Using Cilioplankton as an Indicator of the Ecological Condition of Aquatic Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
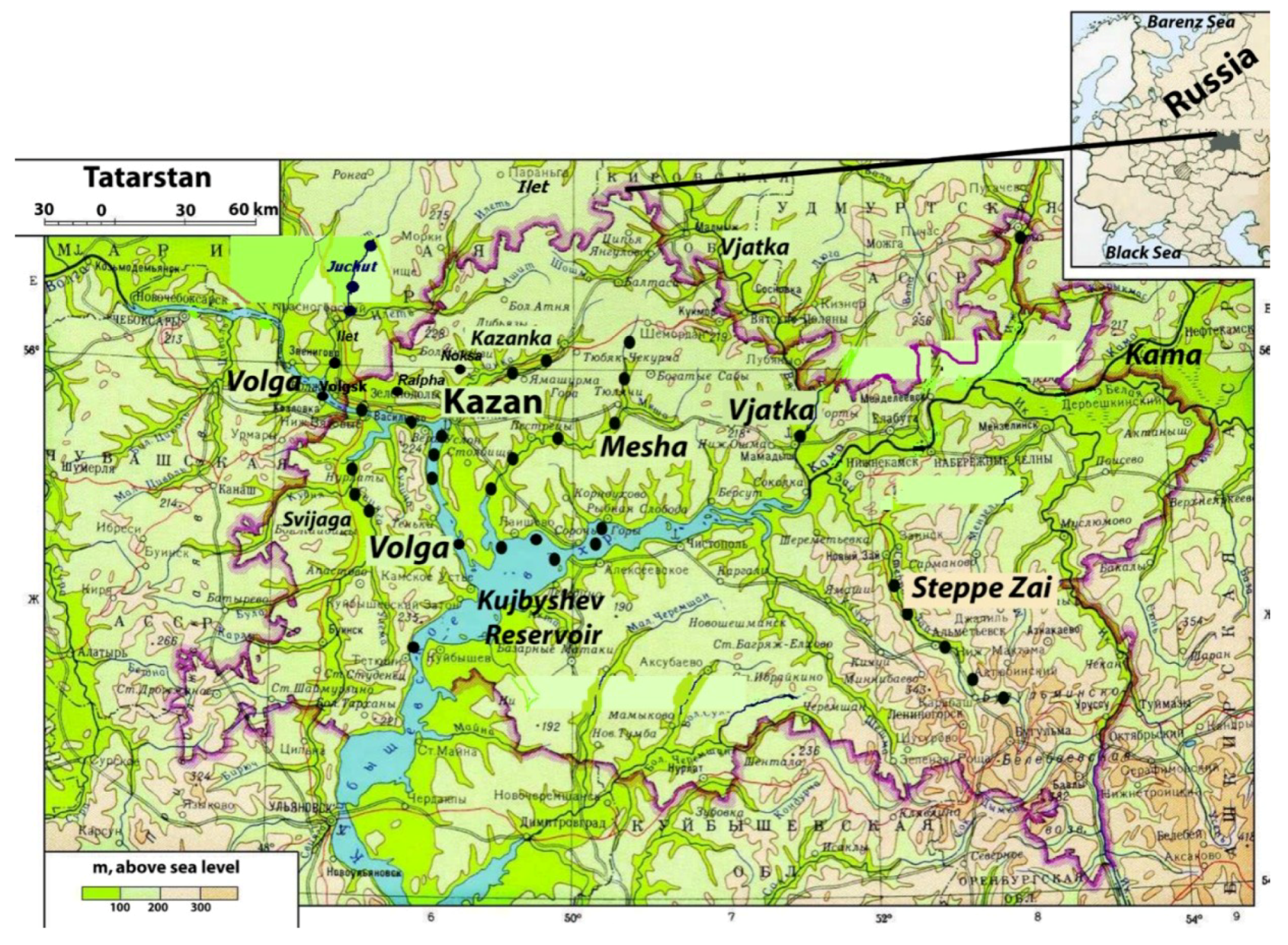
2.1. Study Area
2.2. Sampling and Analysis Methods
2.3. Data Analysis Methods
3. Results
3.1. Hydrochemical Features of the Objects of Study
3.2. Features of the Development of Ciliates
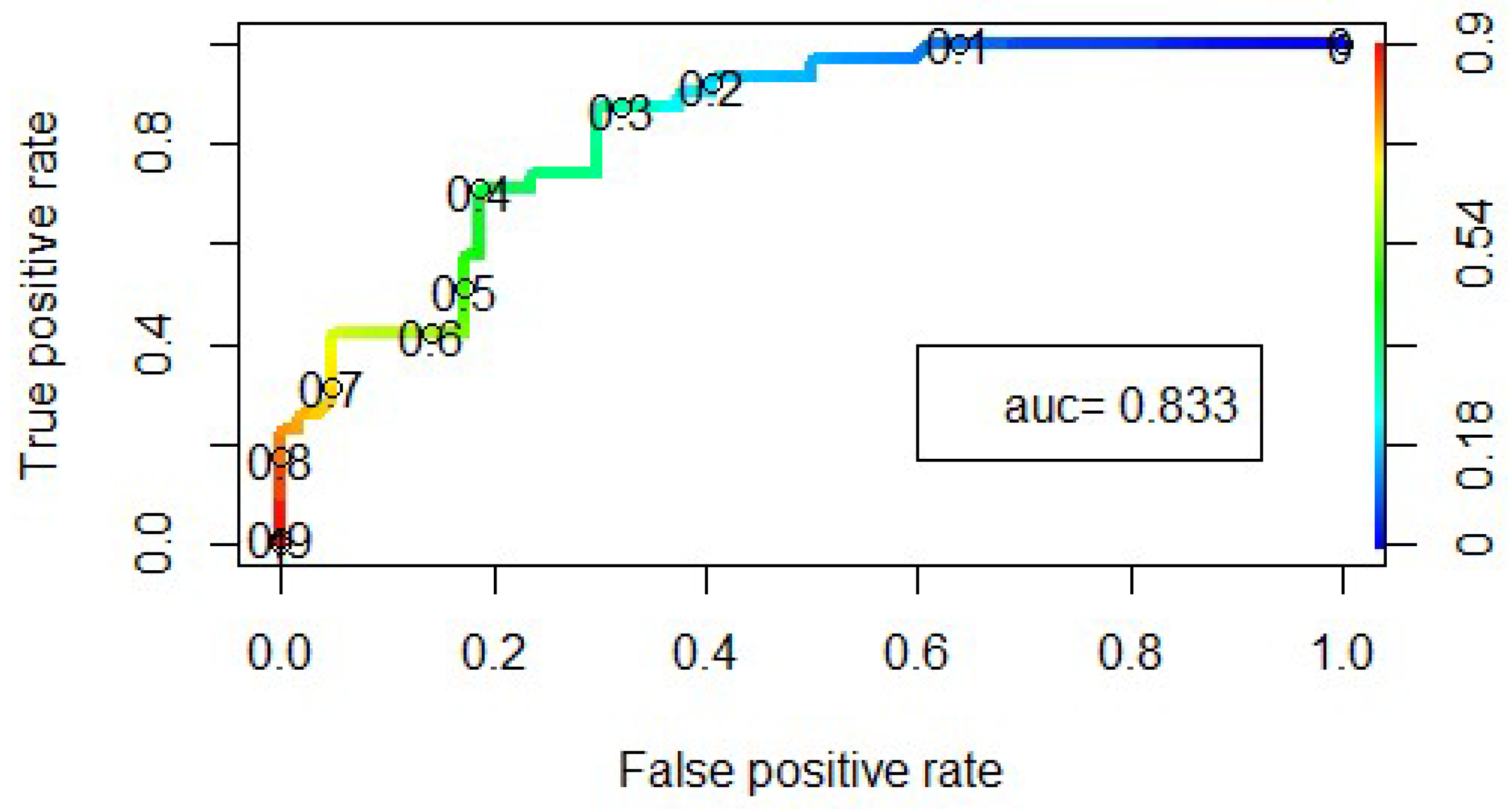
3.3. Statistical Analysis Logistic Regression Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Water Body | Ilet | Yushut | Kazanka | Svijaga | Mesha | Steppe Zai |
|---|---|---|---|---|---|---|
| pH | 7.53–8.03 7.7 ± 0.1 | 7.36–8.0 7.63 ± 0.08 | 7.0–8.6 7.8 ± 0.3 | 7.5–8.2 8.1 ± 0.7 | 7.35–8.35 8.0 ± 0.4 | 7.3–8.5 7.9 ± 0.06 |
| O2 | 7.26–12.4 9.15 ± 2.1 | 6.18–10.67 8.64 ± 0.49 | 7.5–14.57 9.5 ± 3.6 | 10.2–13.1 10.9 ± 1.7 | 6.79–8.14 8.0 ± 1.1 | 2.63–12.53 8.9 ± 0.1 |
| Cl− | 7.09–11.7 10.5 ± 1.1 | 0.0–9.0 6.8 ± 1.2 | 15.1–53.2 9.2 ± 3.2 | 10.0–19.9 24.4 ± 5.7 | 0.0–10.5 11.11 ± 1.36 | 15.2–413.0 156.7 ± 7.2 |
| SO42− | 134.5–694.0 455.8 ± 100.37 | 19.0–619.4 101.3 ± 64.9 | 59.3–835.7 536.1 ± 247.2 | 65.0–200.0 125.50 ± 12.56 | 62.4–192.1 120.8 ± 66.2 | 3.3–345.8 129.9 ± 4.2 |
| Mineralization | 444.1–1135.3 955.1 ± 129.9 | 162.4–251.4 271.8 ± 61.7 | 351.3–1456.7 1082.8 ± 96.4 | 478.0–630.0 659.5 ± 65.9 | 245.0–810.5 460.9 ± 80.8 | 429.2–1479.9 770.8 ± 42.4 |
| Hardness | 11.1–18.0 14.5 ± 1.1 | 2.8–12.8 6.56 ± 1.66 | 3.9–93.0 14.6 ± 4.5 | 3.0–4.0 8.4 ± 1.1 | 3.0–9.3 5.7 ± 1.1 | 4.6–14.3 9.2 ± 0.5 |
| HCO3− | 192.2–281.9 336.3 ± 15.9 | 115.9–149.5 139.65 ± 4.98 | 34.9–350.5 240.4 ± 63.1 | 278.2–366.1 247.8 ± 114.7 | 134.2–363.1 218.9 ± 17.6 | 68.7–497.3 326.9 ± 8.9 |
| COD | 10.6–12.1 12.1 ± 0.6 | 11.0–33.3 22.43 ± 2.38 | 11.0–39.8 23.4 ± 0.6 | 1.4–28.6 16.6 ± 2.4 | 8.1–19.1 13.3 ± 1.1 | 8.7–42.0 24.1 ± 0.5 |
| BOD5 | 0.42–1.53 1.1 ± 0.2 | 0.65–2.09 1.26 ± 0.16 | 1.7–7.9 3.74 ± 0.12 | 1.7–3.2 2.5 ± 0.1 | 1.0–1.79 1.1 ± 0.1 | 0.29–7.04 2.22 ± 0.9 |
