Nuclear CaMKII Isoforms as Regulators of Transcription: From Developmental to Pathological Persistence
Abstract
1. Introduction
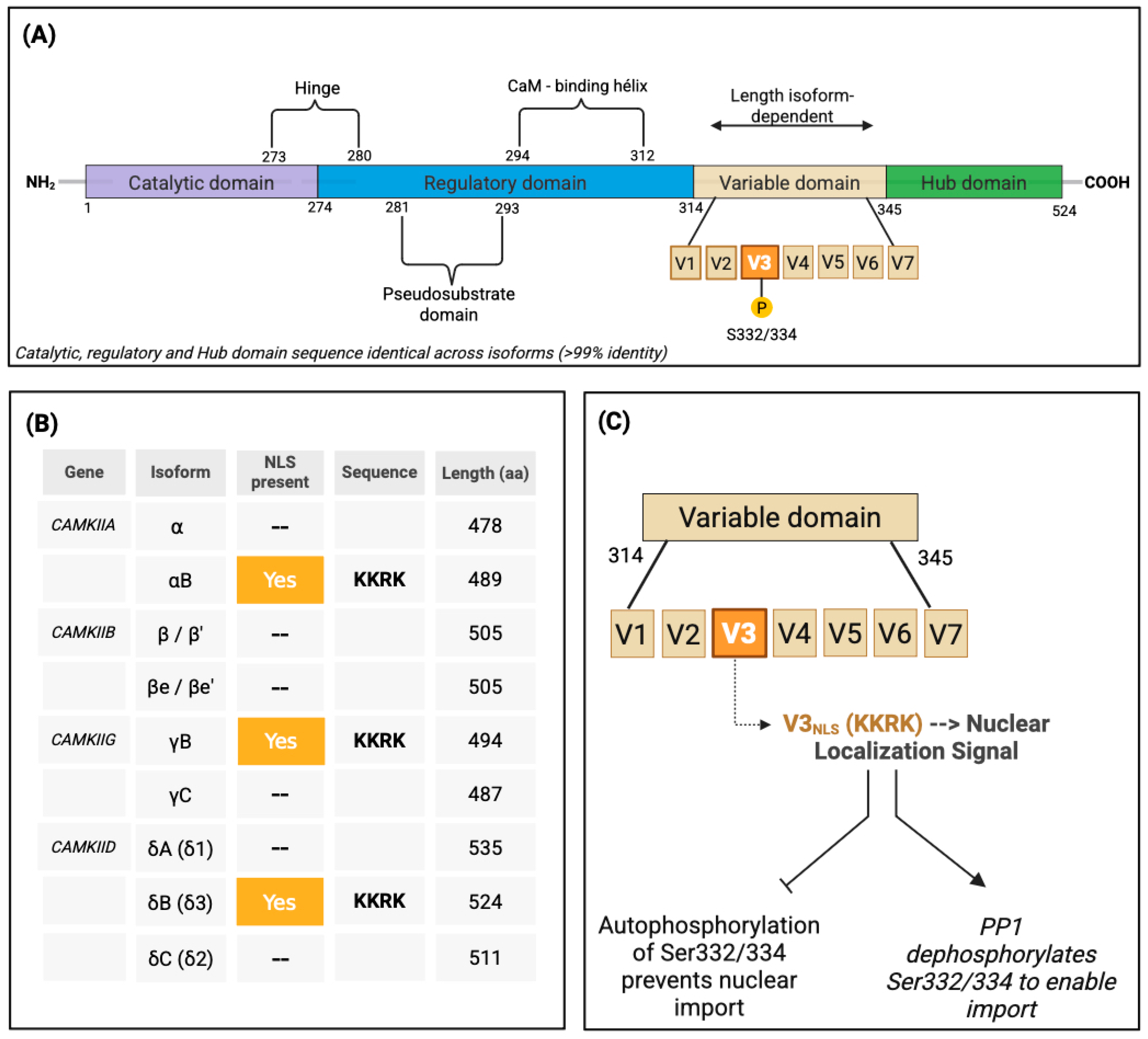
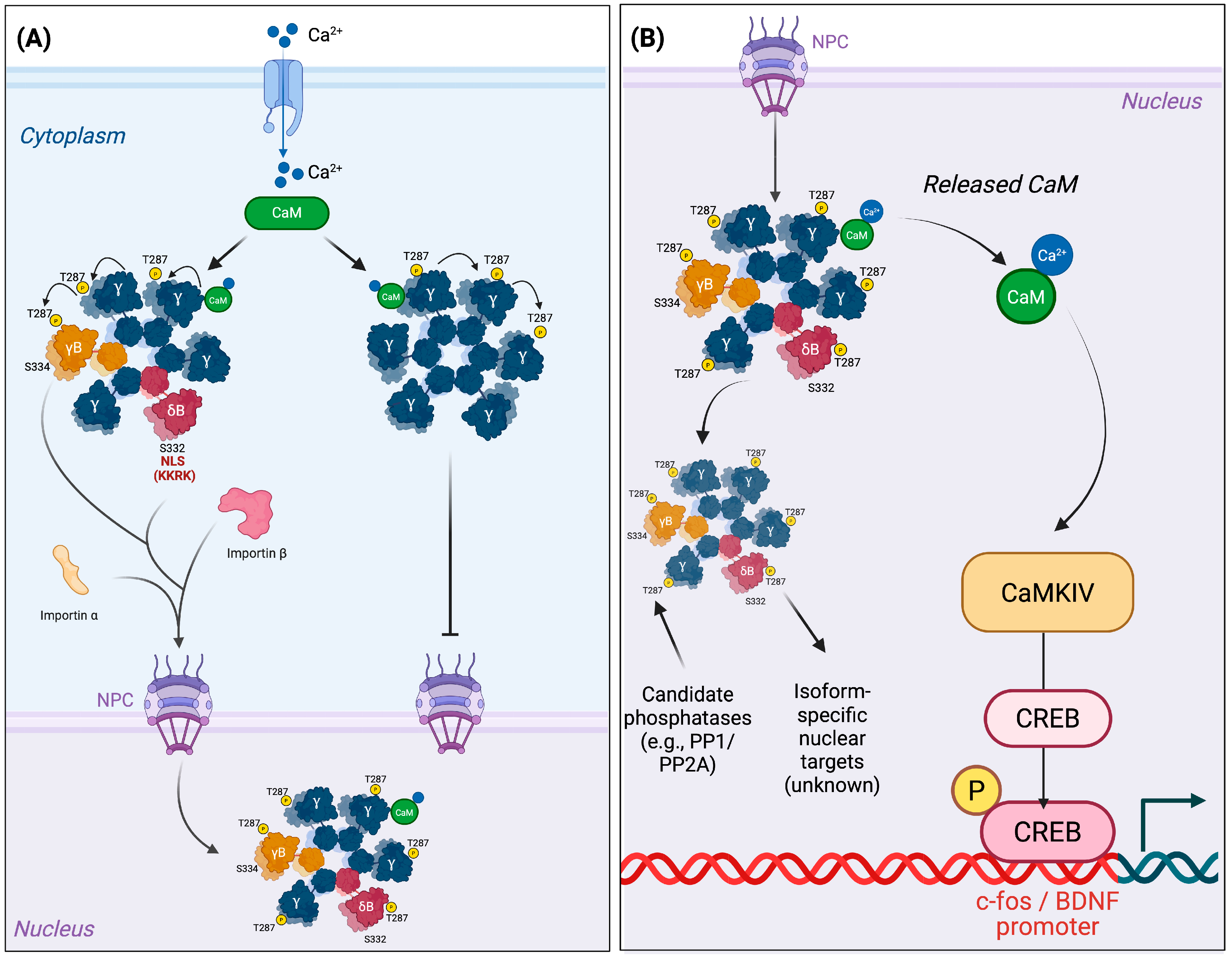
2. Nuclear Trafficking Logic: Isoforms, Assembly, and CaM Shuttling
2.1. Nuclear Localization by Design: The NLS Motif and Its Control
2.2. Assembly Dictates Destiny: The Holoenzyme as a Nuclear Unit
2.3. Nuclear Export and Dampening Mechanisms of CaMKII
3. Nuclear Functions in Physiology: Isoform-Specific Signaling Beyond the Cytosol
3.1. Transcriptional Decoding: CREB, Coactivators, and Immediate Early Genes
3.2. Modulation of Transcription Factors: Precision Tuning by Nuclear CaMKII
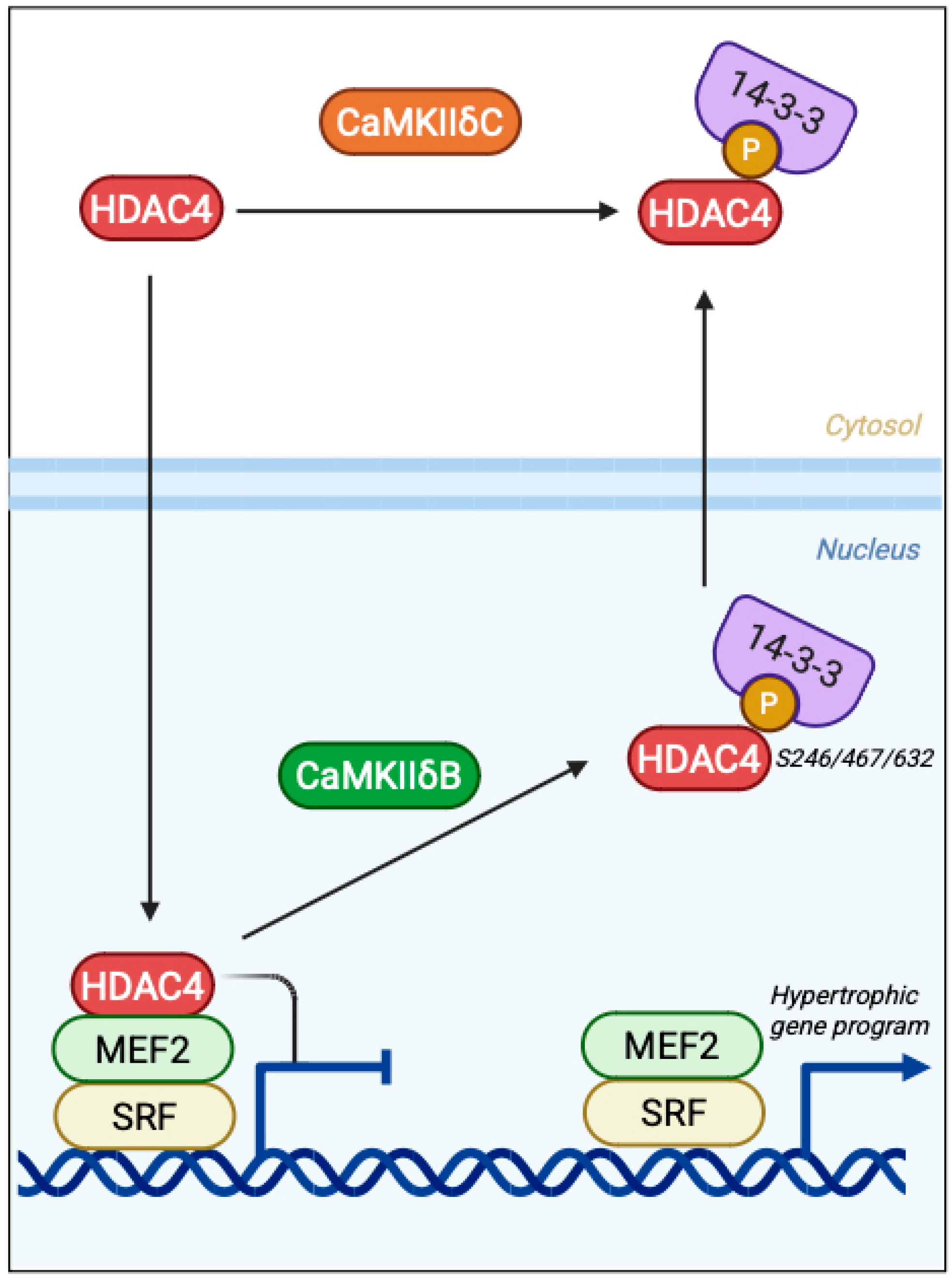
3.3. Epigenetic Control via Class IIa HDAC Phosphorylation
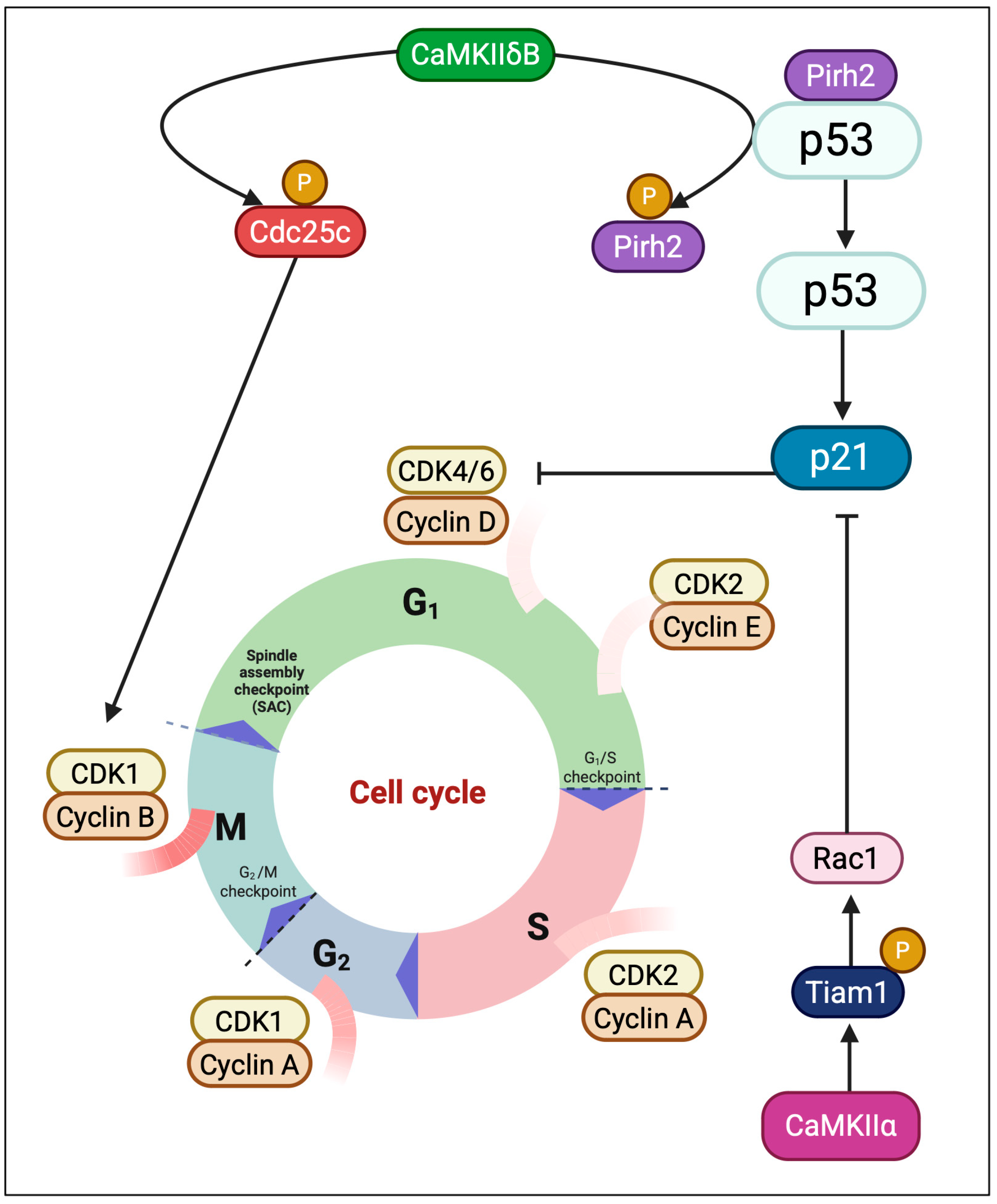
3.4. Cell Cycle Modulation: CaMKII at the Proliferation–Checkpoint Interface
3.5. Lineage Specification and Developmental Patterning
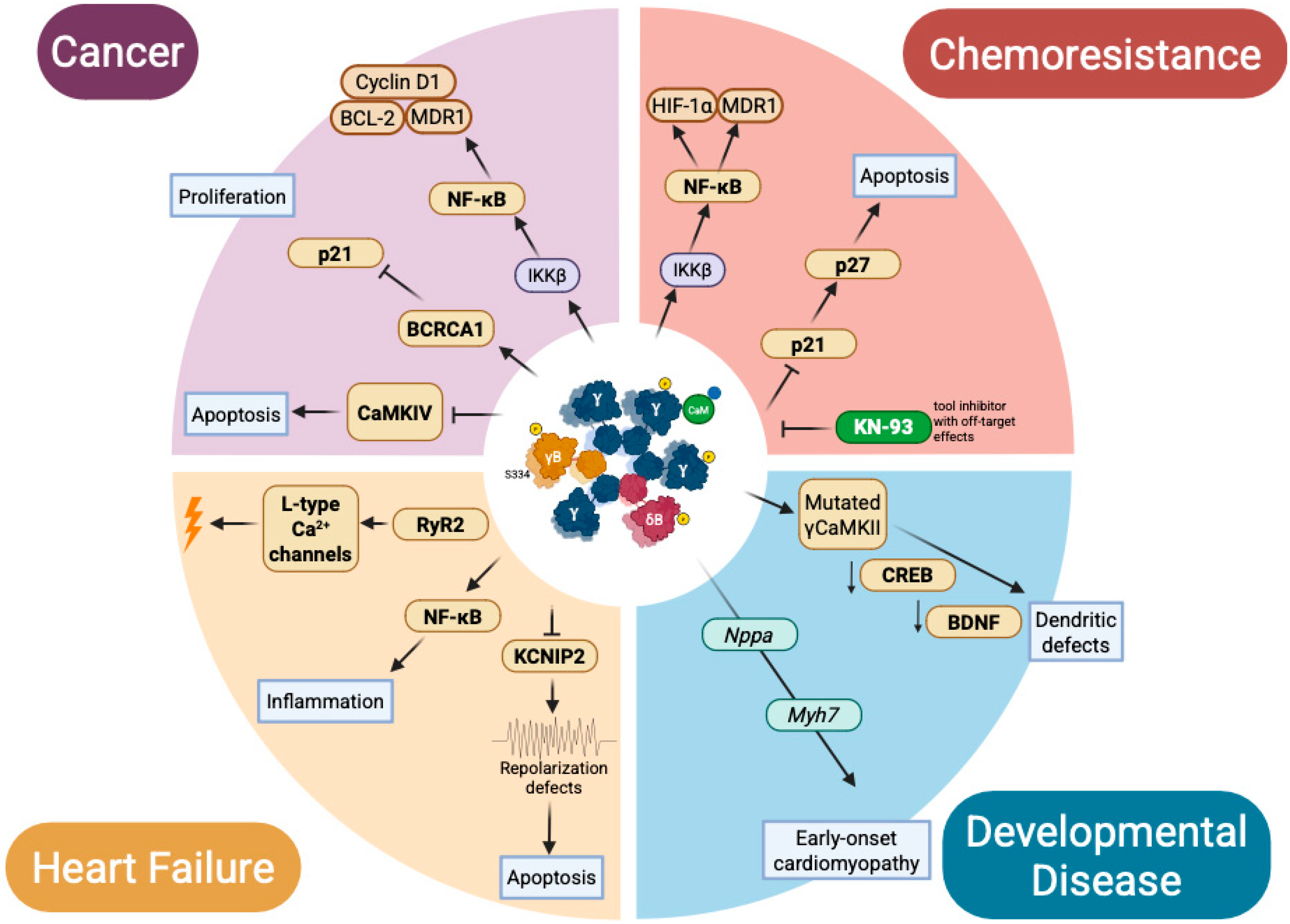
4. Nuclear CaMKII in Pathology: Isoform-Specific Mislocalization, Misregulation, and Molecular Rewiring
4.1. Transcriptional Oncoprotein: Nuclear CaMKII in Cancer
4.2. Developmental Mis-Specification: CaMKII in Neurocardiac Lineage Programs
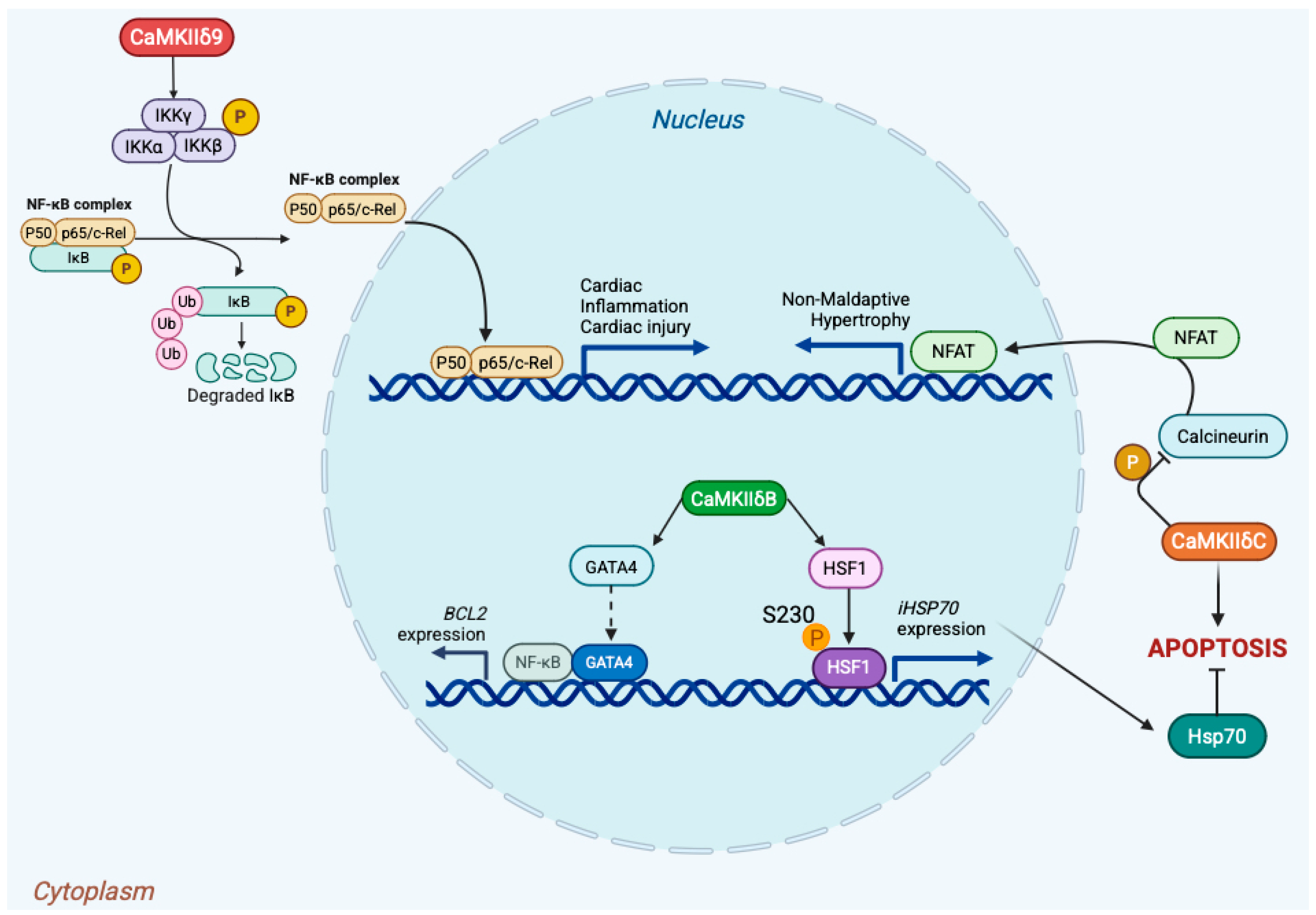
4.3. Cardiac Bifurcation: Nuclear CaMKII at the Edge of Adaptation and Failure
4.4. Chemoresistance and Transcriptional Escape: Nuclear CaMKII in Therapy Failure
4.5. Epigenetic Miswiring and Transcriptional Memory in Chronic Disease
5. Unresolved Dimensions and Strategic Directions: Charting the Nuclear Landscape of CaMKII
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC/C | Anaphase-Promoting Complex/Cyclosome |
| Arc | Activity-regulated cytoskeleton-associated protein |
| ASF/SF2 (SRSF1) | Alternative Splicing Factor/Splicing Factor 2 (Serine/arginine-rich splicing factor 1) |
| BDNF | Brain-Derived Neurotrophic Factor |
| CaM | Calmodulin |
| CaMK | Ca2+/calmodulin-dependent protein kinase |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| CDK | Cyclin-Dependent Kinase |
| CBP | CREB-Binding Protein |
| CML | Chronic Myeloid Leukemia |
| CREB | cAMP Response Element-Binding protein |
| DCM | Dilated Cardiomyopathy |
| EC | Excitation–Contraction |
| eNOS | Endothelial Nitric Oxide Synthase |
| ERK | Extracellular signal-Regulated Kinase |
| FOXO3a | Forkhead Box O3a |
| GB | Glioblastoma |
| GIT1 | G-protein-coupled Receptor Kinase-Interacting Protein 1 |
| HDAC | Histone Deacetylase |
| HIF-1α | Hypoxia-Inducible Factor 1-alpha |
| HSF1 | Heat Shock Factor 1 |
| HSP70 | Heat Shock Protein 70 |
| IKKβ | IκB Kinase beta |
| iHSP70 | Inducible Heat Shock Protein 70 |
| KLF2 | Krüppel-Like Factor 2 |
| KN-93/KN-62 | CaMKII pharmacological inhibitor |
| KChIP2 | Kv Channel-Interacting Protein 2 |
| MEF2 | Myocyte Enhancer Factor 2 |
| MDR1 | Multidrug Resistance Protein 1 (P-glycoprotein) |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NES | Nuclear Export Signal |
| NLS | Nuclear Localization Signal |
| NSCLC | Non-Small Cell Lung Cancer |
| O-GlcNAc | O-linked β-N-acetylglucosamine |
| PGC-1α | Peroxisome proliferator-activated receptor Gamma Coactivator 1-alpha |
| Pirh2 | p53-induced protein with a RING-H2 domain (E3 ubiquitin ligase) |
| PKD | Protein Kinase D |
| PP1 | Protein Phosphatase 1 |
| RyR2 | Ryanodine Receptor 2 |
| Ser/Thr | Serine/Threonine |
| SR | Splicing Regulator |
