Understanding the Permafrost–Hydrate System and Associated Methane Releases in the East Siberian Arctic Shelf
Abstract
1. Introduction
2. Major Components of the ESAS Permafrost–Hydrate System
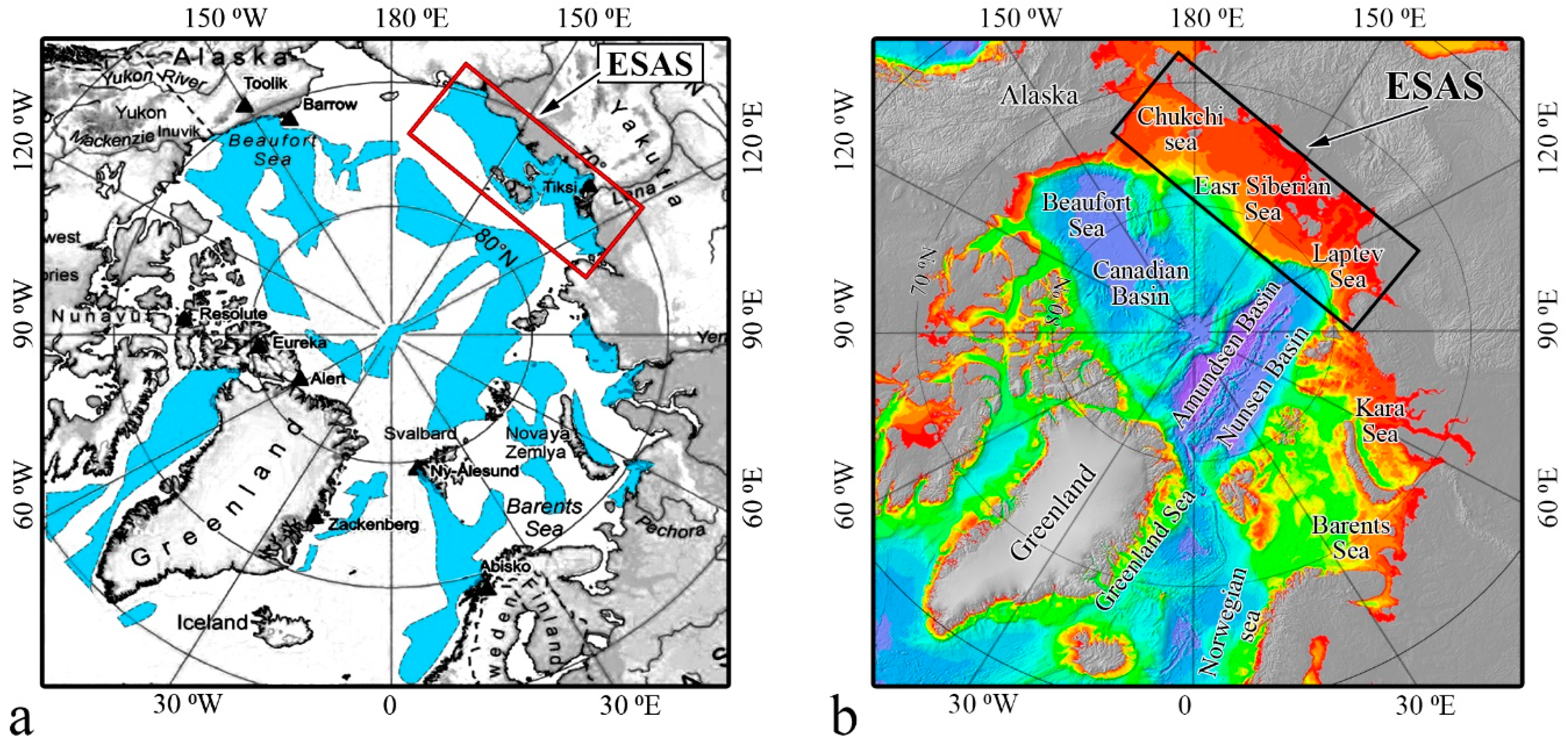
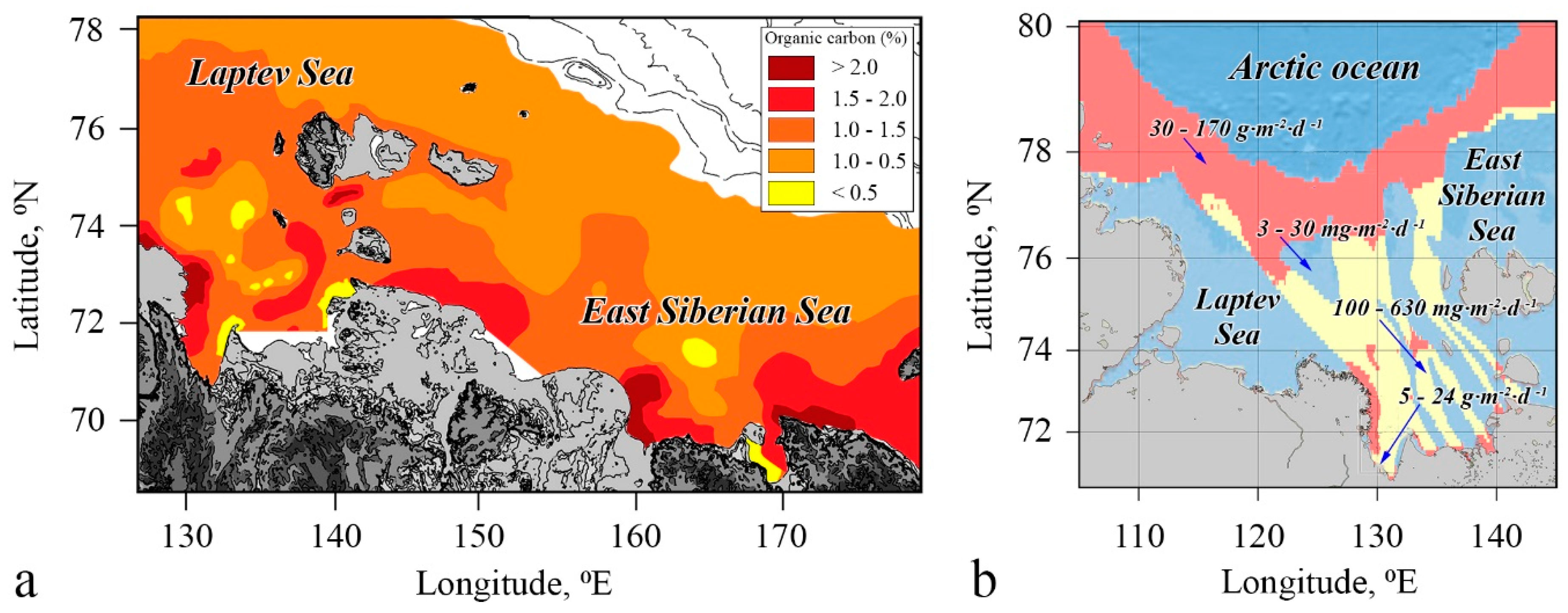
2.1. Specific Features of the ESAS Environment
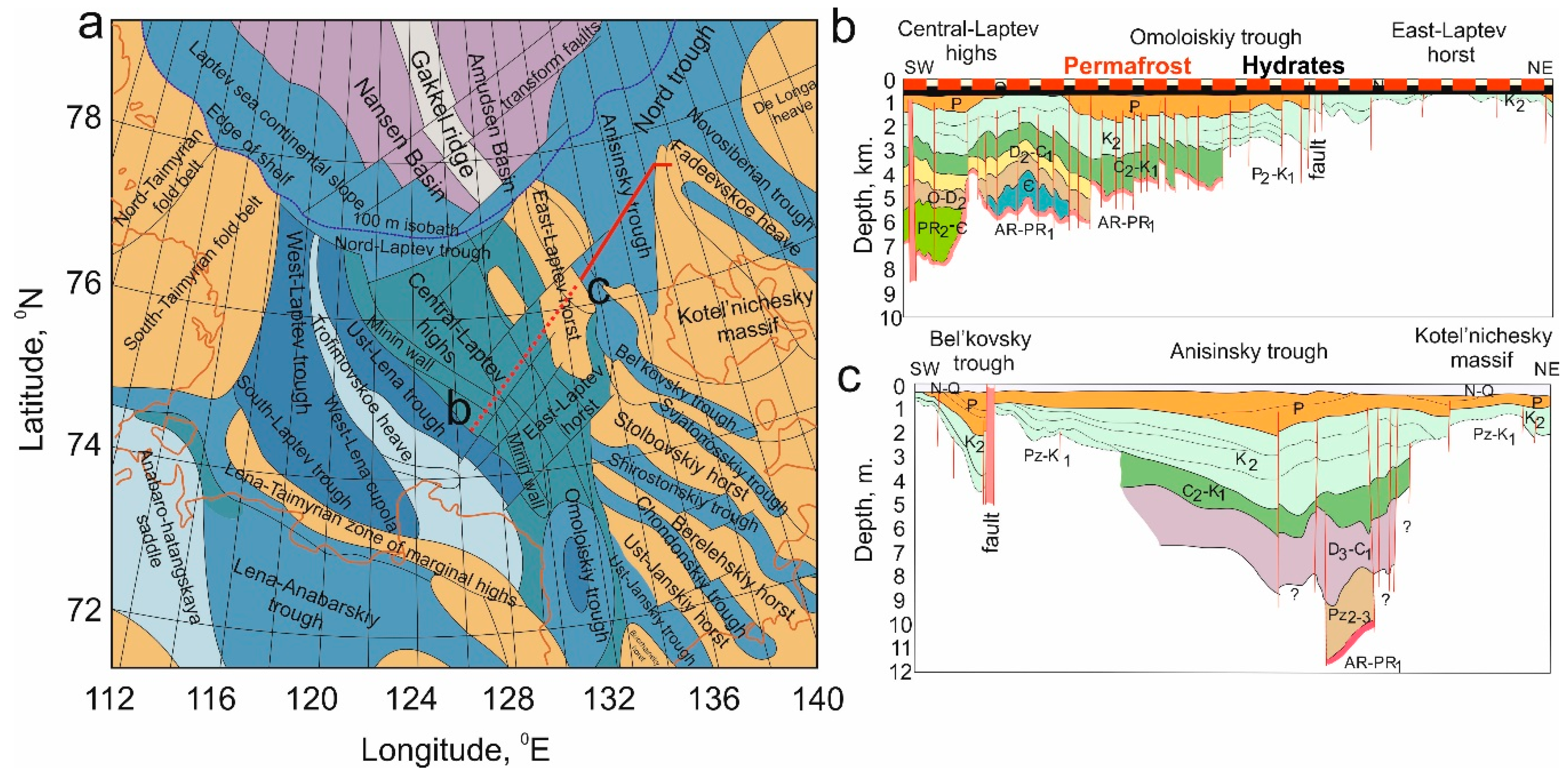
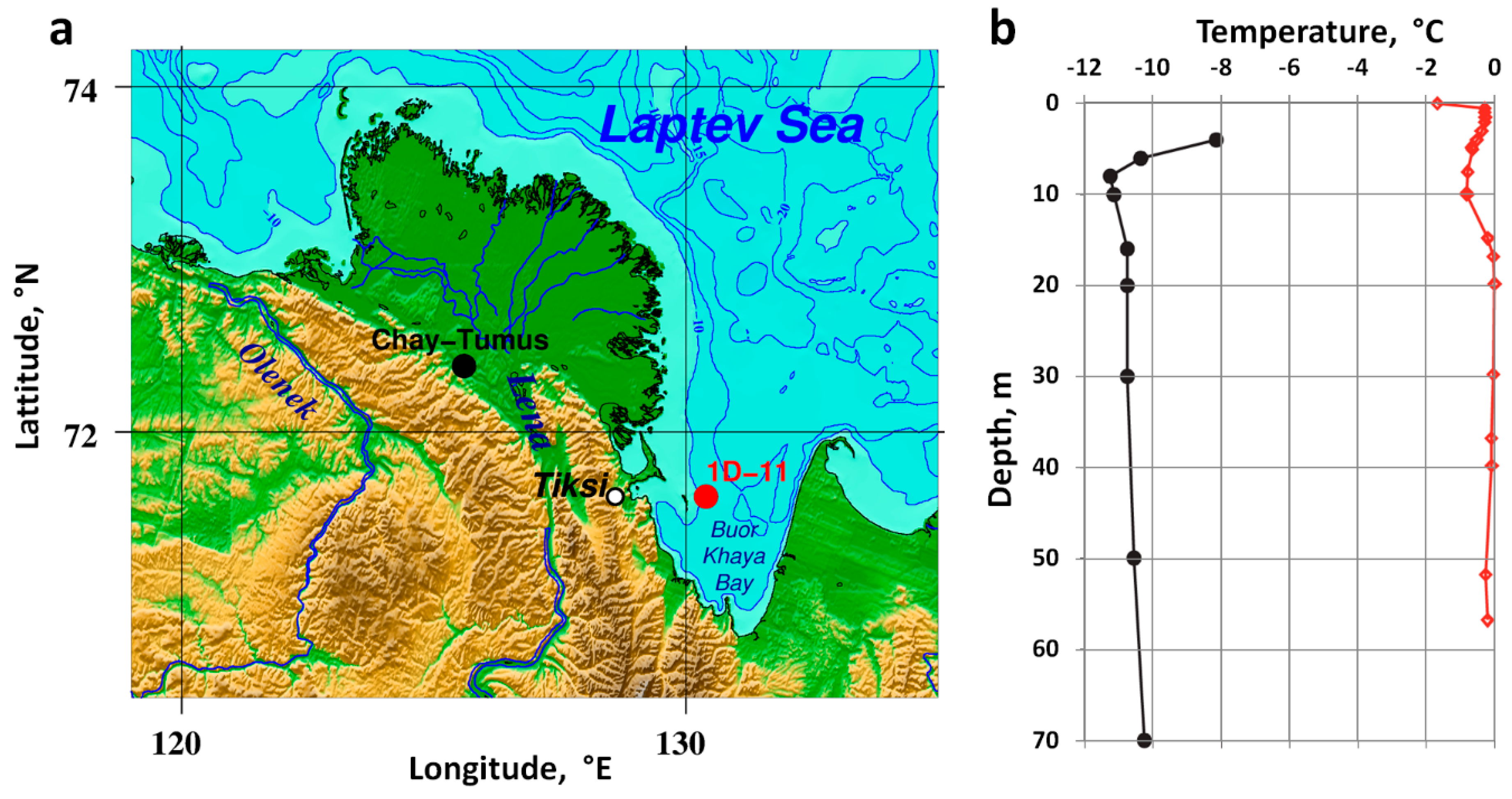
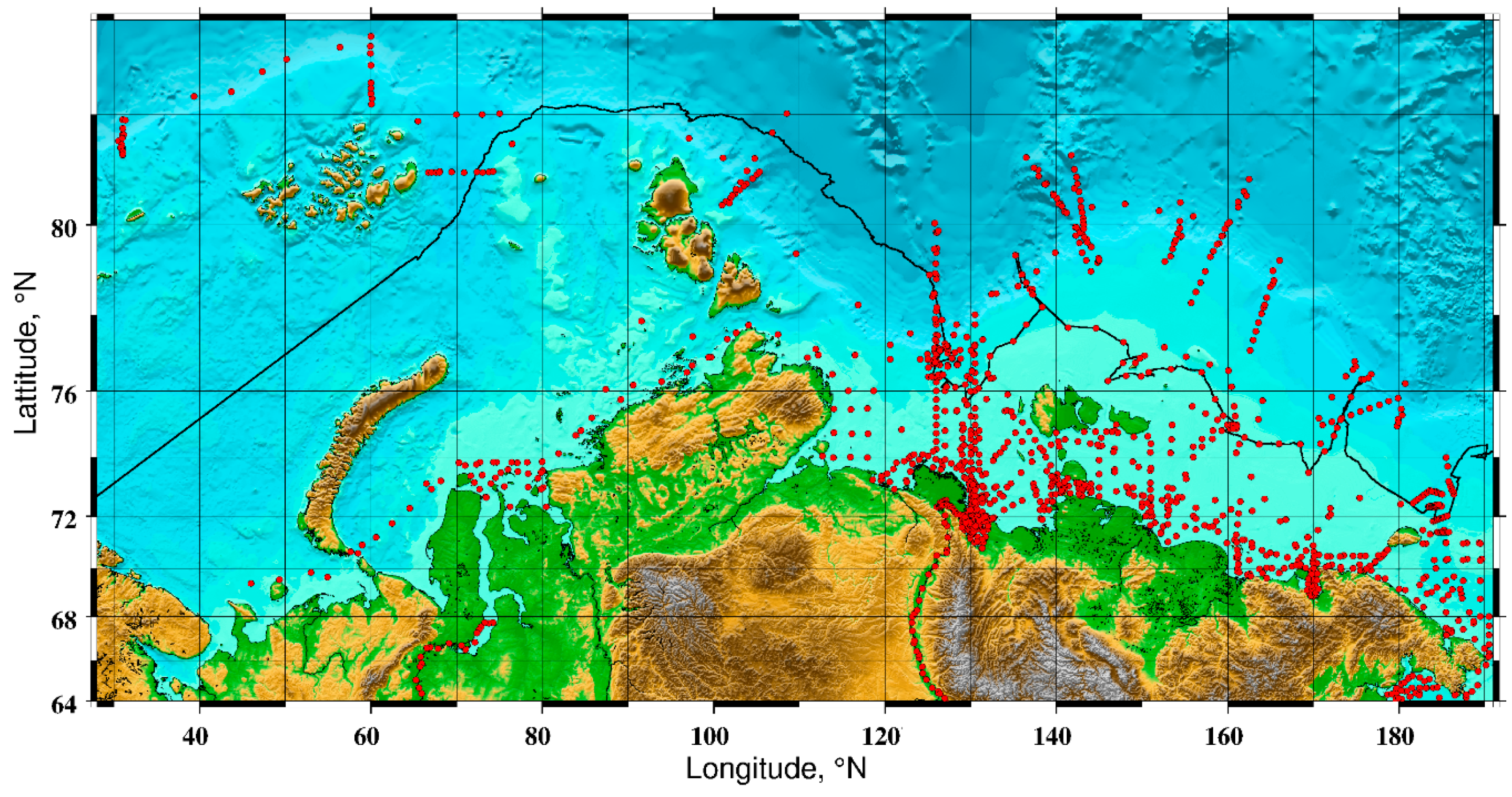
2.2. Current State of Subsea Permafrost
3. Arctic Hydrates in the ESAS
3.1. History of the Topic
3.2. Mechanism of Arctic Hydrate Origination
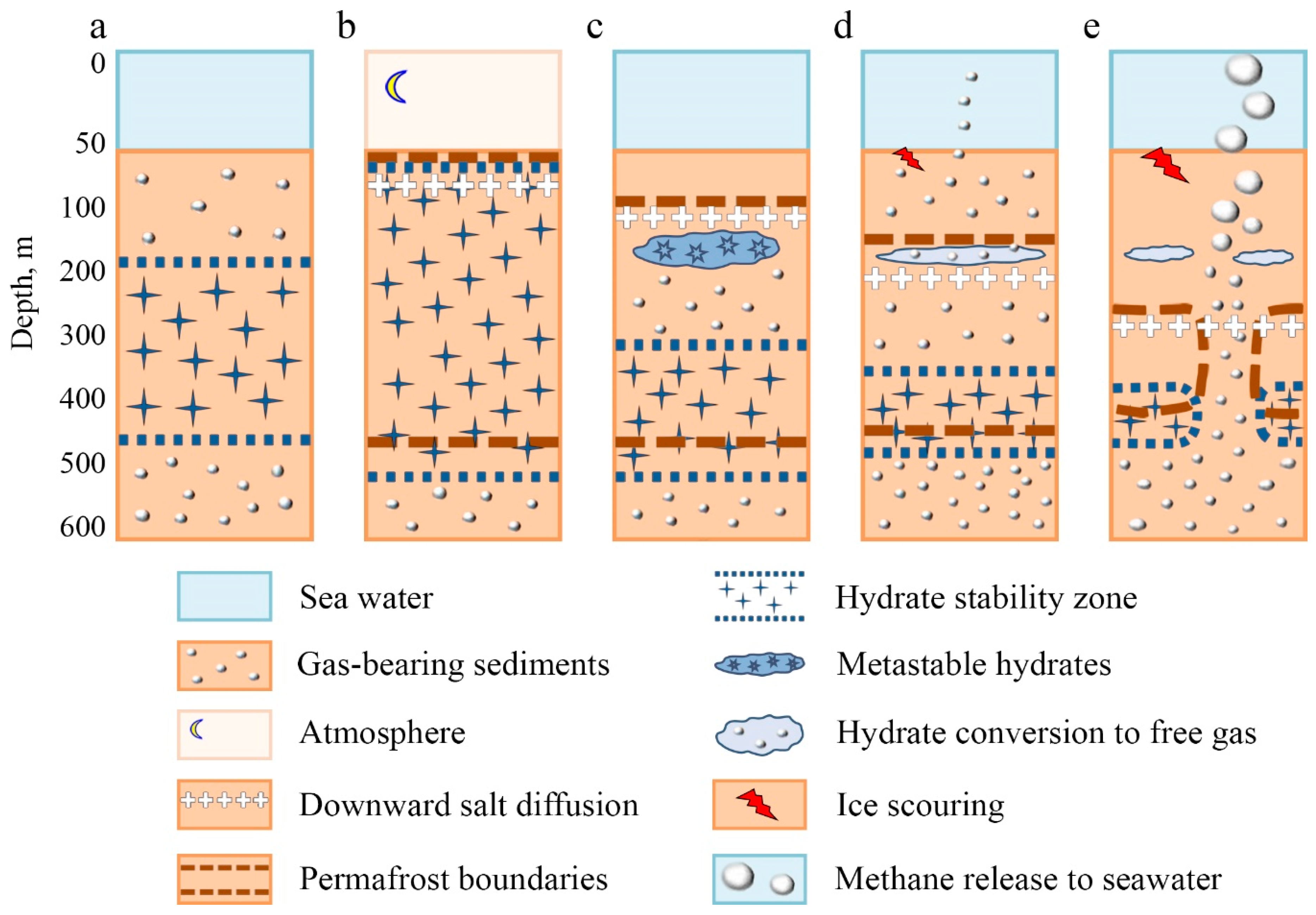
3.3. Principal Scheme of the Permafrost–Hydrates System
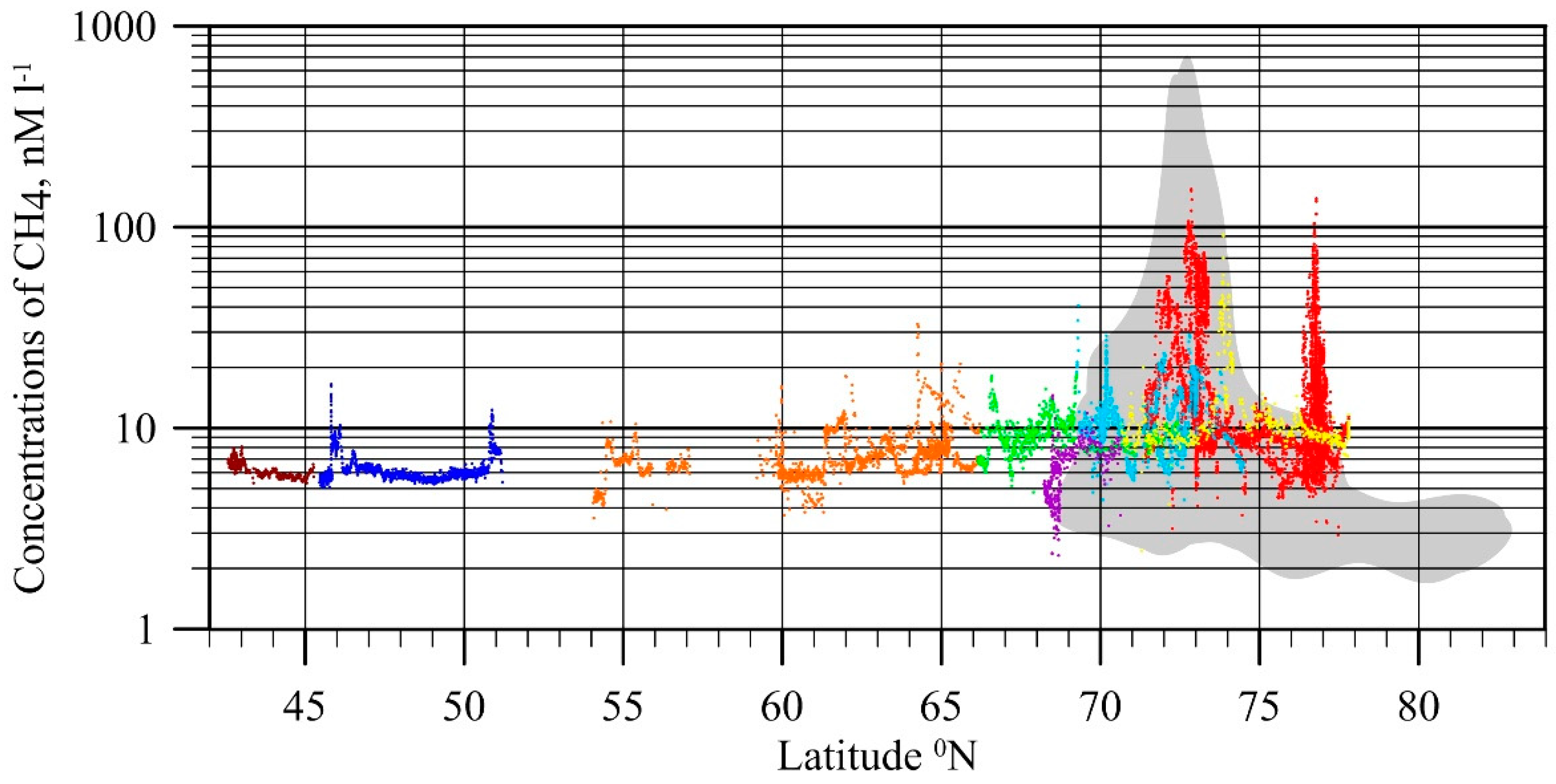
4. Specific Features of CH4 Releases in the ESAS (1994–2017)
4.1. Flux Assessment Based on Observational Data
4.2. Flux Attribution to Permafrost State and Source Contribution
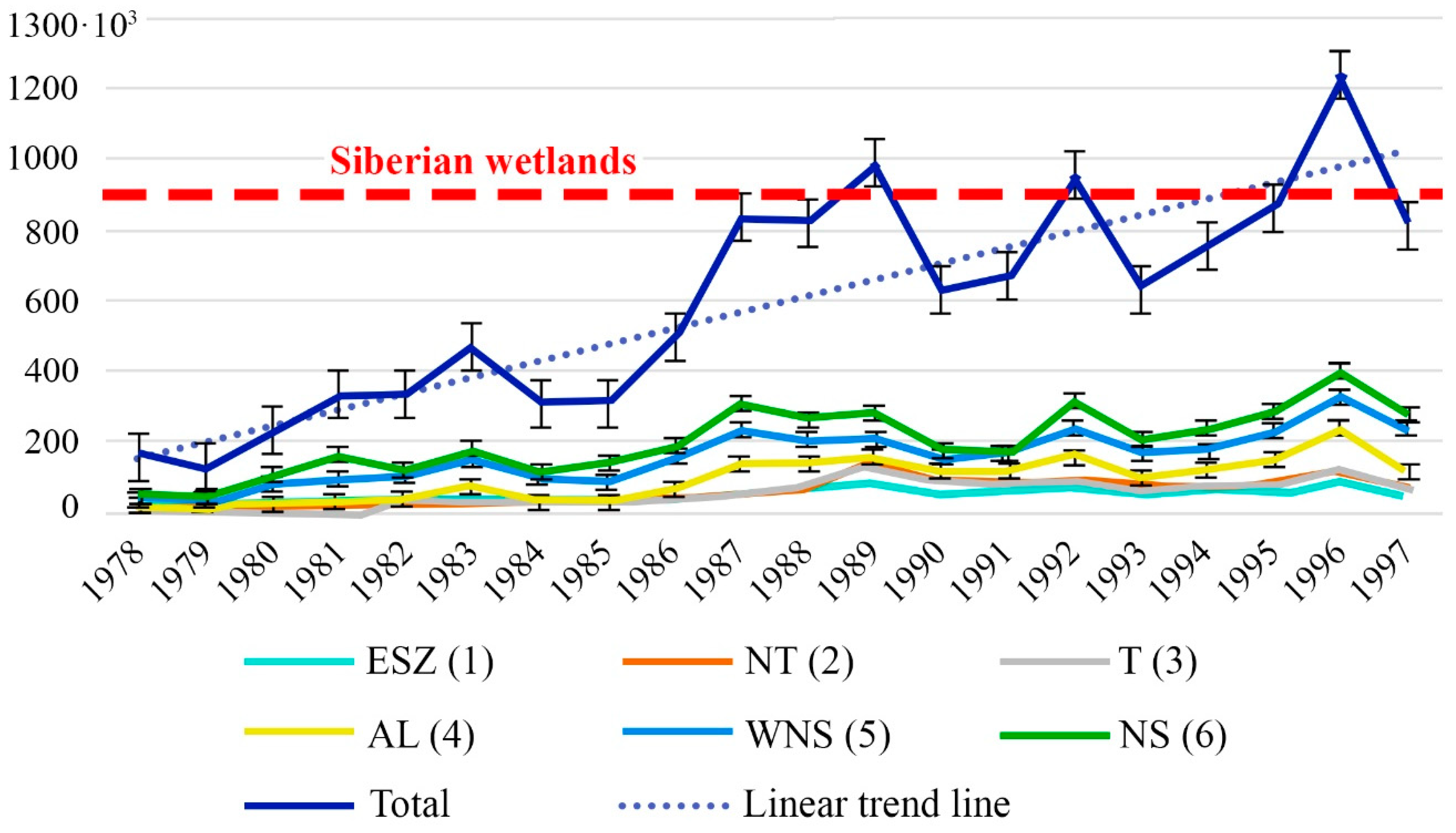
4.3. Factors Controlling CH4 Emissions
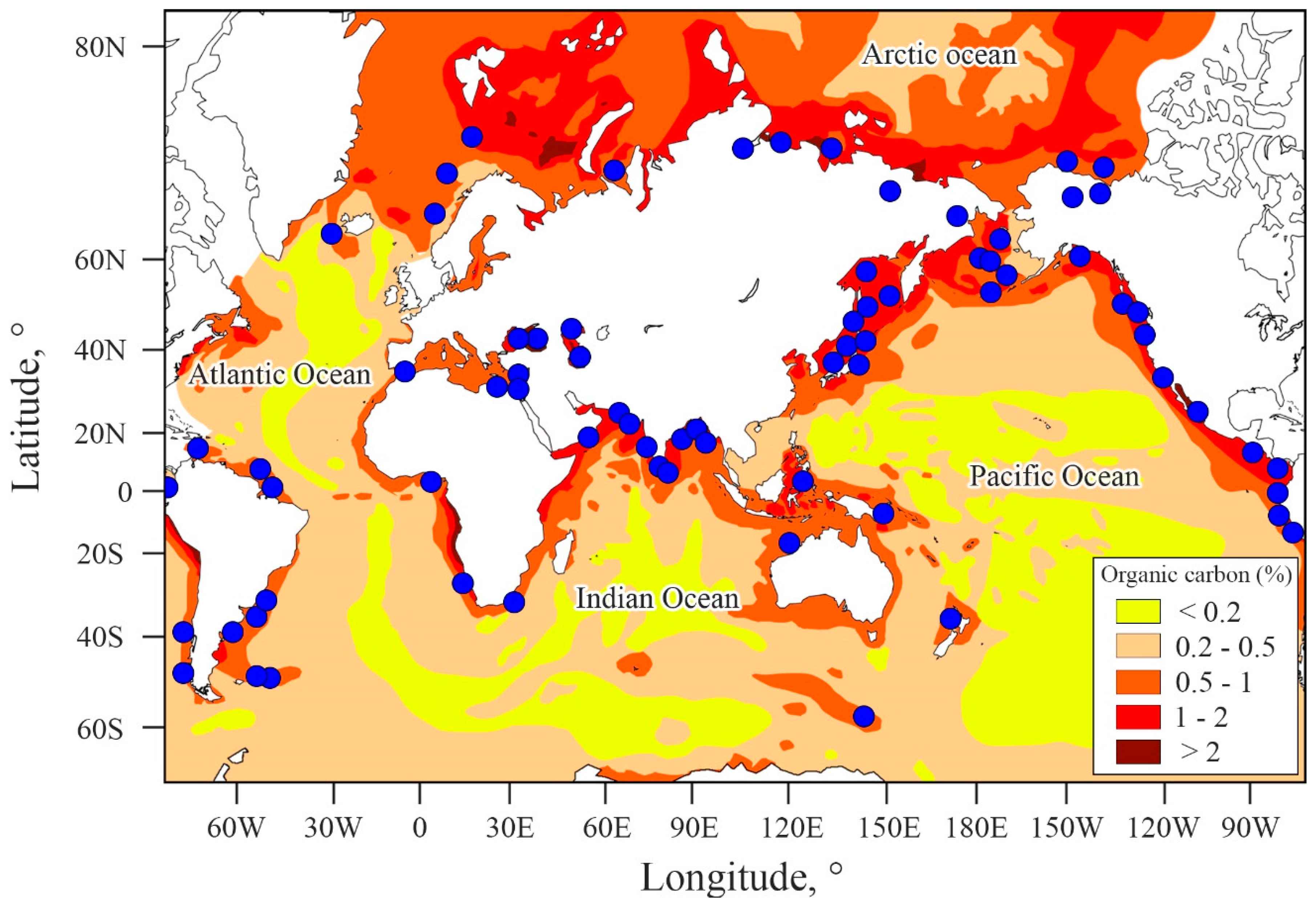
4.4. Contribution of the ESAS to Global Hydrate Pool
5. Discussion and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. The Scientific Basis; Cambridge University Press: New York, NY, USA, 2007; 640p. [Google Scholar]
- Gruber, N. Warming up, turning sour, losing breath: Ocean biogeochemistry under global change. Philos. Trans. R. Soc. A 2011, 369, 1980–1996. [Google Scholar] [CrossRef] [PubMed]
- Friedlingstein, P.; Cox, P.; Betts, R.; Bopp, L.; von Bloh, W.; Brovkin, V.; Cadule, P.; Doney, S.; Eby, M.; Fung, I.; et al. Climate–carbon cycle feedback analysis: Results from the C4MIP model intercomparison. J. Clim. 2006, 19, 3337–3353. [Google Scholar] [CrossRef]
