Experimental Assessment of the Sealing Potential of Hydrated Solgel for the Remediation of Leaky Reservoirs
Abstract
:1. Introduction
2. Materials and Methods
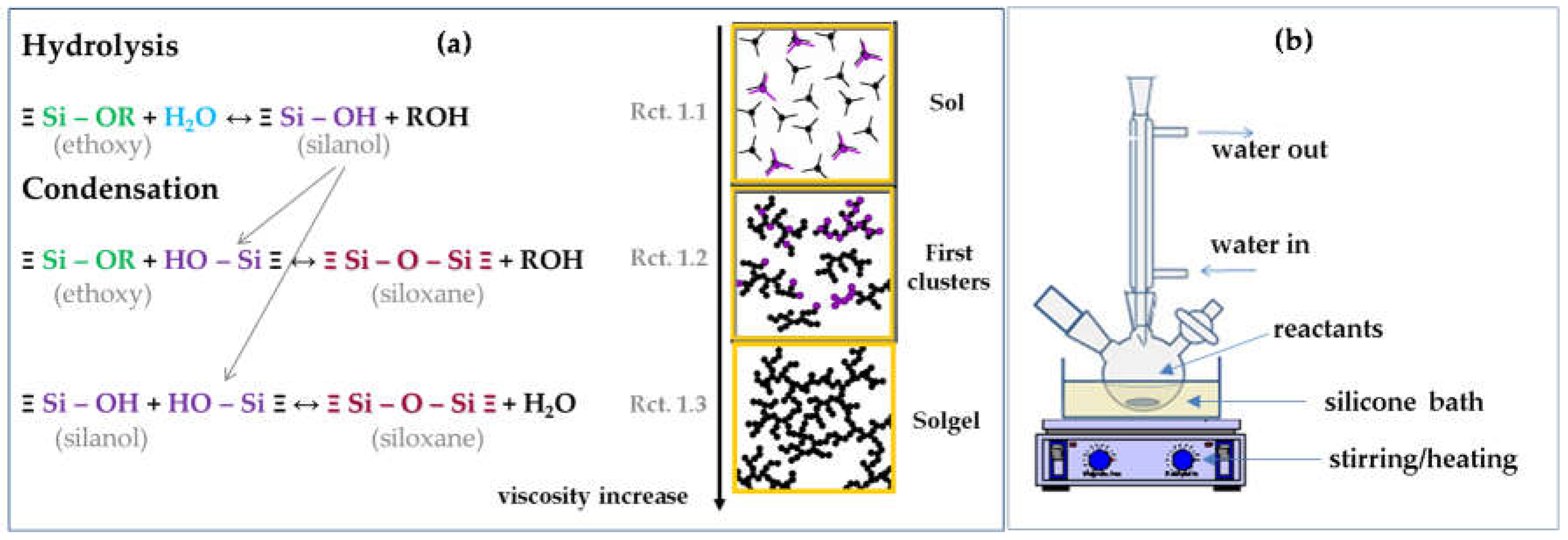
2.1. Solgel Chemistry and Synthesis
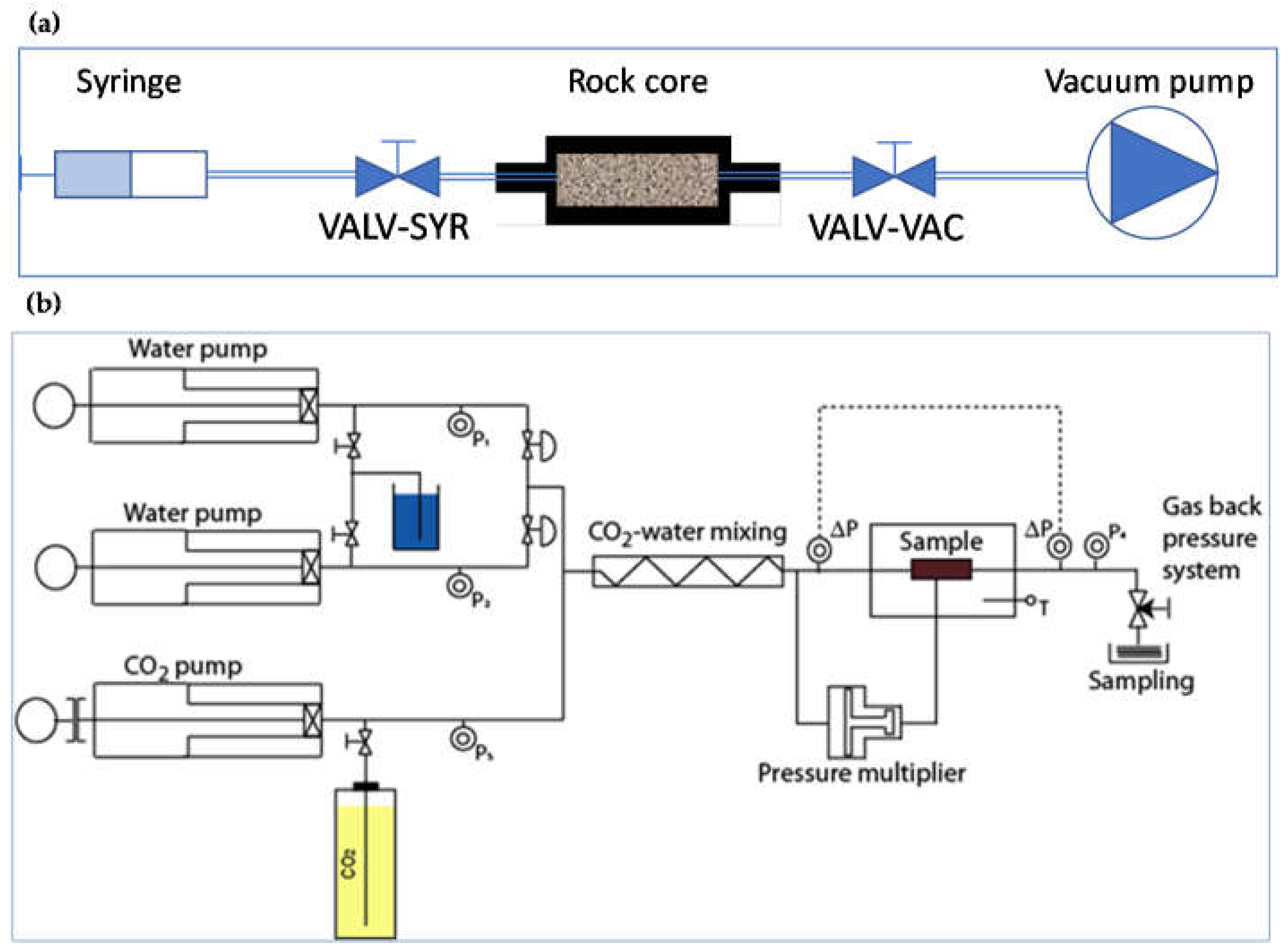
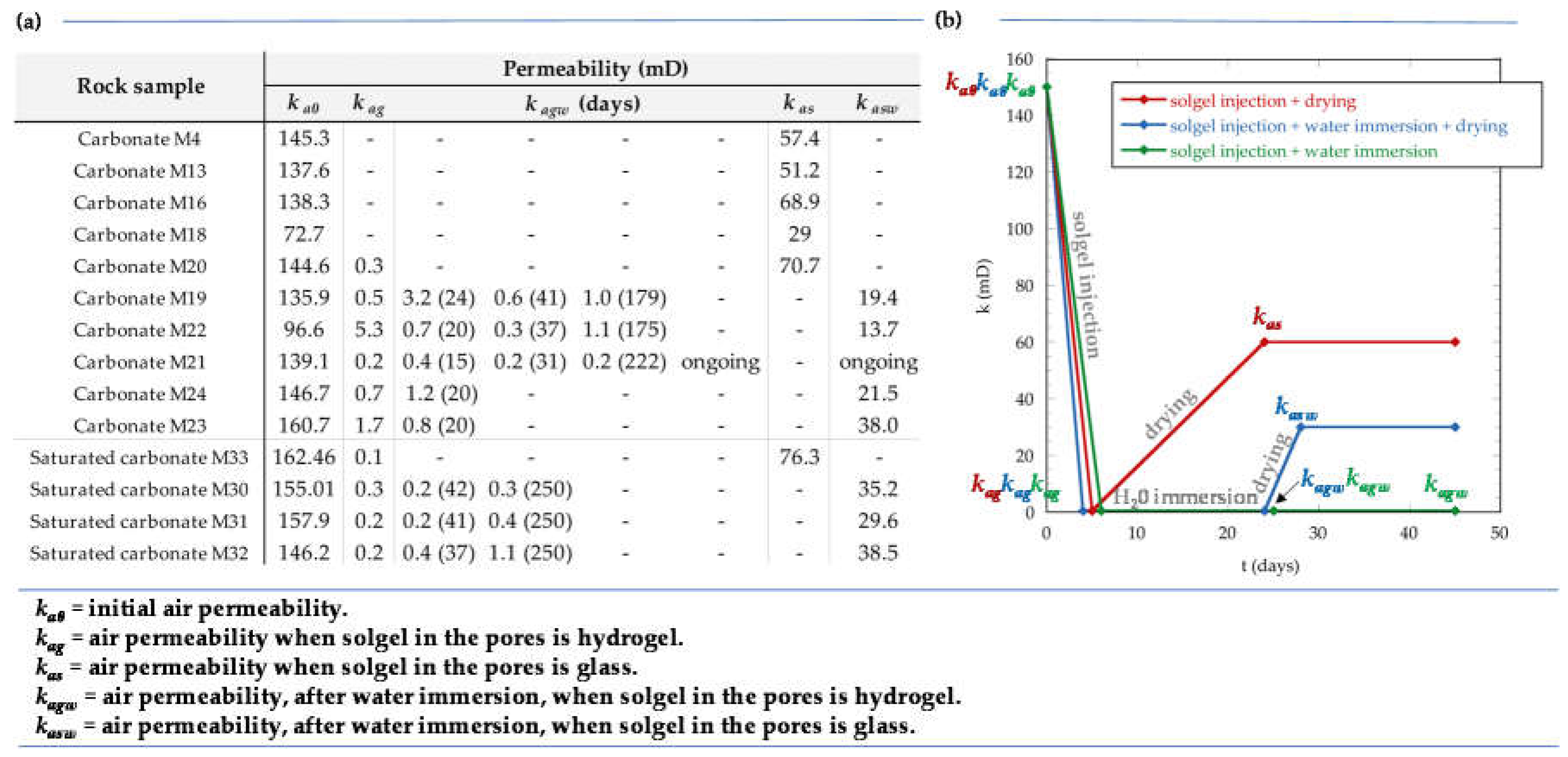