| SR Ca2+ | Sarcoplasmic Reticulum Calcium |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| T-ALL | T-cell Acute Lymphoblastic Leukemia |
| TAK1 | Transforming growth factor-β-Activated Kinase 1 |
| Tiam1 | T-lymphoma invasion and metastasis-inducing protein 1 |
| TRAIL | TNF-related apoptosis-inducing ligand |
| NFAT | Nuclear Factor of Activated T-cells |
| NLK | Nemo-Like Kinase |
| δB/δC/δ9 | CaMKIIδ splice variants (nuclear B, cytosolic C, variant 9) |
References
- Anderson, M.E.; Braun, A.P.; Schulman, H.; Premack, B.A. Multifunctional Ca2+/calmodulin-dependent protein kinase mediates Ca2+-induced enhancement of the L-type Ca2+ current in rabbit ventricular myocytes. Circ. Res. 1994, 75, 854–861. [Google Scholar] [CrossRef]
- Srinivasan, M.; Edman, C.F.; Schulman, H. Alternative splicing introduces a nuclear localization signal that targets multifunctional CaM kinase to the nucleus. J. Cell Biol. 1994, 126, 839–852. [Google Scholar] [CrossRef]
- Brocke, L.; Srinivasan, M.; Schulman, H. Developmental and regional expression of multifunctional Ca2+/calmodulin-dependent protein kinase isoforms in rat brain. J. Neurosci. 1995, 15, 6797–6808. [Google Scholar] [CrossRef]
- Myers, J.B.; Zaegel, V.; Coultrap, S.J.; Miller, A.P.; Bayer, K.U.; Reichow, S.L. The CaMKII holoenzyme structure in activation-competent conformations. Nat. Commun. 2017, 8, 15742. [Google Scholar] [CrossRef]
- Backs, J.; Song, K.; Bezprozvannaya, S.; Chang, S.; Olson, E.N. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J. Clin. Investig. 2006, 116, 1853–1864. [Google Scholar] [CrossRef]
- Ling, H.; Gray, C.B.; Zambon, A.C.; Grimm, M.; Gu, Y.; Dalton, N.; Purcell, N.H.; Peterson, K.; Brown, J.H. Ca2+/Calmodulin-dependent protein kinase II δ mediates myocardial ischemia/reperfusion injury through nuclear factor-κB. Circ. Res. 2013, 112, 935–944. [Google Scholar] [CrossRef]
- Yao, Y.; Li, F.; Zhang, M.; Jin, L.; Xie, P.; Liu, D.; Zhang, J.; Hu, X.; Lv, F.; Shang, H.; et al. Targeting CaMKII-δ9 Ameliorates Cardiac Ischemia/Reperfusion Injury by Inhibiting Myocardial Inflammation. Circ. Res. 2022, 130, 887–903. [Google Scholar] [CrossRef]
- Zhang, T.; Johnson, E.N.; Gu, Y.; Morissette, M.R.; Sah, V.P.; Gigena, M.S.; Belke, D.D.; Dillmann, W.H.; Rogers, T.B.; Schulman, H.; et al. The cardiac-specific nuclear δB isoform of Ca2+/calmodulin-dependent protein kinase II induces hypertrophy and dilated cardiomyopathy associated with increased protein phosphatase 2A activity. J. Biol. Chem. 2002, 277, 1261–1267. [Google Scholar] [CrossRef]
- Awad, S.; Al-Haffar, K.M.; Marashly, Q.; Quijada, P.; Kunhi, M.; Al-Yacoub, N.; Wade, F.S.; Mohammed, S.F.; Al-Dayel, F.; Sutherland, G.; et al. Control of histone H3 phosphorylation by CaMKII δ in response to haemodynamic cardiac stress. J. Pathol. 2015, 235, 606–618. [Google Scholar] [CrossRef]
- Bhattacharyya, M.; Lee, Y.K.; Muratcioglu, S.; Qiu, B.; Nyayapati, P.; Schulman, H.; Groves, J.T.; Kuriyan, J. Flexible linkers in CaMKII control the balance between activating and inhibitory autophosphorylation. Elife 2020, 9, 53670. [Google Scholar] [CrossRef]
- Brown, C.N.; Bayer, K.U. Studying CaMKII: Tools and standards. Cell Rep. 2024, 43, 113982. [Google Scholar] [CrossRef]
- Cohen, S.M.; Suutari, B.; He, X.; Wang, Y.; Sanchez, S.; Tirko, N.N.; Mandelberg, N.J.; Mullins, C.; Zhou, G.; Wang, S.; et al. Calmodulin shuttling mediates cytonuclear signaling to trigger experience-dependent transcription and memory. Nat. Commun. 2018, 9, 2451. [Google Scholar] [CrossRef]
