The Influence of Olive Orchards Copper-Based Fungicide Use, in Soils and Sediments—The Case of Aetoliko (Etoliko) Lagoon Western Greece
Abstract
1. Introduction
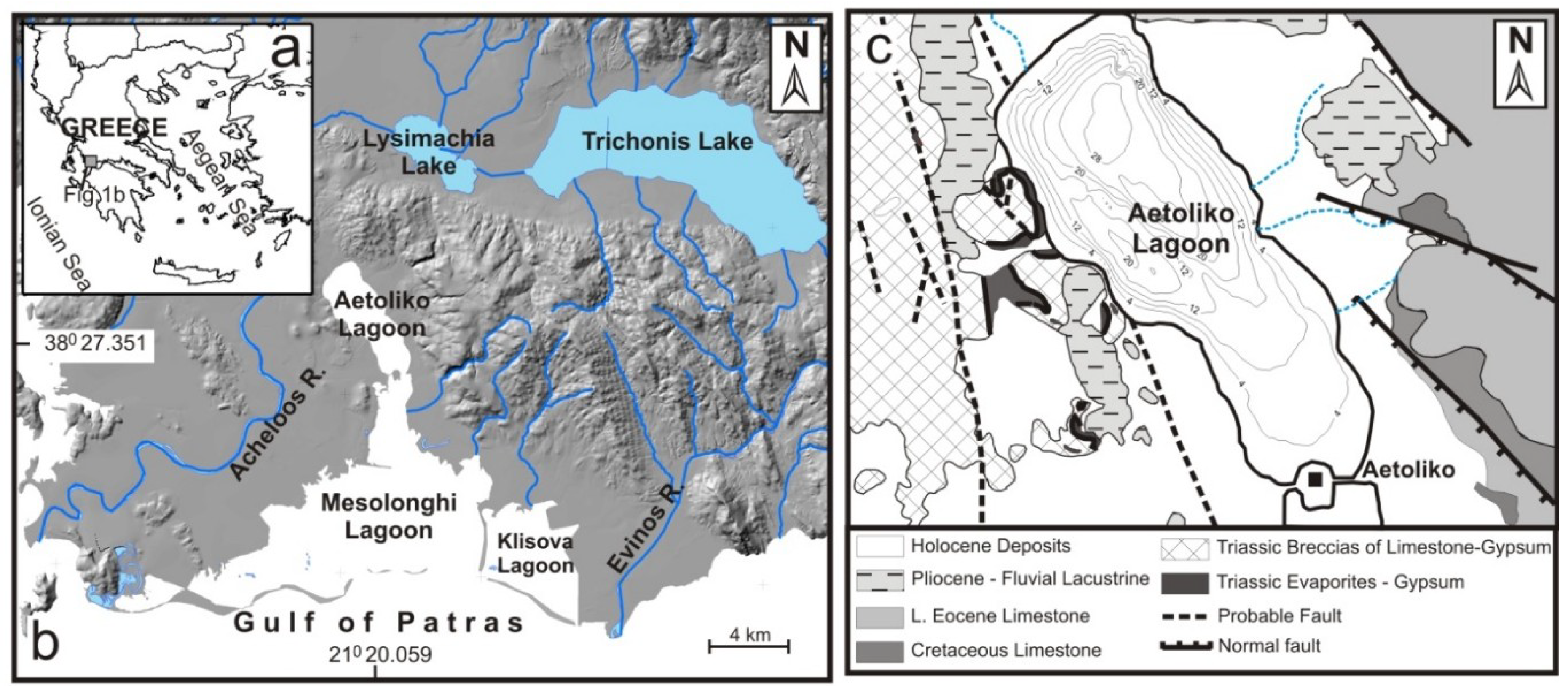
2. Study Area
3. Materials and Methods
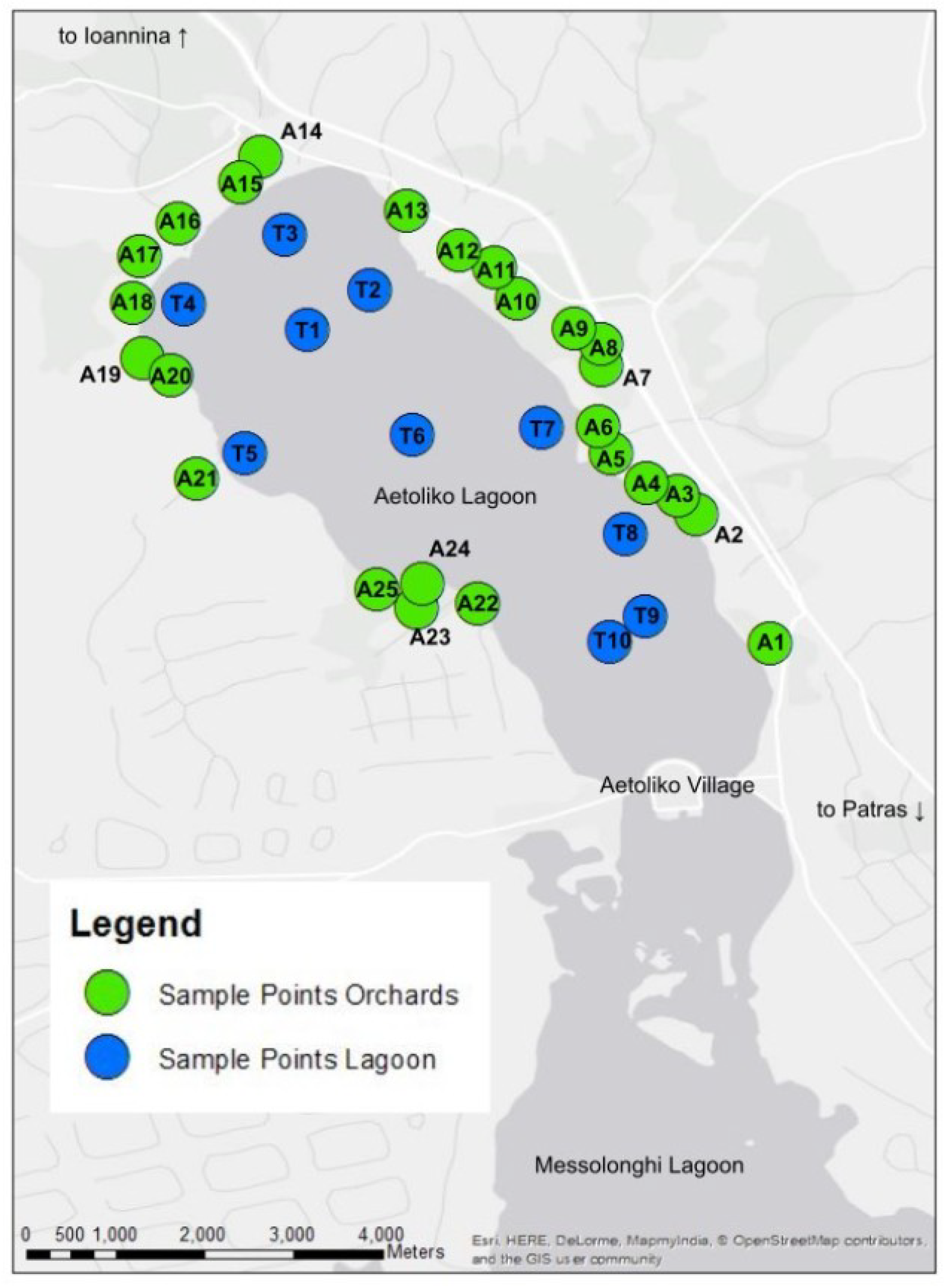
3.1. Sampling the Olive Orchards Soil and Lagoon Sediments
3.2. Analytical Procedure
4. Results
4.1. Soil and Lagoon Sediment Characteristics
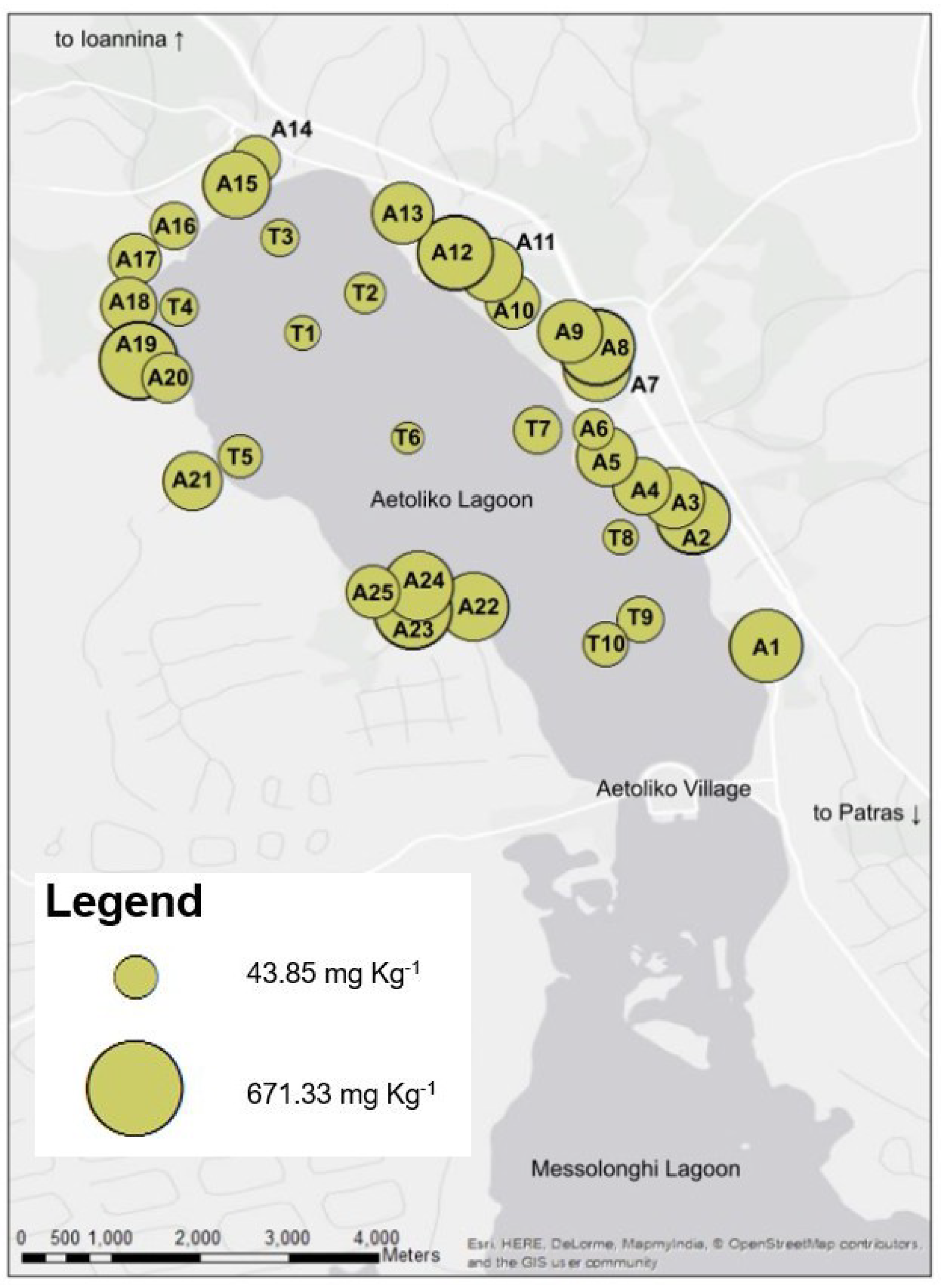
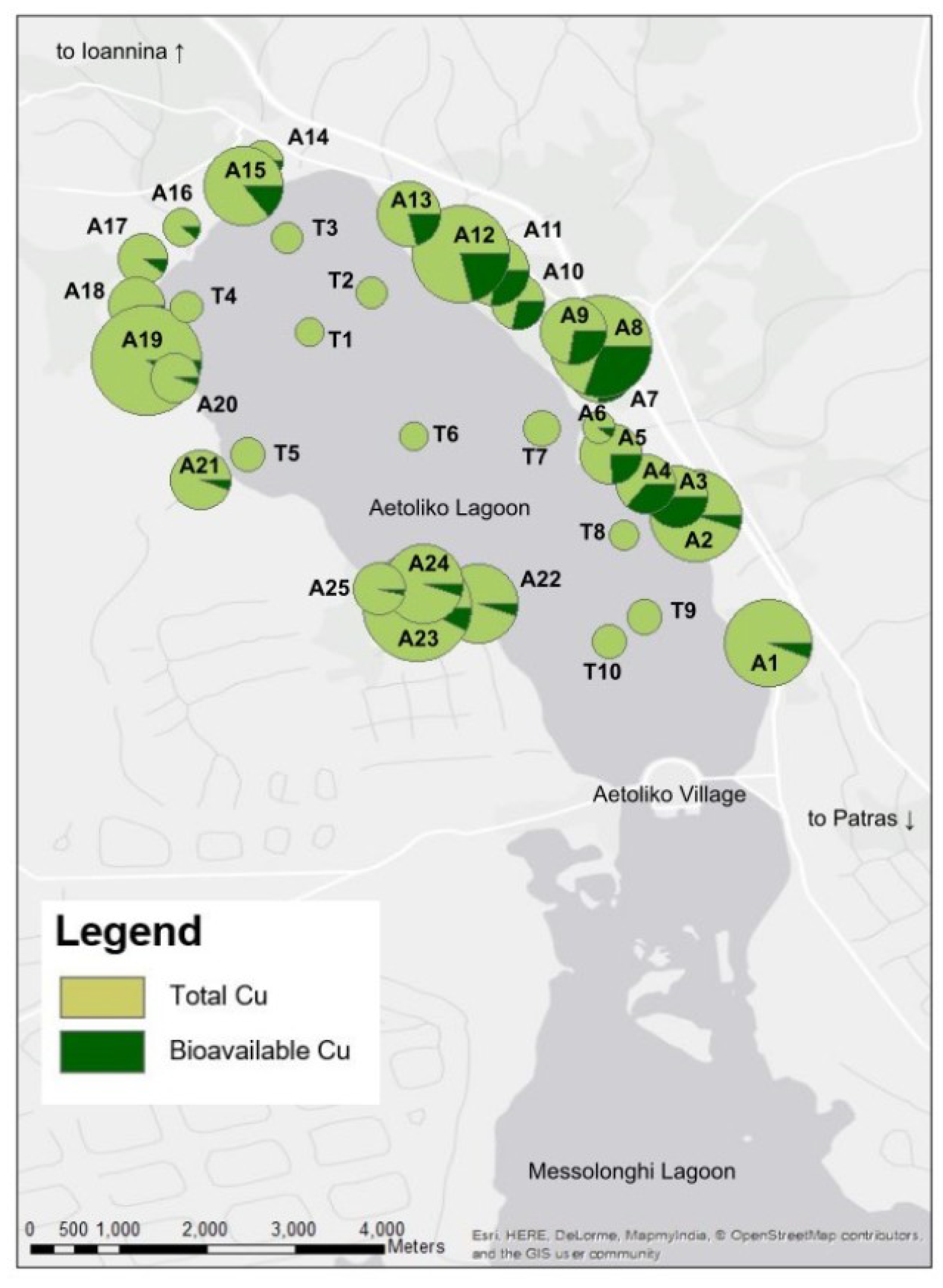
4.2. Total and Bioavailable Copper Concentration
4.3. Statistical Analysis of Soils and Sediments Data
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Sand | Silt | Clay | TC | TOC | TN | CaCO3 | pH | Cu-DTPA | Cu-Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sand | 1 | −0.99045 * | −0.81793 * | −0.1005 | −0.0717 | −0.17978 | −0.18977 | −0.09099 | −0.22792 | −0.05966 |
| Silt | 1 | 0.73081 * | 0.07875 | 0.05386 | 0.1795 | 0.18238 | 0.11357 | 0.23607 | 0.04824 | |
| Clay | 1 | 0.16897 | 0.13022 | 0.14105 | 0.17852 | −0.02343 | 0.14335 | 0.09406 | ||
| TC | 1 | 0.59605 * | 0.67265 * | 0.5263 * | 0.47702 * | 0.06207 | 0.16341 | |||
| TOC | 1 | 0.86597 * | −0.17336 | −0.0062 | 0.51283 * | 0.55847 * | ||||
