The Influence of Thermal Differences and Variation of Cl–F–OH Ratios on Cu-Ni-PGE Mineralization in the Contact Aureole of the South Kawishiwi Intrusion, Duluth Complex
Abstract
1. Introduction
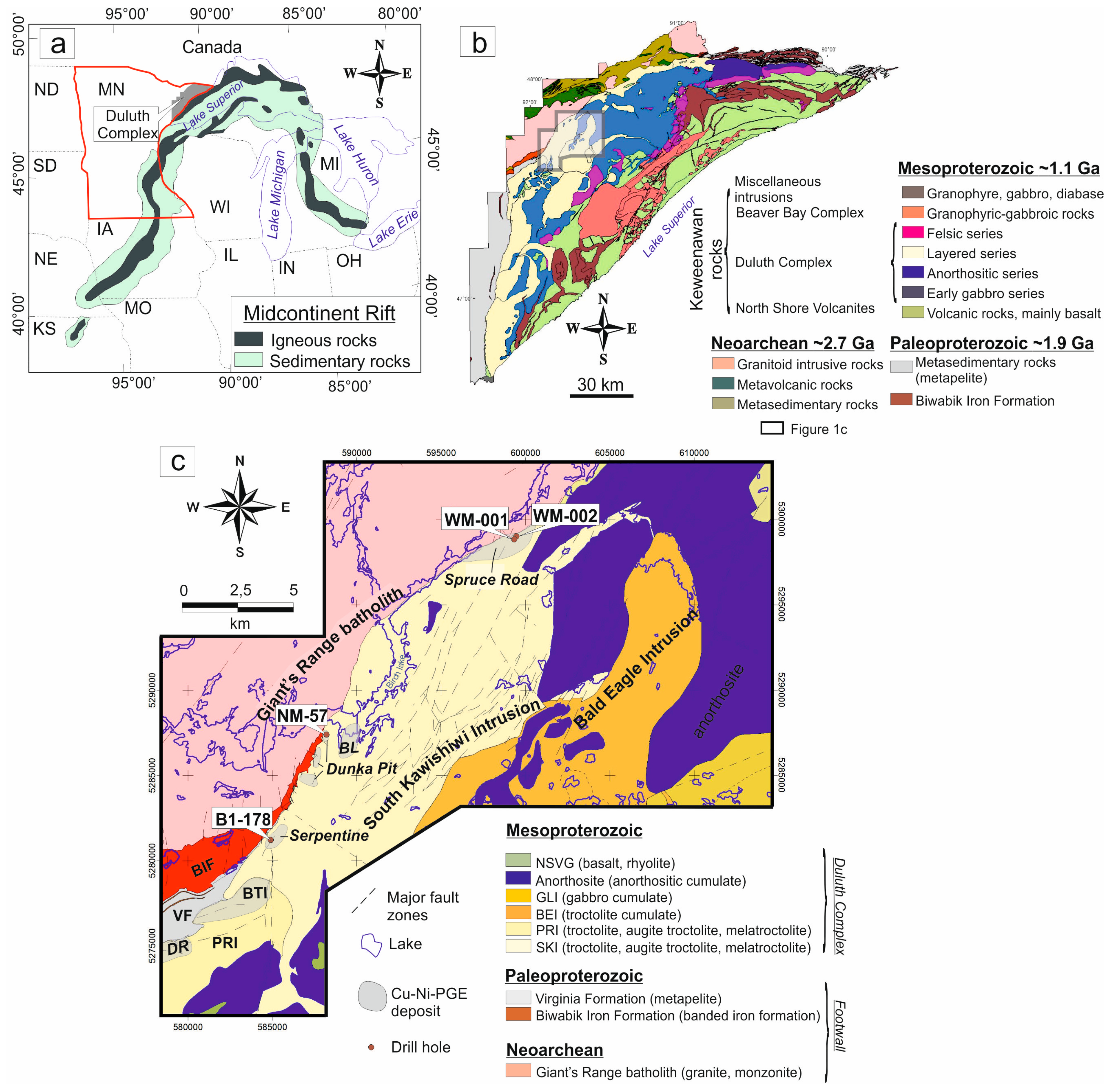
2. Regional Geology
2.1. Setting and Composition of the Duluth Complex
2.2. Geology and Mineralization of the South Kawishiwi Intrusion
2.3. Composition of the Footwall Beneath the South Kawishiwi Intrusion
3. Sampling and Methodology
4. Results
4.1. Petrography
4.1.1. Unmineralized Charnockite at the Dunka Pit Deposit (NM-57 Drill Core)
4.1.2. Mineralized Charnockite at the Spruce Road Deposit (WM-002 Drill Core)
- (1)
- Ortho- and clinopyroxene have well-crystallized crystal faces against quartz and feldspars that occur between plagioclase porphyroblasts (Figure 4a). Euhedral crystal faces against the quartz and feldspar that crystallized from the partial melt prove that pyroxene grew during the contact metamorphism.