| N-NH4+ | 0.05–0.17 0.10 ± 0.01 | 0.16–0.33 0.19 ± 0.02 | 0.0–3.8 0.351 ± 0.05 | 0.1–0.6 0.4 ± 0.06 | 0.0–0.48 0.06 ± 0.14 | 0.0–4.8 0.78 ± 0.06 |
| N-NO2− | 0.0–0.01 0.01 ± 0.0 | 0.01–0.02 0.01 ± 0.0 | 0.0–0.05 0.02 ± 0.0 | 0.0–0.2 0.1 ± 0.02 | 0.01–0.19 0.081 ± 0.088 | 0.0–0.7 0.12 ± 0.01 |
| N-NO3− | 0.17–2.69 1.160.55 | 0.01–3.28 0.53 ± 0.28 | 0.0–1.43 0.37 ± 0.04 | 0.4–7.4 4.0 ± 0.8 | 0.07–1.93 1.04 ± 0.13 | 0.06–10.04 2.67 ± 0.13 |
| PO43− | 0.0–0.12 0.04 ± 0.02 | 0.013–0.12 0.04 ± 0.01 | 0.0–0.1 0.02 ± 0.0 | 0.1–0.6 0.3 ± 0.06 | 0.0–0.19 0.05±0.02 | 0.0–1.65 0.24 ± 0.02 |
| Fe | 0.06–0.28 0.13 ± 0.04 | 0.07–0.51 0.24 ± 0.06 | 0.0–0.36 0.05 ± 0.01 | 0.0–0.2 0.10 ± 0.03 | 0.0–0.27 0.02 ± 0.02 | 0.01–1.1 0.05 ± 0.0 |
| Cu* | 4.6–8.3 6.12 ± 0.75 | 0.1–2.7 1.0 ± 0.37 | 0.0–22.5 4.07 ± 0.37 | 2.0–7.0 3.4 ± 0.58 | 0.0–6.3 2.2 ± 1.21 | 0.0–9.7 2.24 ± 0.09 |
| Mn* | 6.2–14.0 8.56 ± 1.41 | 7.7–126.0 41.9 ± 11.7 | 2.4–3.5 2.7 ± 0.27 | 12.1–171.7 84.8 ± 16.7 | 1.4–94.0 14.3 ± 7.4 | 3.7–142.8 28.2 ± 3.9 |
| TPH | 0.0–0.09 0.04 ± 0.02 | 0.004–0.021 0.016 ± 0.00 | 0.0–0.27 0.09 ± 0.01 | 0.0–0.6 0.2 ± 0.07 | 0.0–0.04 0.01 ± 0.0 | 0.0–0.09 0.03 ± 0.0 |
| Phenols | 0.0–0.0 0.0 ± 0.0 | 0.0–0.001 0.0 ± 0.0 | 0.0–0.004 0.001 ± 0.00 | 0.0–0.0 0.0 ± 0.0 | 0.0–0.0 0.0 ± 0.0 | 0.0–0.005 0.0 ± 0.0 |
| SCIWP | 2.66 | 2.73 | 4.4 | 3.64 | 3.6 | 4.1 |
| Water quality | Polluted | Polluted | Dirty | Highly polluted | Highly polluted | Dirty |
| Water Body | Vjatka | Noksa | Raifa Lake | Kujbyshev Reservoir | MPPM |
|---|---|---|---|---|---|
| pH | 7.71–8.47 8.05 ± 0.09 | 7.27–8.25 7.80 ± 0.14 | 7.3–9.2 8.29 ± 0.17 | 6.5–8.65 7.7 ± 0.1 | 7.05–7.64 7.29 ± 0.07 |
| O2 | 6.57–13.59 9.68 ± 0.3 | 4.44–11.38 7.89 ± 1.19 | 6.3–13.8 8.67 ± 0.64 | 5.59–13.6 9.7 ± 0.6 | 0.0–3.05 0.95 ± 0.43 |
| Cl− | 1.1–56.7 8.32 ± 1.32 | 33.2–51.1 43.87 ± 3.06 | 10.0–17.3 11.9 ± 0.82 | 9.2–78.7 22.3 ± 2.2 | 10.2–37.4 25.52 ± 3.37 |