| TN | 1 | −0.10629 | 0.16641 | 0.52482 * | 0.54797 * | |||||
| CaCO3 | 1 | 0.50533 * | −0.28592 | −0.23362 | ||||||
| pH | 1 | −0.0839 | −0.14546 | |||||||
| Cu-DTPA | 1 | 0.48889 * | ||||||||
| Cu-Total | 1 |
| Sand | Silt | Clay | TC | TOC | TN | TS | CaCO3 | Cu-DTPA | Cu-Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sand | 1 | 0.65887 * | −0.76889 * | 0.22134 | −0.66149 * | −0.59118 | −0.25112 | 0.2644 | 0.02156 | −0.28028 |
| Silt | 1 | −0.98758 * | 0.21212 | −0.12936 | −0.3155 | 0.31642 | 0.57585 | −0.20466 | −0.5398 | |
| Clay | 1 | −0.22653 | 0.24812 | 0.39165 | −0.21649 | −0.54468 | 0.16945 | 0.51735 | ||
| TC | 1 | 0.05311 | 0.07932 | 0.08571 | −0.52268 | 0.14493 | −0.1055 | |||
| TOC | 1 | 0.83865 * | 0.67199 * | −8.36 × 10−4 | −0.5327 | −0.35097 | ||||
| TN | 1 | 0.31596 | −0.00878 | −0.64473 * | −0.26396 | |||||
| TS | 1 | 0.19281 | −0.22402 | −0.4549 | ||||||
| CaCO3 | 1 | −0.55568 | −0.56165 | |||||||
| Cu-DTPA | 1 | 0.79088 * | ||||||||
| Cu-Total | 1 |
References
- Wightwick, A.; Walters, R.; Allinson, G.; Reichman, S.; Menzies, N. Environmental Risks of Fungicides Used in Horticultural Production Systems. In Fungicides; Carisse, O., Ed.; InTech: London, UK, 2010; Chapter 14; pp. 