- (2)
- Quartz occurs with cuspate crystal faces interstitially between plagioclase porphyroblasts (Figure 4b).
- (3)
- Plagioclase forms mosaic textured aggregates, particularly in samples adjacent to the intrusion (Figure 4c). According to the experiments on plagioclases at 2 kbar pressure, this texture indicates dry partial melting at high temperature (880 °C). Overgrowth textures, which occur on corroded and strained residual plagioclase crystals, are also abundant in the entire section.
- (4)
- Partial melt films and pools that crystallized to quartz + plagioclase appear between feldspar porphyroblasts (Figure 4d,e).
- (5)
- Large euhedral biotite (500–1000 μm) crystals, dominant in the distal part of the studied drill core (50–100 m from the intrusion-footwall contact), have smooth crystal faces against quartz, indicating growth of the biotite II in the presence of melt phase.
- (6)
- Symplectitic intergrowth of quartz and plagioclase.
4.1.3. Unmineralized Metapelitic Hornfels at the Serpentine Deposit (B1-178 Drill Core)
4.2. Mineral Chemistry
4.2.1. Pyroxene
4.2.2. Quartz
4.2.3. Biotite
4.2.4. Apatite
4.3. Two-Pyroxene, Titanium-in-Quartz, and Biotite-Apatite Thermometry
5. Discussion
5.1. Petrographic Evidence and Evaluation of the Temporal-Spatial Extent of the Partial Melting
5.2. Evaluation of Temperature During Partial Melting
clinopyroxene + orthopyroxene + plagioclase + magnetite + melt
5.3. Variation of Log(fHF/fHCl)fluid and Log(fH2O/fHF)fluid of Metamorphic Fluids in the Footwall Based on Halogen Concentrations of Biotite
5.4. Variation of Halogen Fugacity of the Syn-Metamorphic Fluids in the Footwall on the Basis of Halogen Concentrations in Apatite
5.5. Implication of the Variation of Halogens in the Early-Retrograde Fluids on the Cu-Ni-PGE Mineralization in the Charnockitic Footwall
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Molnár, F. Pd-Pt-Au rich sulphide ores in footwalls of layered mafic-ultramafic igneous complexes. Proc. Natl. Acad. Sci. USA 2013, 198, 121–125. [Google Scholar]
- Iljina, M.J.; Lee, C.A. PGE Deposits in the Marginal Series of Layered Intrusions. In Exploration in Platinum-Group Elements Deposit; Mungall, J., Ed.; Mineralogical Association of Canada Short Course Series Volume; Mineralogical Association of Canada: Quebec City, QC, Canada, 2005; Volume 35, pp. 75–96. [Google Scholar]
- Péntek, A.; Molnár, F.; Watkinson, D.H.; Jones, P.C.; Mogessie, A. Partial melting and melt segregation in footwall units within the contact aureole of the Sudbury Igneous Complex (North and East Ranges, Sudbury structure), with implications for their relationship to footwall Cu-Ni-PGE mineralization. Int. Geol. Rev. 2011, 53, 291–325. [Google Scholar] [CrossRef]
- Péntek, A.; Molnár, F.; Tuba, G.; Watkinson, D.H.; Jones, P.C. The significance of partial melting processes in hydrothermal low sulfide Cu–Ni–PGE mineralization within the footwall of the Sudbury Igneous Complex, Ontario, Canada. Econ. Geol. 2013, 108, 59–78. [Google Scholar] [CrossRef]