| SO42− | 5.0–133.5 43.97 ± 4.77 | 107.6–552.4 265.8 ± 80.6 | 7.1–29.0 13.18 ± 1.97 | 12.6–414.3 79.2 ± 14.1 | 47.6–480.3 175.64 ± 59.24 |
| Mineralization | 112.9–511.0 277.26 ± 17.98 | 546.9–1211.9 787.7 ± 94.5 | 170.6–263.4 218.6 ± 11.86 | 119.3–607.2 308.7 ± 17.9 | 333.8–1021.4 526.11 ± 93.64 |
| Hardness | 1.2–5.2 2.94 ± 0.20 | 7.6–13.04 9.44 ± 0.77 | 1.36–3.4 2.45 ± 0.18 | 1.6–7.8 3.5 ± 0.2 | 3.0–6.2 4.43 ± 0.39 |
| HCO3− | 59.5–244.6 156.07 ± 10.56 | 311.2–755.1 443.75 ± 67.02 | 86.1–249.0 143.5 ± 13.46 | 10.6–308.2 133.7 ± 9.7 | 146.4–281.9 215.84 ± 18.65 |
| COD | 16.0–43.8 25.07 ± 1.20 | 10.0–24.1 20.02 ± 2.62 | 16.9–35.9 24.9 ± 1.8 | 7.3–47.7 29.4 ± 2.3 | 37.7–208.3 94.3 ± 21.5 |
| COD5 | 0.66–4.73 2.1019 | 1.58–1.93 1.74 ± 0.08 | 0.5–7.1 2.73 ± 0.49 | 0.3–4.29 3.1 ± 0.7 | 11.36–48.27 28.01 ± 6.06 |
| N-NH4+ | 0.0–0.91 0.32 ± 0.04 | 0.05–0.42 0.19 ± 0.06 | 0.018–0.43 0.019 ± 0.04 | 0.0–1.17 0.35 ± 0.07 | 0.54–2.19 1.25 ± 0.21 |
| N-NO2− | 0.0–0.03 0.01 ± 0.00 | 0.029–0.096 0.06 ± 0.01 | 0.0–0.041 0.038 ± 0.03 | 0.0–0.19 0.03 ± 0.01 | 0.0–0.02 0.008 ± 0.00 |
| N-NO3− | 0.01–1.41 0.30 ± 0.06 | 0.99–9.46 3.56 ± 1.57 | 0.0–0.9 0.19 ± 0.09 | 0.0–1.3 0.45 ± 0.26 | 0.0–9.46 1.79 ± 1.42 |
| PO43− | 0.0–0.05 0.02 ± 0.00 | 0.0–0.28 0.11 ± 0.04 | 0.0–0.18 0.05 ± 0.01 | 0.0–0.29 0.05 ± 0.01 | 0.0–0.081 0.016 ± 0.01 |
| Fe | 0.01–0.85 0.21 ± 0.04 | 0.11–0.20 0.14 ± 0.03 | 0.02–0.35 0.19 ± 0.03 | 0.0–0.62 0.13 ± 0.02 | 0.136–0.237 0.189 ± 0.01 |
| Cu* | 0.0–6.6 2.62 ± 0.25 | 0.0–4.7 3.13 ± 1.57 | 0.0–14.0 3.15 ± 1.0 | 0.0–30.5 2.98 ± 0.46 | 2.9–7.5 4.25 ± 0.57 |
| Mn* | 5.6–52.2 25.37 ± 4.62 | 14.0–20.0 18.0 ± 2.0 | 0.0–59.0 12.1 ± 5.3 | 0.0–280.0 49.2 ± 9.4 | 7.0–303.0 160.74 ± 38.70 |
| TPH | 0.0–0.09 0.03 ± 0.00 | 0.0–0.19 0.06 ± 0.01 | 0.0–0.02 0.008 ± 0.0 | 0.0–0.5 0.099 ± 0.030 | 0.059–0.76 0.27 ± 0.11 |
| Phenols | 0.0–0.004 0.001 ± 0.00 | 0.0–0.001 0.0 ± 0.0 | 0.001–0.005 0.001 ± 0.00 | 0.0–0.004 0.001 ± 0.00 | 0.0034–0.18 0.073 ± 0.03 |
