Adsorption of Pb and Cu from Aqueous Solutions by Raw and Heat-Treated Attapulgite Clay
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Mineralogical Composition of Raw and Heat-Treated Clay
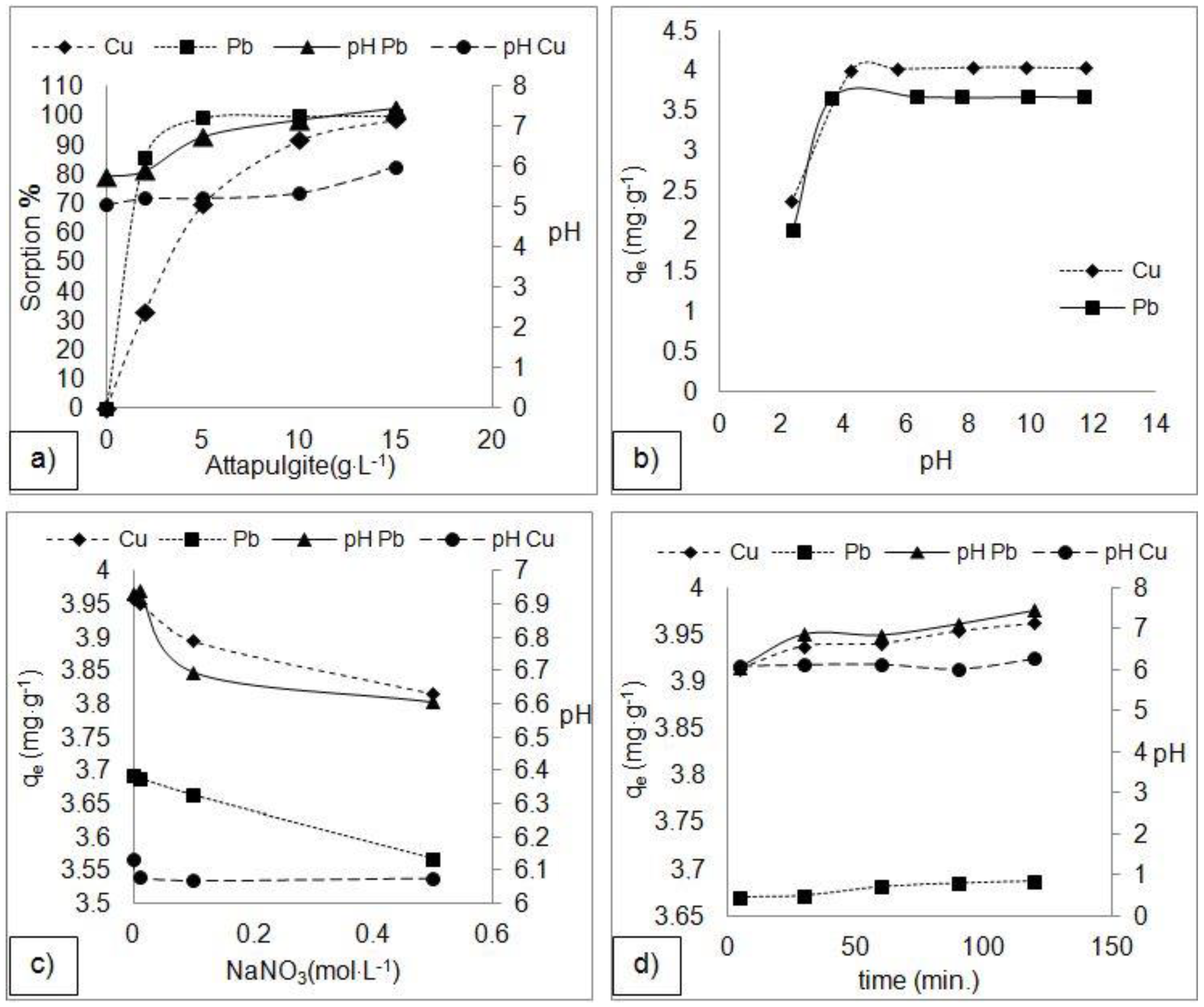
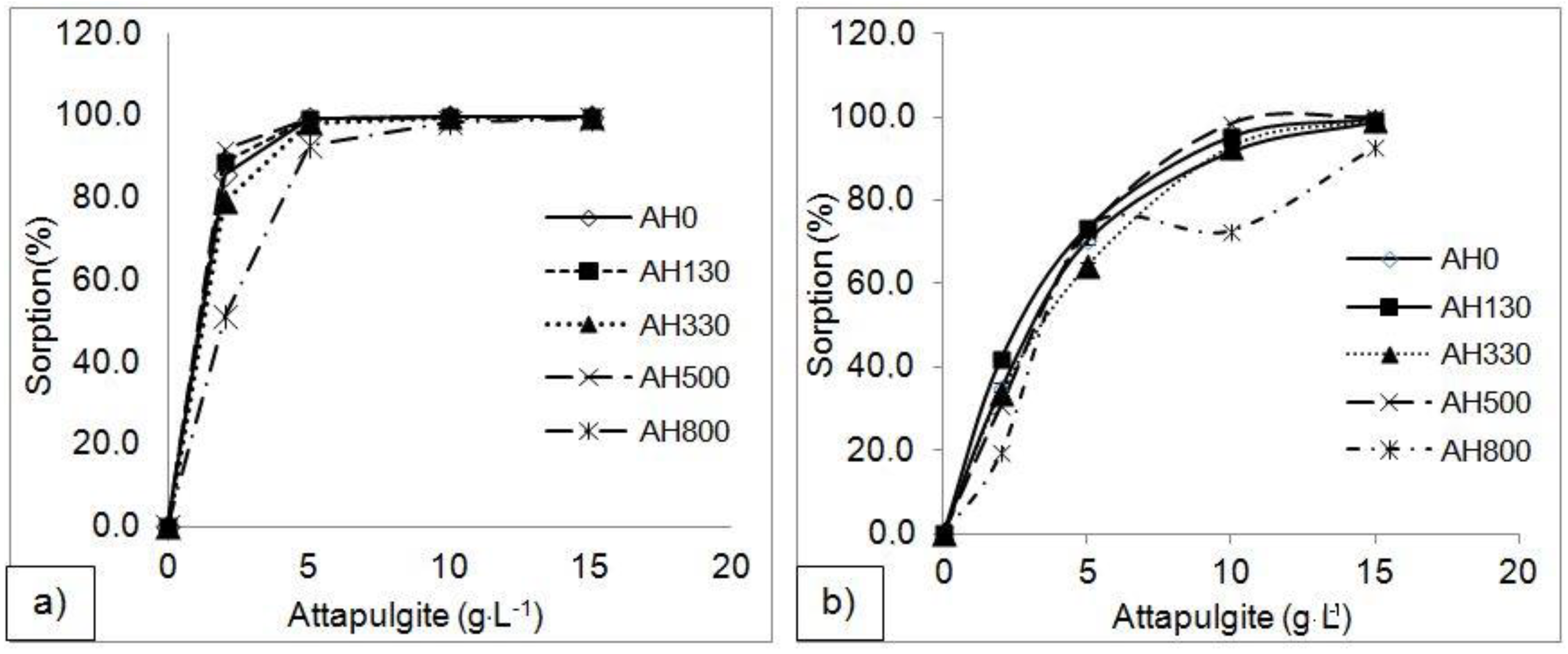
3.2. Adsorption Experiment Results
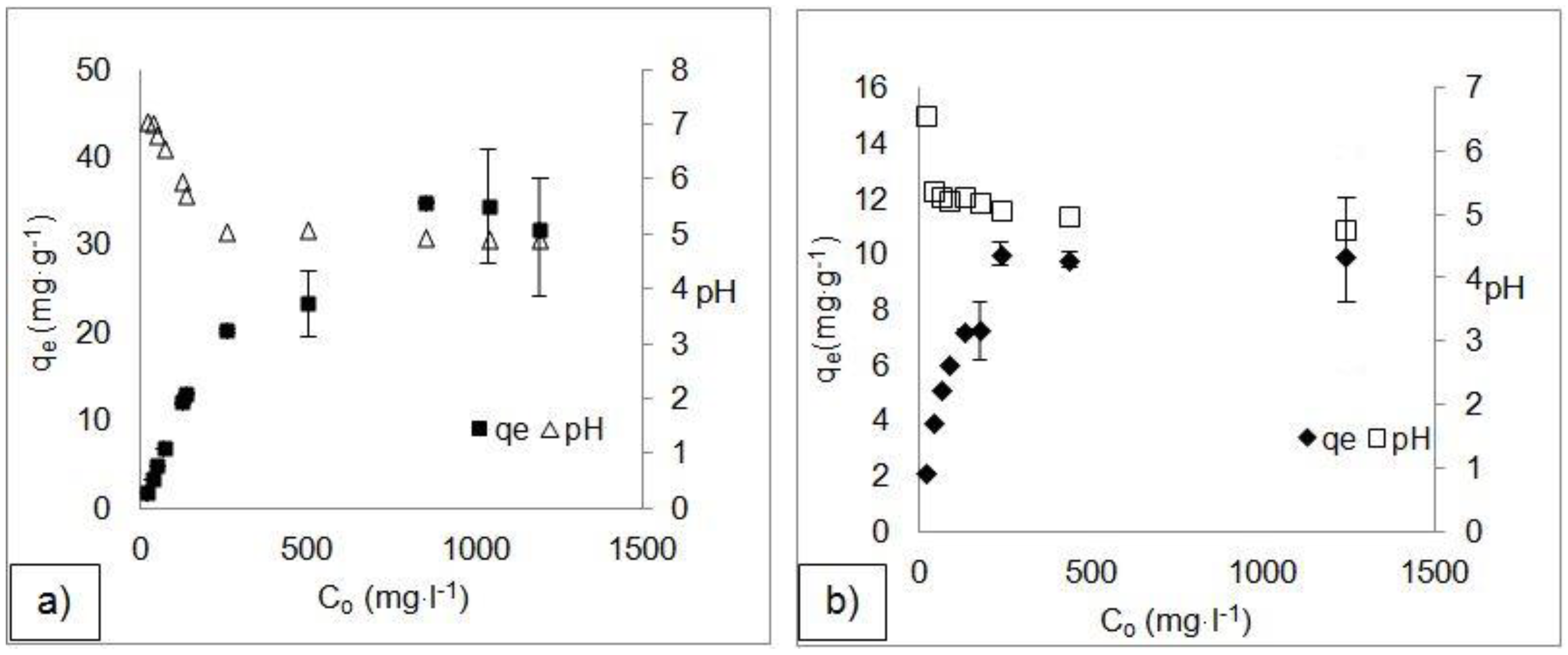
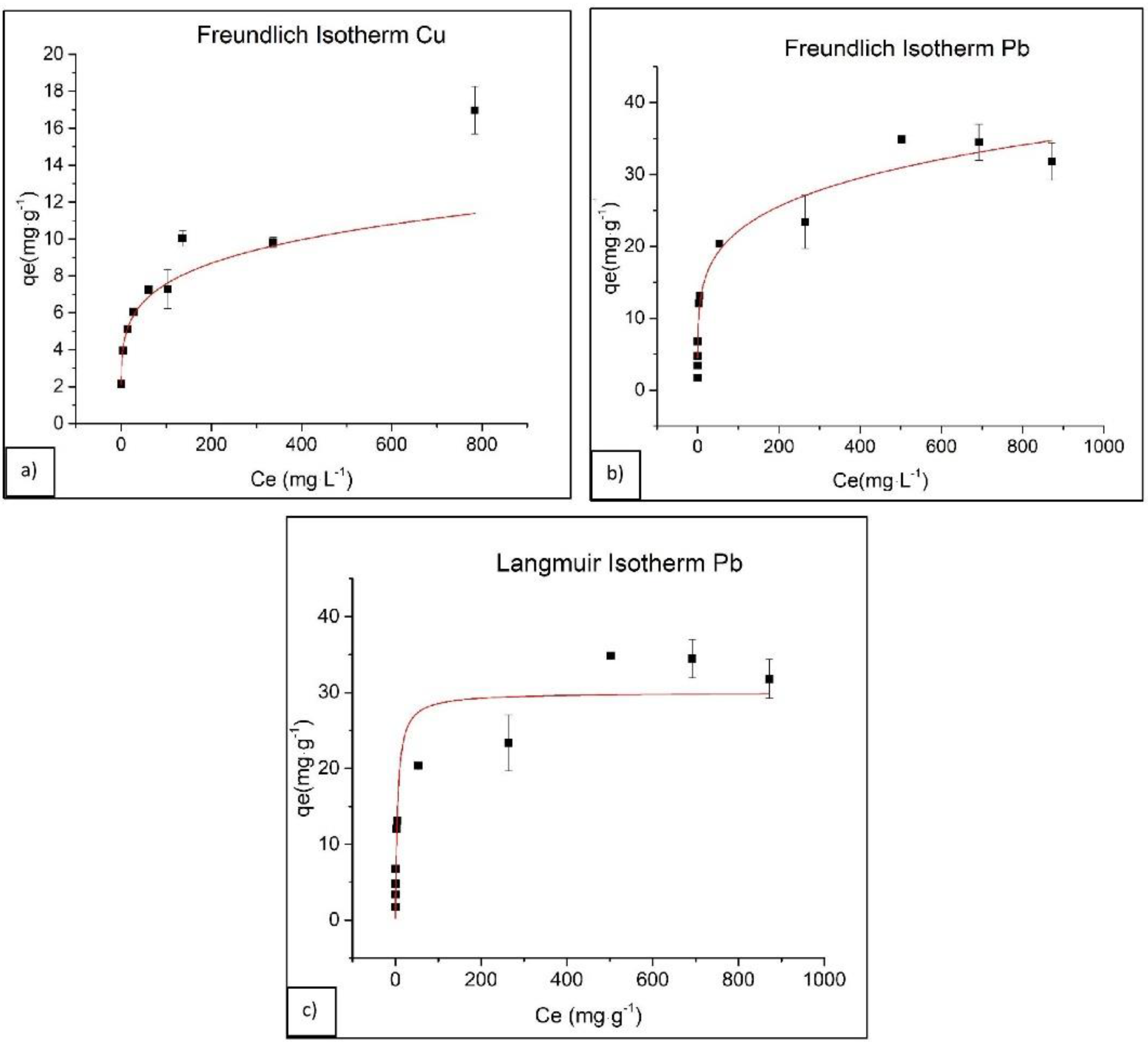
3.3. Adsorption Isotherms
4. Discussion
4.1. Effect of Experimental Parameters on Adsorption Efficiency
4.2. Comparison of Greek Attapulgite Sorption Efficiency with Other Clay Materials
4.3. Uncertainty Issues Affecting the Adsorption Modelling Process
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rouff, A.A.; Reeder, R.J.; Fisher, N.S. Pb (II) sorption with calcite: A radiotracer study. Aquat. Geochem. 2002, 8, 203–228. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I.; Martinex, M.; Miralles, N.; Poch, J.; Serarols, J. Sorption of Pb (II), Ni (II), Cu (II), and Cd (II) from aqueous solution by olive stone waste. Sep. Purif. Technol. 2006, 50, 132–140. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Y.; Wang, A. Removal of Cu (II) from aqueous solution by adsorption onto acid-activated attapulgite. J. Hazard. Mater. 2007, 149, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Sauve, S.; Martinez, C.E.; McBride, M.B.; Hendershot, W.H. Adsorption of free lead by pedogenic oxides, ferrihydrite and leaf compost. Soil Sci. Soc. Am. J. 2000, 64, 595–599. [Google Scholar] [CrossRef]
- Galan, E. Properties and Applications of Attapulgite-Sepiolite Clays. Clay Miner. 1996, 31, 443–453. [Google Scholar] [CrossRef]
- Singer, A. Palygorskite and sepiolite group minerals. Miner. Soil Environ. 1989, 829–872. [Google Scholar]
- El-mofty, S.E.; Ashour, F.H.; El-Shall, H. Adsorption Mechanism of Toxic Metal Ions by Clay (Attapulgite). In Proceedings of the Twelfth International Water Technology Conference, IWTC12 2008, Alexandria, Egypt, 1 January 2008; pp. 403–414. [Google Scholar]
- Chiari, G.; Giustetto, R.; Ricchiardi, G. Crystal structure refinements of attapulgite and Maya Blue from molecular modelling and powder synchrotron diffraction. Eur. J. Mineral. 2003, 15, 21–33. [Google Scholar] [CrossRef]