- Koven, C.D.; Ringeval, B.; Friedlingstein, P.; Ciais, P.; Cadule, P.; Khvorostyanov, D.; Krinner, G.; Tarnocai, C. Permafrost carbon-climate feedbacks accelerate global warming. Proc. Natl. Acad. Sci. USA 2011, 108, 14769–14774. [Google Scholar] [CrossRef] [PubMed]
- Soloviev, V.A.; Ginzburg, G.D.; Telepnev, E.V.; Mikhaluk, Y.N. Cryothermia and Gas Hydrates in the Arctic Ocean; Sevmorgeologia: Leningrad, Russia, 1987; 150p, (Published in Russian). [Google Scholar]
- Kvenvolden, K.A. Methane hydrates and global climate. Glob. Biogeochem. Cycles 1988, 2, 221–229. [Google Scholar] [CrossRef]
- Thornton, B.F.; Wik, M.; Crill, P.M. Double-counting challenges the accuracy of high-latitude methane inventories. Geophys. Res. Lett. 2016, 43, 12569–12577. [Google Scholar] [CrossRef]
- Ruppel, C.D.; Kessler, J.D. The interaction of climate change and methane hydrates. Rev. Geophys. 2017, 55, 126–168. [Google Scholar] [CrossRef]
- Pohlman, J.W.; Greinert, J.; Ruppel, C.; Silyakova, A.; Vielstädte, L.; Casso, M.; Mienert, J.; Bünz, S. Enhanced CO2 uptake at a shallow Arctic Ocean seep field overwhelms the positive warming potential of emitted methane. Proc. Natl. Acad. Sci. USA 2017, 114, 5355–5360. [Google Scholar] [CrossRef]
- Gramberg, I.S.; Kulakov, Y.N.; Pogrebitskiy, Y.Y.; Sorokov, D.S. Arctic Oil and Gas Super Basin; X World Petroleum Congress: London, UK, 1983; pp. 93–99. [Google Scholar]
- Romanovskii, N.N.; Hubberten, H.-W.; Gavrilov, A.V.; Eliseeva, A.A.; Tipenko, G.S. Offshore permafrost and gas hydrate stability zone on the shelf of East Siberian Seas. Geo-Mar. Lett. 2005, 25, 167–182. [Google Scholar] [CrossRef]
- Romanovskii, N.N.; Hubberten, H.-W.; Gavrilov, A.V. Thermokarst and land-ocean interaction, Laptev Sea region, Russia. Permafr. Periglac. Process. 2000, 11, 137–152. [Google Scholar] [CrossRef]
