Multiple Asparaginase Infusions Cause Increasingly Severe Acute Hyperammonemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pigs and Procedures
2.2. Analysis of Samples
2.3. Statistics
3. Results
3.1. General Observations
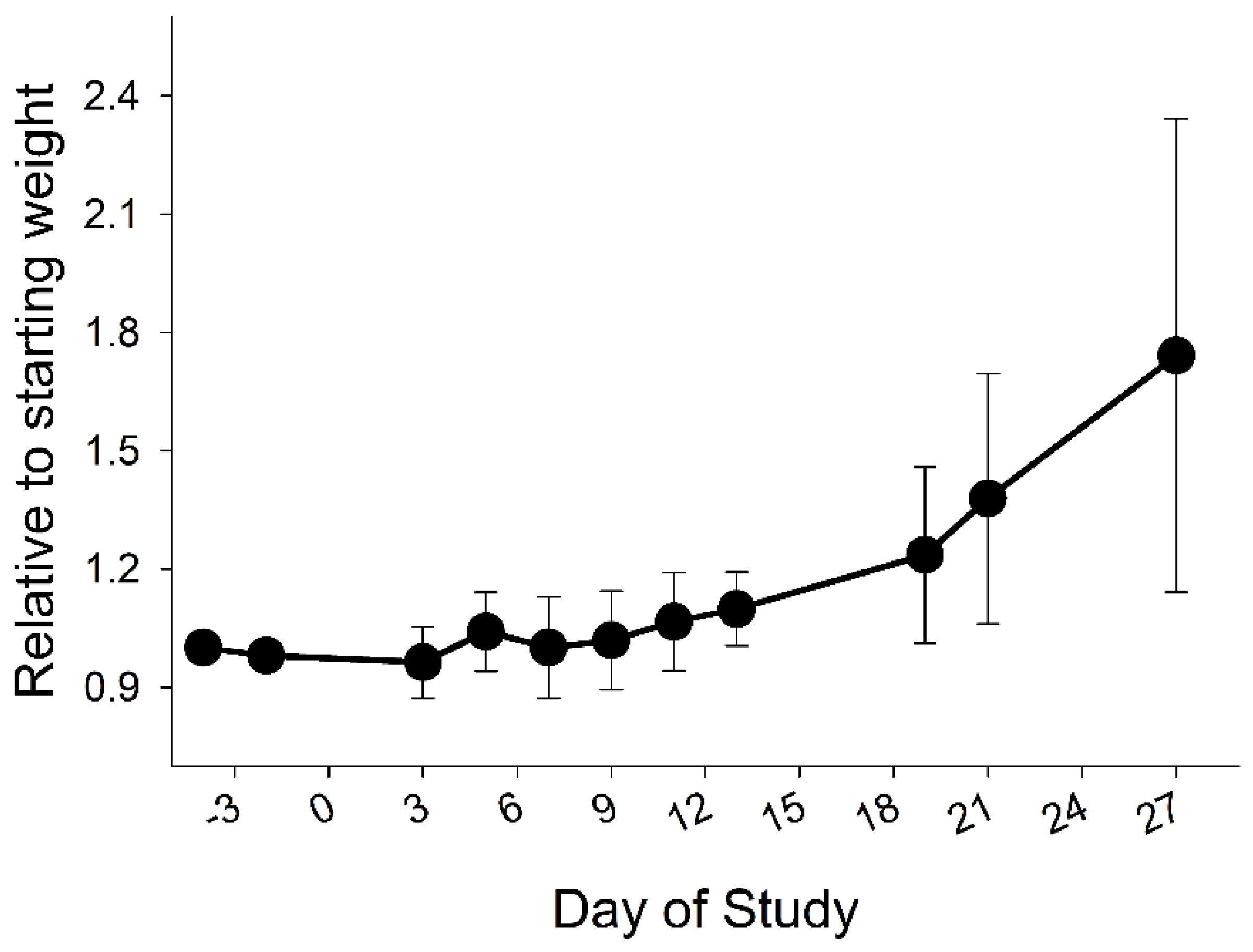
3.2. Growth
3.3. Infusion Reactions
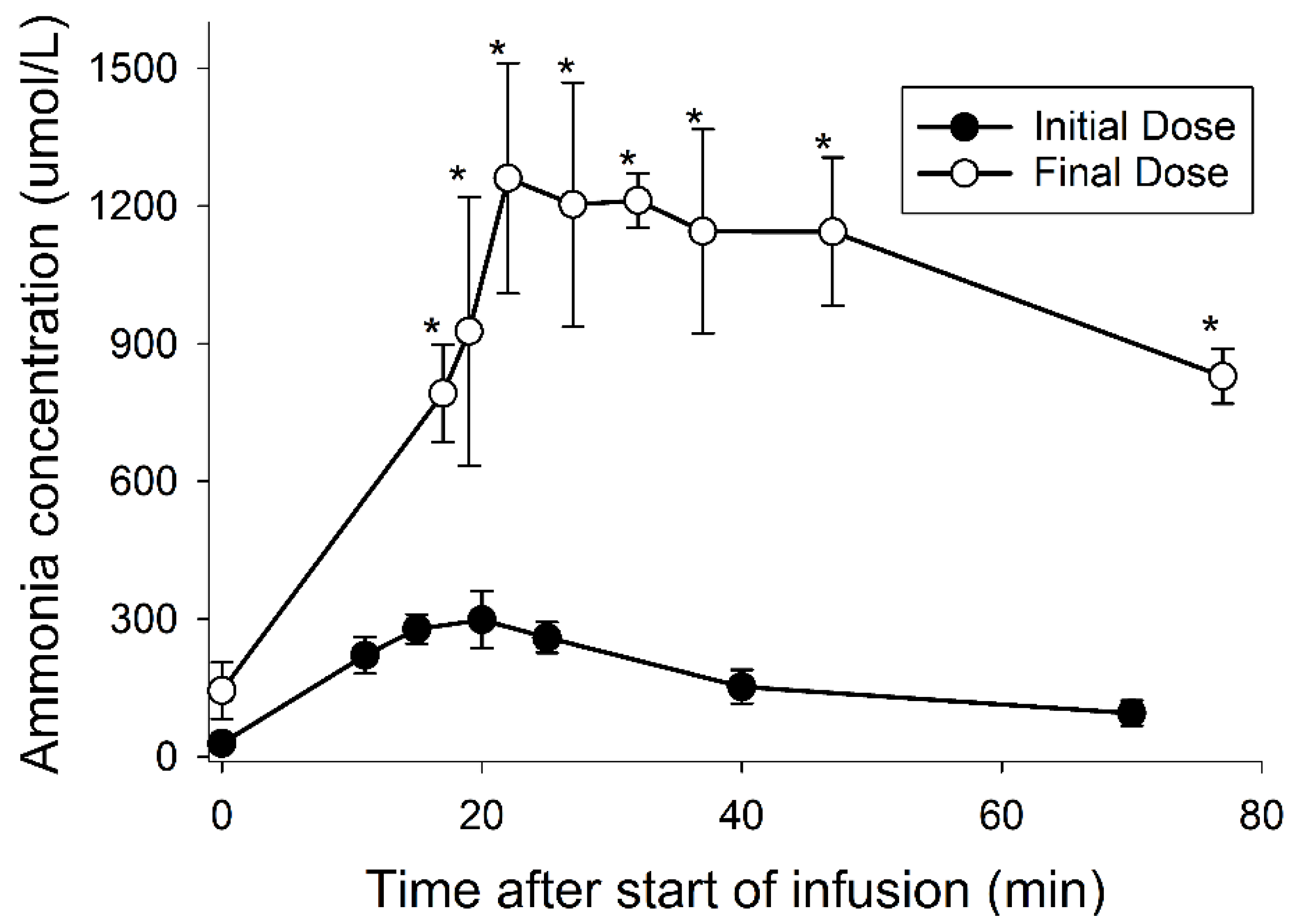
3.4. Ammonia
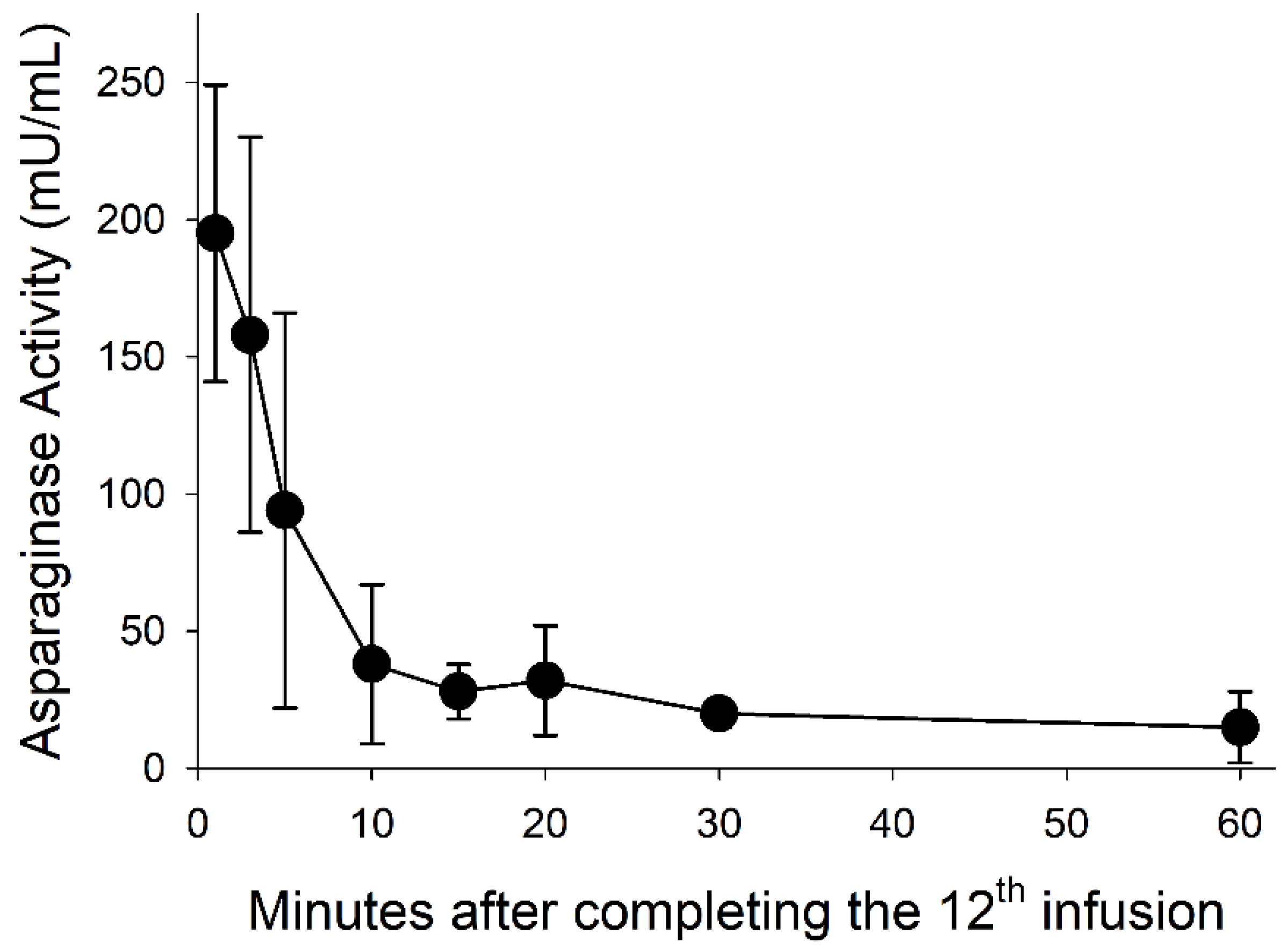
3.5. Asparaginase Activity
4. Discussion
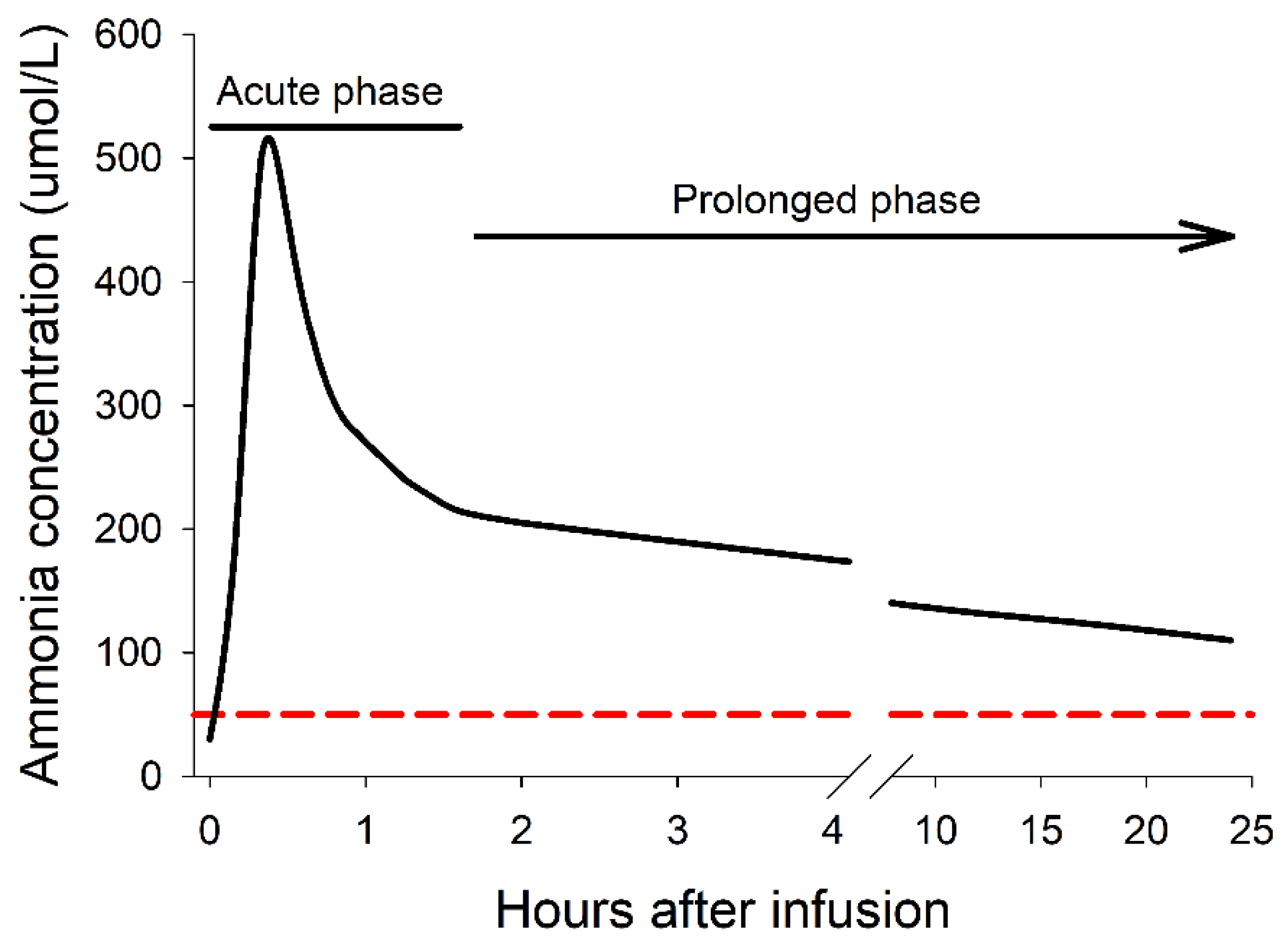
4.1. The Acute and Prolonged Phases of Hyperammonemia
4.2. Asparaginase Hypersensitivity Overlaps with Acute Hyperammonemia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanoshima, R.; Khan, A.; Biala, A.K.; Trueman, J.N.; Drögemöller, B.I.; Wright, G.E.B.; Hasbullah, J.S.; Groeneweg, G.S.S.; Ross, C.J.D.; Carleton, B.C. Analyses of Adverse Drug Reactions-Nationwide Active Surveillance Network: Canadian Pharmacogenomics Network for Drug Safety Database. J. Clin. Pharmacol. 2019, 59, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Heitink-Pollé, K.M.; Prinsen, B.H.; de Koning, T.J.; van Hasselt, P.M.; Bierings, M.B. High incidence of symptomatic hyperammonemia in children with acute lymphoblastic leukemia receiving pegylated asparaginase. JIMD Rep. 2013, 7, 103–108. [Google Scholar] [CrossRef]
- Gupta, S.; Wang, C.; Raetz, E.A.; Schore, R.; Salzer, W.L.; Larsen, E.C.; Maloney, K.W.; Mattano, L.A., Jr.; Carroll, W.L.; Winick, N.J.; et al. Impact of Asparaginase Discontinuation on Outcome in Childhood Acute Lymphoblastic Leukemia: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2020, 38, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Turgay Yagmur, I.; Guzelkucuk, Z.; Yarali, N.; Ozyoruk, D.; Toyran, M.; Civelek, E.; Ozbek, N.Y.; Dibek Misirlioglu, E. Evaluation of hypersensitivity reactions to cancer chemotherapeutic agents in pediatric patients. Ann. Allergy Asthma Immunol. 2020, 124, 350–356. [Google Scholar] [CrossRef]