| SCIWP | 3.6 | 3.34 | 3.45 | 4.63 | 11.35 |
| Water quality | Highly polluted | Highly polluted | Highly polluted | Dirty | Extremely dirty |
References
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Directive 2000/60/EC of the European Parliament ... Action in the Field of Water Policy. Available online: https://www.eea.europa.eu/policy-documents/directive-2000-60-ec-of (accessed on 29 October 2019).
- Morduhaj-Boltovskaya, E.D.; Sorokin, Y.I. Pitanie infuzorij vodoroslyami i bakteriyami (Feeding of ciliates on algae and bacteria). Tr. Inst. Biol. Vnutr. Vod. 1965, 8, 12–14. (In Russian) [Google Scholar]
- Mamaeva, N.V. Ciliates of the Volga River Basin; Nauka: Moscow, Russia, 1979; p. 150. (In Russian) [Google Scholar]
- Mamaeva, N.V.; Kopylov, A.I. To study the nutrition of freshwater infusoria. Cell Tissue Biol. 1978, 20, 472–476. (In Russian) [Google Scholar]
- Obolkina, L.A. Planktonic Ciliates of Lake Baikal. Hydrobiologia 2006, 568, 193–199. (In Russian) [Google Scholar] [CrossRef]
- Porter, K.G.; Sherr, E.B.; Sherr, B.F.; Pace, M.; Sanders, R.W. Protozoa in Planktonic Food Webs1, 2. J. Protozool. 1985, 32, 409–415. [Google Scholar] [CrossRef]
- Berninger, U.G.; Finlay, B.J.; Canter, H.M. The Spatial Distribution and Ecology of Zoochlorellae-Bearing Ciliates in a Productive Pond 1. J. Protozool. 1986, 33, 557–563. [Google Scholar] [CrossRef]
- Berninger, U.G.; Finlay, B.J.; Kuuppo-Leinikki, P. Protozoan control of bacterial abundances in freshwater. Limnol. Oceanogr. 1991, 36, 139–147. [Google Scholar] [CrossRef]
- Beaver, J.R.; Crisman, T.L. The role of ciliated protozoa in pelagic freshwater ecosystems. Microb. Ecol. 1989, 17, 111–136. [Google Scholar] [CrossRef]
- Foissner, W.; Berger, H.; Schaumdurg, J. Identification and Ecology of Limnetic Plancton Ciliates; Bayerisches Landesamtes für Wasserwirtschaft Munchen: Munchen, Germany, 1999; p. 793. [Google Scholar]