- Hyndman, R.D.; Dallimore, S.R. Natural gas hydrates studies in Canada. Recorder 2001, 26, 11–20. [Google Scholar]
- Soloviev, V.A. Gas-hydrate-prone areas of the ocean and gas-hydrate accumulations. In Proceedings of the Sixth International Conference on Gas in Marine Sediments, St. Petersburg, Russia, 5–9 September 2000. [Google Scholar]
- Kvenvolden, K.A.; Lilley, M.D.; Lorenson, T.D.; Barnes, P.W.; McLaughlin, E. The Beaufort Sea continental shelf as a seasonal source of atmospheric methane. Geophys. Res. Lett. 1993, 20, 2459–2462. [Google Scholar] [CrossRef]
- Shakhova, N.; Semiletov, I.; Panteleev, G. The distribution of methane on the East Siberian Arctic shelves: Implications for the marine methane cycle. Geophys. Res. Lett. 2005, 32, L09601. [Google Scholar] [CrossRef]
- Damm, E.; Schauter, U.; Rudels, B.; Haas, C. Excess of bottom-released methane in an Arctic shelf sea polynya in winter. Cont. Shelf Res. 2007. [Google Scholar] [CrossRef]
- Westbrook, G.K.; Thatcher, K.E.; Rohling, E.J.; Piotrowski, A.M.; Pälike, H.; Osborne, A.H.; Nisbet, E.G.; Minshull, T.A.; Lanoisellé, M.; James, R.H.; et al. Escape of methane gas from the seabed along the West Spitsbergen continental margin. Geophys. Res. Lett. 2009, 36, L15608. [Google Scholar] [CrossRef]
- Shakhova, N.; Semiletov, I.; Salyuk, A.; Yusupov, V.; Kosmach, D.; Gustafsson, O. Extensive methane venting to the atmosphere from the sediments of the east Siberian Arctic Shelf. Science 2010, 327, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Shakhova, N.; Semiletov, I.; Leifer, I.; Sergienko, V.; Salyuk, A.; Kosmach, D.; Chernykh, D.; Stubbs, C.; Nicolsky, D.; Tumskoy, V.; et al. Ebullition and storm-induced methane release from the East Siberian Arctic Shelf. Nat. Geosci. 2014, 7. [Google Scholar] [CrossRef]