273–304. ISBN 978-953-307-266-1. Available online: http://www.intechopen.com/books/fungicides/environmental-risks-of-fungicides-used-in-horticulturalproduction-systems (accessed on 19 June 2019).
- European Commission Economic Analysis of the Olive Sector. Directorate-General for Agriculture and Rural Development. 2012, pp. 1–10. Available online: http://ec.europa.eu/agriculture/olive-oil/economic-analysis_en.pdf (accessed on 19 June 2019).
- Gjekanoviky, A.; Bizmpiroulas, A.; Rotsios, K. Export Success Factors for Table Olives: The Perception of Greek Exporting Firms. Procedia Econ. Finance 2015, 33, 584–594. Available online: http://linkinghub.elsevier.com/retrieve/pii/S2212567115017402 (accessed on 19 June 2019).
- Weissteiner, C.J.; Strobl, P.; Sommer, S. Assessment of Status and Trends of Olive Farming Intensity in EU-Mediterranean Countries Using Remote Sensing Time Series and Land Cover Data. Ecol. Indic. 2011, 11, 601–610. [Google Scholar] [CrossRef]
- Roca, L.F.; Moral, J.; Viruega, J.R.; Avila, A.; Oliveira, R.; Trapero, A. Copper Fungicides in the Control of Olive Diseases. Olea 2007, 26, 48–50. Available online: http://olivediseases.com/media/vera_spain_cooper.pdf (accessed on 19 June 2019).