- Molnár, F.; Watkinson, D.H.; Jones, P.C. Multiple hydrothermal processes in footwall units of the North Range, Sudbury Igneous Complex, Canada, and implications for the genesis of vein–type Cu–Ni–PGE deposits. Econ. Geol. 2001, 96, 1645–1670. [Google Scholar] [CrossRef]
- Tuba, G.; Molnár, F.; Watkinson, D.H.; Jones, P.C. Characterization of hydrothermal vein and alteration assemblages associated with ’low–sulfide’ footwall Cu–Ni–PGE mineralization and regional hydrothermal processes, North and East Ranges, Sudbury structure, Canada. Soc. Econ. Geol. Spéc. Publ. 2010, 15, 573–598. [Google Scholar]
- Tuba, G.; Molnár, F.; Ames, D.A.; Péntek, A.; Watkinson, D.H.; Jones, P.C. Multi–stage hydrothermal processes involved in ‘low–sulfide’ Cu(–Ni)–PGE mineralization in the footwall of the Sudbury Igneous Complex (Canada): Amy Lake PGE zone, East Range. Miner. Deposita 2013, 49, 7–47. [Google Scholar] [CrossRef]
- Thériault, R.D.; Barnes, S.-J.; Severson, M.J. Origin of Cu–Ni–PGE mineralization in the Partridge River Intrusion, Duluth Complex, Minnesota. Econ. Geol. 2000, 95, 929–943. [Google Scholar]
- Barnes, J.S.; Cruden, R.A.; Arndt, N.; Saumur, M.B. The mineral system approach applied to Ni—Cu—PGE sulphide deposits. Ore Geol. Rev. 2015, 76, 296–316. [Google Scholar] [CrossRef]
- Saumur, B.M.; Cruden, A.R. Ingress of magmatic Ni-Cu sulphide liquid into surrounding brittle rocks: Physical & structural controls. Ore Geol. Rev. 2017, 90, 439–445. [Google Scholar]
- Holwell, D.A.; McDonald, I.; Armitage, P.E.B. Platinum-group mineral assemblages in the Platreef at the Sandsloot Mine, northern Bushveld Complex, South Africa. Miner. Mag. 2006, 70, 83–101. [Google Scholar] [CrossRef]
- Patelke, R. Exploration Drill Hole Lithology, Geologic Unit, Copper–Nickel Assay, and Location Database for the Keweenawan Duluth Complex, Northeastern Minnesota; Technical Report NRRI/TR–2003/21; University of Minnesota Duluth, Natural Resources Research Institute: Duluth, MN, USA, 2003; 97p. [Google Scholar]
- Peterson, D.M. Ore Deposit Modeling and Mineral Exploration Criteria for Footwall Copper-Nickel-Platinum Group Element Mineralization in the Duluth Complex; Report; Minnesota Department of Natural Resources, Division of Minerals: Carson City, NV, USA, 1997; Volume 317, pp. 1–40. [Google Scholar]
- Samalens, N.; Barnes, S.-J.; Sawyer, E.W. The role of black shales as a source of sulfur and semimetals in magmatic nickel-copper deposits: Example from the Partridge River Intrusion, Duluth Complex, Minnesota, USA. Ore Geol. Rev. 2017, 81, 173–187. [Google Scholar] [CrossRef]
- Peterson, D.M. Nokomis Deposit—Duluth Metals. In Cu–Ni–PGE Deposits in Mafic Intrusions of the Lake Superior Region, Guidebook for Post–Symposium Field Trip B2, Proceedings of the 11th International Platinum Symposium, Sudbury, ON, Canada, 21–24 June 2010; Miller, J.D., Smyk, M.C., Hollings, P.N., Eds.; Ontario Geological Survey: Sudbury, ON, Canada; pp. 97–116.