- Giustetto, R.; Xamena, F.X.L.; Ricchiardi, G.; Borgiga, S.; Danim, A.; Gobetto, R.; Chirotii, M.R. Maya Blue: A computational and spectroscope study. J. Phys. Chem. B 2005, 109, 19360–19368. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.H.; Li, Z.; Yu, T.L.; Munkhbayer, S.; Kuo, T.H.; Hung, Y.C.; Lin, K.H. Sorptive removal of tetracycline from water by attapulgite. J. Hazard. Mater. 2009, 165, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ayuso, E.; García-Sánchez, A. Palygorskite as a feasible amendment to stabilize heavy metal polluted soils. Environ. Pollut. 2003, 125, 337–344. [Google Scholar] [CrossRef]
- Zotiadis, V.; Argyraki, A.; Theologou, E. A Pilot Scale Application of Attapulgitic Clay for Stabilization of Toxic Elements in Contaminated Soil. J. Geotech. Geoenviron. Eng. 2012, 138, 633–637. [Google Scholar] [CrossRef]
- Yin, H.; Han, M.; Tang, W. Phosphorus sorption and supply from eutrophic lake sediment amended with thermally-treated calcium-rich attapulgite and a safety evaluation. Chem. Eng. J. 2016, 285, 671–678. [Google Scholar] [CrossRef]