- Shakhova, N.; Semiletov, I.; Sergienko, V.; Lobkovsky, L.; Yusupov, V.; Salyuk, A.; Salomatin, A.; Chernykh, D.; Kosmach, D.; Panteleev, G.; et al. The East-Siberian Arctic Shelf: Towards further assessment of permafrost-related methane fluxes and role of sea ice. Philos. Trans. R. Soc. A 2015, 373, 20140451. [Google Scholar] [CrossRef] [PubMed]
- Shakhova, N.; Semiletov, I.; Gustafsson, O.; Sergienko, V.; Lobkovsky, L.; Dudarev, O.; Tumskoy, V.; Grigoriev, M.; Mazurov, A.; Salyuk, A.; et al. Current rates and mechanisms of subsea permafrost degradation in the East Siberian Arctic Shelf. Nat. Commun. 2017, 8, 15872. [Google Scholar] [CrossRef]
- Romanovskii, N.N.; Hubberten, H.-W. Results of permafrost modeling of the lowlands and shelf of the Laptev Sea region, Russia. Permafr. Periglac. Process. 2001, 12, 191–202. [Google Scholar] [CrossRef]
- Jakobsson, M. Hypsometry and volume of the Arctic Ocean and its constituent seas. Geochem. Geophys. Geosyst. 2002, 3, 1029. [Google Scholar] [CrossRef]
- Shakhova, N.; Semiletov, I. Methane release and coastal environment in the East Siberian Arctic shelf. J. Mar. Syst. 2007, 66, 227–243. [Google Scholar] [CrossRef]
- Delisle, G. Temporal variability of subsea permafrost and gas hydrate occurrences as a function of climate change in the Laptev Sea, Siberia. Polarforschung 2000, 68, 221–225. [Google Scholar]
- Semiletov, I.P. On aquatic sources and sinks of CO2 and CH4 in the Polar Regions. J. Atmos. Sci. 1999, 56, 286–306. [Google Scholar] [CrossRef]