2.2. Permeability Tests
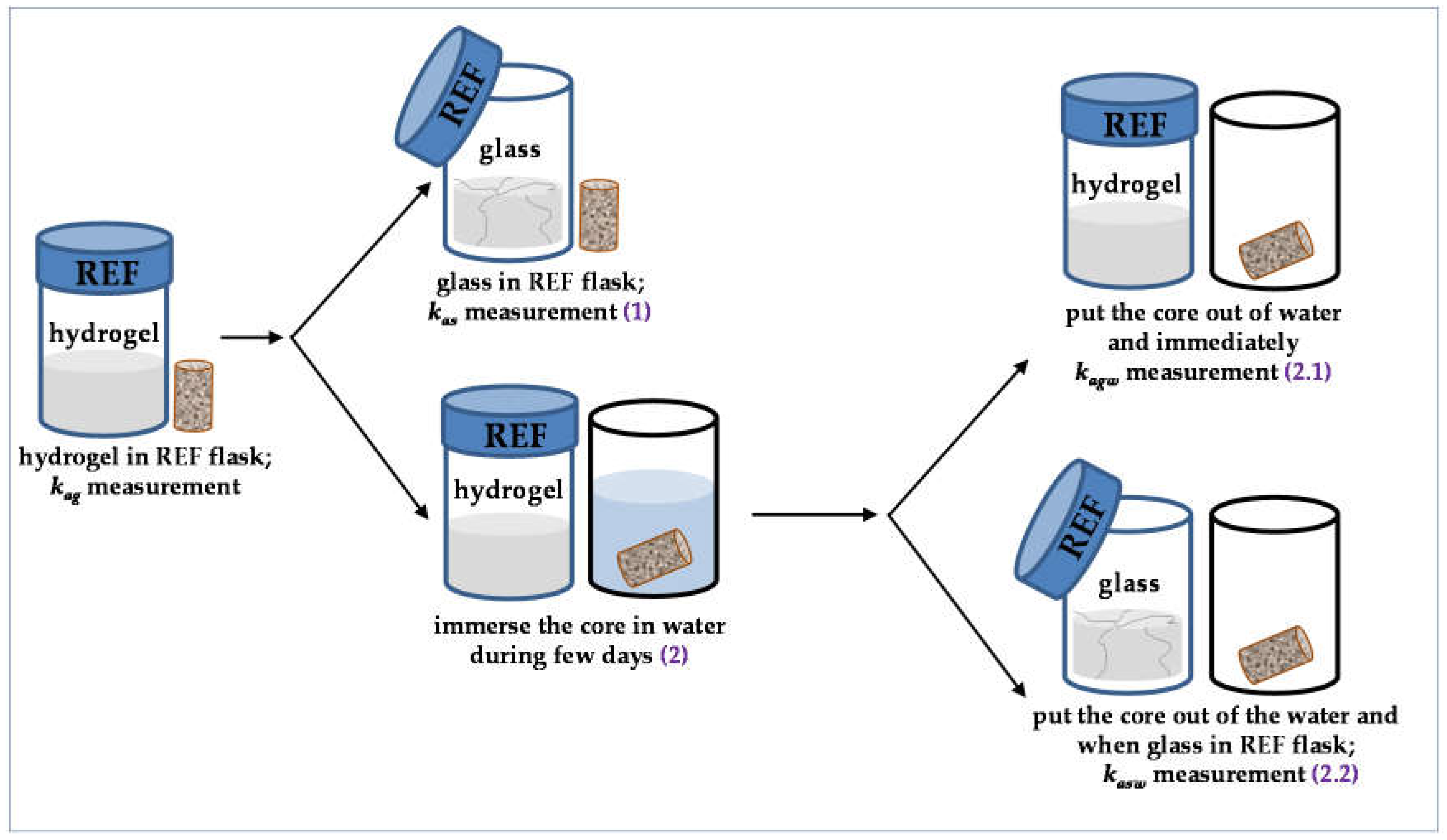
- (1)
- the reference flask was opened, promoting hydrogel solidification, and the corresponding rock permeability (kas) was measured when the hydrogel became glass in the flask.
- (2)
- the core sample was immersed in water during some time (few days). After this time, either (2.1) the core was put out of the water and rock permeability (kagw) was immediately measured or (2.2) the core was put out of the water, the reference flask was opened, and rock permeability (kasw) was measured when the hydrogel became glass in the reference flask.
