Abstract
The faxinal management system is an endangered agro-silvopastoral system which forms part of the local traditional management in the Paraná federal state (Brazil). Significant changes in land management since the 1970s caused farmers to look for alternatives to increase the productivity of their farms. The introduction of new pasture species is causing land degradation problems, of which soil erosion is the most important challenge. Therefore, in this study, we assessed the environmental consequences of introducing exotic pasture species, such as Brachiaria decumbens. To achieve this goal, ten erosion plots were installed with exotic and native pastures (Paspalum notatum Flüggé) to quantify soil and water losses in paired plots. Total rainfall per event, soil properties (soil cover, texture, organic matter, bulk density, porosity, and soil penetration resistance), and pasture production were also estimated. Our results showed a decrease in organic matter and porosity and an increase of the bulk density in the exotic pasture plots. Soil erosion monitoring showed higher soil losses for the exotic cultivated plots (359.8 g m−2 or 3.6 mg ha−1) than for the native plots (90.7 g m−2 or 0.91 mg ha−1). The highest percentage of bare soil surfaces and compaction coincided with the highest soil erosion rates measured in the exotic pastures. However, the mean fodder production in the exotic plots was almost five times higher (987 kg DM ha−1) than in the native ones (204 kg DM ha−1). These findings confirm that farmers have an internal conflict. They want to optimize the production of fodder, but this leads to high soil erosion rates and reduces soil fertility in the medium- and long-term. The traditional, less productive pastoral system is more sustainable from an environmental and cultural point of view. However, this system may not be sustainable from an economic point of view.
1. Introduction
The Brazilian faxinal system is a local agrosilvopastoral system originating from the clearing of Araucaria angustifolia forests. This system was introduced by the Spanish and Portuguese colonizers in the 17th and 18th centuries, and continued by mainly Ukrainian and Polish peasants that immigrated to Paraná federal state (Southern Brazil) since the 19th century, after the abolition of slavery [1]. This traditional system covered 20% of the total land surface of Paraná state (ca. 200,000 km2) in the mid-twentieth century [2]. However, since the 1970s, its land surface and social importance has gradually reduced due to several reasons, such as agricultural intensification, rural exodus, juridical problems with land tenure, and the introduction of exotic species [3].
The faxinal system is a good example of mutualism between private owners who share common valuable resources. Similar land systems that are experiencing the same threats of breakdown of the traditional management system can be identified in other parts of the world. Turner et al. [4] studied the case of the acequias system in New Mexico (USA) where agriculture-based irrigation communities have survived for centuries, but are now at risk because of urbanization, economic development, and the lack of social cohesion [5].
Several activities with a low environmental impact have traditionally coexisted in the faxinal system where forests of A. angustifolia and native pastures are the ecological basement of this human-induced ecosystem. The system consists of extensively raising livestock in communal lands, while wood for self-consumption is extracted from native forests (common tenure), and some commercial crops, such as mate herb (Ilex paraguariensis), are cultivated in privately-owned areas. Thus, it is one system in which a community of farmers, who are individual owners of some inner pieces of land for agricultural purposes, jointly manage the native forest and pasture lands (i.e., each farmer is the owner of some herds of animals and he/she has the right to use the communal lands following the rules of each community [6]).
After some changes in land management since the 1970s, the remaining pasture lands with scattered trees have passed from being the largest land use to be a marginal practice in areas which are unsuitable for cultivation (size < 2 ha, slope > 20%) [7]. They are usually managed by smallholders involving family labor for extensive livestock husbandry of cattle and goats for self-consumption and equines for daily agricultural activities [8]. In the last years, many of these farmers have also increased the extension of their pasture lands aimed at increasing their livestock numbers, particularly cattle (i.e., the effects of the agricultural intensification on the faxinal system are evident even in the most traditional forms of agriculture).
Since many of these above-mentioned farms have problems in guaranteeing acceptable levels of profit needed to maintain livelihoods, farmers have progressively started to introduce exotic pasture species (e.g., Brachiaria decumbens, B. brizantha, etc.) in order to reach higher levels of productivity [9]. B. decumbens is a well-adapted species to a wide range of climate types, i.e., it can reach a high production even in years with an irregular interannual distribution of rainfall [10]. Local species, such as Paspalum notatum, are well-adapted to burning and low temperatures. Its rooting capacity is concentrated in the first centimeters promoting a good grass cover [11]. Usually, it is accompanied by a mismanagement of land resources because there are no regulations for animal stocking rates and it is not common to follow any form of animal rotation-based grazing system. All these factors lead to serious soil and pasture degradation at different scales [12] among other negative environmental consequences, such as soil compaction and soil erosion.
The environmental impact of grazing in rangelands and grasslands has been widely studied, particularly on soil properties [13], pasture production [14], losses of nutrients [15], and organic matter [16]. Soil and water losses in pasture lands have been also studied worldwide [17,18,19]. The effect of grass species on soil and water losses has received little attention, at least in Brazil. Nevertheless, Turner et al. [20] have shown how replacing perennials with annual species has had detrimental effects in many watersheds studied in the United States. In any case, these kinds of studies are still scarce in the faxinal system of Southern Brazil, where the effect of the introduction of exotic species has not been properly assessed. The main goal of this study was, therefore, to assess the impact of exotic pastures (Brachiaria decumbens), in comparison to native species (Paspalum notatum Flüggé), on soil properties, productivity, and soil and water losses in a representative faxinal farm. We hypothesize that the introduction of new pasture species is causing serious problems of soil erosion and, consequently, a decline in pasture productivity in the long-term.