- Fan, J.; He, Z.; Lena, Q.M.; Stoffella, P.J. Accumulation and availability of copper in citrus grove soils as affected by fungicide application. J. Soils Sedim. 2011, 11, 639–648. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Kenngott, K.G.J.; Azeroual, M.; Schafer, R.B.; Schaumann, G.E. Fractionation of copper and uranium in organic and conventional vineyard soils and adjacent stream sediments studied by sequential extraction. J. Soils Sedim. 2017, 17, 1092–1100. [Google Scholar] [CrossRef]
- Scheffer, F.; Schachtschabel, P. Lehrbuch der Bodenkunde; Spektrum Akademischer Verlag: Heidelberg, Germany, 2010; p. 573. [Google Scholar]
- Ministry of Environment of Finland—MEF. Government Decree on the Assessment of Soil Contamination and Remediation Needs. 2007, Volume 214, pp. 1–6. Available online: http://www.finlex.fi/en/laki/kaannokset/2007/en20070214.pdf (accessed on 19 June 2019).
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Hellenic Statistical Authority. Areas of Compact Plantations, by Region and Regional Unities. 2016. Tables 5a, 9, 18. Available online: http://www.statistics.gr/ (accessed on 19 June 2019).
- Dafis, S.; Papastergiadou, S.; Georghiou, K.; Babalonas, D.; Georgiadis, T.; Papageorgiou, M.; Lazaridou, E.; Tsiaoussi, V. Directive 92/43/EEC the Greek “Habitat” Project NATURA 2000: An Overview, English ed.; Commission of the European Communities DG XI, the Goulandris Natural History Museum—Greek Biotope/Wetland Centre: Athens, Greece, 1997. [Google Scholar]
- Gianni, A.; Zacharias, I. Modeling the hydrodynamic interactions of deep anoxic lagoons with their source basins. Estuar. Coast. Shelf Sci. 2012, 110, 157–167. [Google Scholar] [CrossRef]
- Gianni, A.; Zamparas, M.; Papadas, I.T.; Kehayas, G.; Deligiannakis, Y.; Zacharias, I. Monitoring and Modeling of Metal Concentration Distributions in Anoxic Basins: Aitoliko Lagoon, Greece. Aquat. Geochem. 2013, 19, 77–95. [Google Scholar] [CrossRef]
- Avramidis, P.; Bekiar, V.; Christodoulou, D.; Papatheodorou, G. Sedimentology and water column stratification in a permanent anoxic Mediterranean lagoon environment, Aetoliko Lagoon, western Greece. Environ. Earth Sci. 2015, 73, 5687–5701. [Google Scholar] [CrossRef]
- Dassenakis, M.; Krasakopoulou, E.; Matzara, B. Chemical Characteristics of Aetoliko lagoon, Greece, after an ecological shock. Mar. Pollut. Bull. 1994, 28, 427–433. [Google Scholar] [CrossRef]
- Leonardos, I.; Sinis, A.I. Fish Mass mortality in the Etolikon lagoon Greece: The role of local Geology. Cybium 1997, 21, 201–206. [Google Scholar]
- Papadas, I.T.; Katerinopoulos, L.; Gianni, A.; Zacharias, I.; Deligiannakis, Y. A theoretical and experimental physicochemical study of sulfur species in the anoxic lagoon of Aitoliko-Greece. Chemosphere 2009, 74, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Chamalaki, A.; Gianni, A.; Kehayias, G.; Zacharias, I.; Tsiamis, G.; Bourtzis, K. Bacterial diversity and hydrography of Etoliko, an anoxic semi-enclosed coastal basin in Western Greece. Ann. Microb. 2014, 64, 661–670. [Google Scholar] [CrossRef]
- Vött, A.; Schriever, A.; Handl, M.; Brückneret, H. Holocene Palaeogeographies of the Eastern Acheloos River Delta and the Lagoon of Etoliko (NW Greece). J. Coast. Res. 2007, 234, 1042–1066. [Google Scholar] [CrossRef]
- Haenssler, E.; Nadeau, M.J.; Vött, A.; Unkel, I. Natural and human induced environmental changes preserved in a Holocene sediment sequence from the Etoliko Lagoon, Greece: New evidence from geochemical proxies. Quat. Int. 2013, 308–309, 89–104. [Google Scholar] [CrossRef]
- Koutsodendris, A.; Brauer, A.; Zacharias, I.; Putyrskaya, V.; Klemt, E.; Sangiorgi, F.; Pross, J. Ecosystem response to human- and climate-induced environmental stress on an anoxic coastal lagoon (Etoliko, Greece) since 1930 AD. J. Paleolimnol. 2015, 53, 255–270. [Google Scholar] [CrossRef]
- Tóth, G.; Jones, A.; Montanarella, L. (Eds.) LUCAS Topsoil Survey Methodology, Data and Results. In European Commission Joint Research Centre; Report EUR 26102 EN; Joint Research Centre: Ispra, Italy, 2013; pp. 1–154. Available online: https://esdac.jrc.ec.europa.eu/ESDB_Archive/eusoils_docs/other/EUR26102EN.pdf (accessed on 19 June 2019).
- Benennung und Beschreibung von Boden und Fels; DIN 4022-1; Beuth Verlag: Berlin, Germany, 1987.
- Folk, R.L.; Ward, W.C. A Study in the Significance of Grain-Size Parameters. J. Sediment. Petrol. 1957, 27, 3–26. [Google Scholar] [CrossRef]
- Gaudette, H.; Flight, W.; Toner, L.; Folger, D. An inexpensive titration method for the determination of organic carbon in recent sediments. J. Sediment. Petrol. 1974, 44, 249–253. [Google Scholar]
- Avramidis, P.; Nikolaou, K.; Bekiari, V. Total Organic Carbon and Total Nitrogen in Sediments and Soils: A Comparison of the Wet Oxidation—Titration Method with the Combustion-infrared Method. Agric. Agric. Sci. Procedia 2015, 4, 425–430. [Google Scholar] [CrossRef]
- Barouchas, P. Automatic Portable and Digital Calcium Carbonate Measuring Instrument Practicable for Soil Samples—A Device Characterized by Incorporated Temperature—Counter Balancing Means, Potential Communication and Location of the Geographical Position of the Measurement, Greek Industrial Property Organization (OBI). Patent No. GR1008089 (B)―2014-01-21; Int. Cl. G01N33/24; G01N7/18 (valid until 20 October 2032), 2014. [Google Scholar]
- Barouchas, P. Automatic Portable and Digital Soil Calcimeter. World Intellectual Property Organization, WIPO Publication No. WO2014060782 A1, 24 April 2014. [Google Scholar]
- Barouchas, P. FOG II—A New Innovative Portable Instrument for the Total Calcium Carbonate Soil Testing. In Proceedings of the 20th World Congress of Soil Science, Jeju, Korea, 8–13 June 2014; pp. 4–106, AF2494. [Google Scholar]
- Love, S. Field Methods for the Analysis of Mud Brick Architecture. J. Field Arch. 2017, 42, 1–13. [Google Scholar] [CrossRef]
- Müller, G.; Gastner, M. The ‘Karbonat e bombe’, a simple device for the determination of carbonate content in sediments, soils and other materials. Neues Jahrbuch für Mineralogie 1971, 10, 466–469. [Google Scholar]
- Jones, G.A.; Kaiteris, P. A vacuum-gasometric technique for rapid and precise analysis of calcium carbonate in sediments and soils. J. Sediment. Petrol. 1983, 53, 655–660. [Google Scholar] [CrossRef]
- Bernas, B. A new method for decomposition and comprehensive analysis of silicates by atomic absorption spectrometry. Anal. Chem. 1968, 40, 1682–1686. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Soil Quality—Determination of pH; DIN ISO 10390; International Organization for Standardization: Geneva, Switzerland, 2005.
