Care Med. 1996, 154, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Hambleton, K.; Connolly, C.M.; Borg, C.; Davies, J.H.; Jeffers, H.P.; Russell, R.E.K.; Bafadhel, M. Comparison of the peripheral blood eosinophil count using near-patient testing and standard automated laboratory measurement in healthy, asthmatic and COPD subjects. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 2771–2775. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; McKenna, S.; Hargadon, B.; Birring, S.; Green, R.; Siva, R.; Berry, M.; Parker, D.; Monteiro, W.; Pavord, I.D.; et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax 2005, 60, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; Monteiro, W.; Ward, R.; Parker, D.; Morgan, M.D.; Wardlaw, A.J.; Pavord, I.D. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: A randomised controlled trial. Lancet 2000, 356, 1480–1485. [Google Scholar] [CrossRef]
- Siva, R.; Green, R.H.; Brightling, C.E.; Shelley, M.; Hargadon, B.; McKenna, S.; Monteiro, W.; Berry, M.; Parker, D.; Wardlaw, A.J.; et al. Eosinophilic airway inflammation and exacerbations of COPD: A randomised controlled trial. Eur. Respir. J. 2007, 29, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, S.; Locantore, N.; Dransfield, M.T.; Barnes, N.C.; Pavord, I.D. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: A secondary analysis of data from two parallel randomised controlled trials. Lancet Respir. Med. 2015, 3, 435–442. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Guasconi, A.; Vestbo, J.; Jones, P.; Agusti, A.; Paggiaro, P.; Wedzicha, J.A.; Singh, D. Blood Eosinophils: A Biomarker of Response to Extrafine Beclomethasone/Formoterol in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 192, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Bafadhel, M.; Peterson, S.; De Blas, M.A.; Calverley, P.M.; Rennard, S.I.; Richter, K.; Fagerås, M. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: A post-hoc analysis of three randomised trials. Lancet Respir. Med. 2018, 6, 117–126. [Google Scholar] [CrossRef]
- Watz, H.; Tetzlaff, K.; Wouters, E.F.; Kirsten, A.; Magnussen, H.; Rodriguez-Roisin, R.; Vogelmeier, C.; Fabbri, L.; Chanez, P.; Dahl, R.; et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: A post-hoc analysis of the WISDOM trial. Lancet Respir. Med. 2016, 4, 390–398. [Google Scholar] [CrossRef]
- Magnussen, H.; Watz, H.; Zimmermann, I.; Macht, S.; Greguletz, R.; Falques, M.; Jarreta, D.; Garcia Gil, E. Peak inspiratory flow through the Genuair inhaler in patients with moderate or severe COPD. Respir. Med. 2009, 103, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.R.; Hurst, J.R.; Frent, S.M.; Larbig, M.; Fogel, R.; Guerin, T.; Banerji, D.; Patalano, F.; Goyal, P.; Pfister, P.; et al. Long-term Triple Therapy De-escalation to Indacaterol/Glycopyrronium in COPD Patients (SUNSET): A Randomized, Double-Blind, Triple-Dummy Clinical Trial. Am. J. Respir. Crit. Care Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.R.; Donaldson, G.C.; Perera, W.R.; Wilkinson, T.M.; Bilello, J.A.; Hagan, G.W.; Vessey, R.S.; Wedzicha, J.A. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006, 174, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhang, J.; He, X.; Hao, Y.; Wang, K.; Gibson, P.G. Sputum inflammatory cell-based classification of patients with acute exacerbation of chronic obstructive pulmonary disease. PLoS ONE 2013, 8, e57678. [Google Scholar] [CrossRef] [PubMed]
- Bafadhel, M.; Clark, T.W.; Reid, C.; Medina, M.J.; Batham, S.; Barer, M.R.; Nicholson, K.G.; Brightling, C.E. Procalcitonin and C-reactive protein in hospitalized adult patients with community-acquired pneumonia or exacerbation of asthma or COPD. Chest 2011, 139, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.L.; Coombs, N.A.; Staples, K.J.; Ostridge, K.K.; Williams, N.P.; Wootton, S.A.; Devaster, J.M.; Aris, E.; Clarke, S.C.; Tuck, A.C.; et al. Impact and associations of eosinophilic inflammation in COPD: Analysis of the AERIS cohort. Eur. Respir. J. 2017, 50, 1700853. [Google Scholar] [CrossRef] [PubMed]
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. Available online: http://goldcopd.org (accessed on 15 June 2018).
- Walters, J.A.; Gibson, P.G.; Wood-Baker, R.; Hannay, M.; Walters, E.H. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2009, CD001288. [Google Scholar]
- NICE. Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care. National Clinical Guideline Centre: London, UK. Available online: http://guidance.nice.org.uk/CG101/Guidance/pdf/English2010 (accessed on 15 June 2018).
