Total-Arterial Revascularization Is Superior in Heart Failure Patients with Reduced Ejection Fraction—A Propensity Score Matched Retrospective Multicenter Analysis
Abstract
1. Introduction
2. Materials and Methods
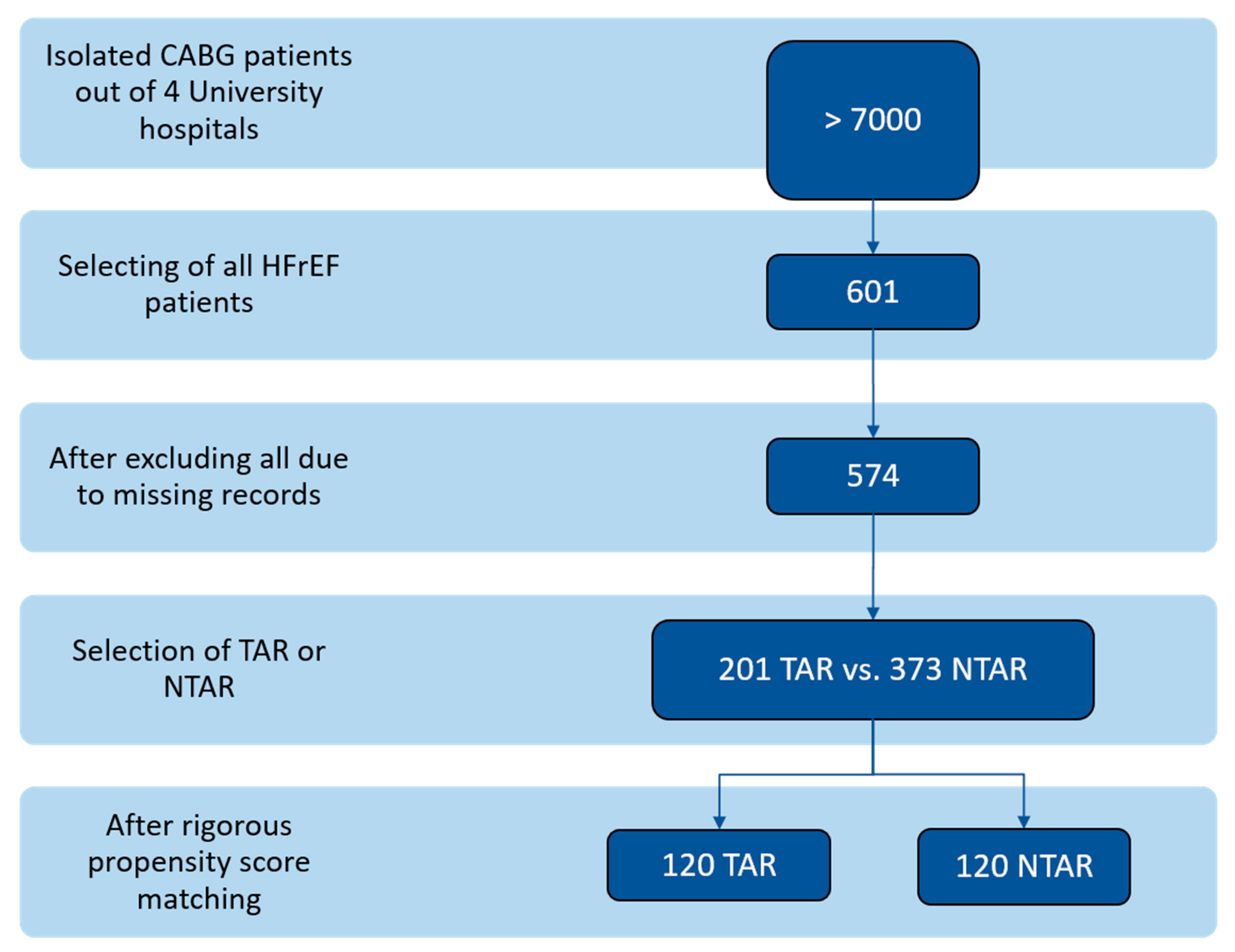
2.1. Study Design and Patient Population
2.2. Primary Outcomes
2.3. Secondary Outcomes