- Heist, E.K.; Srinivasan, M.; Schulman, H. Phosphorylation at the nuclear localization signal of Ca2+/calmodulin-dependent protein kinase II blocks its nuclear targeting. J. Biol. Chem. 1998, 273, 19763–19771. [Google Scholar] [CrossRef]
- Strack, S.; McNeill, R.B.; Colbran, R.J. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 2000, 275, 23798–23806. [Google Scholar] [CrossRef]
- Shioda, N.; Sawai, M.; Ishizuka, Y.; Shirao, T.; Fukunaga, K. Nuclear Translocation of Calcium/Calmodulin-dependent Protein Kinase IIδ3 Promoted by Protein Phosphatase-1 Enhances Brain-derived Neurotrophic Factor Expression in Dopaminergic Neurons. J. Biol. Chem. 2015, 290, 21663–21675. [Google Scholar] [CrossRef]
- Bhattacharyya, M.; Stratton, M.M.; Going, C.C.; McSpadden, E.D.; Huang, Y.; Susa, A.C.; Elleman, A.; Cao, Y.M.; Pappireddi, N.; Burkhardt, P.; et al. Molecular mechanism of activation-triggered subunit exchange in Ca2+/calmodulin-dependent protein kinase II. Elife 2016, 5, e13405. [Google Scholar] [CrossRef]
- De Koninck, P.; Schulman, H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 1998, 279, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Hudmon, A.; Schulman, H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem. J. 2002, 364, 593–611. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Groth, R.D.; Cohen, S.M.; Emery, J.F.; Li, B.; Hoedt, E.; Zhang, G.; Neubert, T.A.; Tsien, R.W. γCaMKII shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell 2014, 159, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, T.C.; Piedras-Renteria, E.S.; Tsien, R.W. α- and βCaMKII: Inverse Regulation by Neuronal Activity and Opposing Effects on Synaptic Strength. Neuron 2002, 36, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Singer, H.A.; Benscoter, H.A.; Schworer, C.M. Novel Ca2+/calmodulin-dependent protein kinase II γ-subunit variants expressed in vascular smooth muscle, brain, and cardiomyocytes. J. Biol. Chem. 1997, 272, 9393–9400. [Google Scholar] [CrossRef]
- Roberts-Craig, F.T.; Worthington, L.P.; O’Hara, S.P.; Erickson, J.R.; Heather, A.K.; Ashley, Z. CaMKII Splice Variants in Vascular Smooth Muscle Cells: The Next Step or Redundancy? Int. J. Mol. Sci. 2022, 23, 7916. [Google Scholar] [CrossRef]
- Wayman, G.A.; Tokumitsu, H.; Davare, M.A.; Soderling, T.R. Analysis of CaM-kinase signaling in cells. Cell Calcium 2011, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shioda, N.; Fukunaga, K. Physiological and Pathological Roles of CaMKII-PP1 Signaling in the Brain. Int. J. Mol. Sci. 2017, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Backs, J.; Olson, E.N. Control of cardiac growth by histone acetylation/deacetylation. Circ. Res. 2006, 98, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Zalcman, G.; Federman, N.; Romano, A. CaMKII Isoforms in Learning and Memory: Localization and Function. Front. Mol. Neurosci. 2018, 11, 445. [Google Scholar] [CrossRef]
- Erickson, J.R.; Pereira, L.; Wang, L.; Han, G.; Ferguson, A.; Dao, K.; Copeland, R.J.; Despa, F.; Hart, G.W.; Ripplinger, C.M.; et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature 2013, 502, 372–376. [Google Scholar] [CrossRef]
- Ma, H.; Li, B.; Tsien, R.W. Distinct roles of multiple isoforms of CaMKII in signaling to the nucleus. Biochim. Biophys. Acta 2015, 1853, 1953–1957. [Google Scholar] [CrossRef]