- Gates, M.A. Quantitative importance of ciliates in the planktonic biomass of lake ecosystems. Hydrobiologia 1984, 108, 233–238. [Google Scholar] [CrossRef]
- Bykova, S.V. Free-living Ciliates in the deep-water part of the Kama river reservoirs. Trans. IBIW 2019, 85, 23–43. (In Russian) [Google Scholar] [CrossRef]
- Weisse, T. Functional diversity of aquatic ciliates. Eur. J. Protistol. 2017, 61, 31–358. [Google Scholar] [CrossRef]
- Weisse, T. Freshwater ciliates as ecophysiological model organisms—lessons from Daphnia, major achievements, and future perspectives Freshwater ciliates as ecophysiological model organisms – lessons from Daphnia, major achievements, and future perspectives. Arch. Hydrobiol. 2006, 167, 371–402. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, W.; Jiang, Y.; Yang, E.J. Use of biofilm-dwelling ciliate communities to determine the environmental quality status of coastal waters. Sci. Total Environ. 2014, 470, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, L.; Tan, Y.; Song, X.; Huang, J. Composition and distribution of ciliates inner and outer of Daya Bay, China. Acta Ecol. Sin. 2010, 30, 288–291. [Google Scholar] [CrossRef]
- Ministry of Ecology of the Republic of Tatarstan. Report on the State of Natural Resources and Environmental Protection of the Republic of Tatarstan in 2002; Ministry of Ecology of the Republic of Tatarstan: Kazan, Russia, 2003; p. 355. (In Russian)
- Institute of Ecology of the Volga Basin. Kuibyshev Reservoir (Scientific Information Guide); Institute of Ecology of the Volga Basin: Tolyatti, Russia, 2008; p. 123. (In Russian) [Google Scholar]
- Nikanorov, A.M.; Zakharov, S.D.; Bryzgalo, V.A.; Zhdanova, G.N. The Rivers of Russia. Part III. Rivers of the Republic of Tatarstan (Hydrochemistry and Hydroecology); Hydrochemical Institute: Rostov-on-Don, Russia, 2010; p. 192. (In Russian) [Google Scholar]