2.4. Surgical Techniques and Perioperative Care Protocols
2.5. Statistical Analysis
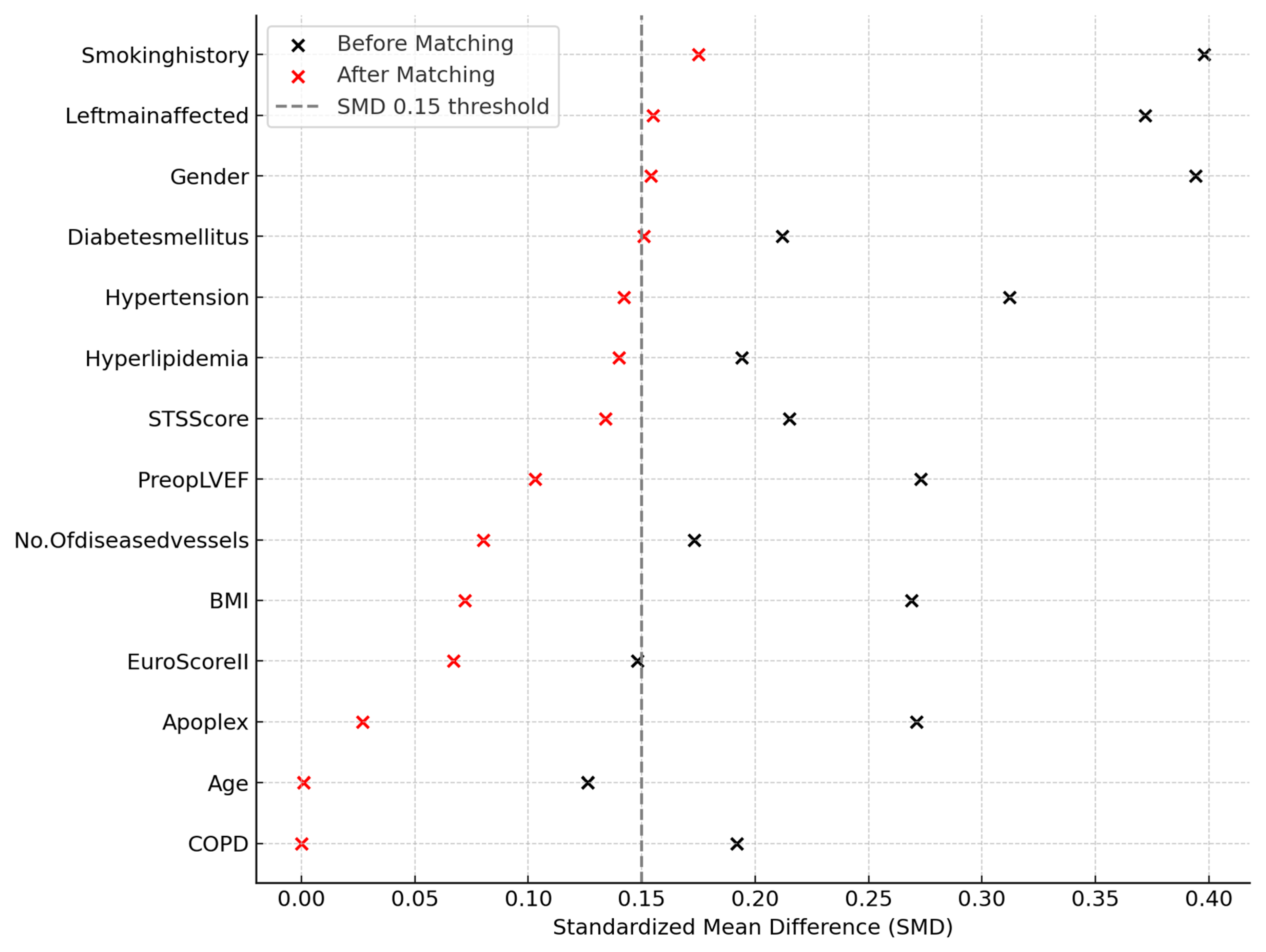
2.6. Propensity Score Estimation
2.7. Matching Procedure
3. Results
3.1. Preoperative Demographic and Clinical Characteristics Following Propensity Score Matching
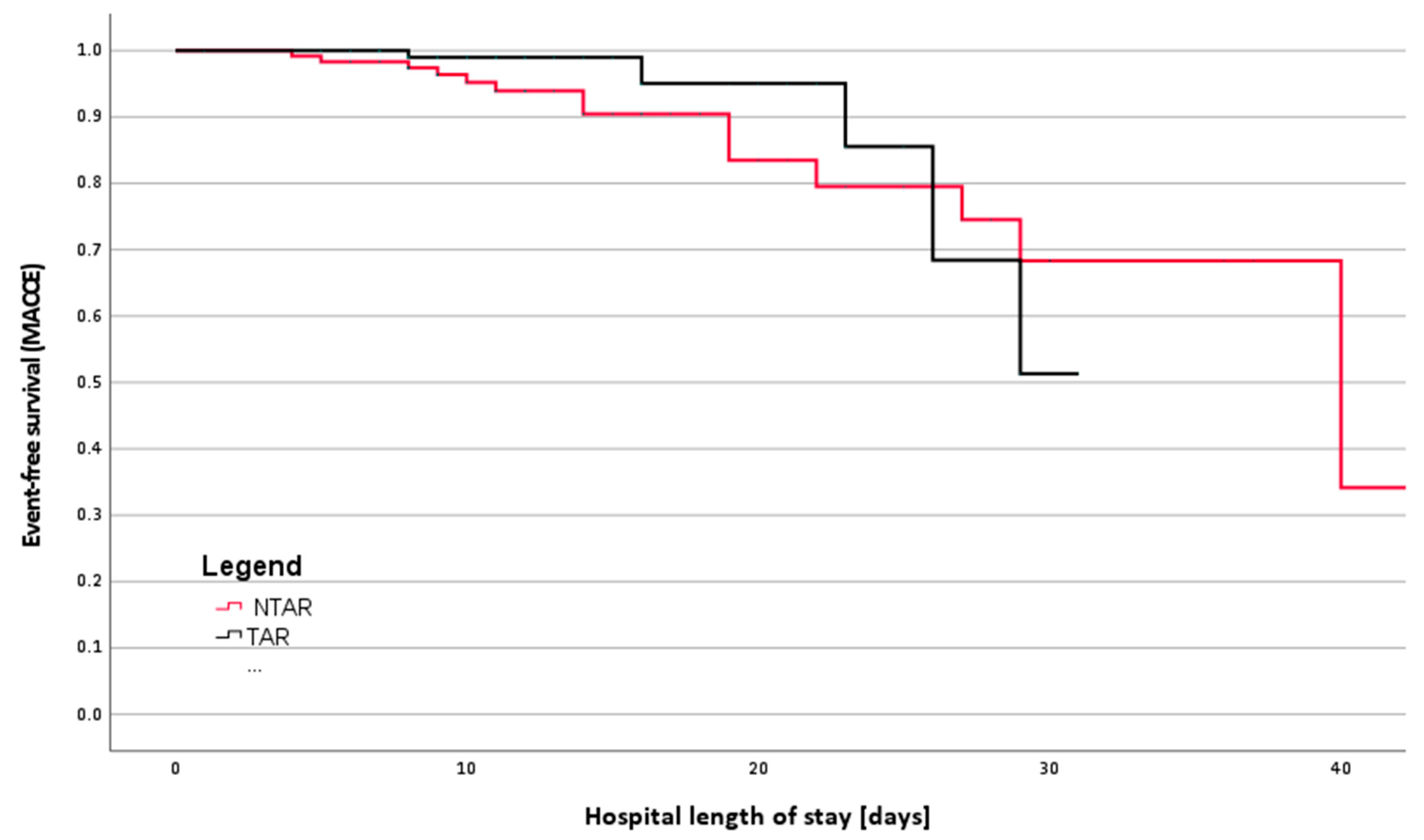
3.2. Primary Outcomes
3.3. Secondary Outcomes
- ICU stay: median 90 h vs. 44.5 h (p < 0.001);
- Hospital stay: median 12 d vs. 10 d (p = 0.002);
- Ventilation time: median 12 h vs. 8 h (p < 0.001).
- Intraoperative: 0.31 ± 0.58 units vs. 0.13 ± 0.45 units (p = 0.028);
- Entire hospital stay: 1.77 ± 2.91 units vs. 0.70 ± 1.33 units (p < 0.001).