- Hoffman, A.; Carpenter, H.; Kahl, R.; Watt, L.F.; Dickson, P.W.; Rostas, J.A.; Verrills, N.M.; Skelding, K.A. Dephosphorylation of CaMKII at T253 controls the metaphase-anaphase transition. Cell. Signal. 2014, 26, 748–756. [Google Scholar] [CrossRef]
- Malik, Z.A.; Stein, I.S.; Navedo, M.F.; Hell, J.W. Mission CaMKIIγ: Shuttle Calmodulin from Membrane to Nucleus. Cell 2014, 159, 235–237. [Google Scholar] [CrossRef][Green Version]
- Yasuda, R.; Hayashi, Y.; Hell, J.W. CaMKII: A central molecular organizer of synaptic plasticity, learning and memory. Nat. Rev. Neurosci. 2022, 23, 666–682. [Google Scholar] [CrossRef]
- Proietti Onori, M.; Koopal, B.; Everman, D.B.; Worthington, J.D.; Jones, J.R.; Ploeg, M.A.; Mientjes, E.; van Bon, B.W.; Kleefstra, T.; Schulman, H.; et al. The intellectual disability-associated CAMK2G p.Arg292Pro mutation acts as a pathogenic gain-of-function. Hum. Mutat. 2018, 39, 2008–2024. [Google Scholar] [CrossRef] [PubMed]
- Akita, T.; Aoto, K.; Kato, M.; Shiina, M.; Mutoh, H.; Nakashima, M.; Kuki, I.; Okazaki, S.; Magara, S.; Shiihara, T.; et al. De novo variants in CAMK2A and CAMK2B cause neurodevelopmental disorders. Ann. Clin. Transl. Neurol. 2018, 5, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Robison, A.J. Emerging role of CaMKII in neuropsychiatric disease. Trends Neurosci. 2014, 37, 653–662. [Google Scholar] [CrossRef]
- Gray, C.B.; Heller Brown, J. CaMKIIδ subtypes: Localization and function. Front. Pharmacol. 2014, 5, 15. [Google Scholar] [CrossRef]
- Duran, J.; Nickel, L.; Estrada, M.; Backs, J.; van den Hoogenhof, M.M.G. CaMKIIδ Splice Variants in the Healthy and Diseased Heart. Front. Cell Dev. Biol. 2021, 9, 644630. [Google Scholar] [CrossRef]
- Little, G.H.; Saw, A.; Bai, Y.; Dow, J.; Marjoram, P.; Simkhovich, B.; Leeka, J.; Kedes, L.; Kloner, R.A.; Poizat, C. Critical role of nuclear calcium/calmodulin-dependent protein kinase IIδB in cardiomyocyte survival in cardiomyopathy. J. Biol. Chem. 2009, 284, 24857–24868. [Google Scholar] [CrossRef]
- Holmberg, C.I.; Hietakangas, V.; Mikhailov, A.; Rantanen, J.O.; Kallio, M.; Meinander, A.; Hellman, J.; Morrice, N.; MacKintosh, C.; Morimoto, R.I.; et al. Phosphorylation of serine 230 promotes inducible transcriptional activity of heat shock factor 1. EMBO J. 2001, 20, 3800–3810. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zhang, Y.; Zheng, M.; Cheng, H.; Zhu, W.; Cao, C.M.; Xiao, R.P. Cardioprotection by CaMKII-δB is mediated by phosphorylation of heat shock factor 1 and subsequent expression of inducible heat shock protein 70. Circ. Res. 2010, 106, 102–110. [Google Scholar] [CrossRef]
- MacDonnell, S.M.; Weisser-Thomas, J.; Kubo, H.; Hanscome, M.; Liu, Q.; Jaleel, N.; Berretta, R.; Chen, X.; Brown, J.H.; Sabri, A.K.; et al. CaMKII negatively regulates calcineurin-NFAT signaling in cardiac myocytes. Circ. Res. 2009, 105, 316–325. [Google Scholar] [CrossRef]
- Lu, J.; McKinsey, T.A.; Zhang, C.L.; Olson, E.N. Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol. Cell 2000, 6, 233–244. [Google Scholar] [CrossRef]
- Zhao, X.; Sternsdorf, T.; Bolger, T.A.; Evans, R.M.; Yao, T.P. Regulation of MEF2 by histone deacetylase 4- and SIRT1 deacetylase-mediated lysine modifications. Mol. Cell. Biol. 2005, 25, 8456–8464. [Google Scholar] [CrossRef]
- Nishino, T.G.; Miyazaki, M.; Hoshino, H.; Miwa, Y.; Horinouchi, S.; Yoshida, M. 14-3-3 regulates the nuclear import of class IIa histone deacetylases. Biochem. Biophys. Res. Commun. 2008, 377, 852–856. [Google Scholar] [CrossRef]