2. Materials and Methods
2.1. Study Area
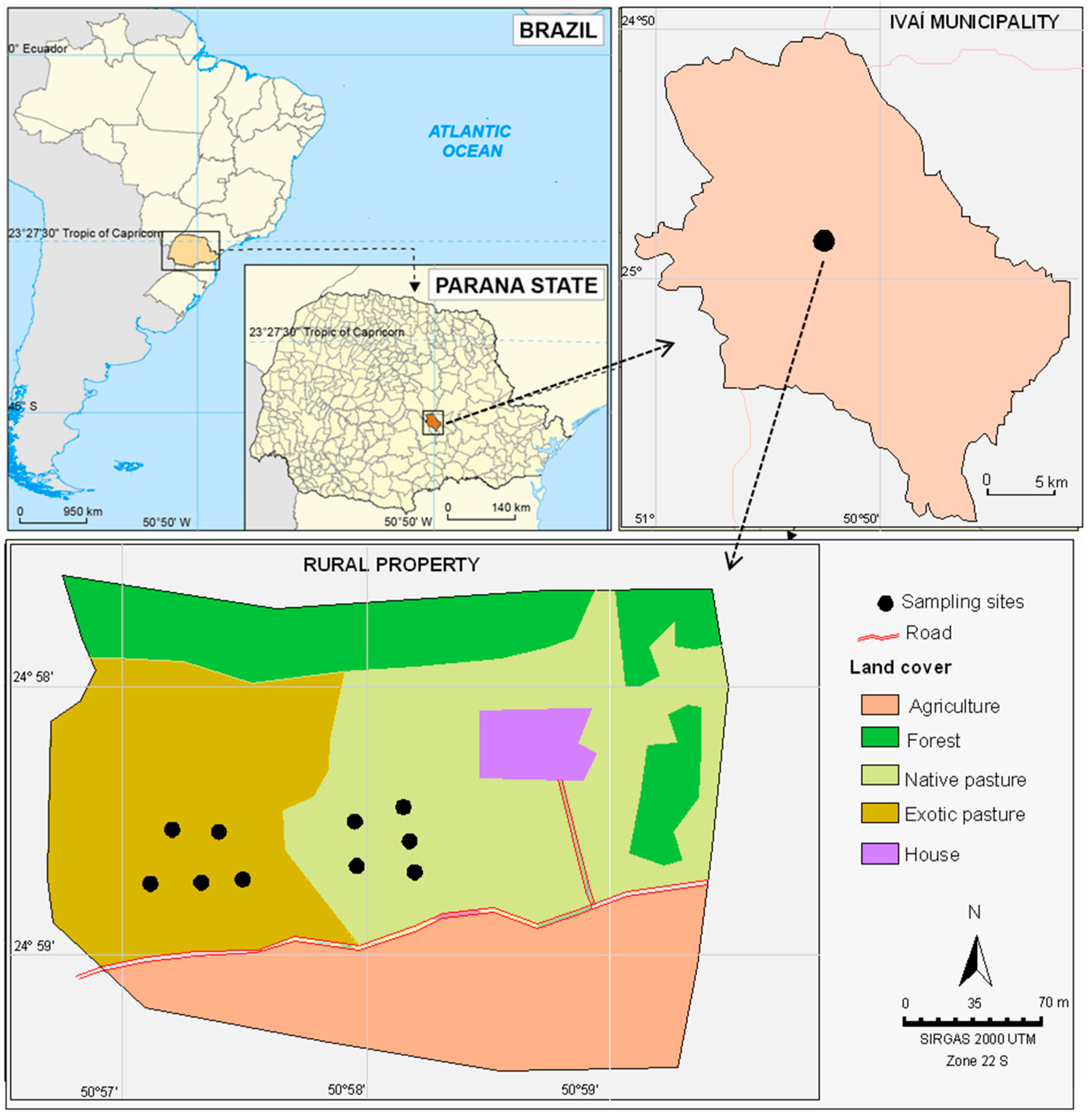
The study was carried out in an 8.0 ha rural property, of which 2.5 ha is exclusively used as pasture land, located in the municipality of Ivaí (Paraná state, Southern Brazil). The rest of the farm is used for agricultural purposes (4.0 ha), such as yerba mate plantations and afforestation of Eucalyptus globulus (1.5 ha) (Figure 1).
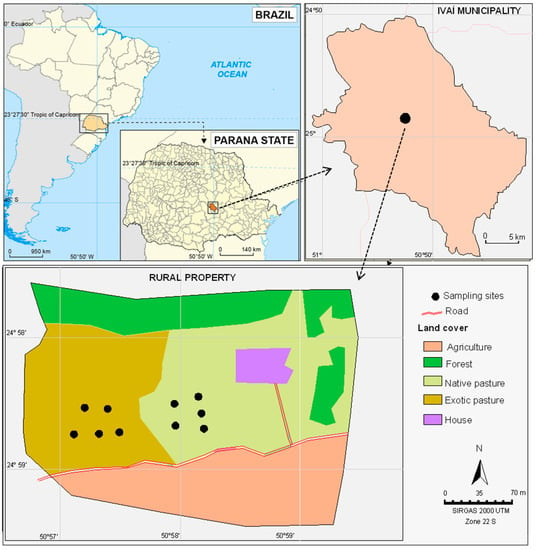
Figure 1.
Geographical location and description of the representative farm used as the study area.
Within the pasture land (2.5 ha), one part (1.4 ha) has been covered by perennial grasses (P. notatum Flüggé) as native pasture since 1995 and, in the other part (1.1 ha), the exotic grass species B. decumbens was introduced after removing the native pastures in 2003. There were no fences between both parts, i.e., animals (10 adult cows with an average weight of 450 kg) had no physical barriers for grazing in both types of pastures.
2.2. Sampling Design and Analysis
Two representative plots (native vs. exotic) were selected with the same slope inclination and similar soil properties in order to allow the comparison of the impact of the grass species only on soil and water losses. To achieve this goal, daily rainfall (measured using a Ville de Paris pluviometer-type devise), the percentage of bare soil surface [21], and soil and water losses after each rainfall event with sediment collectors [22] were quantified. A total number of five Gerlach troughs of 2 m2 (1 m width × 2 m length) were installed in each plot from July 2015 to June 2016. In Table 1, number (N) and dates of each rainfall event with soil erosion sampling were added.

Table 1.
Rainfall and soil erosion sampling dates during the studied period.