3. Results and Discussion
3.1. Key-Parameters Controlling Solgel Applicability
3.2. Sealant Capacity of the Hydrated Gel
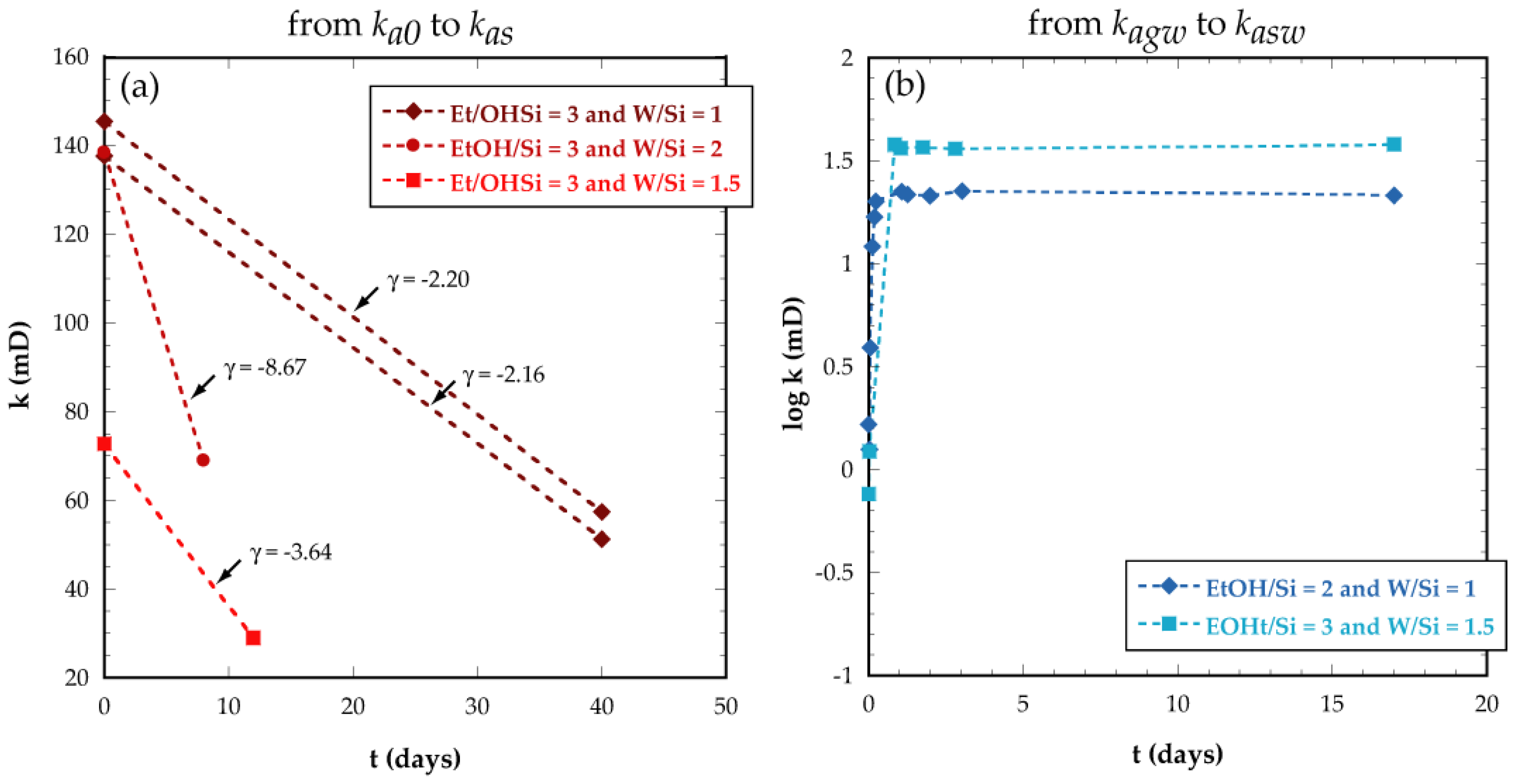
3.2.1. Air Permeability Tests
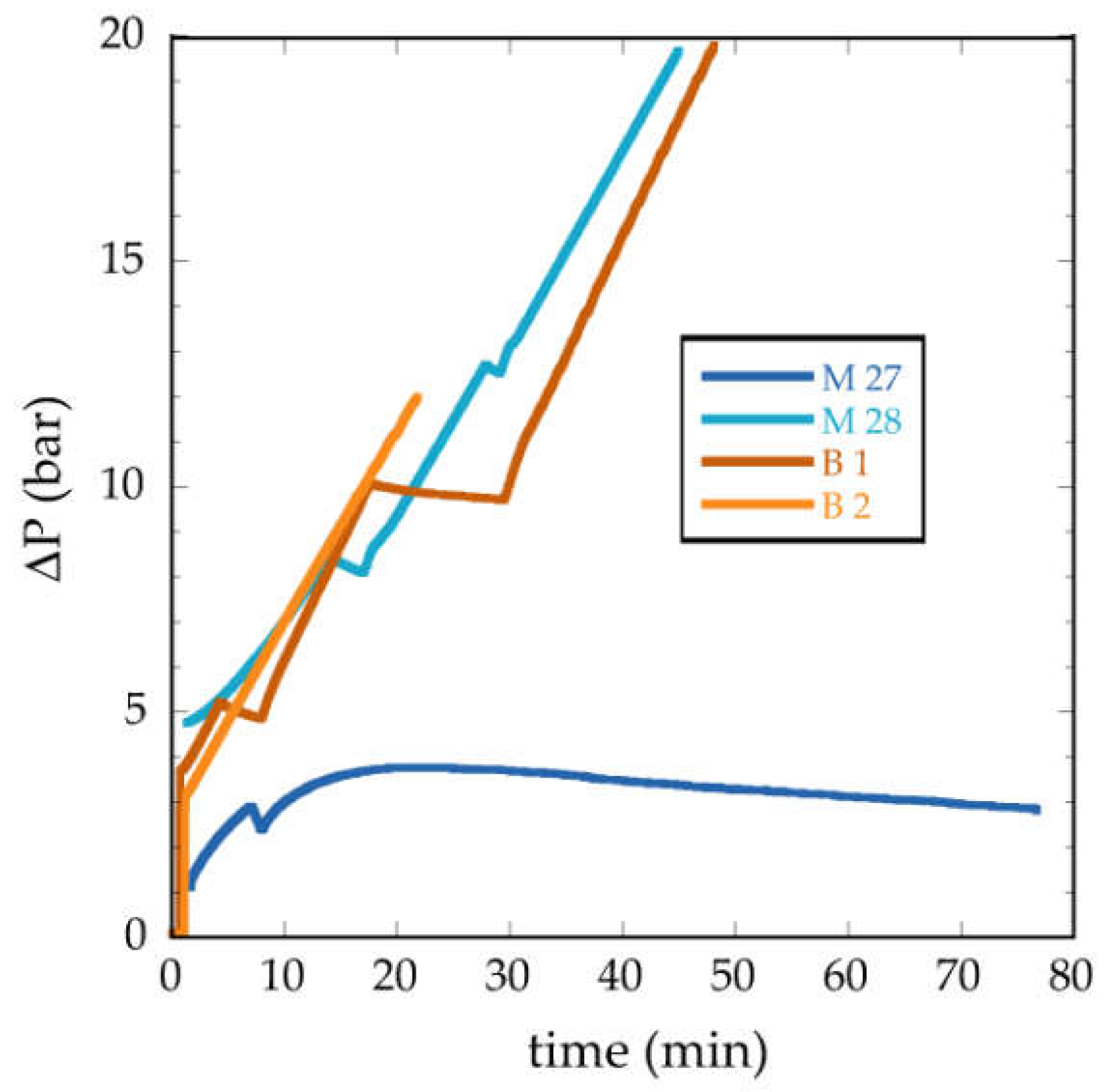
3.2.2. Water Permeability Tests