- Pang, J.; Yan, C.; Natarajan, K.; Cavet, M.E.; Massett, M.P.; Yin, G.; Berk, B.C. GIT1 mediates HDAC5 activation by angiotensin II in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 892–898. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Parra, M.; Verdin, E.; Bassel-Duby, R.; Olson, E.N. Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc. Natl. Acad. Sci. USA 2008, 105, 7738–7743. [Google Scholar] [CrossRef]
- Rasmussen, G.; Rasmussen, C. Calmodulin-dependent protein kinase II is required for G1/S progression in HeLa cells. Biochem. Cell Biol. 1995, 73, 201–207. [Google Scholar] [CrossRef]
- Patel, R.; Holt, M.; Philipova, R.; Moss, S.; Schulman, H.; Hidaka, H.; Whitaker, M. Calcium/calmodulin-dependent phosphorylation and activation of human Cdc25-C at the G2/M phase transition in HeLa cells. J. Biol. Chem. 1999, 274, 7958–7968. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, J.R.; Dikovskaya, D.; Clarke, P.R. Regulation of Cdc2/cyclin B activation in Xenopus egg extracts via inhibitory phosphorylation of Cdc25C phosphatase by Ca2+/calmodulin-dependent protein [corrected] kinase II. Mol. Biol. Cell 2003, 14, 4003–4014. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Chung, L.W.; Siegal, G.P.; Zayzafoon, M. α-CaMKII controls the growth of human osteosarcoma by regulating cell cycle progression. Lab. Investig. 2007, 87, 938–950. [Google Scholar] [CrossRef]
- Chai, S.; Xu, X.; Wang, Y.; Zhou, Y.; Zhang, C.; Yang, Y.; Yang, Y.; Xu, H.; Xu, R.; Wang, K. Ca2+/calmodulin-dependent protein kinase IIγ enhances stem-like traits and tumorigenicity of lung cancer cells. Oncotarget 2015, 6, 16069–16083. [Google Scholar] [CrossRef] [PubMed]
- Devanaboyina, M.; Kaur, J.; Whiteley, E.; Lin, L.; Einloth, K.; Morand, S.; Stanbery, L.; Hamouda, D.; Nemunaitis, J. NF-κB Signaling in Tumor Pathways Focusing on Breast and Ovarian Cancer. Oncol. Rev. 2022, 16, 10568. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Yao, Z.; Hou, D.; Wu, Z.; Zhu, W.G.; Wu, M. Phosphorylation of Pirh2 by calmodulin-dependent kinase II impairs its ability to ubiquitinate p53. EMBO J. 2007, 26, 3062–3074. [Google Scholar] [CrossRef] [PubMed]
- Backs, J.; Stein, P.; Backs, T.; Duncan, F.E.; Grueter, C.E.; McAnally, J.; Qi, X.; Schultz, R.M.; Olson, E.N. The γ isoform of CaM kinase II controls mouse egg activation by regulating cell cycle resumption. Proc. Natl. Acad. Sci. USA 2010, 107, 81–86. [Google Scholar] [CrossRef]
- Medvedev, S.; Stein, P.; Schultz, R.M. Specificity of calcium/calmodulin-dependent protein kinases in mouse egg activation. Cell Cycle 2014, 13, 1482–1488. [Google Scholar] [CrossRef]
- Reber, S.; Over, S.; Kronja, I.; Gruss, O.J. CaM kinase II initiates meiotic spindle depolymerization independently of APC/C activation. J. Cell Biol. 2008, 183, 1007–1017. [Google Scholar] [CrossRef]
- Rostas, J.A.P.; Skelding, K.A. Calcium/Calmodulin-Stimulated Protein Kinase II (CaMKII): Different Functional Outcomes from Activation, Depending on the Cellular Microenvironment. Cells 2023, 12, 401. [Google Scholar] [CrossRef]
- Cook, S.G.; Bourke, A.M.; O’Leary, H.; Zaegel, V.; Lasda, E.; Mize-Berge, J.; Quillinan, N.; Tucker, C.L.; Coultrap, S.J.; Herson, P.S.; et al. Analysis of the CaMKIIα and β splice-variant distribution among brain regions reveals isoform-specific differences in holoenzyme formation. Sci. Rep. 2018, 8, 5448. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Szábo, G.; Sun, Q.Q. Distribution of CaMKIIα expression in the brain in vivo, studied by CaMKIIα-GFP mice. Brain Res. 2013, 1518, 9–25. [Google Scholar] [CrossRef]