- Kahl, A. Urtiere oder Protozoa. I. Wimpertiere oder Ciliata (Infusoria). Die Tierwelt Angrenzenden Meerest. 1935, 30, 651–886. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949; p. 117. [Google Scholar]
- Pantle, R.; Buck, H. Die biologische Uberwachung der Gewasser und die Darstellung der Ergebnisse. Gas Wasserfach 1955, 96, 604. [Google Scholar]
- Sladeček, V. System of Water Quality from Biological Point of View; Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1973; p. 218. [Google Scholar]
- FGBU “GKHI”. Organization and Conduct of Monitoring Observations of the State and Pollution of Surface Water, Guidance; FGBU “GKHI”: Rostov-on-Don, Russia, 2016; p. 100. (In Russian) [Google Scholar]
- FGBU “GKHI”. Method for a Comprehensive Assessment of the Degree of Pollution of Surface Waters by Hydrochemical Indicators, Guidance; FGBU “GKHI”: Rostov-on-Don, Russia, 2016; p. 50. (In Russian) [Google Scholar]
- Hosmer, D.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Giglio, O.D.; Barbuti, G.; Trerotoli, P.; Brigida, S.; Calabrese, A.; Vittorio, G.D.; Lovero, G.; Caggiano, G.; Uricchio, V.F.; Montagna, M.T. Microbiological and hydrogeological assessment of groundwater in southern Italy. Environ. Monit. Assess. 2016, 188, 638. [Google Scholar] [CrossRef]
- Jerves-Cobo, R.; Everaert, G.; Iñiguez-Vela, X.; Córdova-Vela, G.; Díaz-Granda, C.; Cisneros, F.; Nopens, I.; Goethals, P.L.M. A Methodology to Model Environmental Preferences of EPT Taxa in the Machangara River Basin (Ecuador). Water 2017, 9, 195. [Google Scholar] [CrossRef]
- Zimmer-Faust, A.G.; Brown, C.A.; Manderson, A. Statistical models of fecal coliform levels in Pacific Northwest estuaries for improved shellfish harvest area closure decision making. Mar. Pollut. Bull. 2018, 137, 360–369. [Google Scholar] [CrossRef]
- The Comprehensive R Archive Network. Available online: https://cran.r-project.org/ (accessed on 28 October 2019).