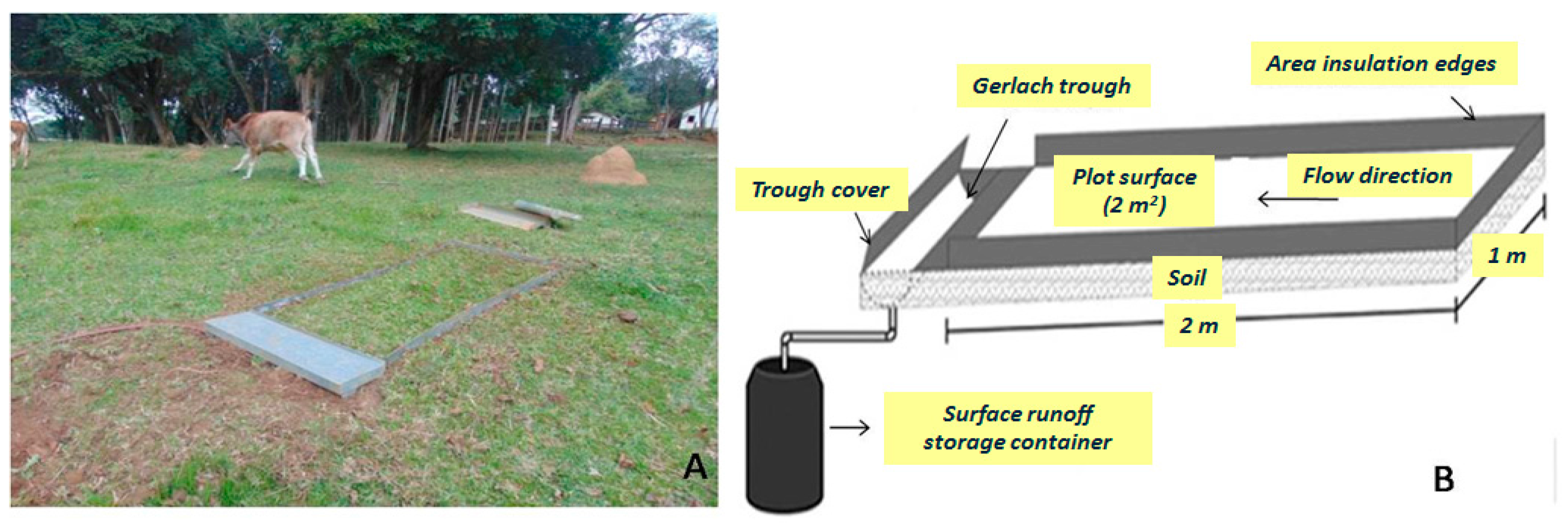
The erosion plots were closed in order to avoid transfers of water and sediments from external areas. Sediment yield and overland flow in each plot after each rainfall event were provisionally stored in 25-L containers. The contents were transported to the laboratory to filter, dry, and weigh the sediments. Animals could graze and freely move within the 10 installed erosion plots (Figure 2).
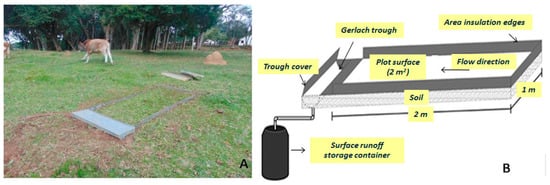
Figure 2.
Details of a parcel installed on native pasture (A) and an illustrative scheme of the parcel (B).
The percentage of bare ground surface was also estimated after each rainfall event using a 1 m2 grid with 100 squares by the same person in each plot. A representative soil profile of each plot was described [23] and classified [24]. A total of 10 bulk density soil samples from 0 to 10 cm depth were randomly collected in the backslope position and mixed in a single bag in order to determine the soil texture [25] and organic matter [26] in the laboratory. Finally, soil penetration resistance as an indicator of soil compaction was measured in each one of the erosion plots (five repetitions) three days after each rainfall event (≈field capacity) using a Humboldt H-4200 stainless steel soil pocket penetrometer.
2.3. Data Analysis
Descriptive statistical analysis, such as the mean and standard deviation, were used to describe the variables of each type of pasture (native vs. exotic). The statistical significance of the comparison of means between groups was conducted by a Tukey test with a level of significance below 5% of probabilities. Statistical procedures were carried out using Statistica 6.0 software package [27]. Soil and water losses were depicted in box plots and a one-way ANOVA was conducted to compare soil and water losses between the plots using SigmaPlot v.13.0 (Systat. Inc., San Jose, CA, USA).