- Overduin, P.P.; Hybberten, H.-W.; Rachold, V.; Romanovskii, N.; Grigoriev, M.; Kasymskaya, M. The evolution and degradation of coastal and offshore permafrost in the Laptev and East Siberian Seas during the last climate cycle. In Coastline Changes: Interrelation of Climate and Geological Processes; Harff, J., Hay, W.W., Tetzlaff, D.M., Eds.; Geological Society of America Special Paper; Geological Society of America: Boulder, CO, USA, 2007; Volume 426, pp. 97–111. [Google Scholar]
- Vonk, J.E.; Sánchez-García, L.; van Dongen, B.E.; Alling, V.; Kosmach, D.; Charkin, A.; Semiletov, I.P.; Dudarev, O.V.; Shakhova, N.; Roos, P.; et al. Activation of old carbon by erosion of coastal and subsea permafrost in Arctic Siberia. Nature 2012, 489, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Khain, V.E.; Filatova, N.I.; Polyakova, I.D. Tectonics, Geodynamics, and Gas-Oil Perspectives in the East-Arctic Seas and Their Continental Margin; Proceedings of the Geologocal Institute of the Russian Academy of Sciences, Nauka Publishing Co.: Moscow, Russia, 2009; 227p, (Published in Russian). [Google Scholar]
- Frenzel, B.; Pécsi, M.; Velichko, A. (Eds.) Atlas of Paleoclimates and Paleoenvironments of the Northern Hemisphere: Late Pleistocene-Holocene; Gustav Fischer: Stuttgart, Germany, 1992; 153p. [Google Scholar]
- Kim, B.; Grikurov, G.; Soloviev, V. High Resolution Seismic Studies in the Laptev Sea Shelf: First Results and Future Needs. In Land-Ocean System in the Siberian Arctic; Kassens, H., Bauch, H.A., Dmitrenko, I.A., Eicken, H., Hubberten, H.W., Melles, M., Thiede, J., Timokhov, L.A., Eds.; Springer: Berlin, Germany, 1999; pp. 683–692. [Google Scholar]
- Taylor, A.; Dallimore, S.; Outcalt, S. Late quaternary history of the Mackenzie-Beaufort region, arctic Canada, from modeling of permafrost temperatures. 1. The onshore–offshore transition. Can. J. Earth Sci. 1996, 33, 52–61. [Google Scholar] [CrossRef]