- Figueiredo, L.; Cole, P.D.; Drachtman, R.A. Asparaginase Erwinia chrysanthemi as a component of a multi-agent chemotherapeutic regimen for the treatment of patients with acute lymphoblastic leukemia who have developed hypersensitivity to E. coli-derived asparaginase. Expert Rev. Hematol. 2016, 9, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Pieters, R.; Hunger, S.P.; Boos, J.; Rizzari, C.; Silverman, L.; Baruchel, A.; Goekbuget, N.; Schrappe, M.; Pui, C.H. L-asparaginase treatment in acute lymphoblastic leukemia: A focus on Erwinia asparaginase. Cancer 2011, 117, 238–249. [Google Scholar] [CrossRef]
- Salzer, W.; Bostrom, B.; Messinger, Y.; Perissinotti, A.J.; Marini, B. Asparaginase activity levels and monitoring in patients with acute lymphoblastic leukemia. Leuk. Lymphoma 2018, 59, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Ko, R.H.; Jones, T.L.; Radvinsky, D.; Robison, N.; Gaynon, P.S.; Panosyan, E.H.; Avramis, I.A.; Avramis, V.I.; Rubin, J.; Ettinger, L.J.; et al. Allergic reactions and antiasparaginase antibodies in children with high-risk acute lymphoblastic leukemia: A children’s oncology group report. Cancer 2015, 121, 4205–4211. [Google Scholar] [CrossRef]
- Kloos, R.Q.; Pieters, R.; Escherich, G.; van der Sluis, I.M. Allergic-like reactions to asparaginase: Atypical allergies without asparaginase inactivation. Pediatr. Blood Cancer 2016, 63, 1928–1934. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, R.S.; Panosyan, E.H.; Seibel, N.L.; Gaynon, P.S.; Avramis, I.A.; Avramis, V.I. Changes of amino acid serum levels in pediatric patients with higher-risk acute lymphoblastic leukemia (CCG-1961). In Vivo 2004, 18, 107–112. [Google Scholar] [PubMed]