- Fan, Q.; Li, Z.; Zhao, H.; Jia, Z.; Xu, J.; Wu, W. Adsorption of Pb (II) on attapulgite from aqueous solution: Effects of pH, ionic strength and temperature. Appl. Clay Sci. 2009, 45, 111–116. [Google Scholar] [CrossRef]
- Potgieter, J.H.; Potgieter-Vermaak, S.S.; Kalibantonga, P.D. Heavy metals removal from solution by attapulgite clay. Miner. Eng. 2006, 19, 463–470. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthes, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Bolster, C.H.; Hornberger, G.M. On the use of linearized Langmuir equations. Soil Sci. Soc. Am. J. 2007, 71, 1796–1806. [Google Scholar] [CrossRef]
- Thompson, A.F.B.; Jackson, K.J. Reactive transport in heterogeneous system: An overview. React. Transp. Porous Media 1996, 34, 269–310. [Google Scholar]
- Wang, W.; Chen, H.; Wang, A. Adsorption characteristics of Cd (II) from aqueous solutions onto activated attapulgite. Sep. Purif. Technol. 2007, 55, 157–164. [Google Scholar] [CrossRef]
- Xu, D.; Shao, D.D.; Chen, C.L.; Ren, A.P.; Wang, X.K. Effect of pH and fulvic acid on sorption and complexation of cobalt onto bare and FA bound MX-80 bentonite. Radiochim. Acta 2006, 94, 97–102. [Google Scholar] [CrossRef]