3. Results
3.1. Soil Properties and Pasture Production
Table 2 shows the mean values of some soil properties and pasture production of each plot (native vs. exotic). Pastures were cultivated in moderately-steep slopes over clay loam-textured soils. Soils were classified as Dystro-ferric Cambisol, with low fertility and high iron contents in the superficial horizons [24]. No significant differences are found in the soil grain size distribution among plots. The native pastures show a higher content of soil organic matter (6.3 g k−1) and a lower bulk density (1.29 g cm−3) than the cultivated pastures (4.8 g k−1 and 1.34 g cm−3). The mean pasture production of exotic pasture was significantly higher (987 kg DM ha−1) than in the native plots (204 kg DM ha−1).

Table 2.
Mean values of soil properties and pasture production of each plot type. Different lower-case letters (a and b) in the same line indicate significant differences (p < 0.05, Tukey test).
3.2. Soil and Water Losses
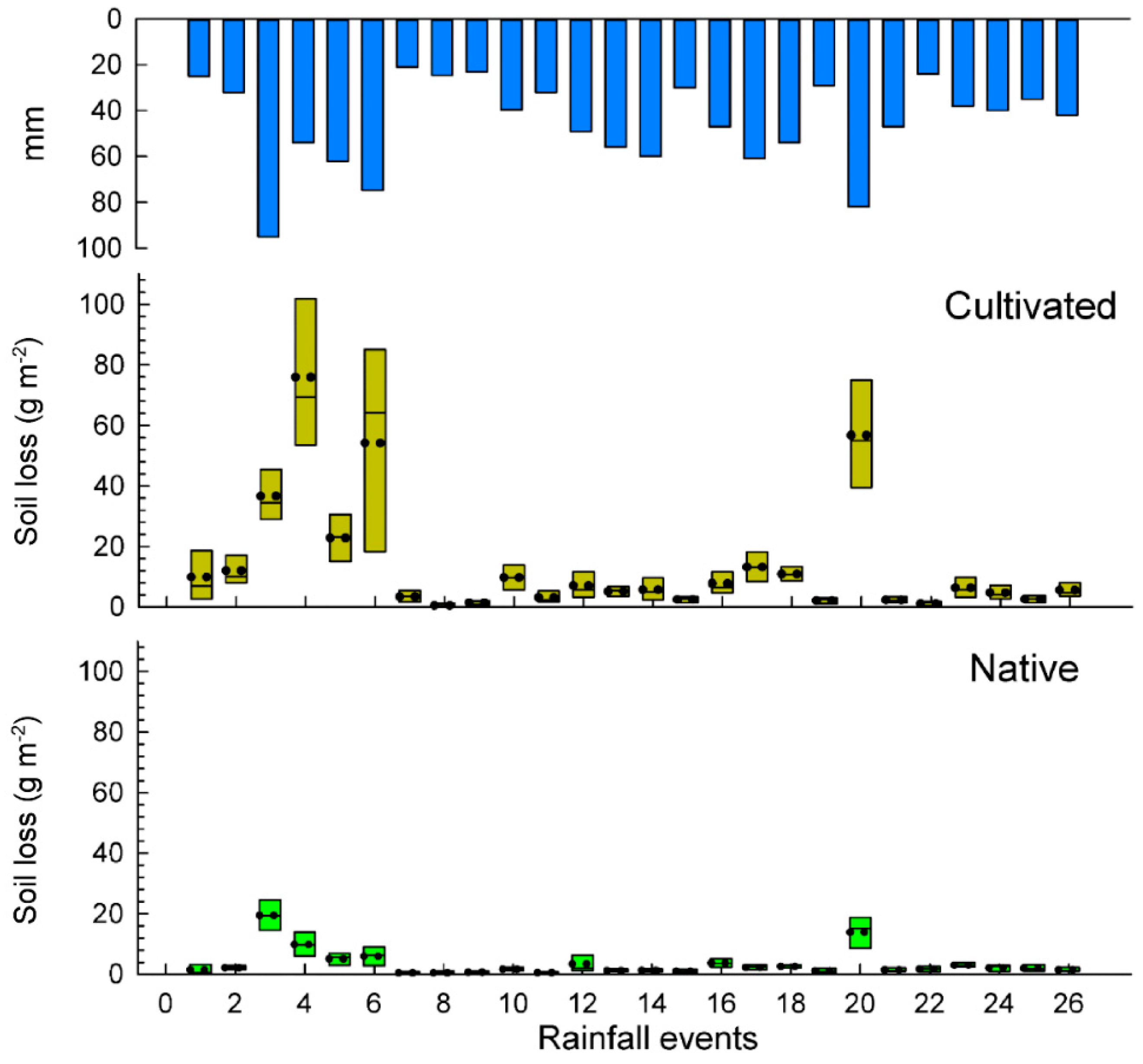
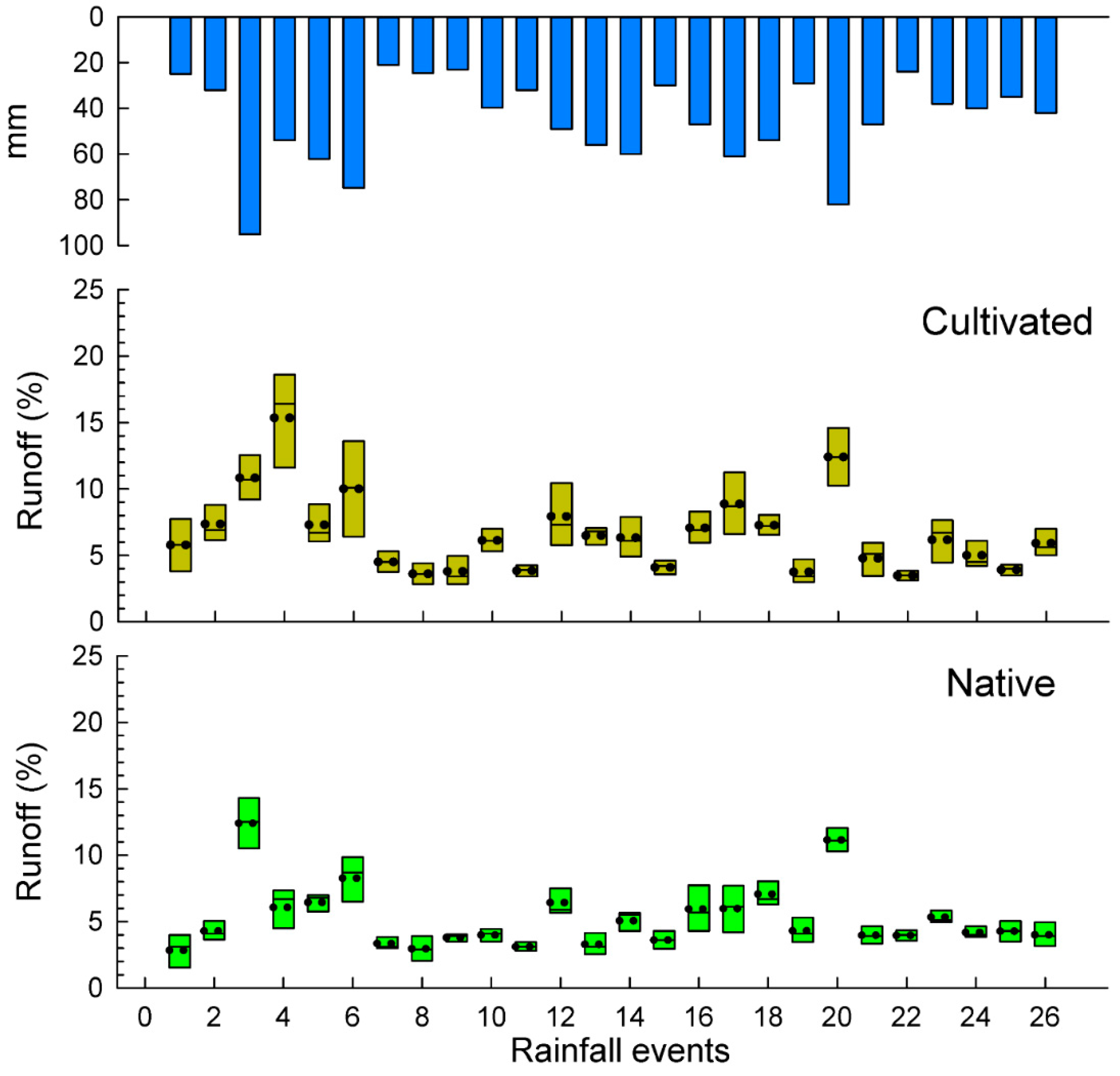
A total number of 26 rainfall events were recorded during the studied period totaling an annual rainfall amount of 1177 mm from 1 July 2015 to 30 June 2016. Soil losses and runoff are depicted in box plots per rainfall event (Figure 3 and Figure 4) and total results are summarized in the Table 2. Some rainfall events (<24 mm) did not cause significant differences in water losses among both plots. The total water losses are significantly higher in the exotic pastures (88.6 L m−2) than in the native ones (71.8 L m−2), which mean an average of 6.6% and 5.2% for the runoff coefficients, respectively. These results show a statistical significance (p < 0.036) for the total average runoff per rainfall event.