- Asselin, B. Immunology of infusion reactions in the treatment of patients with acute lymphoblastic leukemia. Future Oncol. 2016, 12, 1609–1621. [Google Scholar] [CrossRef]
- Gossai, N.; Richards, M.; Boman, L.; Messinger, Y.; Gernbacher, S.; Perkins, J.; Bostrom, B. Symptomatic Hyperammonemia With Erwinia chrysanthemi-derived Asparaginase in Pediatric Leukemia Patients. J. Pediatr. Hematol. Oncol. 2018, 40, 312–315. [Google Scholar] [CrossRef]
- Strickler, N.; Balabanov, S.; Casauro, K.; Schanz, U.; Manz, M.G.; Gerber, B. Acute central nervous system complications and ammonium levels in adult patients with acute lymphoblastic leukemia receiving l-asparaginase. Leuk. Lymphoma 2018, 59, 855–862. [Google Scholar] [CrossRef]
- Santos, A.C.; Land, M.G.P.; Lima, E.C. Ammonia level as a proxy of asparaginase inactivation in children: A strategy for classification of infusion reactions. J. Oncol. Pharm. Pract. 2022, 28, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, W.; Urban, C.; Lackner, H. Clinical experiences with adrenaline as therapy and prevention of E. coli-L-asparaginase-induced anaphylaxis. Klin. Padiatr. 1992, 204, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.; Winchester, K.; Towerman, A.; Gettinger, K.; Carey, C.; Timmermann, K.; Langley, R.; Browne, E. From the Children’s Oncology Group: Evidence-Based Recommendations for PEG-Asparaginase Nurse Monitoring, Hypersensitivity Reaction Management, and Patient/Family Education. J. Pediatr. Oncol. Nurs. 2017, 34, 387–396. [Google Scholar] [CrossRef]