- He, M.; Zhu, Y.; Yang, Y.; Han, B.; Zhang, Y. Adsorption of cobalt (II) ions from aqueous solutions by palygorskite. Appl. Clay Sci. 2011, 54, 292–296. [Google Scholar] [CrossRef]
- Kypritidou, Z.; Argyraki, A.; Chryssikos, G.; Stamatakis, M. Interaction of clay materials with lead in aqueous solutions. Bull. Geol. Soc. Greece 2017, 50, 2221–2230. [Google Scholar] [CrossRef]
- Tsampodimou, M.; Bukas, V.J.; Stathopoulou, E.T.; Gionis, V.; Chryssikos, G.D. Near-infrared investigation of folding sepiolite. Am. Mineral. 2015, 100, 195–202. [Google Scholar] [CrossRef]
- Argyraki, A.; Boutsi, Z.; Zotiadis, V. Towards sustainable remediation of contaminated soil by using diasporic bauxite: Laboratory experiments on soil from the sulfide mining village of Stratoni, Greece. J. Geochem. Explor. 2017, 183, 214–222. [Google Scholar] [CrossRef]
- Zhang, H.; Tong, Z.; Wei, T.; Tang, Y. Sorption characteristics of Pb (II) on alkaline Ca-bentonite. Appl. Clay Sci. 2012, 65, 21–23. [Google Scholar] [CrossRef]