- Sawyer, E.W. Report on Thin Sections from DDH WM–1, Spruce Road Cu–Ni Deposit, South Kawishiwi Intrusion, Duluth Complex; Report of Investigations, NRRI/RI–2002/13; University of Minnesota Duluth, Natural Resources Research Institute: Duluth, MN, USA, 2002; 21p. [Google Scholar]
- Sawyer, E.W. The inception and growth of leucosomes: Microstructure at the start of melt segregation in migmatites. J. Metamorph. Petrol. 2014, 32, 695–712. [Google Scholar] [CrossRef]
- Benkó, Z.; Molnár, F.; Mogessie, A.; Severson, M.; Hauck, S.A.; Arehart, G.A. Cu-Ni-Au-PGE transport in the partially molten charnockitic footwall of the Spruce Road deposit, South Kawishiwi Intrusion, Duluth Complex, Minnesota, USA. Mineral Deposit Research for a High-Tech World. In Proceedings of the 12th SGA Biennial Meeting, Uppsala, Sweden, 12–15 August 2013; pp. 952–955. [Google Scholar]
- Benkó, Z.; Molnár, F.; Mogessie, A.; Severson, M.J.; Hauck, S.A.; Arehart, G.A. Cu-Ni-PGE mineralization of felsic dikes along the Grano fault in the Bathtub Intrusion (Duluth Complex, Minnesota, USA). Mineral deposit research for a high-tech world. In Proceedings of the 12th SGA Biennial Meeting, Uppsala, Sweden, 12–15 August 2013; pp. 948–951. [Google Scholar]
- Peterson, D.M. Shaded Relief Map of the Basal Contact Surface of the South Kawishiwi Intrusion Duluth Complex, Northeastern Minnesota; NRRI/MAP-2002/02; Natural Resources Research Institute: Duluth, MN, USA, 2002. [Google Scholar]
- Hovis, S.T. Observations on Cu-Ni Mineralization in the Giants Range Batholith Footwall to the South Kawishiwi Intrusion, Duluth Complex, Northeastern Minnesota; Report of Investigations NRRI/TR-2003/24; University of Minnesota Duluth, Natural Resources Research Institute: Duluth, MN, USA, 2003; p. 36. [Google Scholar]
- Benkó, Z.; Mogessie, A.; Molnár, F.; Severson, M.; Hauck, S.A.; Raic, G.A. Partial melting processes and Cu-Ni-PGE Mineralization in the footwall of the South Kawishiwi intrusion at the Spruce Road Deposit, Duluth Complex, Minnesota. Econ. Geol. 2015, 110, 1269–1293. [Google Scholar] [CrossRef]
- Ballhaus, C.G.; Stumpfl, E.F. Sulfide and platinum mineralization in the Merensky Reef: Evidence from hydrous silicates and fluid inclusions. Contrib. Miner. Petrol. 1986, 94, 193–204. [Google Scholar] [CrossRef]
- Hanley, J.J. The aqueous geochemistry of the Platinum Group Elements (PGE) in surficial, low-T hydrothermal and high-T magmatic-hydrothermal environments. In Exploration for Platinum Group Element Deposits; Mungall, J., Ed.; Short Course Series Volume; Mineralogical Association of Canada: Oulu, Finland, 6–7 August 2005; Volume 35, pp. 35–56. [Google Scholar]
- Naney, M.T. Phase equilibria of rock forming ferromagnesian silicates in granitic systems. Am. J. Sci. 1983, 283, 993–1033. [Google Scholar] [CrossRef]
- Lindsley, D.H. Pyroxene thermometry. Am. Miner. 1983, 68, 477–493. [Google Scholar]
- Wark, D.A.; Watson, E.B. TitaniQ: A titanium-in-quartz geothermometer. Contrib. Miner. Petrol. 2006, 152, 743–754. [Google Scholar] [CrossRef]
- Zhu, C.; Sverjensky, D.A. F-Cl-OH partitioning between biotite and apatite. Geochim. Cosmochim. Acta 1992, 55, 3435–3467. [Google Scholar] [CrossRef]
- Van Schmus, W.R.; Hinze, W.J. The Midcontinent Rift System. Annu. Rev. Earth Planet. Sci. 1985, 13, 345–383. [Google Scholar] [CrossRef]