4. Discussion
5. Limitations and Future Perspective
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| ANOVA | analysis of variance |
| BIMA | bilateral internal mammary artery |
| CABG | coronary artery bypass grafting |
| CAM-ICU | Confusion Assessment Method for the Intensive Care Unit |
| CI | confidence interval |
| COPD | chronic obstructive pulmonary disease |
| ECLS | extracorporeal life support |
| EuroScore II | European System for Cardiac Operative Risk Evaluation II |
| Fish | Fisher´s exact test |
| HFrEF | heart failure with reduced ejection fraction |
| ICU | intensive care unit |
| IQR | interquartile range |
| LIMA | left internal mammary artery |
| LOS | length of stay |
| MACCE | major adverse cardiac and cerebrovascular events |
| MW | Mann-Whitney U test |
| NTAR | non-total arterial revascularization |
| ONCAB | on-pump coronary artery bypass |
| OPCAB | off-pump coronary artery bypass |
| OR | odds ratio |
| PSM | propensity score matching |
| RBC | red blood cell |
| SD | standard deviation |
| SMD | standardized mean difference |
| STS Score | Society of Thoracic Surgeons Predicted Risk of Mortality Score |
| TAR | total arterial revascularization |
References
- Bakaeen, F.G.; Gaudino, M.; Whitman, G.; Doenst, T.; Ruel, M.; Taggart, D.P.; Stulak, J.M.; Benedetto, U.; Anyanwu, A.; Chikwe, J.; et al. 2021: The American Association for Thoracic Surgery Expert Consensus Document: Coronary artery bypass grafting in patients with ischemic cardiomyopathy and heart failure. J. Thorac. Cardiovasc. Surg. 2021, 162, 829–850.e1. [Google Scholar] [CrossRef]
- Pei, J.; Wang, X.; Xing, Z.; Zheng, K.; Hu, X. Short-term and long-term outcomes of revascularization interventions for patients with severely reduced left ventricular ejection fraction: A meta-analysis. ESC Heart Fail. 2021, 8, 634–643. [Google Scholar] [CrossRef]
- Rocha, R.V.; Tam, D.Y.; Karkhanis, R.; Wang, X.; Austin, P.C.; Ko, D.T.; Gaudino, M.; Royse, A.; Fremes, S.E. Long-term Outcomes Associated With Total Arterial Revascularization vs Non-Total Arterial Revascularization. JAMA Cardiol. 2020, 5, 507–514. [Google Scholar] [CrossRef]
- Tatoulis, J.; Buxton, B.F.; Fuller, J.A.; Royse, A.G. Total arterial coronary revascularization: Techniques and results in 3,220 patients. Ann. Thorac. Surg. 1999, 68, 2093–2099. [Google Scholar] [CrossRef]
- Damgaard, S.; Wetterslev, J.; Lund, J.T.; Lilleor, N.B.; Perko, M.J.; Kelbaek, H.; Madsen, J.K.; Steinbruchel, D.A. One-year results of total arterial revascularization vs. conventional coronary surgery: CARRPO trial. Eur. Heart J. 2009, 30, 1005–1011. [Google Scholar] [CrossRef]
- Kane, L.T.; Fang, T.; Galetta, M.S.; Goyal, D.K.C.; Nicholson, K.J.; Kepler, C.K.; Vaccaro, A.R.; Schroeder, G.D. Propensity Score Matching: A Statistical Method. Clin. Spine Surg. 2020, 33, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Rustenbach, C.J.; Reichert, S.; Radwan, M.; Doll, I.; Mustafi, M.; Nemeth, A.; Marinos, S.L.; Berger, R.; Baumbach, H.; Zdanyte, M.; et al. On- vs. Off-Pump CABG in Heart Failure Patients with Reduced Ejection Fraction (HFrEF): A Multicenter Analysis. Biomedicines 2023, 11, 3043. [Google Scholar] [CrossRef]
- Schober, P.; Vetter, T.R. Kaplan-Meier Curves, Log-Rank Tests, and Cox Regression for Time-to-Event Data. Anesth. Analg. 2021, 132, 969–970. [Google Scholar] [CrossRef]