- USDA. Natural Resources Conservation Service. Soil Quality Indicators: pH. U.S. Department of Agriculture, 1998. Available online: https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_052208.pdf (accessed on 19 June 2019).
- Tiller, K.G.; Merry, R.H. Copper Pollution of Agricultural Soils. In Proceedings of the Golden Jubilee International Symposium on Copper in Soils and Plants, Murdoch University, Perth, Western Australia, 7–9 May 1981; Academic Press: Sydney, Australia, 1981; p. 119. [Google Scholar]
- McBride, M.B. Forms and Distribution of Copper in Solid and Solution Phases of Soil. In Proceedings of the Golden Jubilee International Symposium on Copper in Soils and Plants, Murdoch University, Perth, Western Australia, 7–9 May 1981; Loneragan, J., Robson, A., Graham, R., Eds.; Academic Press: Sydney, Australia, 1981; pp. 25–45. [Google Scholar]
- Vavoulidou, E.; Avramides, E.J.; Papadopoulos, P.; Dimirkou, A.; Charoulis, A.; Konstantinidou-Doltsinis, S. Copper Content in Agricultural Soils Related to Cropping Systems in Different Regions of Greece. Comm. Soil Sci. Plant Anal. 2005, 36, 759–773. [Google Scholar] [CrossRef]
- Carlon, C. Derivation Methods of Soil Screening Values in Europe; EUR 22805-EN; A Review and Evaluation of National Procedures Towards Harmonization; European Commission, Joint Research Centre: Ispra, Italy, 2007; 306p, Available online: https://esdac.jrc.ec.europa.eu/ESDB_Archive/eusoils_docs/other/EUR22805.pdf (accessed on 19 June 2019).
- Van der Voet, E.; Salminen, R.; Eckelman, M.; Mudd, G.; Norgate, T.; Hischier, R. Environmental Risks and Challenges of Anthropogenic Metals Flows and Cycles; A Report of the Working Group on the Global Metal Flows to the International Resource Panel; UNEP: Nairobi, Kenya, 2013; p. 231. [Google Scholar]
- MacDonald, D.D.; Ingersoll, C.G. Tools for Assessing Contaminated Sediments in Freshwater, Estuarine, and Marine Ecosystems. In Sedimentology for Aqueous Systems; Chapter 7; Poleto, C., Charlesworth, S., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; 210p. [Google Scholar]
- Perin, G.; Bonardi, M.; Fabris, R.; Simoncini, B.; Manente, S.; Tosi, L.; Scotto, S. Heavy metal pollution in central Venice Lagoon bottom sediments: Evaluation of the metal bioavailability by geochemical speciation procedure. Environ. Technol. 1997, 18, 593–604. [Google Scholar] [CrossRef]
- Pekey, H.; Karakas, D.; Ayberk, S.; Tolun, L.; Bakoglu, M. Ecological risk assessment using trace elements from surface sediments of Izmit Bay (Northeastern Marmara Sea) Turkey. Mar. Pollut. Bull. 2004, 48, 946–953. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.J. Ageing of Metals in Soils Changes Bioavailability; Fact Sheet on Environmental Risk Assessment No. 5; International Council on Mining and Metals: London, UK, 2001. [Google Scholar]