- Miller, J.D.; Severson, M.J. Geology of the Duluth Complex. In Geology and Mineral Potential of the Duluth Complex and Related Rocks of Northeastern Minnesota; Miller, J.D., Green, J.C., Jr., Severson, M.J., Chandler, V.W., Hauck, S.A., Peterson, D.M., Wahl, T.E., Eds.; Report of Investigations; Minnesota Geological Survey: Saint Paul, MN, USA, 2002. [Google Scholar]
- Severson, M.J.; Hauck, S.A. Finish Logging of Duluth Complex Drill Core (and a Reinterpretation of the Geology at the Mesaba (Babbitt) Deposit); Technical Report NRRI/TR–2008/17; University of Minnesota Duluth, Natural Resources Research Institute: Duluth, MN, USA, 2008; 68p. [Google Scholar]
- Ojakangas, R.W.; Morey, G.B.; Green, J.C. The Mesoproterozoic Midcontinent Rift System, Lake Superior Region, USA. Sediment. Geol. 2001, 141–142, 421–442. [Google Scholar] [CrossRef]
- Naldrett, A.J. Magmatic sulfide depoits; Springer Verlag Berlin: Heidelberg, Germany, 2010; pp. 1–17. [Google Scholar]
- Severson, M.J. Igneous Stratigraphy of the South Kawishiwi Intrusion, Duluth Complex, Northwestern Minnesota; Report of Investigations, NRRI/TR-93/34; University of Minnesota Duluth, Natural Resources Research Institute: Duluth, MN, USA, 1994; p. 210. [Google Scholar]
- Gál, B.; Molnár, F.; Peterson, M.D. Cu-Ni-PGE mineralization in the South Filson Creek Area, South Kawishiwi intrusion, Duluth Complex: Mineralization styles, magmatic and hydrothermal processes. Econ. Geol. 2011, 106, 481–509. [Google Scholar] [CrossRef]
- Green, J.C. Lower Precambrian rocks of the Gabbro Lake Quadrangle, Northeastern Minnesota. Minn. Geol. Surv. Spéc. Publ. Ser. 1970, 13, 96. [Google Scholar]
- Sims, P.K.; Viswanathan, S. Giants Range batholiths In Geology of Minnesota. A Centennial Volume; Sims, P.K., Morey, G.B., Eds.; Minnesota Geological Survey: Saint Paul, MN, USA, 1972; pp. 120–139. [Google Scholar]
- Gál, B.; Molnár, F.; Guzmics, T.; Mogessie, A.; Szabó, C.; Peterson, D.M. Segregation of magmatic fluids and their potential in the mobilization of platinum–group elements in the South Kawishiwi intrusion, Duluth Complex, Minnesota—Evidence from petrography, apatite geochemistry and coexisting fluid and melt inclusions. Ore Geol. Rev. 2013, 54, 59–80. [Google Scholar] [CrossRef]
- Benkó, Z.; Mogessie, A.; Molnár, F.; Krenn, K.; Poulson, S.R.; Hauck, S.A.; Severson, M.; Arehart, G.A. Hydrothermal alteration and Cu–Ni–PGE mobilization in the charnockitic rocks of the footwall of the South Kawishiwi intrusion, Duluth Complex, USA. Ore Geol. Rev. 2015, 67, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Lucente, M.E.; Morey, G.B. Stratigraphy and sedimentology of the Lower Proterozoic Virginia Formation, northern Minnesota. Minn. Geol. Surv. Rep. Investig. 1983, 28, 28. [Google Scholar]
- Zanko, L.M.; Severson, M.J.; Ripley, E.M. Geology and Mineralization of the Serpentine Copper-Nickel Deposit; Report of Investigations, NRRI/TR-93/52; University of Minnesota Duluth, Natural Resources Research Institute: Duluth, MN, USA, 1994. [Google Scholar]
- Arcuri, T.; Ripley, E.M.; Hauck, S.A. Sulfur and oxygen isotope studies of the interaction between peelitic xenoliths and basaltic magma at the Babbitt and Serpentine Cu-Ni deposits, Duluth Complex, Minnesota. Econ. Geol. 1998, 93, 1063–1075. [Google Scholar] [CrossRef]
- Ripley, E.; Li, C. Sulfide saturation in mafic magmas: Is external sulfur required for magmatic Ni-Cu-(PGE) ore genesis? Econ. Geol. 2013, 108, 45–58. [Google Scholar] [CrossRef]
- Mogessie, A.; Stumpfl, E.F. Platinum-group element and stable isotope geochemistry of PGM-bearing troctolitic rocks of the Duluth Complex, Minnesota. Aust. J. Earth Sci. 1992, 39, 315–325. [Google Scholar] [CrossRef]