- Sheikhhosseini, A.; Shirvani, M.; Shariatmadari, H. Competitive sorption of nickel, cadmium, zinc and copper on attapulgite and sepiolite silicate clay minerals. Geoderma 2013, 192, 249–253. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Lugli, C.; Poppi, L. Kinetics of heavy-metal removal and recovery in sepiolite. Appl. Clay Sci. 2000, 16, 45–57. [Google Scholar] [CrossRef]
- Shahmohammadi-Kalalagh, S.; Babazadeh, H.; Nazemi, A.H.; Manshouri, M. Isotherm and kinetic studies on adsorption of Pb, Zn and Cu by kaolinite. Caspian J. Environ. Sci. 2011, 9, 243–255. [Google Scholar]
- Chen, H.; Wang, A. Kinetic and isothermal studies of lead ion adsorption onto palygorskite clay. J. Colloid Interface Sci. 2007, 307, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Koretsky, C. The significance of surface complexation reactions in hydrologic systems: A geochemist’s perspective. J. Hydrol. 2000, 230, 127–171. [Google Scholar] [CrossRef]
- Fendorf, S.E.; Sparks, D.L. Methods of Soil Analysis. Soil Sci. Soc. Am. 1996, 5, 377. [Google Scholar]
- Argyraki, A. Garden soil and house dust as exposure media for lead uptake in the mining village of Stratoni, Greece. Environ. Geochem. Health 2014, 36, 677–692. [Google Scholar] [CrossRef] [PubMed]
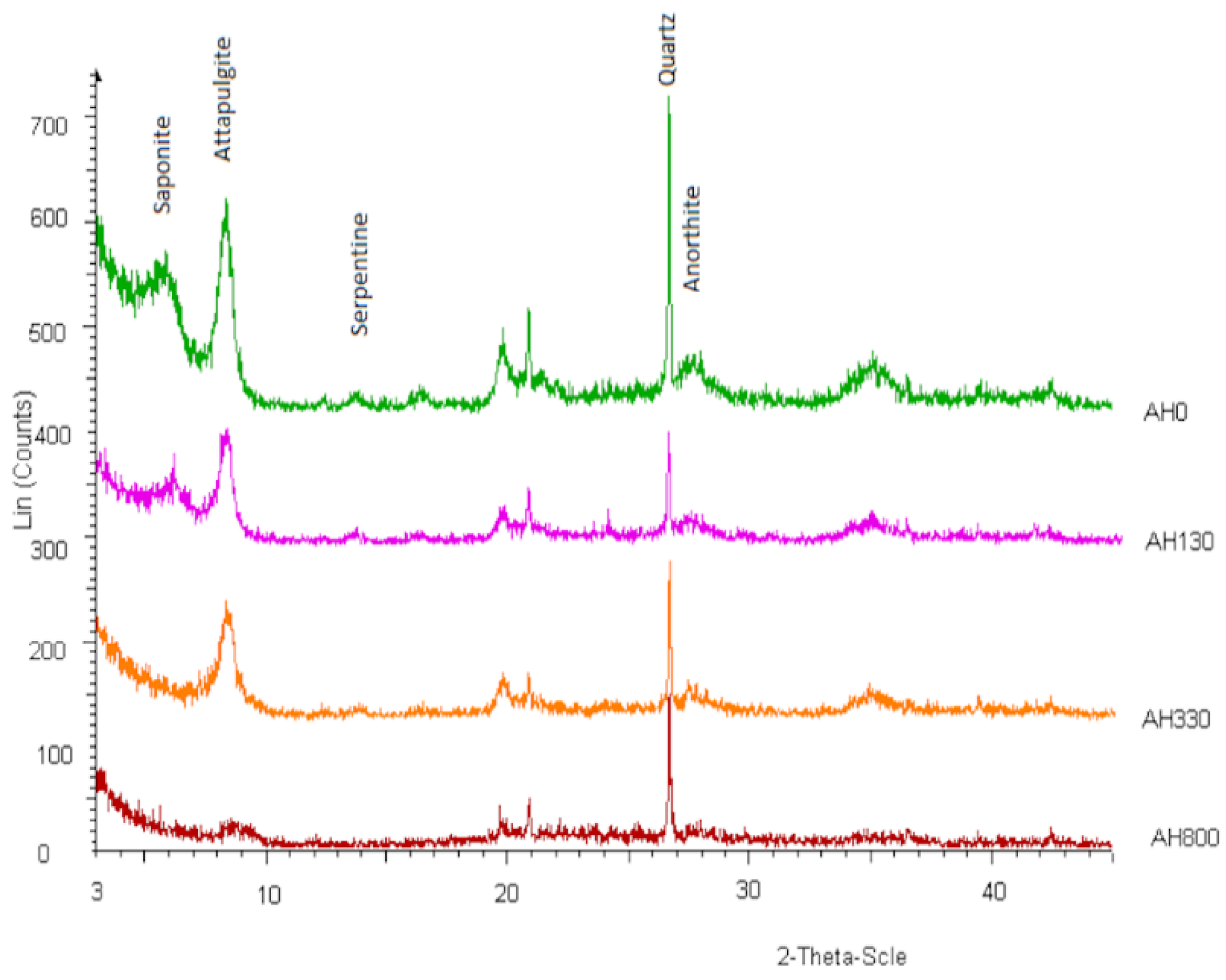