- Tong, W.H.; Pieters, R.; de Groot-Kruseman, H.A.; Hop, W.C.; Boos, J.; Tissing, W.J.; van der Sluis, I.M. The toxicity of very prolonged courses of PEGasparaginase or Erwinia asparaginase in relation to asparaginase activity, with a special focus on dyslipidemia. Haematologica 2014, 99, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Halford, Z.; Park, M. Hypersensitivity rates in pediatric patients receiving intravenous or intramuscular asparaginase agents. J. Hematol. Oncol. Pharm. 2020, 10, 7–12. [Google Scholar]
- Santos, A.C.D.; Land, M.G.P.; Silva, N.P.D.; Santos, K.O.; Lima-Dellamora, E.D.C. Reactions related to asparaginase infusion in a 10-year retrospective cohort. Rev. Bras. Hematol. Hemoter. 2017, 39, 337–342. [Google Scholar] [CrossRef]
- Speas, A.L.; Lyles, S.E.; Wirth, K.A.; Fahey, C.E.; Kow, K.; Lejeune, A.T.; Milner, R.J. Plasma ammonia concentration after L-asparaginase therapy in 27 dogs with high-grade lymphoma or leukemia. J. Vet. Emerg. Crit. Care 2018, 28, 130–139. [Google Scholar] [CrossRef]
- Steiner, M.; Attarbaschi, A.; Kastner, U.; Dworzak, M.; Haas, O.A.; Gadner, H.; Mann, G. Distinct fluctuations of ammonia levels during asparaginase therapy for childhood acute leukemia. Pediatr. Blood Cancer 2007, 49, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Jörck, C.; Kiess, W.; Weigel, J.F.; Mütze, U.; Bierbach, U.; Beblo, S. Transient hyperammonemia due to L-asparaginase therapy in children with acute lymphoblastic leukemia or non-Hodgkin lymphoma. Pediatr. Hematol. Oncol. 2011, 28, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Cauli, O.; Gomez-Pinedo, U.; Agusti, A.; Hernandez-Rabaza, V.; Garcia-Verdugo, J.M.; Felipo, V. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology 2010, 139, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Stock, W.; Douer, D.; DeAngelo, D.J.; Arellano, M.; Advani, A.; Damon, L.; Kovacsovics, T.; Litzow, M.; Rytting, M.; Borthakur, G.; et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: Recommendations of an expert panel. Leuk. Lymphoma 2011, 52, 2237–2253. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zhang, X.; Zhang, Y.; Li, J.; Xing, H. Ammonia exposure causes the disruption of the solute carrier family gene network in pigs. Ecotoxicol. Environ. Saf. 2021, 210, 111870. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.A.; Su, Y.; Lavie, A. Design and Characterization of Erwinia Chrysanthemi l-Asparaginase Variants with Diminished l-Glutaminase Activity. J. Biol. Chem. 2016, 291, 17664–17676. [Google Scholar] [CrossRef]
- Flynn, N.E.; Knabe, D.A.; Mallick, B.K.; Wu, G. Postnatal changes of plasma amino acids in suckling pigs. J. Anim. Sci. 2000, 78, 2369–2375. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef]
- Panetta, J.C.; Liu, Y.; Swanson, H.D.; Karol, S.E.; Pui, C.H.; Inaba, H.; Jeha, S.; Relling, M.V. Higher plasma asparaginase activity after intramuscular than intravenous Erwinia asparaginase. Pediatr. Blood Cancer 2020, 67, e28244. [Google Scholar] [CrossRef]
- Sassen, S.D.; Mathôt, R.A.; Pieters, R.; Kloos, R.Q.; de Haas, V.; Kaspers, G.J.; van den Bos, C.; Tissing, W.J.; Te Loo, M.; Bierings, M.B.; et al. Population pharmacokinetics of intravenous Erwinia asparaginase in pediatric acute lymphoblastic leukemia patients. Haematologica 2017, 102, 552–561. [Google Scholar] [CrossRef]
- Czogała, M.; Balwierz, W.; Sztefko, K.; Rogatko, I. Clinical utility of ammonia concentration as a diagnostic test in monitoring of the treatment with L-asparaginase in children with acute lymphoblastic leukemia. Biomed. Res. Int. 2014, 2014, 945860. [Google Scholar] [CrossRef] [PubMed]