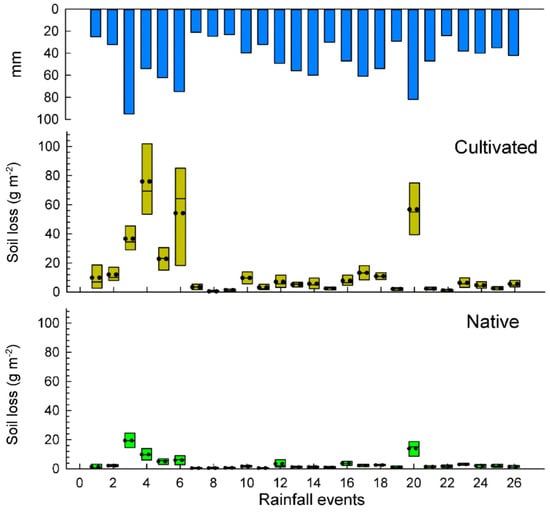
Figure 3.
Box plots of soil losses in cultivated and nature pasture plots.
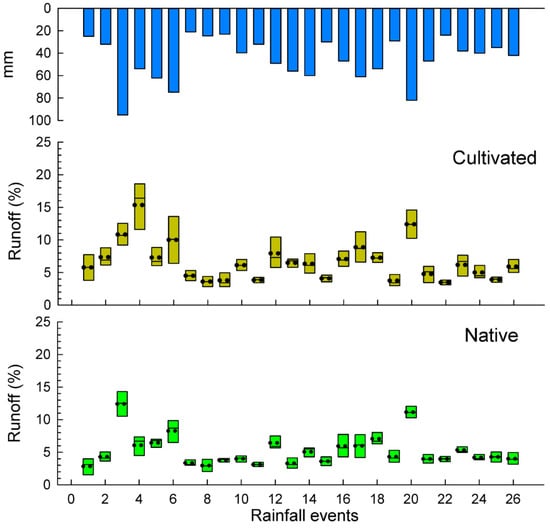
Figure 4.
Box plots of runoff coefficients in cultivated and nature pasture plots.
Cultivated pastures recorded a total soil loss of 364.8 g m-2 (3.6 mg ha−1) which meant it was 4.0 times higher than in native pastures, which registered 90.7 g m−2 (0.91 mg ha−1). After applying a Tukey test, significant differences (p < 0.001) were obtained for the total average soil loss per rainfall event in the two pasture types.
Rainfall events lower than 30 mm did not show significant differences in soil loss (the mean value of each event) for the two types of pastures, however, the mean value was three times higher for the exotic pasture (native 1.0 g m−2 vs. exotic 3.0 g m−2). However, rainfall events higher than 30 mm showed significant differences between native and exotic pastures, reaching 4.4 g m−2 and 18.1 g m−2, respectively.
A significant contrast was found in the rainiest event (E3: 95 mm), where soil losses in the native pastures were only 12.4 g m−2 and, on the other plots, had up to three times higher soil losses (36.7 g m−2) for the cultivation of B. decumbens.
Finally, in Table 3 the average values of sediment concentration of 1.2 and 4.5 g L−1 can be observed for the nature and exotic pasture, respectively. Additionally, they showed significant differences (p < 0.001) among them.

Table 3.
Mean values of soil erosion parameters; Rc: runoff coefficient, SC: sediment concentration.
3.3. Bare Soil and Penetration Resistance
Data of the percentage of bare ground and soil penetration resistance as an indicator of the soil compaction degree can be observed in the Table 4. In general, exotic pastures show a higher soil compaction than the native species. In native pasture plots, a total average of 2.73 ± 0.32 MPa was measured. The maximum value reached is 3.5 MPa, and 2.4 MPa is the minimum measured. On the other hand, for exotic pastures, values as high as 3.25 ± 0.25 MPa were found for the mean soil penetration. The highest value found was 3.7 MPa and the lowest one was 2.9 MPa. The Tukey test to observe the statistical differences among plots proved the two sites to be statistical significantly different (p < 0.001).

Table 4.
Mean values of bare soil (%) and penetration resistance (SPR).
The percentage of bare ground was estimated after each rainfall event (n = 26). As average plots with exotic pastures recorded a mean value (19.16% ± 2.31) much higher than in plots with native pastures (9.99% ± 1.66). The percentage of bare ground in exotic plots ranged from 15 to 24%; meanwhile, in the native ones, these ranged from 7 to 13%. The data variability was not so high (CV < 20%) in both cases.
3.4. Relationships between Variables
Table 5 shows the coefficients of correlations among all the studied variables. Bare soil looked to be the most influencing factor on soil loss in the exotic pastures (r = 0.452, p < 0.05); meanwhile, penetration resistance had the highest influence on soil loss in the native pastures (r = 0.532, p < 0.01) although it was not significant in the exotic ones. Rainfall was positively correlated with soil and water losses in both pastures (native and exotic), but these coefficients were higher in the native pasture (r = 0.795, p < 0.01; soil loss). Furthermore, penetration resistance was positively correlated with bare soil, being particularly high in the exotic pastures (r = 0.879, p < 0.001). The percentage of bare soil was a key influencing factor on soil and water losses, as well as on penetration resistance on exotic pastures.

Table 5.
Pearson’s correlation coefficients among soil erosion parameters, rainfall, bare soil percentage, and penetration resistance of both types of pastures. Significant correlations have been highlighted in bold. *, **, *** mean significant at p < 0.05, < 0.01, and < 0.001, respectively.