- Hudmon, A.; Lebel, E.; Roy, H.; Sik, A.; Schulman, H.; Waxham, M.N.; De Koninck, P. A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association. J. Neurosci. 2005, 25, 6971–6983. [Google Scholar] [CrossRef]
- Xu, X.; Yang, D.; Ding, J.H.; Wang, W.; Chu, P.H.; Dalton, N.D.; Wang, H.Y.; Bermingham, J.R., Jr.; Ye, Z.; Liu, F.; et al. ASF/SF2-regulated CaMKIIδ alternative splicing temporally reprograms excitation-contraction coupling in cardiac muscle. Cell 2005, 120, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Quijada, P.; Hariharan, N.; Cubillo, J.D.; Bala, K.M.; Emathinger, J.M.; Wang, B.J.; Ormachea, L.; Bers, D.M.; Sussman, M.A.; Poizat, C. Nuclear Calcium/Calmodulin-dependent Protein Kinase II Signaling Enhances Cardiac Progenitor Cell Survival and Cardiac Lineage Commitment. J. Biol. Chem. 2015, 290, 25411–25426. [Google Scholar] [CrossRef]
- Cohen, T.J.; Choi, M.C.; Kapur, M.; Lira, V.A.; Yan, Z.; Yao, T.P. HDAC4 regulates muscle fiber type-specific gene expression programs. Mol. Cells 2015, 38, 343–348. [Google Scholar] [CrossRef]
- McKinsey, T.A.; Zhang, C.L.; Lu, J.; Olson, E.N. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 2000, 408, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, M.J.; Wu, H.; Arnold, M.A.; Shelton, J.M.; Backs, J.; McAnally, J.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J. Clin. Investig. 2007, 117, 2459–2467. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, B.; Fasoli, A.; Ko, C.Y.; Van, B.W.; Alim, C.C.; Shen, E.Y.; Ciccozzi, M.M.; Tapa, S.; Ripplinger, C.M.; Erickson, J.R.; et al. CaMKII Serine 280 O-GlcNAcylation Links Diabetic Hyperglycemia to Proarrhythmia. Circ. Res. 2021, 129, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, T.; Meng, Z.; Gan, Y.; Xu, X.; Lou, G.; Li, H.; Gan, X.; Zhou, H.; Tang, J.; et al. CaMKII γ, a critical regulator of CML stem/progenitor cells, is a target of the natural product berbamine. Blood 2012, 120, 4829–4839. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, J.; Ma, X.; Kim, B.W.; Wang, H.; Li, J.; Pan, Y.; Xu, Y.; Ding, L.; Yang, L.; et al. Stabilization of the c-Myc Protein by CAMKIIγ Promotes T Cell Lymphoma. Cancer Cell 2017, 32, 115–128.e7. [Google Scholar] [CrossRef]
- Yang, L.; Wu, B.; Wu, Z.; Xu, Y.; Wang, P.; Li, M.; Xu, R.; Liang, Y. CAMKIIγ is a targetable driver of multiple myeloma through CaMKIIγ/Stat3 axis. Aging 2020, 12, 13668–13683. [Google Scholar] [CrossRef]
- Han, J.M.; Kim, Y.J.; Jung, H.J. Discovery of a New CaMKII-Targeted Synthetic Lethal Therapy against Glioblastoma Stem-like Cells. Cancers 2022, 14, 1315. [Google Scholar] [CrossRef]
- Mishra, S.; Gray, C.B.; Miyamoto, S.; Bers, D.M.; Brown, J.H. Location matters: Clarifying the concept of nuclear and cytosolic CaMKII subtypes. Circ. Res. 2011, 109, 1354–1362. [Google Scholar] [CrossRef]
- Toko, H.; Takahashi, H.; Kayama, Y.; Oka, T.; Minamino, T.; Okada, S.; Morimoto, S.; Zhan, D.Y.; Terasaki, F.; Anderson, M.E.; et al. Ca2+/calmodulin-dependent kinase IIδ causes heart failure by accumulation of p53 in dilated cardiomyopathy. Circulation 2010, 122, 891–899. [Google Scholar] [CrossRef]