- Xu, H.; Jiang, Y.; Xu, G. Identifying functional species pool of planktonic protozoa for discriminating water quality status in marine ecosystems. Ecol. Indic. 2016, 62, 306–311. [Google Scholar] [CrossRef]
- Zhong, X.; Xu, G.; Xu, H. Use of multiple functional traits of protozoa for bioassessment of marine pollution. Mar. Pollut. Bull. 2017, 119, 33–38. [Google Scholar] [CrossRef]
- Luna-Pabello, V.M.; Mayén, R.; Olvera-Viascan, V.; Saavedra, J.; Durán de Bazúa, C. Ciliated protozoa as indicators of a wastewater treatment system performance. Biol. Wastes 1990, 32, 81–90. [Google Scholar] [CrossRef]
- Hu, B.; Qi, R.; Yang, M. Systematic analysis of microfauna indicator values for treatment performance in a full-scale municipal wastewater treatment plant. J. Environ. Sci. 2013, 25, 1379–1385. [Google Scholar] [CrossRef]
- Puigagut, J.; Salvadó, H.; García, J. Short-term harmful effects of ammonia nitrogen on activated sludge microfauna. Water Res. 2005, 39, 4397–4404. [Google Scholar] [CrossRef] [PubMed]
- Madoni, P.; Romeo, M.G. Acute toxicity of heavy metals towards freshwater ciliated protists. Environ. Pollut. 2006, 141, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, L.I.; Zhukov, A.V.; Tribun, M.M. Species composition, seasonal dynamics and morphological and ecological features of aerotenk ciliofauna of water treatment plans. J. Water Chem. Ecol. 2011, 12, 56–62. (In Russian) [Google Scholar]
- Liepa, R.A.; Druvietis, I.Y.; Melberg, A.G. The effect of various concentrations of nutrients on the structure of microplankton in controlled ecosystems. Exp. Aquat. Toxicol. 1984, 9, 171–184. (In Russian) [Google Scholar]
- Kondratyeva, T.A. The effect of nitrogen load on the structural characteristics of the infusor community. Bull. Kazan Technol. Uni. 2013, 3, 164–168. [Google Scholar]
- Kondrateva, T.A. Ecological modifications of water bodies’ cilioplankton of the Republic of Tatarstan. J. Water Chem. Ecol. 2016, 4, 10–16. (In Russian) [Google Scholar]
- Xu, Y.; Fan, X.; Warren, A.; Zhang, L.; Xu, H. Functional diversity of benthic ciliate communities in response to environmental gradients in a wetland of Yangtze Estuary, China. Mar. Pollut. Bull. 2018, 127, 726–732. [Google Scholar] [CrossRef]
- Xu, Y.; Stoeck, T.; Forster, D.; Ma, Z.; Zhang, L.; Fan, X. Environmental status assessment using biological traits analyses and functional diversity indices of benthic ciliate communities. Mar. Pollut. Bull. 2018, 131, 646–654. [Google Scholar] [CrossRef]
| Parameter | Units | Mean Value | Standard Deviation | Min Value | Max Value | Median Value |
|---|---|---|---|---|---|---|
| Temperature | °C | 15.51 ± 0.24 | 0.15 | 30.6 | 15.0 | |
| pH | 8.04 ± 0.02 | 7.27 | 9.20 | 8.05 | ||
| Dissolved oxygen (DO) | mg/L | 9.33 ± 0.07 | 2.63 | 14.67 | 9.31 | |
| Total solids (TSol) | mg/L | 11.01 ± 0.24 | 1.10 | 38.00 | 9.55 | |
| Mineralization | mg/L | 624.5 ± 14.6 | 112.9 | 1479.9 | 536.9 | |
| Hardness | mEq/L | 6.54 ± 0.16 | 1.20 | 18.0 | 5.90 | |
| Hydrocarbonates | mg/L | 220.7 ± 4.4 | 54.9 | 755.1 | 219.7 | |
| Phosphates | mg/L | 0.12 ± 0.01 | 0.00 | 1.65 | 0.05 | |
| N-Ammonia | mg/L | 0.49 ± 0.03 | 0.00 | 4.80 | 0.30 | |