- Overduin, P.; Wetterich, S.; Günther, F.; Grigoriev, M.N.; Grosse, G.; Schirrmeister, L.; Hubberten, H.W.; Makarov, A.S. Coastal dynamics and submarine permafrost in shallow water of the central Laptev Sea, East Siberia. Cryosphere 2016, 10, 1449–1462. [Google Scholar] [CrossRef]
- Shakhova, N.E.; Nicolsky, D.; Semiletov, I.P. On the current state of sub-sea permafrost in the East-Siberian Shelf testing of modeling results by observational data. Trans. Russ. Acad. Sci. (Dokl. Earth Sci.) 2009, 429, 1518–1521, (translated in English by Springer). [Google Scholar] [CrossRef]
- Nicolsky, D.; Shakhova, N. Modeling sub-sea permafrost in the East-Siberian Arctic Shelf: The Dmitry Laptev Strait. Environ. Res. Lett. 2010, 5. [Google Scholar] [CrossRef]
- Nicolsky, D.; Romanovsky, V.; Romanovskii, N.; Kholodov, A.; Shakhova, N.; Semiletov, I. Modeling sub-sea permafrost in the East Siberian Arctic Shelf: The Laptev Sea region. J. Geophys. Res. 2012, 117, F03028. [Google Scholar] [CrossRef]
- Grigoriev, N.F. Mnogoletnemerzlie Porodi Primorskoy zoni Yakutii; Nauka: Moscow, Russia, 1986; 123p, (Published in Russian). [Google Scholar]
- Van Everdinger, R.O. Multi-Language Glossary of Permafrost and Related Ground-Ice Terms; International Permafrost Association, The Arctic Institute of North America, The University of Calgary: Calgary, AB, Canada, 2005. [Google Scholar]
- Grigoriev, M.N.; Razumov, S.O.; Kunitsky, V.V.; Spektor, V.B. Dynamics of the Russian East Arctic coast: Major factors, regularities and tendencies. Earth Cryosphere 2006, 10, 74–94. Available online: http://www/izdatgeo.ru/pdf/krio/2006-4/74 (accessed on 4 April 2019).