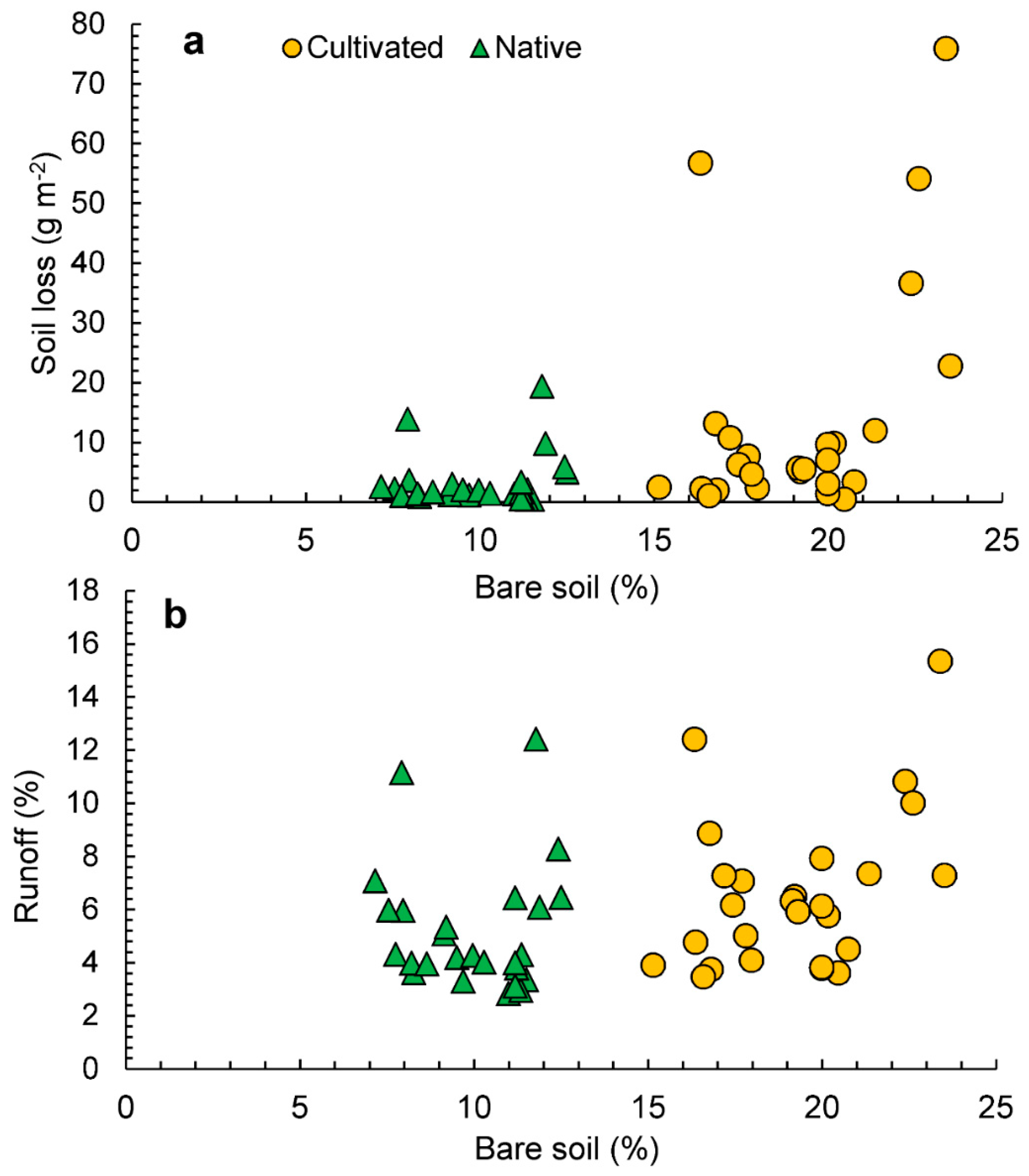
Figure 5a shows the scatterplot of bare soil against soil losses. In the native pastures, we observed soil losses lower than 10 g m−2, which is produced under a soil exposure ranging from 9 to 24%. In the cultivated pastures, soil losses increased as the percentage of bare soil increased. Soil losses up to 20 g m−2 are generated with a percentage of bare soil lower than 20%. The highest losses are recorded when soil exposure reached percentages from 30 to 35%.
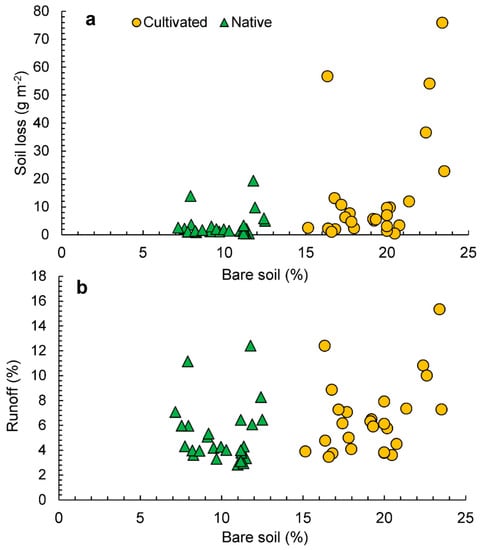
Figure 5.
Scatterplots of bare soil, soil losses (a), and runoff (b) in cultivated and native pastures.
In Figure 5b, scatterplots of soil bare ground against runoff coefficient by groups are depicted. Rainfall is highly correlated with water losses in both cases: native (r = 0.895, p < 0.001) and exotic (r = 0.627, p < 0.001). In the latter case, some data variability is found in rainfall events lower than 30 mm ranging from 25 to 35% rainfall runoff coefficient. Rainfall events higher 60 mm are able to generate significant runoff coefficients in both cases (see Figure 4). The water loss dynamics condition soil loss production. Figure 5b shows the scatterplot of water losses against soil losses in both cases. In both the native, as well as the cultivated, pastures there is a high correlation between water and soil losses (r = 0.773, p < 0.001 for native, and r = 0.570 for cultivated, pastures).
4. Discussion
We have compared values obtained from nearby plots which were located on similar topographical and edaphic conditions. Nevertheless, the effects of cultivating, or not, exotic pasture species since 2003 can be now recognized. The cultivated area recorded a higher bulk density and content of soil organic matter 1.5% lower than in native plots. This fact could be due to the necessity of some tillage during the process of seeding. These values partially agree with those showed by Neil and Davidson [28] who found gains or losses of C depending on the type of Brachiaria.
The most outstanding effects of cultivating pastures were observed in soil losses because exotic plots recorded losses up to four times higher than in the native plots. Native species of perennial grasses, such as P. notatum Flüggé, are highly valuable in terms of soil protection by its capacity of generating large covered patches at the soil surface level due to its rooting capacity [29]. It has stems of the short rhizome type and prostrates aerial, flat, and green or reddish shoots which grow sympodially, quickly covering the area where the plant is installed. Furthermore, this species is heat-tolerant (tropical areas), and it can grow in soils with low fertility and water deficit (semi-arid areas), as well as under overgrazing conditions [30]. Nevertheless, farmers are reluctant to continue using this pasture because its productivity is up to five times lower than other exotic species.
Regarding the water losses, the runoff coefficient was not significantly different between both types: native 5.7% vs. exotic 8.7%. Although these values are slightly higher than those reported by Vadas et al. [15] in comparable conditions, nevertheless, our results suggest moderate rainfall (25–39 mm) has a negative effect in terms of water losses in the cultivated pastures of B. decumbens. Moreover, if bulk density and penetration resistance are higher and, consequently, porosity is lower, therefore, the infiltration will be lower and runoff higher. This causes more transport capacity and more soil loss in the cultivated sites.