- Mountouris, A.; Voutsas, E.; Tassios, D. Bioconcentration of heavy metals in aquatic environments: The importance of bioavailability. Mar. Pollut. Bull. 2002, 44, 1136–1141. [Google Scholar] [CrossRef]
- Bryan, G.W.; Langston, W.J. Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries: A review. Environ. Pollut. 1992, 76, 89–131. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters, 2nd ed.; John Wiley & Sons Ltd.: New York, NY, USA, 1981. [Google Scholar]
- Dutta, J.; Mishra, A.K. Influence of the presence of heavy metals on the behaviour of bentonites. Environ. Earth Sci. 2016, 75. [Google Scholar] [CrossRef]
- Lukman, S.; Essa, M.H.; Nuthu, D.; Muazu, N.; Bukhari, A.; Basheer, C. Adsorption and desorption of heavy metals onto natural clay material: Influence of initial pH. J. Environ. Sci. Technol. 2013, 6, 1–15. [Google Scholar] [CrossRef]
- Sipos, P.; Kis, V.K.; Baldzs, R.; Toth, A.; Kovacs, I.; Nemeth, T. Contribution of individual pure or mixed-phase mineral particles to metal sorption in soils. Geoderma 2018, 324, 1–8. [Google Scholar] [CrossRef]
- Medhi, H.; Bhattacharyya, K.G. Kinetic and mechanistic studies on adsorption of Cu(II) in aqueous medium onto montmorillonite K10 and its modified derivative. New J. Chem. 2017, 41, 13533–13552. [Google Scholar] [CrossRef]
- El Ass, K. Adsorption of cadmium and copper onto natural clay: Isotherm, kinetic and thermodynamic studies. Glob. NEST J. 2018, 20, 198–207. [Google Scholar]
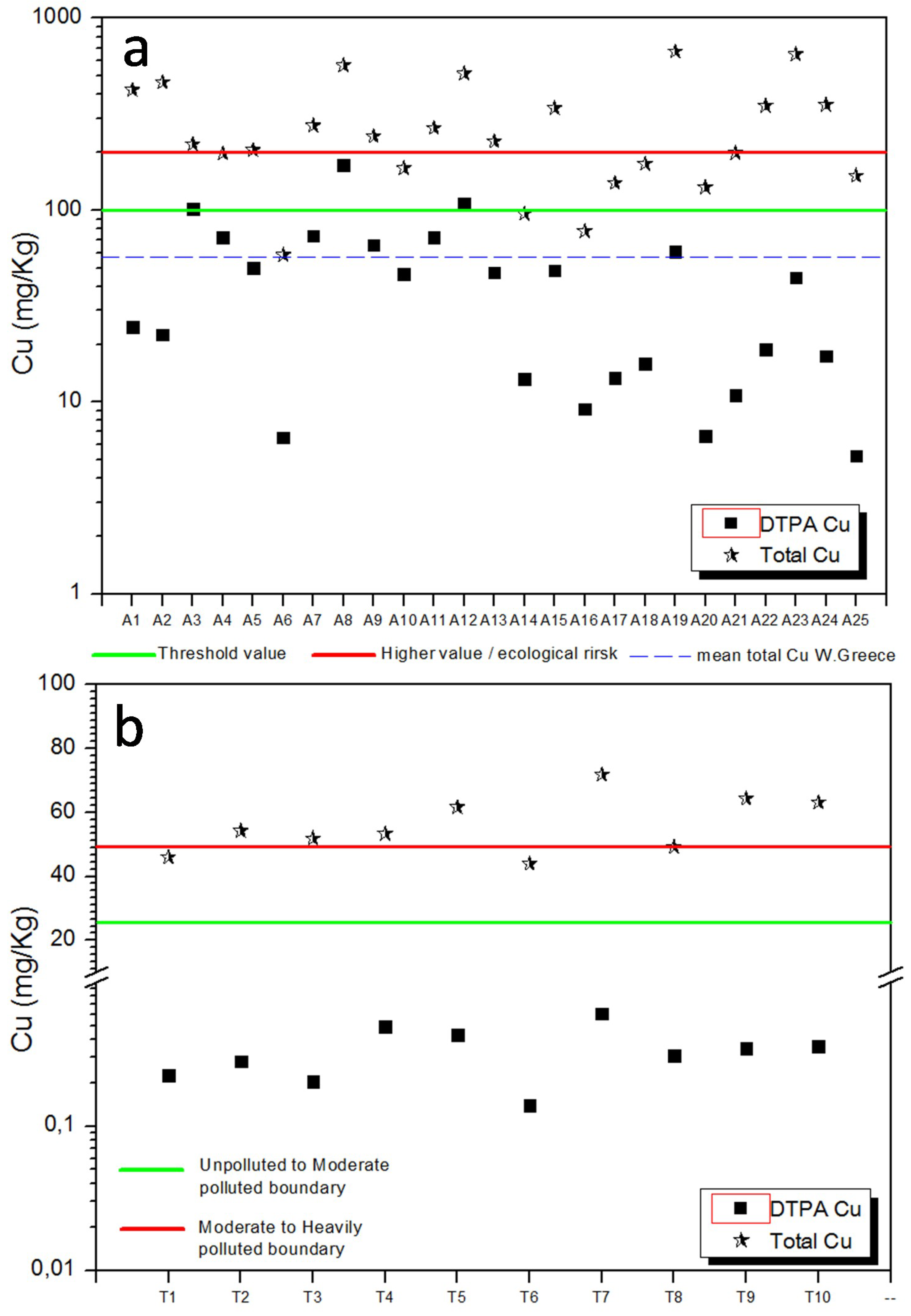
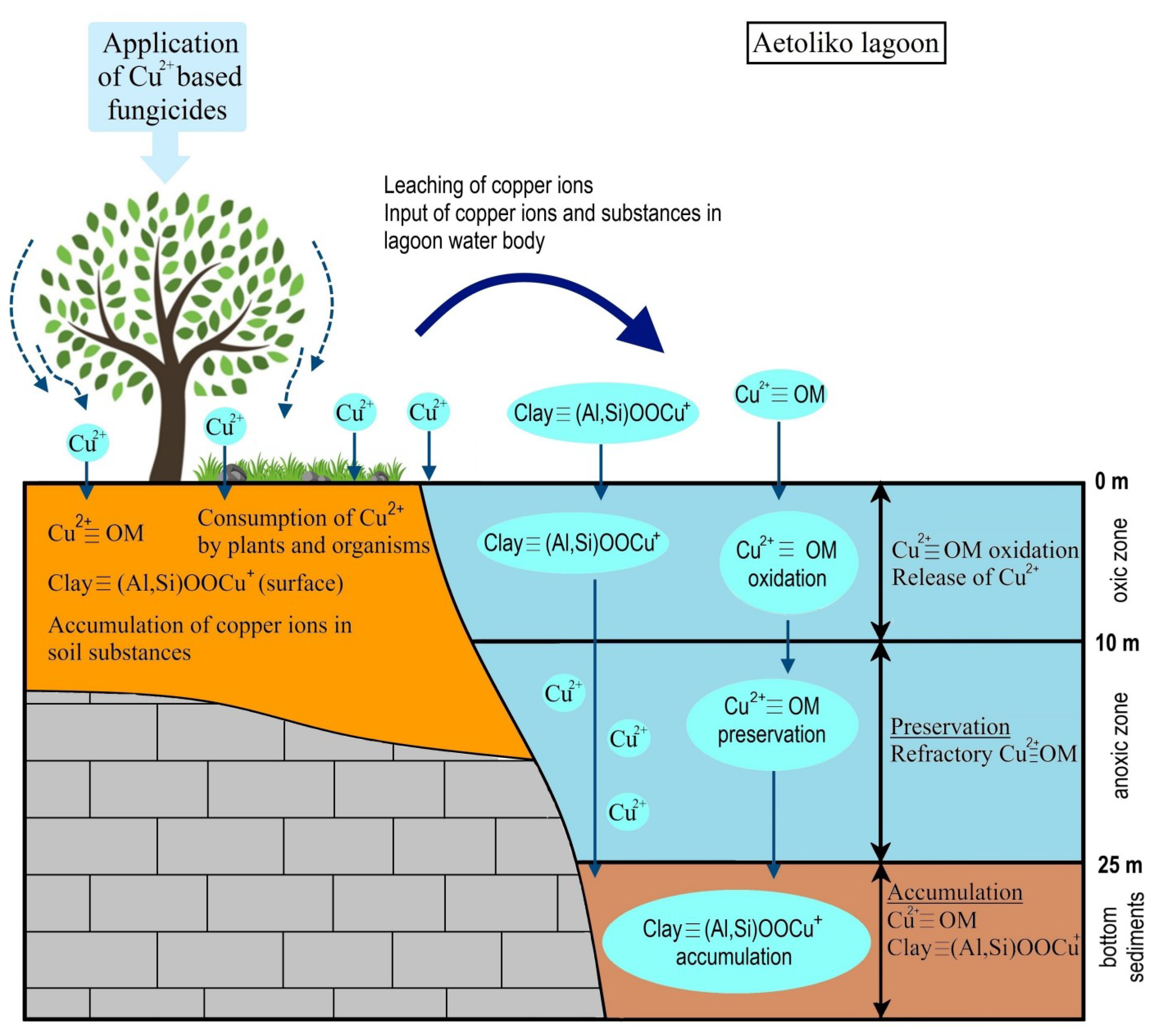
| Sampling Stations | Coordinates | Grain Size Analysis | Statistical Parameters (Φ) | TC (%) | TOC (%) | TN (%) | CaCO3 (%) | pH | Cu-DTPA (mg/Kg) | Cu-Total (mg/Kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Longitude | Latitude | Sand | Silt | Clay | Mean | Sorting | Skewness | Kurtosis | ||||||||
| A1 | 38°27.085 | 21°21.809 | 12.04 | 77.68 | 10.29 | 5.90 | 2.00 | 0.18 | 1.02 | 5.24 | 2.83 | 0.74 | 2.40 | 7.25 | 24.60 | 422.50 |
| A2 | 38°27.868 | 21°21.362 | 32.57 | 57.99 | 9.44 | 5.24 | 2.49 | 0.10 | 0.83 | 5.01 | 3.09 | 0.63 | 0.00 | 5.48 | 22.45 | 463.44 |
| A3 | 38°27.982 | 21°21.248 | 31.59 | 58.87 | 9.53 | 5.27 | 2.50 | 0.06 | 0.86 | 3.60 | 2.80 | 0.55 | 0.00 | 6.35 | 101.50 | 220.38 |
| A4 | 38°28.059 | 21°21.059 | 25.89 | 64.06 | 10.04 | 5.49 | 2.48 | −0.01 | 0.92 | 3.84 | 2.87 | 0.49 | 0.00 | 5.94 | 72.05 | 197.04 |
| A5 | 38°28.244 | 21°20.845 | 22.61 | 66.62 | 10.77 | 5.73 | 2.34 | 0.02 | 0.87 | 3.84 | 2.96 | 0.53 | 1.20 | 6.54 | 49.86 | 205.63 |
| A6 | 38°28.405 | 21°20.770 | 13.53 | 73.81 | 12.66 | 6.14 | 2.26 | 0.04 | 1.06 | 1.61 | 0.34 | 0.25 | 7.10 | 7.38 | 6.52 | 58.37 |
| A7 | 38°28.774 | 21°20.787 | 22.98 | 64.36 | 12.66 | 5.72 | 2.73 | −0.08 | 1.03 | 3.89 | 2.98 | 0.54 | 0.00 | 6.67 | 73.36 | 275.50 |
| A8 | 38°28.905 | 21°20.780 | 19.18 | 65.25 | 15.57 | 6.07 | 2.76 | −0.10 | 1.08 | 5.10 | 3.30 | 0.75 | 0.00 | 6.22 | 171.20 | 567.04 |