- Wilcox, R.R. Robust regression: Testing global hypotheses about the slopes when there is multicollinearity or heteroscedasticity. Br. J. Math. Stat. Psychol. 2019, 72, 355–369. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.U.; Dorman, N.M.; Christiansen, S.L.; Hoorn, E.J.; Ingelfinger, J.R.; Inker, L.A.; Levin, A.; Mehrotra, R.; Palevsky, P.M.; et al. Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020, 97, 1117–1129. [Google Scholar] [CrossRef]
- Taggart, D.P.; D’Amico, R.; Altman, D.G. Effect of arterial revascularisation on survival: A systematic review of studies comparing bilateral and single internal mammary arteries. Lancet 2001, 358, 870–875. [Google Scholar] [CrossRef]
- Schwann, T.A.; Tatoulis, J.; Puskas, J.; Bonnell, M.; Taggart, D.; Kurlansky, P.; Jacobs, J.P.; Thourani, V.H.; O’Brien, S.; Wallace, A.; et al. Worldwide Trends in Multi-arterial Coronary Artery Bypass Grafting Surgery 2004–2014: A Tale of 2 Continents. Semin. Thorac. Cardiovasc. Surg. 2017, 29, 273–280. [Google Scholar] [CrossRef]
- Sun, L.Y.; Gaudino, M.; Chen, R.J.; Bader Eddeen, A.; Ruel, M. Long-Term Outcomes in Patients with Severely Reduced Left Ventricular Ejection Fraction Undergoing Percutaneous Coronary Intervention Vs Coronary Artery Bypass Grafting. JAMA Cardiol. 2020, 5, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Carson, P.; Wertheimer, J.; Miller, A.; O’Connor, C.M.; Pina, I.L.; Selzman, C.; Sueta, C.; She, L.; Greene, D.; Lee, K.L.; et al. The Stich Trial (Surgical Treatment for Ischemic Heart Failure): Mode-of-Death Results. JACC Heart Fail. 2013, 1, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, B.; Verma, S.; Mazine, A.; Tam, D.Y.; Juni, P.; Puskas, J.D.; Murugavel, S.; Friedrich, J.O. Impact of total arterial revascularization on long term survival: A systematic review and meta-analysis of 130,305 patients. Int. J. Cardiol. 2017, 233, 29–36. [Google Scholar] [CrossRef]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–744. [Google Scholar] [CrossRef]
- Shahian, D.M.; Jacobs, J.P.; Badhwar, V.; Kurlansky, P.A.; Furnary, A.P.; Cleveland, J.C., Jr.; Lobdell, K.W.; Vassileva, C.; Wyler von Ballmoos, M.C.; Thourani, V.H.; et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 1-Background, Design Considerations, and Model Development. Ann. Thorac. Surg. 2018, 105, 1411–1418. [Google Scholar] [CrossRef]
- Taggart, D.P.; Benedetto, U.; Gerry, S.; Altman, D.G.; Gray, A.M.; Lees, B.; Gaudino, M.; Zamvar, V.; Bochenek, A.; Buxton, B.; et al. Bilateral versus Single Internal-Thoracic-Artery Grafts at 10 Years. N. Engl. J. Med. 2019, 380, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Sheikhy, A.; Fallahzadeh, A.; Forouzannia, K.; Pashang, M.; Tajdini, M.; Momtahen, S.; Mansourian, S.; Shirzad, M.; Sadeghian, S.; Hosseini, K. Off-pump versus on-pump coronary artery bypass graft surgery outcomes in patients with severe left ventricle dysfunction: Inverse probability weighted study. BMC Cardiovasc. Disord. 2022, 22, 488. [Google Scholar] [CrossRef]
- Gaudino, M.F.L.; Taggart, D.P.; Fremes, S.E. The ROMA trial: Why it is needed. Curr. Opin. Cardiol. 2018, 33, 622–626. [Google Scholar] [CrossRef]
- Buttar, S.N.; Yan, T.D.; Taggart, D.P.; Tian, D.H. Long-term and short-term outcomes of using bilateral internal mammary artery grafting versus left internal mammary artery grafting: A meta-analysis. Heart 2017, 103, 1419–1426. [Google Scholar] [CrossRef]