Soil losses were significantly higher in the cultivated pastures than in the native plot. This latter recorded value of soil loss (151.8 g m−2) is similar to those published by da Rocha Junior [19] in mixed livestock-crop farming (108.0 g m−2). Nonetheless, the main soil loss found in cultivated pastures (359.8 g m−2) is almost double that of the main soil loss for pasture land estimated by Panagos et al. [31] in Europe (202.0 g m−2).The higher soil and water losses quantified in cultivated pastures can be due to their inherent characteristics. A higher soil loss was observed in more clayey soils. Thus, a greater capacity to withstand erosion through binding forces (clay) can become more erosive if those binds are broken.
Cultivated pastures of B. decumbens were more productive (Table 1) than the native pastures. It probably produced a higher concentration of animals grazing in this part of the farm. In this case, higher water and soil loss rates can be explained as a consequence of a higher grazing pressure [32] since animal trampling and defoliation causes a reduction in the number of plants, biomass availability, and litter cover that protects soils and provides organic matter for plants [33].
The cultivated pastures showed a higher percentage of bare ground surfaces (24.8 ± 6.7%) than the native pastures (13.4 ± 3.8%). These values are lower than those quantified by Zhou et al. [13] in China. Bartley et al. [34] reported soil and water losses between 6 and 9 times higher in (pasture land) hills with large patches of bare soil than in other comparable vegetation covered hills at the surface level. This reduction in vegetation cover causes a decrease of soil moisture because more water is lost by surface runoff. Exposed soil is more vulnerable to splash effects provoking a higher soil detachment. Soil porosity is also reduced making the capacity of soils to infiltrate water and soil aeration more difficult.
Soil compaction looked to be the most influencing factor on soil loss in the native pastures. Since soil was relatively well-covered throughout the year, animal trampling may have provoked the soil and water losses [35,36,37]. Nevertheless, larger surfaces of bare ground in spring time should be considered as a good indicator of soil compaction and a serious problem for farmers in terms of pasture productivity [14,38,39].
Our findings clearly show the two sides of the coin linked to the introduction of new pasture species. From a conservationist point of view, soils are altered and, consequently, soil, water, and nutrient losses are higher in the exotic pasture than in the native pastures. However, from an economic profitability point of view, farmers need to produce more biomass to avoid extra expenses in fodder supply acquisition. Therefore, we recommend looking for a balanced management approach that guarantees sustainable use of pastures with appropriate inputs by farmers. Solutions may focus on promoting rotational grazing systems adjusting animal stocking rates to land capacity for pasture production, and finding ways of fertilizing that can enhance the productivity of native species.
5. Conclusions
The introduction of exotic species of pastures, such as Brachiaria decumbens, replacing well-adapted local species, such as Paspalum notatum Flüggé, in the faxinal system of Southern Brazil induces increased soil erosion. Native species properly protect soil due to their carpet effect even under conditions of heavy grazing. Nevertheless, the newly-introduced cultivated pastures reached a production up to five times higher than the native ones. It can be very positive in terms of incomes for farmers, but it is indirectly promoting practices considered as land mismanagement, such as overgrazing and overstocking. Our findings suggest farmers must find a balance between productivist practices, such as the introduction of new pastures, and conservationist techniques, such as rotational grazing, reduction of animal stocking rates, or seek alternative improvements to increase the productivity of native pastures.
Author Contributions
The Brazilian group conceived and designed the experiments, performed the experiments, analyzed the data and contributed reagents/materials/analysis tools. V.A., M.P.F., A.C., S.D.K. and J.R.-C. wrote the paper and made the figures and tables.
Acknowledgments
The study was financially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil—grant number 457595/2014-0 MCTI/CNPQ/Universal 14/2014—Faixa A). We would also like to thank the anonymous reviewers for their useful comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Campigoto, J.A.; Bona, A.N. A Hermenêutica e a origem dos faxinais. Revista de História Regional 2010, 14, 127–153. [Google Scholar] [CrossRef]
- Gonçalves Cunha, L.A. Desenvolvimento Rural e Desenvolvimento Territorial: O Caso do Paraná Tradicional. Ph.D. Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2003. [Google Scholar]
- Watzlawick, L.F.; de Albuquerque, J.M.; Redin, C.G.; Longhi, R.V.; Longhi, S.J. Estrutura, diversidade e distribuição espacial da vegetação arbórea na Floresta Ombrófila Mista em Sistema Faxinal, Rebouças (PR). Ambiência 2011, 7, 415–427. [Google Scholar] [CrossRef][Green Version]
- Turner, B.L.; Tidwell, V.; Fernald, A.; Rivera, J.A.; Rodriguez, S.; Guldan, S.; Ochoa, C.; Hurd, B.; Boykin, K.; Cibils, A. Modeling acequia irrigation systems using system dynamics: Model development, evaluation, and sensitivity analyses to investigate effects of socio-economic and biophysical feedbacks. Sustainability 2016, 8, 1019. [Google Scholar] [CrossRef]
- Gunda, T.; Turner, B.; Tidwell, V.C. The influential role of sociocultural feedbacks on vommunity-managed irrigation system behaviors during times of water stress. Water Resour. Res. 2018, 54, 1–18. [Google Scholar]
- Chang, M.Y. Sistema Faxinal: Uma Forma de Organização Camponesa em Desagregação no Centro-sul do Paraná; Boletim Técnico IAPAR nº22: Londrina, Brazil1, 1988; p. 124. [Google Scholar]
- Thomaz, E.L.; Antoneli, V. Rain interception in a secondary fragment of araucaria forest with Faxinal, Guarapuava-PR. CERNE 2015, 21, 363–369. [Google Scholar] [CrossRef]
- Galvão Leite, D.M.; Cherumbim, A.A. Caracterização da criação animal em Sistema Faxinal. Rev. Bras. Agroecol. 2009, 4, 3959–3962. [Google Scholar]