| A9 | 38°28.997 | 21°20.616 | 35.11 | 55.62 | 9.28 | 5.02 | 2.66 | 0.07 | 0.90 | 3.01 | 2.27 | 0.48 | 0.00 | 6.36 | 65.83 | 242.35 |
| A10 | 38°29.177 | 21°20.276 | 54.18 | 39.85 | 5.96 | 3.90 | 2.69 | 0.24 | 1.01 | 2.88 | 2.13 | 0.41 | 0.00 | 6.26 | 46.52 | 165.31 |
| A11 | 38°29.365 | 21°20.135 | 54.38 | 37.12 | 8.50 | 3.92 | 3.10 | 0.27 | 0.84 | 4.40 | 2.83 | 0.53 | 0.50 | 6.73 | 72.22 | 267.60 |
| A12 | 38°29.468 | 21°19.926 | 33.46 | 57.53 | 9.01 | 4.95 | 2.82 | −0.04 | 0.95 | 6.97 | 4.04 | 0.87 | 1.30 | 6.88 | 107.60 | 514.46 |
| A13 | 38°29.713 | 21°19.607 | 58.25 | 34.63 | 7.12 | 3.50 | 3.10 | 0.29 | 0.84 | 6.50 | 3.59 | 0.72 | 2.70 | 7.07 | 47.19 | 228.39 |
| A14 | 38°30.037 | 21°18.714 | 28.83 | 58.03 | 13.14 | 5.42 | 2.91 | −0.03 | 0.95 | 2.16 | 1.59 | 0.39 | 0.00 | 6.38 | 13.18 | 95.65 |
| A15 | 38°29.884 | 21°18.598 | 51.15 | 40.20 | 8.65 | 3.89 | 3.24 | 0.16 | 0.79 | 6.09 | 3.12 | 0.71 | 1.90 | 7.31 | 48.41 | 339.78 |
| A16 | 38°29.633 | 21°18.219 | 52.35 | 40.34 | 7.31 | 3.81 | 2.98 | 0.19 | 0.86 | 3.19 | 0.66 | 0.36 | 11.60 | 7.59 | 9.19 | 77.67 |
| A17 | 38°29.435 | 21°17.983 | 23.23 | 62.98 | 13.78 | 5.76 | 2.86 | −0.04 | 1.11 | 8.91 | 2.50 | 0.52 | 41.50 | 7.41 | 13.32 | 138.37 |
| A18 | 38°29.155 | 21°17.939 | 33.46 | 56.40 | 10.13 | 5.06 | 2.95 | −0.06 | 0.96 | 7.41 | 2.24 | 0.51 | 31.50 | 7.53 | 15.80 | 174.28 |
| A19 | 38°28.816 | 21°18.004 | 13.31 | 68.58 | 18.11 | 6.59 | 2.75 | −0.14 | 1.38 | 5.71 | 3.39 | 0.60 | 8.20 | 7.43 | 60.70 | 671.33 |
| A20 | 38°28.710 | 21°18.173 | 51.27 | 38.64 | 10.09 | 3.93 | 3.39 | 0.20 | 0.70 | 8.63 | 3.22 | 0.64 | 0.00 | 7.38 | 6.68 | 131.24 |
| A21 | 38°28.083 | 21°18.332 | 12.64 | 66.78 | 20.58 | 6.44 | 2.45 | 0.11 | 0.81 | 3.94 | 2.58 | 0.52 | 0.00 | 4.85 | 10.81 | 198.20 |
| A22 | 38°27.327 | 21°20.035 | 63.72 | 28.76 | 7.52 | 3.37 | 3.01 | 0.50 | 0.86 | 4.00 | 3.40 | 0.52 | 2.30 | 6.80 | 18.78 | 349.40 |
| A23 | 38°27.303 | 21°19.660 | 59.02 | 34.70 | 6.28 | 3.56 | 2.60 | 0.32 | 1.06 | 3.46 | 2.32 | 0.46 | 0.00 | 5.70 | 44.54 | 647.61 |
| A24 | 38°27.448 | 21°19.699 | 73.65 | 21.44 | 4.91 | 2.64 | 2.54 | 0.50 | 1.20 | 2.63 | 1.90 | 0.39 | 0.00 | 5.85 | 17.35 | 353.73 |
| A25 | 38°27.414 | 21°19.426 | 63.57 | 30.24 | 6.19 | 3.25 | 2.82 | 0.36 | 1.00 | 2.62 | 1.56 | 0.38 | 0.00 | 5.59 | 5.25 | 150.68 |
| Min | 12.04 | 21.44 | 4.91 | 2.64 | 2.00 | −0.14 | 0.70 | 1.61 | 0.34 | 0.25 | 0.00 | 4.85 | 5.25 | 58.37 | ||
| Max | 73.65 | 77.68 | 20.58 | 6.59 | 3.39 | 0.50 | 1.38 | 8.91 | 4.04 | 0.87 | 41.50 | 7.59 | 171.20 | 671.33 | ||
| Sampling Stations | Coordinates | Grain Size Analysis (%) | Statistical Parameters (Φ) | TC (%) | TOC (%) | TN (%) | TS (%) | CaCO3 (%) | Cu-DTPA (mg/Kg) | Cu-Total (mg/Kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Longitude | Latitude | Sand | Silt | Clay | Mean | Sorting | Skewness | Kurtosis | ||||||||