- Waljee, A.K.; Rogers, M.A.; Lin, P.; Singal, A.G.; Stein, J.D.; Marks, R.M.; Ayanian, J.Z.; Nallamothu, B.K. Short term use of oral corticosteroids and related harms among adults in the United States: Population based cohort study. BMJ 2017, 357, j1415. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.A.; Kullar, R.; Goldstein, E.J.C.; Gilchrist, M.; Nathwani, D.; Cheng, A.C.; Cairns, K.A.; Escandon-Vargas, K.; Villegas, M.V.; Brink, A.; et al. A global call from five countries to collaborate in antibiotic stewardship: United we succeed, divided we might fail. Lancet Infect Dis. 2017, 17, e56–e63. [Google Scholar] [CrossRef]
- Walters, J.A.; Tan, D.J.; White, C.J.; Gibson, P.G.; Wood-Baker, R.; Walters, E.H. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2014, 9, Cd001288. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, D.J.; Jarrett, H.; Steurer-Stey, C.A.; Garcia-Aymerich, J.; Puhan, M.A. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2012, 12, Cd010257. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Soter, S.; Barta, I.; Antus, B. Predicting sputum eosinophilia in exacerbations of COPD using exhaled nitric oxide. Inflammation 2013, 36, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Bafadhel, M.; Greening, N.J.; Harvey-Dunstan, T.C.; Williams, J.E.; Morgan, M.D.; Brightling, C.E.; Hussain, S.F.; Pavord, I.D.; Singh, S.J.; Steiner, M.C. Blood Eosinophils and Outcomes in Severe Hospitalized Exacerbations of COPD. Chest 2016, 150, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Salturk, C.; Karakurt, Z.; Adiguzel, N.; Kargin, F.; Sari, R.; Celik, M.E.; Takir, H.B.; Tuncay, E.; Sogukpinar, O.; Ciftaslan, N.; et al. Does eosinophilic COPD exacerbation have a better patient outcome than non-eosinophilic in the intensive care unit? Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Steer, J.; Gibson, J.; Bourke, S.C. The DECAF Score: Predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax 2012, 67, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Stolz, D.; Christ-Crain, M.; Bingisser, R.; Leuppi, J.; Muller, C.; Huber, P.; Muller, B.; Tamm, M. Antibiotic treatment of exacerbations of COPD: A randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007, 131, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.M.; Snijders, D.; de Graaff, C.S.; Vlaspolder, F.; Jansen, H.M.; Boersma, W.G. Antibiotics in addition to systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 181, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Van Velzen, P.; Ter Riet, G.; Bresser, P.; Baars, J.J.; van den Berg, B.T.J.; van den Berg, J.W.K.; Brinkman, P.; Dagelet, J.W.F.; Daniels, J.M.A.; Groeneveld-Tjiong, D.R.G.L.; et al. Doxycycline for outpatient-treated acute exacerbations of COPD: A randomised double-blind placebo-controlled trial. Lancet Respir Med. 2017, 5, 492–499. [Google Scholar] [CrossRef]
- Rohde, G.G.; Koch, A.; Welte, T. Randomized double blind placebo-controlled study to demonstrate that antibiotics are not needed in moderate acute exacerbations of COPD—The ABACOPD study. BMC Pulm. Med. 2015, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Pancholi, M.; Venge, P.; Lomas, D.A.; Barer, M.R.; Johnston, S.L.; Pavord, I.D.; et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: A randomized placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2012, 186, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Bafadhel, M.; Davies, L.; Calverley, P.M.; Aaron, S.D.; Brightling, C.E.; Pavord, I.D. Blood eosinophil guided prednisolone therapy for exacerbations of COPD: A further analysis. Eur. Respir. J. 2014, 44, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Sivapalan, P.; Moberg, M.; Eklof, J.; Janner, J.; Vestbo, J.; Laub, R.R.; Browatzki, A.; Armbruster, K.; Wilcke, J.T.; Seersholm, N.; et al. A multi-center randomized, controlled, open-label trial evaluating the effects of eosinophil-guided corticosteroid-sparing therapy in hospitalised patients with COPD exacerbations—The CORTICO steroid reduction in COPD (CORTICO-COP) study protocol. BMC Pulm. Med. 2017, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Bafadhel, M.; Beasley, R.; Bel, E.H.; Faner, R.; Gibson, P.G.; Louis, R.; McDonald, V.M.; Sterk, P.J.; Thomas, M.; et al. Precision medicine in airway diseases: Moving to clinical practice. Eur. Respir. J. 2017, 50, 1701655. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camp, J.; Cane, J.L.; Bafadhel, M. Shall We Focus on the Eosinophil to Guide Treatment with Systemic Corticosteroids during Acute Exacerbations of COPD?: PRO. Med. Sci. 2018, 6, 74. https://doi.org/10.3390/medsci6030074
Camp J, Cane JL, Bafadhel M. Shall We Focus on the Eosinophil to Guide Treatment with Systemic Corticosteroids during Acute Exacerbations of COPD?: PRO. Medical Sciences. 2018; 6(3):74. https://doi.org/10.3390/medsci6030074
Chicago/Turabian StyleCamp, James, Jennifer L. Cane, and Mona Bafadhel. 2018. "Shall We Focus on the Eosinophil to Guide Treatment with Systemic Corticosteroids during Acute Exacerbations of COPD?: PRO" Medical Sciences 6, no. 3: 74. https://doi.org/10.3390/medsci6030074
APA StyleCamp, J., Cane, J. L., & Bafadhel, M. (2018). Shall We Focus on the Eosinophil to Guide Treatment with Systemic Corticosteroids during Acute Exacerbations of COPD?: PRO. Medical Sciences, 6(3), 74. https://doi.org/10.3390/medsci6030074