- Martinkoski, L.; Vogel, G.F.; Jadoski, S.O.; Watzlawick, L.F. Soil physical quality under silvopastoral management and secondary forest. Floresta Ambiente 2017, 24, e20160282. [Google Scholar]
- Loch, D. Brachiaria decumbens (Signal grass)—A review with particular reference to Australia. Trop. Grassl. 1977, 11, 141–157. [Google Scholar]
- Wilson, J.R.; Hill, K.; Cameron, D.; Shelton, H.M. The growth of Paspalum notatum under the shade of a Eucalyptus grandis plantation canopy or in full sun. Trop. Grassl. 1990, 24, 24–28. [Google Scholar]
- Martha Júnior, G.B.; Corsi, M. Pastagens no Brasil: Situação atual e perspectivas. Preços Agrícolas 2001, 15, 3–6. [Google Scholar]
- Zhou, Z.; Gan, Z.; Shangguan, Z.; Dong, Z. Effects of grazing on soil physical properties and soil erodibility in semiarid grassland of the Northern Loess Plateau (China). CATENA 2010, 82, 87–91. [Google Scholar] [CrossRef]
- Pulido, M.; Schnabel, S.; Lavado Contador, J.F.; Lozano-Parra, J.; González, F. The impact of heavy grazing on soil quality and pasture production in rangelands of SW Spain. Land Degrad. Dev. 2018, 29, 219–230. [Google Scholar] [CrossRef]
- Vadas, P.A.; Busch, D.L.; Powell, J.M.; Brink, G.E. Monitoring runoff from cattle-grazed pastures for a phosphorus loss quantification tool. Agric. Ecosyst. Environ. 2015, 199, 124–131. [Google Scholar] [CrossRef]
- Pulido-Fernández, M.; Schnabel, S.; Lavado-Contador, J.F.; Miralles Mellado, I.; Ortega Pérez, R. Soil organic matter of Iberian open woodland rangelands as influenced by vegetation cover and land management. CATENA 2013, 109, 13–24. [Google Scholar] [CrossRef]
- Teague, W.; Dowhower, S.; Baker, S.; Ansley, R.; Kreuter, U.; Conover, D.; Waggoner, J. Soil and herbaceous plant responses to summer patch burns under continuous and rotational grazing. Agric. Ecosyst. Environ. 2010, 137, 113–123. [Google Scholar] [CrossRef]
- Van Oudenhoven, A.P.; Veerkamp, C.J.; Alkemade, R.; Leemans, R. Effects of different management regimes on soil erosion and surface runoff in semi-arid to sub-humid rangelands. J. Arid Environ. 2015, 121, 100–111. [Google Scholar] [CrossRef]
- Da Rocha Junior, P.R.; Andrade, F.V.; de Sá Mendonça, E.; Donagemma, G.K.; Fernandes, R.B.A.; Bhattharai, R.; Kalita, P.K. Soil, water, and nutrient losses from management alternatives for degraded pasture in Brazilian Atlantic Rainforest biome. Sci. Total Environ. 2017, 583, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Fuhrer, J.; Wuellner, M.; Menendez, H.M.; Dunn, B.H.; Gates, R. Scientific case studies in land-use driven soil erosion in the central United States: Why soil potential and risk concepts should be included in the principles of soil health. Int. Soil Water Conserv. Res. 2018, 6, 63–78. [Google Scholar] [CrossRef]
- Antoneli, V.; Thomaz, E.L. Comparação de infiltração de água no solo mensurada em período seco e úmido, em diferentes usos da terra na bacia do arroio Boa Vista, Guamiranga, Paraná. Ambiência 2009, 5, 301–318. [Google Scholar]
- Gerlach, T. Hillslope troughs for measuring sediment movement. Révue Géomorphologie Dynamique 1967, 4, 173. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Guidelines for Soil Description, 4th ed.; FAO: Rome, Italy, 2006; p. 97. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; p. 203. [Google Scholar]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Walkley, A.; Black, L.A. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Statsoft. STATISTICA (Data Analysis Software System), Version 6. 2001. Available online: www.statsoft.com (accessed on 7 May 2018).
- Neill, C.; Davidson, E. Soil carbon accumulation or loss following deforestation for pasture in the Brazilian Amazon. In Global Climate Change and Tropical Ecosystems; Lal, R., Kimble, J.M., Stewart, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 197–211. [Google Scholar]
- Ye, C.; Guo, Z.; Li, Z.; Cai, C. The effect of Bahiagrass roots on soil erosion resistance of Aquults in subtropical China. Geomorphology 2017, 285, 82–93. [Google Scholar] [CrossRef]
- Agharkar, M.; Lomba, P.; Altpeter, F.; Zhang, H.; Kenworthy, K.; Lange, T. Stable expression of AtGA2ox1 in a low-input turfgrass (Paspalum notatum Flugge) reduces bioactive gibberellin levels and improves turf quality under field conditions. Plant Biotechnol. J. 2007, 5, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Panagos, P.; Borrelli, P.; Meusburger, K.; Alewell, C.; Lugato, E.; Montanarella, L. Estimating the soil erosion cover-management factor at the European scale. Land Use Policy 2015, 48, 38–50. [Google Scholar] [CrossRef]
- Stavi, I.; Ungar, E.D.; Lavee, H.; Sarah, P. Grazing-induced spatial variability of soil bulk density and content of moisture, organic carbon and calcium carbonate in a semi-arid rangeland. CATENA 2008, 75, 288–296. [Google Scholar] [CrossRef]
- da Silva, A.P.; Imhoff, S.; Corsi, M. Evaluation of soil compaction in an irrigated short-duration grazing system. Soil Tillage Res. 2003, 70, 83–90. [Google Scholar] [CrossRef]
- Bartley, R.; Roth, C.H.; Ludwig, J.; McJanett, D.; Liedloff, A.; Corfield, J.; Hawdon, A.; Abbott, B. Runoff and erosion from Australia’s tropical semi-arid rangelands: Influence of ground cover for differing space and time scales. Hydrol. Process. 2006, 20, 3317–3333. [Google Scholar] [CrossRef]
- Mulholland, B.; Fullen, M.A. Cattle trampling and soil compaction on loamy sands. Soil Use Manag. 1991, 7, 189–193. [Google Scholar] [CrossRef]
- Evans, R. Soil erosion in the UK initiated by grazing animals. Geography 1997, 17, 127–141. [Google Scholar] [CrossRef]
- Bilotta, G.S.; Brazier, R.E.; Haygarth, P.M. The impacts of grazing animals on the quality of soils, vegetation, and surface waters in intensively managed grasslands. Adv. Agron. 2007, 94, 237–280. [Google Scholar]
- Pulido, M.; Schnabel, S.; Contador, J.F.L.; Lozano-Parra, J.; Gómez-Gutiérrez, Á.; Brevik, E.C.; Cerdà, A. Reduction of the frequency of herbaceous roots as an effect of soil compaction induced by heavy grazing in rangelands of SW Spain. CATENA 2017, 158, 381–389. [Google Scholar] [CrossRef]
- Pulido, M.; Schnabel, S.; Contador, J.F.L.; Lozano-Parra, J.; Gómez-Gutiérrez, Á. Selecting indicators for assessing soil quality and degradation in rangelands of Extremadura (SW Spain). Ecol. Indic. 2017, 74, 49–61. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).