- Uchinoumi, H.; Yang, Y.; Oda, T.; Li, N.; Alsina, K.M.; Puglisi, J.L.; Chen-Izu, Y.; Cornea, R.L.; Wehrens, X.H.T.; Bers, D.M. CaMKII-dependent phosphorylation of RyR2 promotes targetable pathological RyR2 conformational shift. J. Mol. Cell. Cardiol. 2016, 98, 62–72. [Google Scholar] [CrossRef]
- Blaich, A.; Welling, A.; Fischer, S.; Wegener, J.W.; Köstner, K.; Hofmann, F.; Moosmang, S. Facilitation of murine cardiac L-type Cav1.2 channel is modulated by calmodulin kinase II-dependent phosphorylation of S1512 and S1570. Proc. Natl. Acad. Sci. USA 2010, 107, 10285–10289. [Google Scholar] [CrossRef]
- Lee, T.S.; Karl, R.; Moosmang, S.; Lenhardt, P.; Klugbauer, N.; Hofmann, F.; Kleppisch, T.; Welling, A. Calmodulin kinase II is involved in voltage-dependent facilitation of the L-type Cav1.2 calcium channel: Identification of the phosphorylation sites. J. Biol. Chem. 2006, 281, 25560–25567. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Zhang, J.; Liu, Y.; Zheng, X.; Ding, F.; Sun, X.; Zhao, M.; Hao, L. The CaMKII phosphorylation site Thr1604 in the CaV1.2 channel is involved in pathological myocardial hypertrophy in rats. Channels 2020, 14, 151–162. [Google Scholar] [CrossRef]
- Panama, B.K.; Latour-Villamil, D.; Farman, G.P.; Zhao, D.; Bolz, S.S.; Kirshenbaum, L.A.; Backx, P.H. Nuclear factor κB downregulates the transient outward potassium current Ito,f through control of KChIP2 expression. Circ. Res. 2011, 108, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Kreusser, M.M.; Backs, J. Integrated mechanisms of CaMKII-dependent ventricular remodeling. Front. Pharmacol. 2014, 5, 36. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhao, R.; Zhe, H. The emerging role of CaMKII in cancer. Oncotarget 2015, 6, 11725–11734. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mora, O.G.; Lahair, M.M.; Evans, M.J.; Kovacs, C.J.; Allison, R.R.; Sibata, C.H.; White, K.S.; McCubrey, J.A.; Franklin, R.A. Inhibition of the CaM-kinases augments cell death in response to oxygen radicals and oxygen radical inducing cancer therapies in MCF-7 human breast cancer cells. Cancer Biol. Ther. 2006, 5, 1022–1030. [Google Scholar] [CrossRef]
- Saldivar-Cerón, H.I.; Villamar-Cruz, O.; Wells, C.M.; Oguz, I.; Spaggiari, F.; Chernoff, J.; Patiño-López, G.; Huerta-Yepez, S.; Montecillo-Aguado, M.; Rivera-Pazos, C.M.; et al. p21-Activated Kinase 1 Promotes Breast Tumorigenesis via Phosphorylation and Activation of the Calcium/Calmodulin-Dependent Protein Kinase II. Front. Cell Dev. Biol. 2021, 9, 759259. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ding, Z.; Peng, Y.; Pan, F.; Li, J.; Zou, L.; Zhang, Y.; Liang, H. HIF-1α inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoprotein. PLoS ONE 2014, 9, e98882. [Google Scholar] [CrossRef]
- Hegyi, B.; Chen-Izu, Y.; Jian, Z.; Shimkunas, R.; Izu, L.T.; Banyasz, T. KN-93 inhibits IKr in mammalian cardiomyocytes. J. Mol. Cell. Cardiol. 2015, 89, 173–176. [Google Scholar] [CrossRef]
- Karls, A.S.; Mynlieff, M. Nonspecific, reversible inhibition of voltage-gated calcium channels by CaMKII inhibitor CK59. Cell Mol. Neurobiol. 2013, 33, 723–729. [Google Scholar] [CrossRef]
- Johnson, C.N.; Pattanayek, R.; Potet, F.; Rebbeck, R.T.; Blackwell, D.J.; Nikolaienko, R.; Sequeira, V.; Le Meur, R.; Radwański, P.B.; Davis, J.P.; et al. The CaMKII inhibitor KN93-calmodulin interaction and implications for calmodulin tuning of Na(V)1.5 and RyR2 function. Cell Calcium 2019, 82, 102063. [Google Scholar] [CrossRef]