- Tsurusawa, M.; Chin, M.; Iwai, A.; Nomura, K.; Maeba, H.; Taga, T.; Higa, T.; Kuno, T.; Hori, T.; Muto, A.; et al. L-Asparagine depletion levels and L-asparaginase activity in plasma of children with acute lymphoblastic leukemia under asparaginase treatment. Cancer Chemother. Pharmacol. 2004, 53, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Battistel, A.P.; Rocha, B.S.D.; Santos, M.T.D.; Daudt, L.E.; Michalowski, M.B. Allergic reactions to asparaginase: Retrospective cohort study in pediatric patients with acute lymphoid leukemia. Hematol. Transfus. Cell Ther. 2021, 43, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Cecconello, D.K.; Magalhães, M.R.; Werlang, I.C.R.; Lee, M.L.M.; Michalowski, M.B.; Daudt, L.E. Asparaginase: An old drug with new questions. Hematol. Transfus. Cell Ther. 2020, 42, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Appel, I.M.; Kazemier, K.M.; Boos, J.; Lanvers, C.; Huijmans, J.; Veerman, A.J.; van Wering, E.; den Boer, M.L.; Pieters, R. Pharmacokinetic, pharmacodynamic and intracellular effects of PEG-asparaginase in newly diagnosed childhood acute lymphoblastic leukemia: Results from a single agent window study. Leukemia 2008, 22, 1665–1679. [Google Scholar] [CrossRef] [PubMed]
- Horvath, T.D.; Chan, W.K.; Pontikos, M.A.; Martin, L.A.; Du, D.; Tan, L.; Konopleva, M.; Weinstein, J.N.; Lorenzi, P.L. Assessment of l-Asparaginase Pharmacodynamics in Mouse Models of Cancer. Metabolites 2019, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.B.; Bridges, J.M. L-Asparaginase activity in human and animal sera. Nature 1968, 217, 758–759. [Google Scholar] [CrossRef]
- Watanabe, S.; Miyake, K.; Ogawa, C.; Matsumoto, H.; Yoshida, K.; Hirabayashi, S.; Hasegawa, D.; Inoue, T.; Kizu, J.; Machida, R.; et al. The ex vivo production of ammonia predicts L-asparaginase biological activity in children with acute lymphoblastic leukemia. Int. J. Hematol. 2009, 90, 347–352. [Google Scholar] [CrossRef]