4. Conclusions
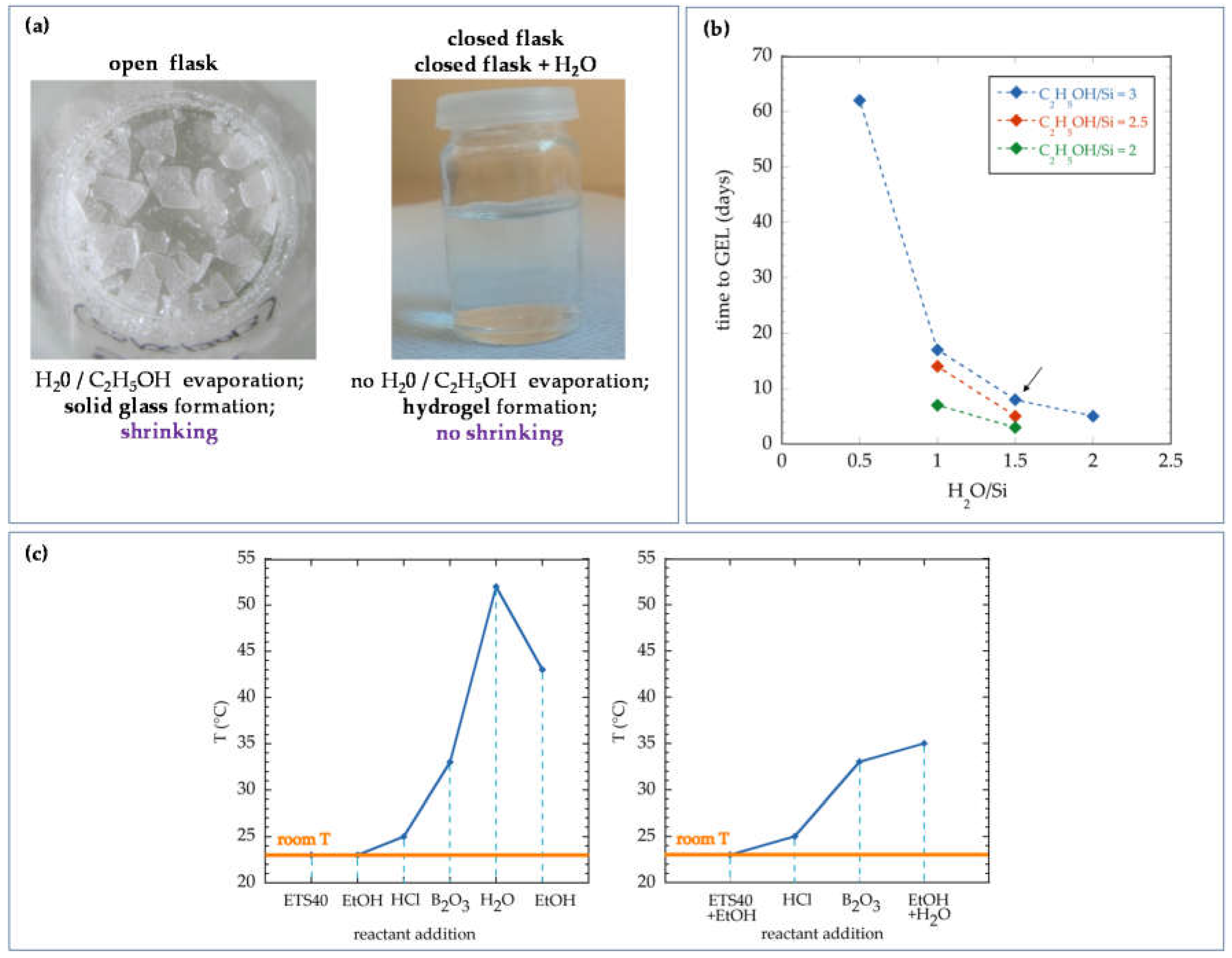
- ETS40-based sol was selected among other silicon alkoxide precursors owing to its affordable price, adequate hydrogel formation time and the ability to produce final materials without fissures.