| T1 | 38.451329° | 21.363612° | 10.29 | 77.68 | 12.04 | 5.90 | 2.00 | 0.18 | 1.02 | 5.24 | 4.51 | 0.77 | 1.73 | 2.40 | 0.23 | 45.96 |
| T2 | 38.464482° | 21.356034° | 9.44 | 57.99 | 32.57 | 5.24 | 2.49 | 0.10 | 0.83 | 5.01 | 4.87 | 0.90 | 1.93 | 0.00 | 0.28 | 54.20 |
| T3 | 38.466344° | 21.354113° | 9.53 | 58.87 | 31.59 | 5.27 | 2.50 | 0.06 | 0.86 | 3.60 | 4.72 | 0.80 | 1.81 | 0.00 | 0.21 | 51.80 |
| T4 | 38.467748° | 21.350825° | 10.04 | 64.06 | 25.89 | 5.49 | 2.48 | −0.01 | 0.92 | 3.84 | 4.05 | 0.60 | 2.08 | 0.00 | 0.49 | 53.35 |
| T5 | 38.470667° | 21.347412° | 10.77 | 66.62 | 22.61 | 5.73 | 2.34 | 0.02 | 0.87 | 3.84 | 3.90 | 0.66 | 1.58 | 1.20 | 0.43 | 61.61 |
| T6 | 38.473452° | 21.346091° | 12.66 | 73.81 | 13.53 | 6.14 | 2.26 | 0.04 | 1.06 | 1.61 | 3.71 | 0.70 | 1.65 | 7.10 | 0.14 | 43.85 |
| T7 | 38.479552° | 21.346429° | 12.66 | 64.36 | 22.98 | 5.72 | 2.73 | −0.08 | 1.03 | 3.89 | 2.42 | 0.55 | 1.16 | 0.00 | 0.60 | 71.87 |
| T8 | 38.481763° | 21.346259° | 15.57 | 65.25 | 19.18 | 6.07 | 2.76 | −0.10 | 1.08 | 5.10 | 2.78 | 0.62 | 0.94 | 0.00 | 0.31 | 49.17 |
| T9 | 38.483272° | 21.343522° | 9.28 | 55.62 | 35.11 | 5.02 | 2.66 | 0.07 | 0.90 | 3.01 | 4.18 | 0.75 | 1.13 | 0.00 | 0.35 | 64.30 |
| T10 | 38.486290° | 21.337759° | 5.96 | 39.85 | 54.18 | 3.90 | 2.69 | 0.24 | 1.01 | 2.88 | 3.98 | 0.78 | 1.15 | 0.00 | 0.36 | 63.15 |
| Min | 5.96 | 39.85 | 12.04 | 3.90 | 2.00 | −0.10 | 0.83 | 1.61 | 2.42 | 0.55 | 0.94 | 0.00 | 0.14 | 43.85 | ||
| Max | 15.57 | 77.68 | 54.18 | 6.14 | 2.76 | 0.24 | 1.08 | 5.24 | 4.87 | 0.90 | 2.08 | 7.10 | 0.60 | 71.87 | ||
| Average | 10.62 | 62.41 | 26.97 | 5.45 | 2.49 | 0.05 | 0.96 | 3.80 | 3.91 | 0.71 | 1.52 | 1.07 | 0.34 | 55.93 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramidis, P.; Barouchas, P.; Dünwald, T.; Unkel, I.; Panagiotaras, D. The Influence of Olive Orchards Copper-Based Fungicide Use, in Soils and Sediments—The Case of Aetoliko (Etoliko) Lagoon Western Greece. Geosciences 2019, 9, 267. https://doi.org/10.3390/geosciences9060267
Avramidis P, Barouchas P, Dünwald T, Unkel I, Panagiotaras D. The Influence of Olive Orchards Copper-Based Fungicide Use, in Soils and Sediments—The Case of Aetoliko (Etoliko) Lagoon Western Greece. Geosciences. 2019; 9(6):267. https://doi.org/10.3390/geosciences9060267
Chicago/Turabian StyleAvramidis, Pavlos, Pantelis Barouchas, Thomas Dünwald, Ingmar Unkel, and Dionisios Panagiotaras. 2019. "The Influence of Olive Orchards Copper-Based Fungicide Use, in Soils and Sediments—The Case of Aetoliko (Etoliko) Lagoon Western Greece" Geosciences 9, no. 6: 267. https://doi.org/10.3390/geosciences9060267
APA StyleAvramidis, P., Barouchas, P., Dünwald, T., Unkel, I., & Panagiotaras, D. (2019). The Influence of Olive Orchards Copper-Based Fungicide Use, in Soils and Sediments—The Case of Aetoliko (Etoliko) Lagoon Western Greece. Geosciences, 9(6), 267. https://doi.org/10.3390/geosciences9060267