- Sawyer, E.W. Atlas of Migmatites; National Research Council of Canada: Ottawa, ON, Canada, 2008. [Google Scholar]
- Bohlen, S.R.; Essene, E.J. A critical evaluation of two–pyroxene thermometry in Adirondack granulites. Lithos 1979, 12, 335–345. [Google Scholar] [CrossRef]
- Glassley, W.E.; Sorensen, K. Constant Ps–T amphibolite to granulite facies transition in Agto (West Greenland) metadolerites: Implications and applications. J. Petrol. 1980, 21, 69–105. [Google Scholar] [CrossRef]
- Pitra, P.; De Waal, S.A. High-temperature, low-pressure metamorphism and development of prograde symplectites, Marble Hall Fragment, Bushveld Complex (South Africa). J. Metamorph. Petrol. 2008, 19, 311–325. [Google Scholar] [CrossRef]
- Zhu, C.; Sverjensky, D.A. Partitioning of F-Cl-OH between minerals and hydrothermal fluids. Geochim. Cosmochim. Acta 1991, 55, 1837–1858. [Google Scholar] [CrossRef]
- Sallet, R. Fluorine as a tool in the petrogenesis of quartz-bearing magmatic associations: Applications of an improved F-OH biotite-apatite thermometer grid. Lithos 2000, 50, 241–253. [Google Scholar] [CrossRef]
- Bath, A.B.; Walshe, J.L.; Cloutier, J.; Verrall, M.; Cleverley, J.S.; Pownceby, M.I.; Macrae, C.M.; Wilson, C.N.; Tunjic, J.; Nortje, G.S.; et al. Biotite and apatite as tools for tracking pathways of oxidized fluids in the Archean East Repulse gold deposit, Australia. Econ. Geol. 2013, 108, 667–690. [Google Scholar] [CrossRef]
- Petcovic, H.L.; Grunder, A.L. Textural and thermal history of partial melting in tonalitic wallrock at the margin of a basalt dike, Wallowa Mountains, Oregon. J. Petrol. 2003, 44, 2287–2313. [Google Scholar] [CrossRef]
- Vielzeuf, D.; Montel, J.M. Partial melting of metagreywackes. Part I: Fluid absent experiments and phase relationships. Contrib. Miner. Petrol. 1994, 98, 257–276. [Google Scholar]
- Montel, J.M.; Vielzeuf, D. Partial melting of metagreywackes Part II. Compositions of minerals and melts. Contrib. Miner. Petrol. 1997, 128, 176–196. [Google Scholar] [CrossRef]
- Patiño Douce, A.E.; Beard, J.S. Dehydration-meting of biotite gneiss and amphibolite from 36 kbar to 16 kbar. J. Petrol. 1995, 36, 707–738. [Google Scholar] [CrossRef]
- Naldrett, A.J. Magmatic Sulfide Deposits; Springer-Verlag Berlin: Heidelberg, Germany, 2010; pp. 1–727. [Google Scholar]
- Duran, C.J.; Barnes, S.J.; Pleše, P.; Kudrna Prašek, M.; Zientek, M.L.; Pagé, P. Fractional crystallization-induced variations in sulfides from the Norilsk-Talnakh mining district (polar Siberia, Russia). Ore Geol. Rev. 2017, 90, 326–351. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.-J.; Prichard, H.M.; Fisher, P.C. Mineralogy and Geochemistry of Cu-Rich Ores from the McCreedy East Ni-Cu-PGE Deposit (Sudbury, Canada): Implications for the Behavior of Platinum Group and Chalcophile Elements at the End of Crystallization of a Sulfide Liquid. Econ. Geol. 2014, 109, 343–366. [Google Scholar] [CrossRef]
- Liu, Y.; Brenan, J. Partitioning of platinum-group elements (PGE) and chalcogens (Se, Te, As, Sb, Bi) between monosulfide-solid solution (MSS), intermediate solid solution (ISS) and sulfide liquid at controlled fO2-fS2 conditions. Geochim. Cosmochim. Acta 2015, 159, 139–161. [Google Scholar] [CrossRef]