- The reactant boron oxide was introduced to the synthesis in order to improve the mechanical strength of the resulting material.
- Given that most of the reactions involved in the solgel synthesis using ETS40 were exothermic, no heating of the mixture while preparing or injecting it is required to maintain the product at the right temperature (between 20 and 40 °C), thus decreasing the complexity of the surface injection system and the final costs.
- The order of addition of reactants to the bulk solution during the solgel synthesis was found to be a critical parameter to control the temperature of the solgel during its preparation and injection. The different protocols do not affect measurably the final properties of the hydrogel.
- The amount of water and alcohol used in the synthesis was optimized in order to prevent the expulsion of excess liquid in the final product, thus decreasing the amount of reactants needed for the solgel synthesis. Furthermore, this formulation allows the maximum concentration of Si in the solgel, thus the maximum density of the hydrogel phase.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsang, C.-F.; Niemi, A. Overview of Processes Occurring during CO2 Geological Storage and Their Relevance to Key Questions of Performance. In Geological Storage of CO2 in Deep Saline Formations. Theory and Applications of Transport in Porous Media, 1st ed.; Niemi, A., Bear, J., Bensabat, J., Eds.; Springer Nature: Dordrecht, The Netherlands, 2017; Volume 29, pp. 15–38. ISBN 978-94-024-0994-9. [Google Scholar]
- Manceau, J.-C.; Hatzignatiou, D.G.; de Lary, L.; Jensen, N.B.; Réveillère, A. Mitigation and remediation technologies and practices in case of undesired migration of CO2 from a geological storage unit-Current status. Int. J. Greenh. Gas Control 2014, 22, 272–290. [Google Scholar] [CrossRef]
- Shipton, Z.K.; Evans, J.P.; Dockrill, B.; Heath, J.; Williams, A.; Kirchner, D.; Kolesar, P.T. Natural Leaking CO2-Charged Systems as Analogs for Failed Geologic Storage Reservoirs. In Carbon Dioxide Capture for Storage in Deep Geological Formations—Results from CO2 Capture Project, 1st ed.; Benson, S.M., Ed.; Elsevier Ltd.: Oxford, UK, 2005; Volume 2, pp. 699–713. ISBN 0-08-044570-5. [Google Scholar]
- Oldenburg, C.M.; Bryant, S.L.; Nicot, J.P. Certification framework based on effective trapping for geologic carbon sequestration. Int. J. Greenh. Gas Control 2009, 3, 444–457. [Google Scholar] [CrossRef] [Green Version]
- Watson, T.; Bachu, S. Evaluation of the potential for gas and CO2 leakage along wellbores. SPE Drill. Complet. 2009, 24. [Google Scholar] [CrossRef]
- Celia, M.A.; Bachu, S.; Nordbotten, J.M.; Gasda, S.E.; Dahle, H.K. Quantitative Estimation of CO2 Leakage from Geological Storage: Analytical Models, Numerical Models, and Data Needs. In Greenhouse Gas Control Technologies, 1st ed.; Wilson, M., Rubin, E.S., Keith, D.W., Gilboy, C.F., Morris, T., Thambimuthu, K., Gale, J., Eds.; Elsevier Ltd.: Oxford, UK, 2005; Volume 1, pp. 663–671. ISBN 9780080539737. [Google Scholar]
- Intergovernmental Panel on Climate Change. Special Report on Carbon Dioxide Capture and Storage, 1st ed.; Cambridge University Press: New York, NY, USA, 2005; ISBN 13978-0-521-86643-9. [Google Scholar]
- Kuuskraa, V.A. Overview of Mitigation and remediation Options for Geological Storage of CO2. In Staff Workshop on Technical Papers; AB1925 Report to Legislature; California Institute for Energy and Environment: Berkeley, CA, USA, 28 June 2007. [Google Scholar]
- Rodrigues, C.F.A.; Dinis, M.A.P.; Lemos de Sousa, M.J. Review of European energy policies regarding the recent “carbon capture, utilization and storage” technologies scenario and the role of coal seams. Environ. Earth Sci. 2015, 74, 2553–2561. [Google Scholar] [CrossRef]
- Bachu, S.; Celia, M. Assessing the Potential for CO2 Leakage, Particularly through Wells, from Geological Storage Sites. In The Science of CO2 Storage; AGU Monograph Series GM148; McPherson, B.J.O.L., Sundquist, E., Eds.; American Geophysical Union: Washington, DC, USA, 2009; pp. 203–216. [Google Scholar]