- Machado, M.C.; Pinheiro da Silva, F. Hyperammonemia due to urea cycle disorders: A potentially fatal condition in the intensive care setting. J. Intensive Care 2014, 2, 22. [Google Scholar] [CrossRef]
- Peters, M.; Zee-Cheng, J.; Kuhl, A.; Orozco, J.; Lally, E.; Schwoerer, J.S. Pegylated asparaginase as cause of fatal hyperammonemia in patients with latent urea cycle disorder. Pediatr. Blood Cancer 2018, 65, e27239. [Google Scholar] [CrossRef] [PubMed]
- Kamal, N.; Koh, C.; Samala, N.; Fontana, R.J.; Stolz, A.; Durazo, F.; Hayashi, P.H.; Phillips, E.; Wang, T.; Hoofnagle, J.H. Asparaginase-induced hepatotoxicity: Rapid development of cholestasis and hepatic steatosis. Hepatol. Int. 2019, 13, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.J.; Rheingold, S.R. Differentiating hypersensitivity versus infusion-related reactions in pediatric patients receiving intravenous asparaginase therapy for acute lymphoblastic leukemia. Leuk. Lymphoma 2017, 58, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Klug Albertsen, B.; Schmiegelow, K.; Schrøder, H.; Carlsen, N.T.; Rosthøj, S.; Avramis, V.I.; Jakobsen, P. Anti-Erwinia asparaginase antibodies during treatment of childhood acute lymphoblastic leukemia and their relationship to outcome: A case-control study. Cancer Chemother. Pharmacol. 2002, 50, 117–120. [Google Scholar] [CrossRef] [PubMed]
| Day of Study | Description | Symptom Severity * | Comments |
|---|---|---|---|
| −7, −5, −3 | Pre-dose | 0 | Controls for baseline ammonia |
| 1 | Initial dose | 0 | |
| 3 | 2nd dose | 0 | |
| 5 | 3rd dose | 1 | Lethargy |
| 7 | 4th dose | 2 | Lethargy, vomiting, discomfort |