| N-Nitrates | mg/L | 1.27 ± 0.07 | 0.00 | 10.04 | 0.58 | |
| N-Nitrites | mg/L | 0.06 ± 0.00 | 0.00 | 0.70 | 0.02 | |
| Biological oxygen demand (BOD5) | mgO2/L | 2.31 ± 0.06 | 0.00 | 7.90 | 1.97 | |
| Chemical oxygen demand (COD) | mgO2/L | 22.89 ± 0.24 | 1.40 | 42.00 | 22.90 | |
| Water Body | Q | N | H | S | Water Quality |
|---|---|---|---|---|---|
| Yushut | 7.0–10.0 8.3 ± 0.9 | 2640.0–2937.0 2783.0 ± 85.91 | 0.7–2.7 1.76 ± 0.23 | 1.91–2.29 2.08 ± 0.04 | Weakly Polluted |
| Ilet | 3.0–11.0 6.6 ± 1.3 | 44.0–400.0 229.60 ± 70.03 | 1.13–2.66 1.81 ± 0.31 | 1.65–2.35 2.04 ± 0.16 | Weakly Polluted - |
| Vjatka | 14.0–16.0 15.0 ± 0.6 | 700.0–3198.0 1726.7 ± 754.6 | 2.26–3.60 2.91 ± 0.39 | 1.88–2.32 2.07 ± 0.13 | Weakly Polluted - |
| Kazanka | 2.0–21.0 9.9 ± 0.7 | 198.0–132000.0 19091.9 ± 5680.9 | 1.73–3.37 2.56 ± 0.29 | 1.84–2.14 2.04 ± 0.05 | Weakly Polluted - |
| Mesha | 5.0–19.0 8.6 ± 0.8 | 200.0–9200.0 2748.6 ± 490.4 | 0.76–3.6 2.34 ± 0.25 | 1.2–3.0 2.12 ± 0.17 | Weakly Polluted - |
| Noksa | 0.0–11.0 4.5 ± 1.7 | 0.0–3176.0 757.92 ± 496.72 | 0.38–2.87 1.39 ± 0.43 | 2.01–2.95 2.33 ± 0.17 | Weakly Polluted |
| Svijaga | 1.0–11.0 5.0 ± 0.8 | 400.0–8000.0 3660.0 ± 1005.8 | 0.0–2.8 1.53 ± 0.29 | 2.0–2.5 2.29 ± 0.06 | Weakly Polluted |
| Steppe Zai | 1.0–14.0 7.1 ± 0.8 | 100.0–6800.0 1857.80 ± 520.19 | 0.0–3.0 2.10 ± 0.18 | 1.7–3.6 2.57 ± 0.11 | Polluted |
| Raifa Lake | 2.0–17.0 11.1 ± 1.4 | 346.5–18414.0 4249.6±1630.1 | 1.28–2.52 1.71 ± 0.41 | 1.31–2.05 1.73±0.22 | Relatively clean - Weakly Polluted |
| Kujbyshev Reservoir | 1.0–14.0 6.5 ± 0.6 | 99.0–4158.0 904.2 ± 218.4 | 0.92–4.5 2.08 ± 0.09 | 1.4–2.9 2.16 ± 0.03 | Weakly Polluted |
| MPPM | 0.0–11.0 3.6 ± 2.3 | 0.0–2628.0 683.2 ± 509.6 | 0.0–2.23 0.64 ± 0.35 | 2.69–3.3 3.01 ± 0.15 | Polluted |
| Estimate | Std. Error | Z-Value | Pr (>|z|) | |
|---|---|---|---|---|
| Intercept | −1.2937 | 0.3410 | −3.79 | 0.0001 |
| Temperature | −0.6995 | 0.3555 | −1.97 | 0.0491 |
| Cl− | 1.1347 | 0.3484 | 3.26 | 0.0011 |
| Mineralization | 1.8498 | 0.6147 | 3.01 | 0.0026 |
| HCO3− | −1.2116 | 0.5539 | −2.19 | 0.0287 |
| BOD5 | 0.9782 | 0.3580 | 2.73 | 0.0063 |
| N-NO3− | 1.3826 | 0.4613 | 3.00 | 0.0027 |
| Fe | −0.8404 | 0.3309 | −2.54 | 0.0111 |
| Total petroleum hydrocarbons | −1.8934 | 0.5276 | −3.59 | 0.0003 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondrateva, T.; Nikonenkova, T.; Stepanova, N. Using Cilioplankton as an Indicator of the Ecological Condition of Aquatic Ecosystems. Geosciences 2019, 9, 464. https://doi.org/10.3390/geosciences9110464
Kondrateva T, Nikonenkova T, Stepanova N. Using Cilioplankton as an Indicator of the Ecological Condition of Aquatic Ecosystems. Geosciences. 2019; 9(11):464. https://doi.org/10.3390/geosciences9110464
Chicago/Turabian StyleKondrateva, Tatiana, Tatiana Nikonenkova, and Nadezhda Stepanova. 2019. "Using Cilioplankton as an Indicator of the Ecological Condition of Aquatic Ecosystems" Geosciences 9, no. 11: 464. https://doi.org/10.3390/geosciences9110464
APA StyleKondrateva, T., Nikonenkova, T., & Stepanova, N. (2019). Using Cilioplankton as an Indicator of the Ecological Condition of Aquatic Ecosystems. Geosciences, 9(11), 464. https://doi.org/10.3390/geosciences9110464