- Harrison, W.D.; Osterkamp, T.E. Heat and mass transport processes in subsea permafrost 1. An analysis of molecular diffusion and its consequences. J. Geophys. Res. 1978, 83, 4707–4712. [Google Scholar] [CrossRef]
- Baker, G.C.; Osterkamp, T.E. Implications of salt fingering processes for salt movement in thawed coarse-grained subsea permafrost. Cold Reg. Sci. Technol. 1988, 5, 45–52. [Google Scholar] [CrossRef]
- Baker, G.C.; Osterkamp, T.E. Salt redistribution during freezing of saline sand column at constant rates. Wat. Resour. Res. 1989, 25, 1825–1831. [Google Scholar] [CrossRef]
- Frederick, J.M.; Buffett, B.A. Taliks in relic submarine permafrost and methane hydrate deposits: Pathways for gas escape under present and future conditions. J. Geophys. Res. Earth Surf. 2014, 119. [Google Scholar] [CrossRef]
- Are, F.E. Shore face of the Arctic seas—A natural laboratory for subsea permafrost dynamics. In Permafrost; Phillips, M., Springman, S.M., Arenson, L.U., Eds.; Swets & Zeitlinger: Lisse, The Netherlands, 2003; pp. 27–32. [Google Scholar]
- Slagoda, E.A. Cryolithogenic Deposits of the Laptev Sea Coastal Lowland: Lithology and Micro-Morphology (Bykovsky Peninsula and Moustach Island); Express: Tyumen, Russia, 2004; 122p, (Published in Russian). [Google Scholar]
- Gunter, F.; Overduin, P.P.; Yakushina, I.A.; Opel, T.; Baranskaya, A.V.; Grigoriev, M.N. Observing Moustakh disappear: Permafrost thaw subsidence and erosion of a ground-ice-rich island in response to arctic summer warming and sea ice reduction. Cryosphere 2015, 9, 151–178. [Google Scholar] [CrossRef]
- Kim, G.; Lee, K.K.; Park, K.S.; Hwang, D.W.; Yang, H.S. Large submarine ground discharge (SGD) from volcanic island. Geophys. Res. Lett. 2003, 30, 2098. [Google Scholar] [CrossRef]
- Burnett, W.C.; Aggarwal, P.K.; Aureli, A.; Bokuniewicz, H.; Cable, J.E.; Charette, M.A.; Kontar, E.; Krupa, S.; Kulkarni, K.M.; Loveless, A.; et al. Quantifying submarine ground discharge in the coastal zone via multiple methods. Sci. Total Environ. 2006, 367, 498–543. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, P.I.; Melnikov, V.P.; Tsarev, V.P.; Degtiarev, B.V.; Mizulina, N.B.; Popov, A.P.; Berezniakov, A.I.; Svetchnikov, A.M. On hydrocarbon generation in permafrost places. Izvestiya AN SSSR (Proc. Sov. Acad. Sci. Geol. Sect) 1989, 2, 118–128. [Google Scholar]
- Dallimore, S.R.; Collett, T.S. Interpermafrost gas hydrates from a deep core hole in the Mackenzie Delta, Northwest Territories, Canada. Geology 1995, 23, 527–530. [Google Scholar] [CrossRef]
- Istomin, V.A. Gas hydrates in the permafrost zone. Gas Ind. Russ. 2006, 4, 16–27. [Google Scholar]
- Yakushev, V.S.; Collett, T.S. Gas hydrates in Arctic regions: Risk to drilling and production. In Proceedings of the Second International Offshore and Polar Engineering Conference, San-Francisco, CA, USA, 14–19 June 1992; Volume 1, pp. 669–673. [Google Scholar]
- Ravdonikas, O.V. Main Results of Hydrogeological Studies of Oil Regions in the Northern West Siberia; Proceedings of NIIGA; Gosgeoltekhizdat: Moscow, Russia, 1962; Volume 129, 194p, (Published in Russian). [Google Scholar]
- Yershov, E.D.; Lebedenko, Y.R.; Chuvilin, E.M.; Istomin, V.S. Features of gas hydrate occurrence in permafrost. USSR Acad. Sci. 1991, 321, 788–791. (in Russian). [Google Scholar]
- Yakushev, V.S.; Chuvilin, E.M. Natural gas and hydrate accumulation within permafrost in Russia. Cold Reg. Sci. Technol. 2000, 31, 189–197. [Google Scholar] [CrossRef]
- Istomin, V.A.; Kwon, V.G.; Rodger, P.M. Aspect of gas hydrates decomposition kinetics and their environmental impacts. Gas Ind. Russ. 2009, 113, 21–27. [Google Scholar]
- Dallimore, S.R.; Chuvilin, E.M.; Yakushev, V.S. 2001. Natural gas in permafrost of northern Canada and Russia: Genesis and forms of existence. In Proc. 2nd Conference of Russian Geocriological Scientists; Moscow State University Press: Moscow, Russia, 2001; Volume 3, pp. 289–294. [Google Scholar]
- Chuvilin, E.M.; Guryeva, O.M. Experimental study of self-preservation effect of gas hydrates in frozen sediments. In Proceedings of the 9th International Conference on Permafrost, Fairbanks, AK, USA, 28 June–3 July 2008; pp. 1–5. [Google Scholar]
- Handa, Y.P. Calorimetric determination of the composition, enthalpies of dissociation and heat capacity in the range 85 to 270 K for clathrate hydrates of xenon and krypton. J. Chem. Thermodyn. 1986, 18, 891–902. [Google Scholar] [CrossRef]
- Dallimore, S.R.; Chuvilin, E.M.; Yakushev, V.S.; Grechishev, V.S.; Ponomarev, E.S.; Pavlov, V.A. Field and laboratory chrakterization of interpermafrost gas hydrates, Mackenzie delta, N.W.T., Canada. In Proceedings of the 2nd International Conference on Natural Gas Hydrates, Toulouse, France, 2–6 June 1996; pp. 525–531. [Google Scholar]
- Chuvilin, E.M.; Yakushev, V.S.; Petrova, E.V. Gas and possible gas hydrates in the permafrost of Bovanenkovo Gas Field, Yamal Peninsula, West Siberia. Polarforschung 2000, 68, 215–219. [Google Scholar]
- Chuvilin, E.; Buchanov, B.; Davletshina, D.; Grebenkin, S.; Istomin, V. Dissociation and Self-Preservation of Gas Hydrates in Permafrost. Geosciences 2018, 8, 431. [Google Scholar] [CrossRef]
- Chuvilin, E.M.; Lupachik, M.V.; Guryeva, O.M. Kinetics research of ice transition into gas hydrates in porous media. In Physics and Chemistry of Ice; Furukawa, Y., Sazaki, G., Uchida, T., Watanabe, N., Eds.; Hokkaido University Press: Sapporo, Japan, 2011; pp. 127–132. [Google Scholar]
- Kwon, T.-H.; Cho, G.-C.; Santamarina, J.C. Gas hydrates dissociation in sediments: Pressure-temperature evolution. Geochem. Geophys. Geosyst. 2008, 9, Q03019. [Google Scholar] [CrossRef]
- Hachikubo, A.; Takeya, S.; Chuvilin, E.; Istomin, V. Preservation phenomena of methane hydrate in porous space. Phys. Chem. Phys. 2011, 13, 17449–17452. [Google Scholar] [CrossRef]
- Takeya, S.; Yoneyama, A.; Ueda, K.; Mimachi, H.; Takahashi, M.; Sano, K.; Hyodo, K.; Takeda, T.; Gotoh, Y. Anomalously preserved clathrate hydrate of natural gas in pellet form at 253 K. J. Phys. Chem. 2012, 116, 13842–13848. [Google Scholar] [CrossRef]
- Riedel, M.; Brent, T.; Taylor, G.; Taylor, A.E.; Hong, J.K.; Jin, Y.K.; Dallimore, S.R. Evidence for gas hydrate occurrences in the Canadian Arctic Beaufort Sea within permafrost-associated shelf and deep-water marine environments. J. Mar. Pet. Geol. 2017, 81, 66–78. [Google Scholar] [CrossRef]
- Chuvilin, E.; Bukhanov, B.; Cheverev, V.; Motenko, R.; Grechishcheva, E. Effect of ice and hydrate formation on thermal conductivity of sediments. In Impact of Thermal Conductivity on Energy Technologies; Shahzad, A., Ed.; IntechOpen: London, UK, 2018; pp. 115–132. [Google Scholar]
- Makogon, Y.F.; Holditch, S.A.; Makogon, T.Y. Natural gas-hydrates—A potential energy source for the 21st Century. J. Pet. Sci. Eng. 2007, 56, 14–31. [Google Scholar] [CrossRef]
- Fleming, K.; Johnston, P.; Zwartz, D.; Yokoyama, Y.; Lambeck, K.; Chappell, J. Refining the eustatic sea-level curve since the last glacial maximum using far- and intermediate-field sites. Earth Planet. Sci. Lett. 1998, 163, 327–342. [Google Scholar] [CrossRef]
- Gwiazda, R.; Paull, C.K.; Dallimore, S.R.; Melling, H.; Jin, Y.K.; Hong, J.K.; Riedel, M.; Lundsten, E.; Anderson, K.; Conway, K. Freshwater seepage into sediments of the shelf, shelf edge and continental slope of the Canadian Beaufort Sea. Geochem. Geophys. Geosyst. 2018. [Google Scholar] [CrossRef]