- Kullerud, G.; Yund, R.A.; Moh, G.H. Phase relations in the Cu–Fe–S, Cu–Ni–S, and Fe–Ni–S systems. In Magmatic Ore Deposits; Wilson, H.D.B., Ed.; Economic Geology Monograph 4; Society of Economic Geologists: Littleton, CO, USA, 1969; pp. 1–366. [Google Scholar]
- Ebel, D.S.; Naldrett, A.J. Fractional crystallization of sulfide ore liquids at high temperature. Econ. Geol. 1996, 91, 607–621. [Google Scholar] [CrossRef]
- Tsujimura, T.; Kitakaze, A. New phase relations in the Cu-Fe-S system at 800 °C; constraint of fractional crystallization of a sulfide liquid. Neues Jahrb. Mineral. Monatshefte 2004, 10, 433–444. [Google Scholar] [CrossRef]
- Zhao, J.; Brugger, J.; Ngothai, Y.; Pring, A. The replacement of chalcopyrite by bornite under hydrothermal conditions. Am. Miner. 2014, 99, 2389–2397. [Google Scholar] [CrossRef]
- Manning, D.A.C. The effect of fluorine on liquidus phase relationships in the system Qz–Ab–Or with excess water at 1 kbar. Contrib. Miner. Petrol. 1981, 76, 206–215. [Google Scholar] [CrossRef]
- Xiong, X.-L.; Zhao, Z.-H.; Zhu, J.-C.; Bing, R. Phase relations in albite granite–H2O–HF system and their petrogenetic applications. Geochem. J. 1999, 33, 199–214. [Google Scholar]
- Aranovich, L.Y.; Newton, R.C.; Manning, C.E. Brine-assisted anatexis: Experimental melting in the system haplogranite-H2O-NaCl-KCl at deep-crustal conditions. Earth. Plan. Sci. Lett. 2013, 374, 111–120. [Google Scholar] [CrossRef]
- Manning, C.E.; Aranovich, L.Y. Brines at high pressure and temperature: Thermodynamic, petrologic and geochemical effects. Precambr. Res. 2014, 253, 6–16. [Google Scholar] [CrossRef]
- Munoz, J.L. Calculation of HF and HCl fugacities from biotite compositions: Revised equations. Geol. Soc. Am. Abstr. Progr. 1992, 24, A221. [Google Scholar]
- Munoz, J.L. F–OH and Cl–OH exchange in micas with applications to hydrothermal ore deposits. In Micas; Bailey, S.W., Ed.; Reviews in Mineralogy; Mineralogical Society of America: Washington, DC, USA, 1987; Volume 13, pp. 469–493. [Google Scholar]
- Korzhinsky, M.A. Apatite solid solutions as indicators of the fugacity of HCl and HF in hydrothermal fluids. Geochem. Int. 1981, 3, 45–60. [Google Scholar]
- Spear, F.S.; Pyle, J.M. Apatite, monazite and xenotime in metamorphic rocks. In Phosphates: Geochemical, Geobiological, and Materials Importance; Kohn, M.J., Rakovan, J., Hughes, J.M., Eds.; Reviews in Mineralogy and Geochemistry 48; Mineralogical Society of America: Washington, DC, USA, 2002; pp. 293–336. [Google Scholar]
- Piccoli, P.M.; Candela, P.A. Apatite in felsic rocks: A model for the estimation of initial halogen contents in the Bishop Tuff (Long Valley) and Tuolumne Intrusive Suite (Sierra Nevada Batholith) Magmas. Am. J. Sci. 1994, 294, 92–135. [Google Scholar] [CrossRef]
- Li, C.; Naldrett, A.J.; Coats, C.J.A.; Johannessen, P. Platinum, palladium, gold and copper-rich stringers at the Strathcona mine, Sudbury: Their enrichment by fractionation of a sulfide liquid. Econ. Geol. 1992, 87, 1584–1598. [Google Scholar] [CrossRef]
- Jago, B.C.; Morrison, G.G.; Little, T.L. Metal zonation patterns and microtextural and micromineralogical evidence for alkali- and halogen rich fluids in the genesis of the Victor Deep and McCreedy East footwall copper orebodies, Sudbury Igneous Complex. Ont. Geol. Surv. Spéc. Vol. 1994, 5, 65–75. [Google Scholar]
- Marshall, D.D.; Watkinson, D.H.; Farrow, C.E.G.; Molnár, F.; Fouillac, A.M. Multiple fluid generations in the Sudbury Igneous Complex: Fluid inclusion, Ar, O, Rb and Sr evidence. Chem. Geol. 1999, 157, 1–19. [Google Scholar] [CrossRef]