- Chen, N.N.; Ma, X.D.; Miao, Z.; Zhang, X.M.; Han, B.Y.; Almaamari, A.A.; Huang, J.M.; Chen, X.Y.; Liu, Y.J.; Su, S.W. Doxorubicin resistance in breast cancer is mediated via the activation of FABP5/PPARγ and CaMKII signaling pathway. Front. Pharmacol. 2023, 14, 1150861. [Google Scholar] [CrossRef]
- Han, J.M.; Jung, H.J. Synergistic Anticancer Effect of a Combination of Berbamine and Arcyriaflavin A against Glioblastoma Stem-like Cells. Molecules 2022, 27, 7968. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, I.A.; Baek, K.I.; Kim, Y.; Park, C.; Jo, H. Flow-induced reprogramming of endothelial cells in atherosclerosis. Nat. Rev. Cardiol. 2023, 20, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ha, C.H.; Jhun, B.S.; Wong, C.; Jain, M.K.; Jin, Z.G. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood 2010, 115, 2971–2979. [Google Scholar] [CrossRef] [PubMed]
- McKinsey, T.A.; Zhang, C.L.; Olson, E.N. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc. Natl. Acad. Sci. USA 2000, 97, 14400–14405. [Google Scholar] [CrossRef]
- Reyes Gaido, O.E.; Pavlaki, N.; Granger, J.M.; Mesubi, O.O.; Liu, B.; Lin, B.L.; Long, A.; Walker, D.; Mayourian, J.; Schole, K.L.; et al. An improved reporter identifies ruxolitinib as a potent and cardioprotective CaMKII inhibitor. Sci. Transl. Med. 2023, 15, eabq7839. [Google Scholar] [CrossRef]
- He, B.J.; Joiner, M.L.; Singh, M.V.; Luczak, E.D.; Swaminathan, P.D.; Koval, O.M.; Kutschke, W.; Allamargot, C.; Yang, J.; Guan, X.; et al. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat. Med. 2011, 17, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaytán-Gómez, A.M.; Ramos-Cortés, C.A.; Suarez-García, R.X.; Martínez-Islas, D.A.; Marroquin-Aguilar, A.T.; Avelino-Vivas, F.; Solis-Galván, D.M.; Laguna-González, A.A.; García-García, B.M.; Minaya-Pérez, E.; et al. Nuclear CaMKII Isoforms as Regulators of Transcription: From Developmental to Pathological Persistence. Med. Sci. 2025, 13, 246. https://doi.org/10.3390/medsci13040246
Gaytán-Gómez AM, Ramos-Cortés CA, Suarez-García RX, Martínez-Islas DA, Marroquin-Aguilar AT, Avelino-Vivas F, Solis-Galván DM, Laguna-González AA, García-García BM, Minaya-Pérez E, et al. Nuclear CaMKII Isoforms as Regulators of Transcription: From Developmental to Pathological Persistence. Medical Sciences. 2025; 13(4):246. https://doi.org/10.3390/medsci13040246
Chicago/Turabian StyleGaytán-Gómez, Areli Marlene, Claudio Adrián Ramos-Cortés, Ricardo Xopan Suarez-García, Diego Alberto Martínez-Islas, Axel Tonatiuh Marroquin-Aguilar, Fernanda Avelino-Vivas, Dafne Montserrat Solis-Galván, Alexis Arturo Laguna-González, Bruno Manuel García-García, Eduardo Minaya-Pérez, and et al. 2025. "Nuclear CaMKII Isoforms as Regulators of Transcription: From Developmental to Pathological Persistence" Medical Sciences 13, no. 4: 246. https://doi.org/10.3390/medsci13040246
APA StyleGaytán-Gómez, A. M., Ramos-Cortés, C. A., Suarez-García, R. X., Martínez-Islas, D. A., Marroquin-Aguilar, A. T., Avelino-Vivas, F., Solis-Galván, D. M., Laguna-González, A. A., García-García, B. M., Minaya-Pérez, E., Quiñones-Lara, E., Muciño-Galicia, A. E., Villamar-Cruz, O., Arias-Romero, L. E., León-Cabrera, S., Armas-López, L., & Saldívar-Cerón, H. I. (2025). Nuclear CaMKII Isoforms as Regulators of Transcription: From Developmental to Pathological Persistence. Medical Sciences, 13(4), 246. https://doi.org/10.3390/medsci13040246