- Paull, C.K.; Ussler, W.; Dallimore, S.R.; Blasco, S.; Lorenson, T.D.; Melling, H.; Medioli, B.; Nixon, F.M.; McLaughlin, F.A. Origin of pingo-like features on the Beaufort Sea shelf and their possible relationship to decomposing methane gas hydrates. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef]
- Shakhova, N.; Sergienko, V.; Semiletov, I. Carbon Cycle in the East Siberian Arctic Seas; Part 1: Methane: Initial Results 1994–2010; Dal’nauka Press: Vladivostok, Russia, 2018; ISBN 978-5-8044-1682-0. 239p, (Published in Russian). [Google Scholar]
- Kosmach, D.; Sergienko, V.; Dudarev, O.; Kurilenko, A.; Gustafsson, O.; Semiletov, I. Methane in the surface waters of the Northern Eurasian seas. Dokl. Chem. 2015, 465, 281–285. [Google Scholar] [CrossRef]
- Sapart, C.J.; Shakhova, N.; Semiletov, I.; Jansen, J.; Szidat, S.; Kosmach, D.; Dudarev, O.; van der Veen, C.; Egger, M.; Sergienko, V.; et al. The origin of methane in the East Siberian Arctic Shelf unraveled with triple isotope analysis. Biogeosciences 2017, 14, 2283–2292. [Google Scholar] [CrossRef]
- Galimov, E.M. The causes of the global variations of carbon isotopic composition in the biosphere. Geochem. Int. 1999, 37, 699–713. [Google Scholar]
- Notz, D. The future of ice sheets and sea ice: Between reversible retreat and unstoppable loss. Proc. Natl. Acad. Sci. USA 2009, 106, 20590–20595. [Google Scholar] [CrossRef]
- Wadhams, P. Arctic ice cover, ice thickness and tipping points. Ambio 2012, 41, 23–33. [Google Scholar] [CrossRef]
- Bussmann, I.; Hackbusch, S.; Schaal, P.; Wichels, A. Methane distribution and oxidation around the Lena Delta in summer 2013. Biogeosciences 2017, 14, 4985–5002. [Google Scholar] [CrossRef]
- Kulakov, M.; Stanovoy, V.; Kirillov, S. Oceanography of the ESS, paper presented at ESSS Workshop. In Proceedings of the ESSS Workshop 2003, Malaga, Spain, 11–18 October 2003; International Arctic Research Center: Fairbanks, AK, USA, 2003. [Google Scholar]
- Smolyanitsky, V.; Karelin, I.; Karklin, V.; Ivanov, B. Sea ice of the Eastern Siberian Sea: Ice conditions, albedo, surface contamination and ice mass exchange. In Proceedings of the Oceanography of the ESS. Paper presented at ESSS Workshop 2003, Malaga, Spain, 11–18 October 2003; International Arctic Research Center: Fairbanks, AK, USA, 2003. [Google Scholar]
- Zakharov, J.M. 2003 Changes of the Arctic sea ice extent in XX century. Meteorol. Gidrol. 2003, 5, 75–86. [Google Scholar]
- Kremenetski, K.V.; Velichko, A.A.; Borisova, O.K.; MacDonald, G.M.; Smith, L.C.; Frey, K.E.; Orlova, L.A. 2003 Peatlands of the Western Siberian lowlands: Current knowledge on zonation, carbon content and Late Quaternary history. Quat. Sci. Rev. 2003, 22, 703–723. [Google Scholar] [CrossRef]
- Drachev, S.S.; Kaul, N.; Beliaev, V.N. Eurasia spreading basin to Laptev Shelf transition: Structural pattern and heat flow. Geophys. J. Int. 2003, 152, 688–698. [Google Scholar] [CrossRef]
- Cramer, B.; Franke, D. Indications for an active petroleum system in the Laptev Sea, NE Siberia. J. Pet. Geol. 2005, 28, 369–384. [Google Scholar] [CrossRef]
- Ruppel, C. Permafrost-Associated Gas Hydrate: Is It Really Approximately 1% of the Global System? J. Chem. Eng. Data 2015, 60, 429–436. [Google Scholar] [CrossRef]
- Vetrov, A.A.; Romankevich, E.A. Carbon Cycle in the Russian Arctic Seas; Springler: Berlin/Heidelberg, Germany, 2004; 233p. [Google Scholar]
- Ginsburg, G.D.; Soloviev, V.A. Submarine Gas Hydrates; VNllOkeangeologia: St. Petersburg, Russia, 1994; 199p, (Published in Russian). [Google Scholar]
- Shakhova, N.; Semiletov, I.; Leifer, I.; Rekant, P.; Salyuk, A.; Kosmach, D. Geochemical and geophysical evidence of methane release from the inner East Siberian Shelf. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef]
- Hinz, K.; Delisle, G.; Block, M. Seismic evidence for depth extent of permafrost in shelf sediments of the Laptev Sea, Russian Arctic. In Proceedings of the Seventh International Conference on Permafrost, Yellowknife, NT, Canada, 23–27 June 1998; Collection Nordicana No. 55. pp. 453–457. [Google Scholar]
- Kleiber, H.; Niessen, F. Late Pleistocene paleorivers channels on the Laptev Sea Shelf—Implications from sub-bottom profiling. In Land-Ocean System in the Siberian Arctic; Kassens, H., Bauch, H.A., Dmitrenko, I.A., Eicken, H., Hubberten, H.W., Melles, M., Thiede, J., Timokhov, L.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 657–665. [Google Scholar]
- Taylor, D.I. Near-shore shallow gas around the UK coast. Cont. Shelf Res. 1992, 12, 1135–1144. [Google Scholar] [CrossRef]
- Bellefleur, G.; Riedel, M.; Huang, J.; Saeki, T.; Milkereit, B.; Ramachandran, K. Seismic characterization of gas hydrate accumulations in a permafrost environment: Lessons learned from Mallik, Northwest Territories, Canada. In Scientific Results from the JOGMEC/NRCan/Aurora Mallik 2007–2008 Gas Hydrate Production Research Well Program, Mackenzie Delta, Northest Territories, Canada; Dallimore, S.R., Yamamoto, K., Wright, J.F., Bellefleur, G., Eds.; Geological Survey of Canada Bulletin; Geological Survey of Canada: Ottawa, ON, Canada, 2012; Volume 601, pp. 1–17. [Google Scholar]
- Hauck, C.; Bach, M.; Hilbich, C. A 4-phase model to quantify subsurface ice and water content in permafrost regions based on geo-physical datasets. In Proceedings of the Ninth International Conference on Permafrost; Kane, D.L., Hinkel, K.M., Eds.; Institute of Northern Engineering, University Alaska: Fairbanks, AK, USA, 2008; Volume 1, pp. 675–680. [Google Scholar]
- Overduin, P.P.; Liebner, S.; Knoblauch, C.; Günther, F.; Wetterich, S.; Schirrmeister, L.; Hubberten, H.-W.; Grigoriev, M.N. Methane oxidation following submarine permafrost degradation: Measurements from a central Laptev Sea shelf borehole. J. Geophys. Res. Bbogeosci. 2015, 120, 965–978. [Google Scholar] [CrossRef]
- Rivkina, E.; Shcherbakova, V.; Laurinavichius, K.; Petrovskaya, L.; Krivushin, K.; Kraev, G.; Pecheritsina, S.; Gilichinsky, D. Biogeochemistry of methane and methanogenic archaea in Permafrost. FEMS Microbiol. Ecol. 2007, 61, 1–15. [Google Scholar] [CrossRef]
- Reeburgh, W.S. Oceanic methane biogeochemistry. Chem. Rev. 2007, 107, 486–513. [Google Scholar] [CrossRef]
- Sasakawa, M.; Tsunogai, U.; Kameyama, S.; Nakagawa, F.; Nojiri, Y.; Tsuda, A. Carbon isotopic characterization for the origin of excess methane in subsurface seawater. J. Geophys. Res. 2008, 113, CO3012. [Google Scholar] [CrossRef]
- Thornton, B.F.; Geibel, M.C.; Crill, P.M.; Humborg, C.; Mörth, C.-M. Methane fluxes from the sea to the atmosphere across the Siberian shelf seas. Geophys. Res. Lett. 2016, 43, 5869–5877. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakhova, N.; Semiletov, I.; Chuvilin, E. Understanding the Permafrost–Hydrate System and Associated Methane Releases in the East Siberian Arctic Shelf. Geosciences 2019, 9, 251. https://doi.org/10.3390/geosciences9060251
Shakhova N, Semiletov I, Chuvilin E. Understanding the Permafrost–Hydrate System and Associated Methane Releases in the East Siberian Arctic Shelf. Geosciences. 2019; 9(6):251. https://doi.org/10.3390/geosciences9060251
Chicago/Turabian StyleShakhova, Natalia, Igor Semiletov, and Evgeny Chuvilin. 2019. "Understanding the Permafrost–Hydrate System and Associated Methane Releases in the East Siberian Arctic Shelf" Geosciences 9, no. 6: 251. https://doi.org/10.3390/geosciences9060251
APA StyleShakhova, N., Semiletov, I., & Chuvilin, E. (2019). Understanding the Permafrost–Hydrate System and Associated Methane Releases in the East Siberian Arctic Shelf. Geosciences, 9(6), 251. https://doi.org/10.3390/geosciences9060251