| 9 | 5th dose | 2 | Same |
| 11 | 6th dose | 3 | |
| 13 | 7th dose | 3 | Same; two with seizures, begin daily prednisolone administration (2 mg/m2 via duodenal catheter) for remainder of study to reduce hypersensitivity |
| 15 | 8th dose | 4 | Severe symptoms; seizures, imbalance, urticaria |
| 16–21 | Recovery period | 0 | No symptoms; eating and gaining weight |
| 22 | 9th dose | 4 | Severe symptoms Begin IV epinephrine (0.05 mg) at the time of each dose |
| 23 | 10th dose (booster) | 2–3 | Mild to moderate symptoms |
| 25 | 11th dose | 3–4 | Same severe symptoms with high respiratory rate, urticaria |
| 26–29 | Recovery period | 0 | No symptoms; eating and gaining weight |
| 30 | 12th and final dose | 4–5 | Severe symptoms with one death |
| Dose | Asparaginase Activity 48 h after Infusion (U/L) |
|---|---|
| 3 | 88 ± 11 |
| 5 | 21 ± 12 |
| 6 | 10 ± 3 |
| 11 | 12 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buddington, R.K.; Buddington, K.K.; Howard, S.C. Multiple Asparaginase Infusions Cause Increasingly Severe Acute Hyperammonemia. Med. Sci. 2022, 10, 43. https://doi.org/10.3390/medsci10030043
Buddington RK, Buddington KK, Howard SC. Multiple Asparaginase Infusions Cause Increasingly Severe Acute Hyperammonemia. Medical Sciences. 2022; 10(3):43. https://doi.org/10.3390/medsci10030043
Chicago/Turabian StyleBuddington, Randal K, Karyl K Buddington, and Scott C. Howard. 2022. "Multiple Asparaginase Infusions Cause Increasingly Severe Acute Hyperammonemia" Medical Sciences 10, no. 3: 43. https://doi.org/10.3390/medsci10030043
APA StyleBuddington, R. K., Buddington, K. K., & Howard, S. C. (2022). Multiple Asparaginase Infusions Cause Increasingly Severe Acute Hyperammonemia. Medical Sciences, 10(3), 43. https://doi.org/10.3390/medsci10030043