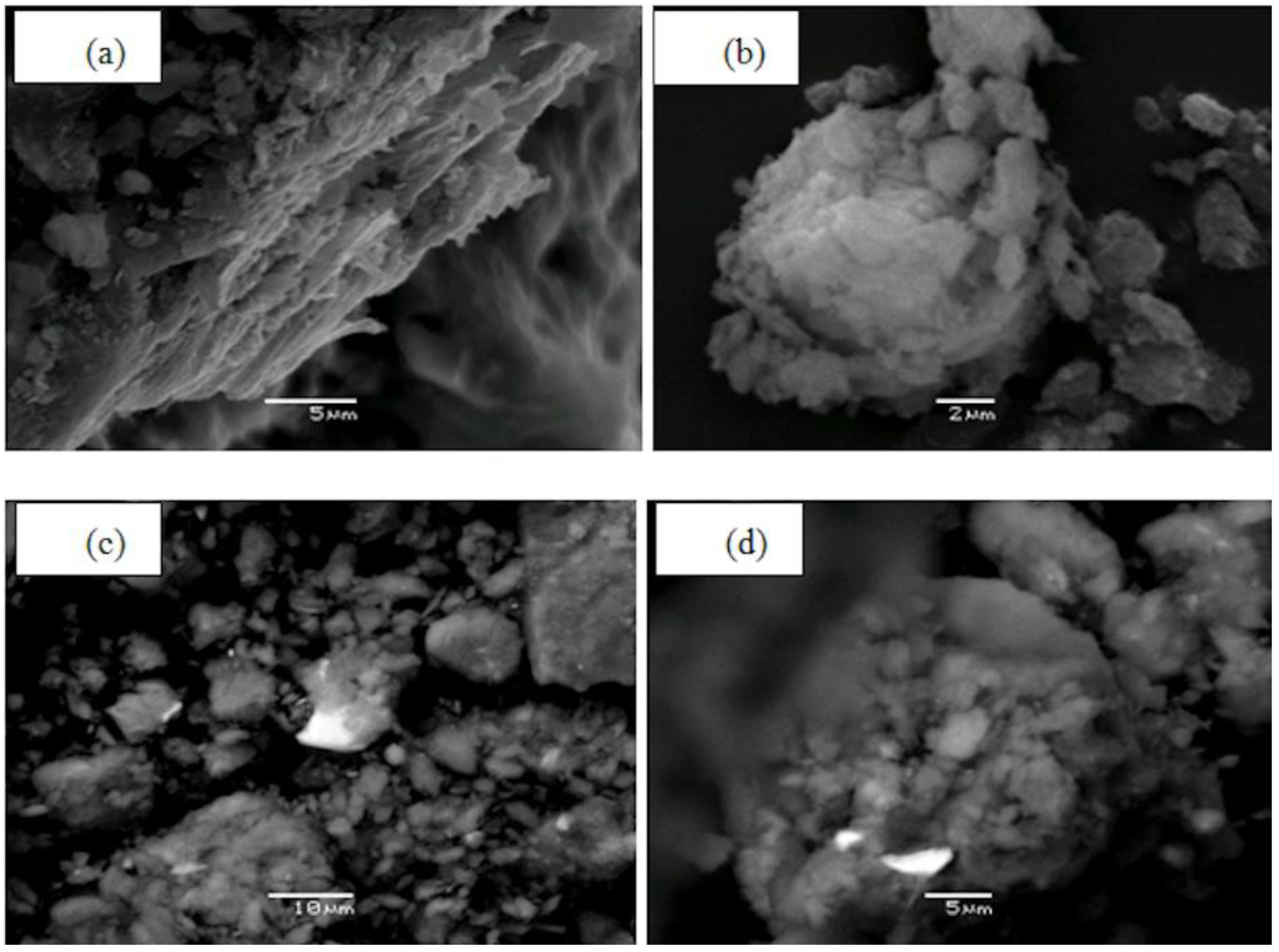

| Oxides | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | P2O5 | Cr2O3 | Ba | LOI | SUM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt % | 54.10 | 9.79 | 8.49 | 0.36 | 6.76 | 0.30 | 0.82 | 0.48 | 0.02 | 0.20 | 0.02 | 18.37 | 99.98 |
| Isotherm Model | Parameter | Pb | Cu | ||
|---|---|---|---|---|---|
| Non-Linear | Linear | Non-Linear | Linear | ||
| Freundlich | 1/n | 0.21 | 0.25 | 0.20 | 0.20 |
| Kf | 8.48 | 7.24 | 3.04 | 3.09 | |
| R2 | 0.965 | 0.933 | 0.997 | 0.976 | |
| Langmuir | Qmax (mg·g−1) | 30 | 33.11 | 4.41 | 7.32 |
| KL (L·mg−1) | 0.198 | 0.077 | 5.77 | 3.04 | |
| R2 | 0.876 | 0.987 | 0.827 | 0.802 | |
| Adsorbent | Qmax (mg·g−1) Pb | Qmax (mg·g−1) Cu | Conditions | Reference |
|---|---|---|---|---|
| Natural kaolinite | 7.75 | 4.42 | 293 K, pH 4.5 | Shahmohammadi-Kalalagh 2011 [28] |
| Natural palygorskite clay | 62.1 | 30.7 | 298 K, pH 7.0 | Potgieter et al., 2006 [15] |
| Natural palygorskite clay | - | 2.36 | 298 K | Sheikhhosseini et al., 2013 [28] |
| Natural palygorskite clay | 104.28 | - | 303 K, pH 6.0 | Chen and Wang 2007 [31] |