- Zhao, D.F.; Edelman, J.J.; Seco, M.; Bannon, P.G.; Wilson, M.K.; Byrom, M.J.; Thourani, V.; Lamy, A.; Taggart, D.P.; Puskas, J.D.; et al. Coronary Artery Bypass Grafting With and Without Manipulation of the Ascending Aorta: A Network Meta-Analysis. J. Am. Coll. Cardiol. 2017, 69, 924–936. [Google Scholar] [CrossRef]
- Greaves, D.; Psaltis, P.J.; Davis, D.H.J.; Ross, T.J.; Ghezzi, E.S.; Lampit, A.; Smith, A.E.; Keage, H.A.D. Risk Factors for Delirium and Cognitive Decline Following Coronary Artery Bypass Grafting Surgery: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e017275. [Google Scholar] [CrossRef]
- Djordjevic, J.; Ngaage, D.L. The relationship between total arterial revascularization and blood transfusion following coronary artery bypass grafting. World J. Surg. 2015, 39, 1288–1293. [Google Scholar] [CrossRef]
- Spadaccio, C.; Nenna, A.; Candura, D.; Rose, D.; Moscarelli, M.; Al-Attar, N.; Sutherland, F. Total arterial coronary artery bypass grafting in patients with preoperative anemia. J. Card. Surg. 2022, 37, 1528–1536. [Google Scholar] [CrossRef]
- Paone, G.; Likosky, D.S.; Brewer, R.; Theurer, P.F.; Bell, G.F.; Cogan, C.M.; Prager, R.L.; Thoracic Membership of the Michigan Society of, and Surgeons Cardiovascular. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann. Thorac. Surg. 2014, 97, 87–93. [Google Scholar] [CrossRef]
- Leal-Noval, S.R.; Rincon-Ferrari, M.D.; Garcia-Curiel, A.; Herruzo-Aviles, A.; Camacho-Larana, P.; Garnacho-Montero, J.; Amaya-Villar, R. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest 2001, 119, 1461–1468. [Google Scholar] [CrossRef]
- Raphael, J.; Hensley, N.B.; Chow, J.; Parr, K.G.; McNeil, J.S.; Porter, S.B.; Taneja, M.; Tanaka, K.; Mazzeffi, M. Red Blood Cell Transfusion and Postoperative Delirium in Hip Fracture Surgery Patients: A Retrospective Observational Cohort Study. Anesthesiol. Res. Pract. 2021, 2021, 8593257. [Google Scholar] [CrossRef]
- Grant, M.C.; Isada, T.; Ruzankin, P.; Whitman, G.; Lawton, J.S.; Dodd, O.J.; Barodka, V.; Johns Hopkins Enhanced Recovery Program for the Cardiac Surgery Working, G. Results from an enhanced recovery program for cardiac surgery. J. Thorac. Cardiovasc. Surg. 2020, 159, 1393–1402.e7. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.J.; Zhao, S.; Tian, D.H.; Taggart, D.P.; Yan, T.D. A meta-analysis comparing bilateral internal mammary artery with left internal mammary artery for coronary artery bypass grafting. Ann. Cardiothorac. Surg. 2013, 2, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, B.; Choong, C.K.; Goldsmith, K.; Gerrard, C.; Nashef, S.A.; Vuylsteke, A. Prolonged stay in intensive care unit is a powerful predictor of adverse outcomes after cardiac operations. Ann. Thorac. Surg. 2012, 94, 109–116. [Google Scholar] [CrossRef]
- Belyayev, L.; Stock, E.M.; Hattler, B.; Bakaeen, F.G.; Kinlay, S.; Quin, J.A.; Haime, M.; Biswas, K.; Zenati, M.A. Complete Coronary Revascularization and Outcomes in Patients Undergoing CABG: Insights from the REGROUP Trial. Am. J. Cardiol. 2024, 217, 127–135. [Google Scholar] [CrossRef]
- Massoth, C.; Zarbock, A.; Meersch, M. Acute Kidney Injury in Cardiac Surgery. Crit. Care Clin. 2021, 37, 267–278. [Google Scholar] [CrossRef]
- Gaudino, M.; Bakaeen, F.G.; Sandner, S.; Aldea, G.S.; Arai, H.; Chikwe, J.; Firestone, S.; Fremes, S.E.; Gomes, W.J.; Bong-Kim, K.; et al. Expert Systematic Review on the Choice of Conduits for Coronary Artery Bypass Grafting: Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) and The Society of Thoracic Surgeons (STS). Ann. Thorac. Surg. 2023, 116, 659–674. [Google Scholar] [CrossRef]