- Benson, S.M.; Hepple, R. Prospects for Early Detection and Options for Remediation of Leakage from CO2 Sequestration Projects. In Carbon Dioxide Capture for Storage in Deep Geologic Formations; Thomas, D.C., Benson, S.M., Eds.; Elsevier Ltd.: Oxford, UK, 2005; Volume 2, ISBN 9780080445700. [Google Scholar]
- Esposito, A.; Benson, S.M. Remediation of possible leakage from geologic CO2 storage reservoirs into groundwater aquifers. Energy Procedia 2011, 4, 3216–3223. [Google Scholar] [CrossRef]
- Loizzo, M.; Akemu, O.A.P.; Jammes, L.; Desroches, J.; Lombardi, S.; Annunziatellis, A. Quantifying the risk of CO2 leakage through wellbores. SPE Drill. Complet. 2011, 26. [Google Scholar] [CrossRef]
- Deremble, L.; Loizzo, M.; Huet, B.; Lecampion, B.; Quesada, D. Stability of a leakage pathway in a cemented annulus. Energy Procedia 2011, 4, 5283–5290. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Strazisar, B.R.; Dzombak, D.A.; Lowry, G.V.; Thaulow, N. Degradation of well cement by CO2 under geologic sequestration conditions. Environ. Sci. Technol. 2007, 41, 4787–4792. [Google Scholar] [CrossRef] [PubMed]
- Abdoulghafour, H.; Luquot, L.; Gouze, P. Characterization of the mechanisms controlling the permeability changes of fractured cements flowed through by CO2-rich brine. Environ. Sci. Technol. 2013, 47, 10332–10338. [Google Scholar] [CrossRef] [PubMed]
- Davidovitch, J. Geopolymer Chemistry and Applications, 4th ed.; Institut Géopolymère: Saint-Quentin, France, 2015; ISBN 9782951482098. [Google Scholar]
- Nasvi, M.M.C.; Gamage, R.P.; Jay, S. Geopolymer as well cement and the variation of its mechanical behavior with curing temperature. Greenh. Gases Sci. Technol. 2012, 2, 46–58. [Google Scholar] [CrossRef]
- Shaw, J.C.; Bramhill, B.; Wardlaw, N.C.; Costerton, J.W. Bacteria fouling in a model core system. Appl. Environ. Microbiol. 1985, 49, 693–701. [Google Scholar] [PubMed]
- Cunningham, A.B.; Gerlach, R.; Spangler, L.; Mitchell, A.C.; Parks, S.; Phillips, A. Reducing the risk of well bore leakage of CO2 using engineered biomineralization barriers. Energy Procedia 2011, 4, 5178–5185. [Google Scholar] [CrossRef]
- Phillips, A.J.; Lauchnor, E.; Eldring, J.; Esposito, R.; Mitchell, A.C.; Gerlach, R.; Cunningham, A.B.; Spangler, L.H. Potential CO2 leakage reduction through biofilm-induced calcium carbonate precipitation. Environ. Sci. Technol. 2013, 47, 142–149. [Google Scholar] [CrossRef] [PubMed]
- DiCarlo, D.A.; Aminzadeh, B.; Roberts, M.; Chung, D.H.; Bryant, S.L.; Huh, C. Mobility control through spontaneous formation of nanoparticle stabilized emulsions. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; An, C.; Mo, D.; Liu, N.; Lee, R. Foam mobility control for nanoparticle-stabilized supercritical CO2 foam. In Proceedings of the Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Aminzadeh, B.; Chung, D.H.; Bryant, S.L.; Huh, C.; Dicarlo, D.A. CO2 leakage prevention by introducing engineered nanoparticles to the in-situ brine. Energy Procedia 2013, 37, 5290–5297. [Google Scholar] [CrossRef]
- Farhadi, H.; Riahi, S.; Ayatollahi, S.; Ahmadi, H. Experimental study of nanoparticle-surfactant-stabilized CO2 foam: Stability and mobility control. Chem. Eng. Res. Des. 2016, 111, 449–460. [Google Scholar] [CrossRef]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery—Theory and Practice, 1st ed.; Gulf Professional Publishing: Burlington, MA, USA, 2010; ISBN 978-1-85617-745-0. [Google Scholar]
- Syed, A.; Pantin, B.; Durucan, S.; Korre, A.; Shi, J.-Q. The use of polymer-gel solutions for remediation of potential CO2 leakage from storage reservoirs. Energy Procedia 2014, 63, 4638–4645. [Google Scholar] [CrossRef]
- Lakatos, I.; Lakatos-Szabó, J.; Tiszai, G.; Palaásthy, G.; Kosztin, B.; Trömböczky, S.; Bodola, M.; Patterman-Farkas, G. Application of silicate-based well treatment techniques at the Hungarian oil fields. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 3–6 October 1999; Society of Petroleum Engineers: Houston, TX, USA. [Google Scholar]