| Natural palygorskite clay | 20.72 | - | 313 K, pH 5.0 | Fan et al., 2009 [14] |
| Natural palygorskite clay | 37.2 | 17.4 | pH 5–6 | Alvarez-Ayuso and Sanchez 2003 [11] |
| Natural Greek palygorskite clay | 30 | 4.41 | 295 K, pH 4.0 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyrgaki, K.; Messini, P.; Zotiadis, V. Adsorption of Pb and Cu from Aqueous Solutions by Raw and Heat-Treated Attapulgite Clay. Geosciences 2018, 8, 157. https://doi.org/10.3390/geosciences8050157
Pyrgaki K, Messini P, Zotiadis V. Adsorption of Pb and Cu from Aqueous Solutions by Raw and Heat-Treated Attapulgite Clay. Geosciences. 2018; 8(5):157. https://doi.org/10.3390/geosciences8050157
Chicago/Turabian StylePyrgaki, Konstantina, Panagiota Messini, and Vassilis Zotiadis. 2018. "Adsorption of Pb and Cu from Aqueous Solutions by Raw and Heat-Treated Attapulgite Clay" Geosciences 8, no. 5: 157. https://doi.org/10.3390/geosciences8050157
APA StylePyrgaki, K., Messini, P., & Zotiadis, V. (2018). Adsorption of Pb and Cu from Aqueous Solutions by Raw and Heat-Treated Attapulgite Clay. Geosciences, 8(5), 157. https://doi.org/10.3390/geosciences8050157