| Variable n (%)|mean (±SD) | Total Cohort | NTAR | TAR | p Value |
|---|---|---|---|---|
| (n = 240) | (n = 120) | (n = 120) | ||
| Demographic Data | ||||
| Age | 67.11 (±9.59) | 66.93 (±9.56) | 67.30 (±9.65) | 0.763 ANOVA |
| Gender | ||||
| Male | 221 (92.1%) | 113 (94.2%) | 108 (90.0%) | 0.232 Chi2 |
| Female | 19 (7.9%) | 7 (5.8%) | 12 (10.0%) | |
| BMI | 28.09 (±4.68) | 27.86 (±4.80) | 28.32 (±4.57) | 0.448 ANOVA |
| Clinical Measurements | ||||
| EuroScore II | 5.49 (±5.44) | 5.62 (±5.52) | 5.37 (±5.37) | 0.718 ANOVA |
| STS Score | 3.01 (±2.50) | 2.81 (±2.50) | 3.21 (±2.49) | 0.214 ANOVA |
| LVEF preop | 32.10 (±6.70) | 31.82 (±6.15) | 32.38 (±7.22) | 0.519 ANOVA |
| Health Status | ||||
| Diabetes | ||||
| OAD | 58 (24.2%) | 33 (27.5%) | 25 (20.8%) | 0.425 Chi2 |
| Insulin dependent | 43 (17.9%) | 22 (18.3%) | 21 (17.5%) | |
| Smoking history | ||||
| Former. | 66 (27.5%) | 33 (27.5%) | 33 (27.5%) | 0.458 Chi2 |
| Active. | 66 (27.5%) | 37 (30.8%) | 29 (24.2%) | |
| Hypertension | 226 (94.2%) | 115 (95.8%) | 111 (92.5%) | 0.271 Chi2 |
| COPD | 54 (22.5%) | 27 (22.5%) | 27 (22.5%) | 1.000 Chi2 |
| Hyperlipidemia | 204 (85.0%) | 105 (87.5%) | 99 (82.5%) | 0.278 Chi2 |
| Apoplexy. pre-operative | 25 (10.4%) | 13 (10.8%) | 12 (10.0%) | 0.833 Chi2 |
| Carotid Stenosis | 37 (15.4%) | 19 (15.8%) | 18 (15.0%) | 0.858 Chi2 |
| Peripheral Vascular Disease | 64 (26.7%) | 32 (26.7%) | 32 (26.7%) | 1.000 Chi2 |
| Renal insufficiency | 86 (36.1%) | 40 (33.3%) | 46 (39.0%) | 0.419 Fish |
| Cardiac Status | ||||
| Left main disease | 89 (37.1%) | 39 (32.5%) | 50 (41.7%) | 0.142 Chi2 |
| 3-Vessel disease | 218 (90.8%) | 109 (90.8%) | 109 (90.8%) | 1.000 Chi2 |
| SR Rhythm pre-op | 187 (77.9%) | 99 (82.5%) | 88 (73.3%) | 0.191 Chi2 |
| NSTEMI | 70 (29.2%) | 32 (26.7%) | 38 (31.7%) | 0.204 Chi2 |
| STEMI | 20 (8.3%) | 11 (9.1%) | 9 (7.5%) | 0.272 Fish |
| Previous PCI | 95 (39.6%) | 46 (38.3%) | 49 (40.8%) | 0.692 Chi2 |
| Type of surgery | ||||
| Elective | 117 (48.8%) | 64 (54.7%) | 53 (45.3%) | 0.155 Chi2 |
| Non-Elective | 123 (51.3%) | 56 (45.5%) | 67 (54.5%) | |
| Off-pump | 120 (50.0%) | 56 (46.6%) | 64 (53.3%) | 0.136 Chi2 |
| Variable | Total Cohort | NTAR | TAR | p Value |
|---|---|---|---|---|
| (n = 240) | (n = 120) | (n = 120) | ||
| Intraoperative Requirement for Transfusion [Units]. mean (±SD) | ||||
| Packed red blood cells | 0.22 (±0.65) | 0.31 (±0.58) | 0.13 (±0.45) | 0.028 ANOVA |
| Pooled thrombocytes | 0.16 (±0.52) | 0.17 (±0.57) | 0.16 (±0.46) | 0.902 ANOVA |
| Fresh-frozen-plasma | 0.18 (±0.89) | 0.23 (±1.04) | 0.13 (±0.72) | 0.429 ANOVA |
| Postoperative (after chest closure) Vasopressor and Inotropic requirements [γ]. mean (95% CI) | ||||
| Epinephrine | 0.015 (0.01–0.02) | 0.018 (0.01–0.03) | 0.01 (0.005–0.02) | 0.008 MW |
| Norepinephrine | 0.12 (0.01–0.13) | 0.17 (0.09–0.14) | 0.10 (0.10–0.13) | 0.028 MW |
| Postoperative Parameters. median (IQR) | ||||
| Hospital LOS (d) | 11 (9–16) | 12 (9–18) | 10 (8–14) | 0.002 MW |
| ICU LOS (h) | 70 (31.75–120) | 90 (48–143) | 44.5 (22–104.75) | <0.001 MW |
| Ventilation (h) | 11 (5–18) | 12 (6.25–24) | 8 (5–15) | <0.001 MW |
| Surgical time (min) | 203.5 (166–246.75) | 208 (166.5–259) | 198.5 (165.25–236.5) | 0.262 MW |
| Complete Revascularization. n (%) | 184 (76.7%) | 98 (81.7%) | 86 (71.7%) | 0.067 Chi2 |
| Transfusion requirements during the entire clinical stay [Units]. mean (±SD) | ||||