- Tittelboom, K.V.; De Belie, N.; De Muynck, W.; Verstraete, W. Use of bacteria to repair cracks in concrete. Cement Concr. Res. 2010, 40, 157–166. [Google Scholar] [CrossRef]
- Blue, A.J. Experimental Evaluations of Selected Sealants to Remediate CO2 Leakage. Master’s Thesis, Missouri University of Science and Technology, Rolla, MO, USA, 2016. [Google Scholar]
- Castañeda-Herrera, C.A.; Black, J.R.; Stevens, G.W.; Haese, R.R. Preliminary experiments for a chemical reactive barrier as a leakage mitigation technology. Energy Procedia 2017, 114, 4140–4146. [Google Scholar] [CrossRef]
- Ito, T.; Xu, T.; Tanaka, H.; Taniuchi, Y.; Okamoto, A. Possibility to remedy CO2 leakage from geological reservoir using CO2 reactive grout. Int. J. Greenh. Gas. Control. 2014, 20, 310–323. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science. The Physics and Chemistry of Sol-Gel Processing, 1st ed.; Academic Press, Inc.: San Diego, CA, USA, 1990; ISBN 0-12-134970-5. [Google Scholar]
- Dimitriev, Y.; Ivanova, Y.; Iordanova, R. History of sol-gel science and technology (Review). J. Univ. Chem. Technol. Metall. 2008, 43, 181–192. [Google Scholar]
- Milea, C.A.; Bogatu, C.; Duta, A. The influence of parameters in silica sol-gel process. Bull. Transilv. Univ. Brasov 2011, 4, 59–66. [Google Scholar]
- Zarzycki, J. Past and present of sol-gel science and technology. J. Sol.-Gel. Sci. Technol. 1997, 8, 17–22. [Google Scholar] [CrossRef]
- Hamd, W. Elaboration Par Voie Sol-Gel et Etude Microstructurale de Gels et de Couches Minces de SnO2. Master’s Thesis, Limoges University, Limoges, France, 14 December 2009. [Google Scholar]
- Barboiu, C.; Sala, B.; Bec, S.; Pavan, S.; Petit, E.; Colomban, P.; Sanchez, J.; de Perthuis, S.; Hittner, D. Structural and mechanical characterizations of microporous silica-boron membranes for gas separation. J. Membr. Sci. 2009, 326, 514–525. [Google Scholar] [CrossRef]
- Gouze, P.; Le Borgne, T.; Leprovost, R.; Lods, G.; Poidras, T.; Pezard, P. Non-Fickian dispersion in porous media: 1. Multiscale measurements using single-well injection withdrawal tracer tests. Water Resour. Res. 2008, 44. [Google Scholar] [CrossRef] [Green Version]
- Siena, M.; Riva, M.; Giamberini, M.; Gouze, P.; Guadagnini, A. Statistical modeling of gas-permeability spatial variability along a limestone core. Spat. Stat. 2017. [Google Scholar] [CrossRef] [Green Version]
- Scheidegger, A.E. The Physics of Flow through Porous Media, 3rd ed.; University of Toronto Press: Toronto, ON, Canada, 1974; ISBN 0802018491. [Google Scholar]
- Klinkenberg, L.J. The permeability of porous media to liquids and gases. In Proceedings of the Drilling and Production Practices, New York, NY, USA, 1 January 1941; American Petroleum Institute: Washington, DC, USA. [Google Scholar]
- Luquot, L.; Gouze, P. Experimental determination of porosity and permeability changes induced by injection of CO2 into carbonate rocks. Chem. Geol. 2009, 265, 148–159. [Google Scholar] [CrossRef]
- Klein, L.C. Sol-Gel processing of silicates. Ann. Rev. Mater. Sci. 1985, 15, 227–248. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Rios, M.; Gouze, P. Experimental Assessment of the Sealing Potential of Hydrated Solgel for the Remediation of Leaky Reservoirs. Geosciences 2018, 8, 290. https://doi.org/10.3390/geosciences8080290
Garcia-Rios M, Gouze P. Experimental Assessment of the Sealing Potential of Hydrated Solgel for the Remediation of Leaky Reservoirs. Geosciences. 2018; 8(8):290. https://doi.org/10.3390/geosciences8080290
Chicago/Turabian StyleGarcia-Rios, Maria, and Philippe Gouze. 2018. "Experimental Assessment of the Sealing Potential of Hydrated Solgel for the Remediation of Leaky Reservoirs" Geosciences 8, no. 8: 290. https://doi.org/10.3390/geosciences8080290
APA StyleGarcia-Rios, M., & Gouze, P. (2018). Experimental Assessment of the Sealing Potential of Hydrated Solgel for the Remediation of Leaky Reservoirs. Geosciences, 8(8), 290. https://doi.org/10.3390/geosciences8080290