| Packed red blood cells | 1.23 (±2.38) | 1.77 (±2.91) | 0.70 (±1.33) | <0.001 ANOVA |
| Pooled thrombocytes | 0.25 (±0.77) | 0.28 (±0.87) | 0.23 (±0.67) | 0.561 ANOVA |
| Fresh-frozen-plasma | 0.28 (±1.10) | 0.38 (±1.27) | 0.18 (±0.89) | 0.178 ANOVA |
| Variable n (%) | Total Cohort | NTAR | TAR | p Value |
|---|---|---|---|---|
| (n = 240) | (n = 120) | (n = 120) | ||
| Resuscitation | 5 (2.1%) | 3 (2.5%) | 2 (1.7%) | 1.000 Fish |
| Resternotomy | 4 (1.7%) | 2 (1.7%) | 2 (1.7%) | 1.000 Fish |
| ECLS | 8 (3.3%) | 6 (5.0%) | 2 (1.7%) | 0.181 Fish |
| AKI | 29 (12.89%) | 13 (10.9%) | 16 (13.4%) | 0.559 Fish |
| Dialysis | 20 (8.3%) | 8 (6.7%) | 12 (10.0%) | 0.484 Fish |
| Delirium | 23 (9.6%) | 17 (14.2%) | 6 (5.0%) | 0.016 Chi2 |
| Sepsis | 9 (3.8%) | 6 (5.0%) | 3 (2.5%) | 0.209 Fish |
| Stroke | 7 (2.9%) | 5 (4.2%) | 2 (1.7%) | 0.446 Fish |
| Postoperative MI | 10 (4.2%) | 6 (5.0%) | 4 (3.33%) | 0.333 Fish |
| Mortality | 9 (3.8%) | 7 (5.8%) | 2 (1.7%) | 0.086 Chi2 |
| MACCE | 22 (9.2%) | 17 (14.2%) | 5 (4.1%) | 0.007 Chi2 |
| Variable | OR | 95% CI | B | p Value (Wald Statistic) |
|---|---|---|---|---|
| Resuscitation | 0.648 | 0.037–11.45 | −0.433 | 0.767 |
| Resternotomy | 1.353 | 0.236–121.42 | 1.678 | 0.292 |
| ECLS | 0.209 | 0.024–1.822 | −1.566 | 0.156 |
| AKI | 0.902 | 0.243–3.352 | −0.104 | 0.877 |
| Dialysis | 1.369 | 0.975–55.694 | 1.997 | 0.103 |
| Delirium | 0.290 | 0.10–1.027 | −1.139 | 0.049 |
| Sepsis | 0.180 | 0.017–1.881 | −1.713 | 0.152 |
| Stroke | 0.143 | 0.302–51.472 | 1.372 | 0.295 |
| Postoperative MI | 0.531 | 0.502–22.095 | 1.203 | 0.213 |
| Mortality | 0.399 | 0.011–0.861 | −2.313 | 0.036 |
| MACCE | 0.643 | 0.094–0.739 | −1.334 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rustenbach, C.J.; Schano, J.; Salewski, C.; Häberle, H.; Ngamsri, K.-C.; Djordjevic, I.; Wendt, S.; Caldonazo, T.; Saqer, I.; Saha, S.; et al. Total-Arterial Revascularization Is Superior in Heart Failure Patients with Reduced Ejection Fraction—A Propensity Score Matched Retrospective Multicenter Analysis. Med. Sci. 2025, 13, 179. https://doi.org/10.3390/medsci13030179
Rustenbach CJ, Schano J, Salewski C, Häberle H, Ngamsri K-C, Djordjevic I, Wendt S, Caldonazo T, Saqer I, Saha S, et al. Total-Arterial Revascularization Is Superior in Heart Failure Patients with Reduced Ejection Fraction—A Propensity Score Matched Retrospective Multicenter Analysis. Medical Sciences. 2025; 13(3):179. https://doi.org/10.3390/medsci13030179
Chicago/Turabian StyleRustenbach, Christian Jörg, Julia Schano, Christoph Salewski, Helene Häberle, Kristian-Christos Ngamsri, Ilija Djordjevic, Stefanie Wendt, Tulio Caldonazo, Ibrahim Saqer, Shekhar Saha, and et al. 2025. "Total-Arterial Revascularization Is Superior in Heart Failure Patients with Reduced Ejection Fraction—A Propensity Score Matched Retrospective Multicenter Analysis" Medical Sciences 13, no. 3: 179. https://doi.org/10.3390/medsci13030179
APA StyleRustenbach, C. J., Schano, J., Salewski, C., Häberle, H., Ngamsri, K.-C., Djordjevic, I., Wendt, S., Caldonazo, T., Saqer, I., Saha, S., Schnackenburg, P., Serna-Higuita, L. M., Doenst, T., Hagl, C., Wahlers, T., Schlensak, C., & Reichert, S. (2025). Total-Arterial Revascularization Is Superior in Heart Failure Patients with Reduced Ejection Fraction—A Propensity Score Matched Retrospective Multicenter Analysis. Medical Sciences, 13(3), 179. https://doi.org/10.3390/medsci13030179