- Hanley, J.J.; Mungall, J.E. Chlorine enrichment and hydrous alteration of the Sudbury breccia hosting footwall Cu-Ni-PGE mineralization at the Fraser mine, Sudbury, Ontario, Canada. Can. Miner. 2003, 41, 857–881. [Google Scholar] [CrossRef]
- Mungall, J.E.; Brenan, J.M. Experimantal evidence for the chalcophile behaviour of the halogens. Can. Miner. 2003, 41, 207–220. [Google Scholar] [CrossRef]
- Wykes, J.; Mavrogenes, J. Hydrous sulfide melting: Experimental evidence for the solubility of H2O in sulfide melts. Econ. Geol. 2005, 100, 157–164. [Google Scholar] [CrossRef]
- Boudreau, A.E.; McCallum, I.S. Infiltration metasomatism in layered intrusions—An example from the Stillwater Complex, Montana. J. Volcanol. Geoth. Res. 1992, 52, 171–183. [Google Scholar] [CrossRef]
- Boudreau, A.E.; Love, C.; Hoatson, D. Halogen geochemistry of the Munni Munni Complex and associated intrusions of the Pilbara Block, W. Australia. Geochim. Cosmochim. Acta 2000, 57, 4467–4477. [Google Scholar] [CrossRef]
- Boudreau, A.E.; Kruger, F.J. Variation in the composition of apatite through the Merensky cyclic unit in the western Bushveld Complex. Econ. Geol. 1990, 85, 737–745. [Google Scholar] [CrossRef]
- Boudreau, A.E. Some geochemical considerations for platinum-group element exploration in layered intrusions. Explor. Min. Geol. 1995, 3, 215–225. [Google Scholar]
- Boudreau, A.E.; Love, C.; Prendergast, M.D. Halogen geochemistry of the Great Dyke, Zimbabwe. Contrib. Miner. Petrol. 1995, 122, 289–300. [Google Scholar] [CrossRef]
- Meurer, W.P.; Boudreau, A.E. Evaluation of models for halogen variations in apatite from layered intrusions using a detailed study of apatite from OB-III and OB-IV of the Middle Banded series, Stillwater Complex, Montana. Contrib. Miner. Petrol. 1996, 123, 225–236. [Google Scholar] [CrossRef]
- Holland, G.; Sherwood-Lollar, B.; Li, L.; Lacrampe-Couloume, G.; Slater, G.F.; Ballentine, C.J. Deep fracture fluids isolated in the crust since the Precambrian era. Nature 2013, 497, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Audétat, A.; Pettke, T.; Heinrich, C.A.; Bodnar, R.J. The composition of magmatic–hydrothermal fluids in barren and mineralized intrusion. Econ. Geol. 2008, 103, 877–908. [Google Scholar] [CrossRef]
- Wood, S.A. The aqueous geochemistry of the platinum-group elements with applications to ore deposits. In The Geology, Geochemistry, Mineralogy and Mineral Beneficiation of Platinum-Group Elements: Canadian Institute of Mining, Metallurgy and Petroleum; Cabri, L.J., Ed.; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 2002; Volume 54, pp. 483–506. [Google Scholar]
- Hanley, J.J.; Pettke, T.; Mungall, J.E.; Spooner, E.T.C. The solubility of platinum and gold in NaCl brines at 1.5 kbar, 600 to 800 °C: A laser ablation ICP-MS pilot study of synthetic fluid inclusions. Geochim. Cosmochim. Acta 2005, 69, 2593–2611. [Google Scholar] [CrossRef]
- Ballhaus, C.G.; Ryan, C.G.; Mernagh, T.P.; Green, D.H. The partitioning of Fe, Ni, Cu, Pt, and Au between sulphide, metal, and fluid phases: A pilot study. Geochim. Cosmochim. Acta 1994, 58, 811–826. [Google Scholar] [CrossRef]
- Molnár, F.; Watkinson, D.H.; Jones, C.; Gatter, I. Fluid inclusion evidence for hydrothermal enrichment of magmatic ore at the contact zone of the 4b Ni-Cu-PGE deposit, Lindsley Mine, Sudbury, Canada. Econ. Geol. 1997, 92, 674–685. [Google Scholar] [CrossRef]
- Gammons, C.H.; Bloom, M.S. Experimental investigation of the hydrothermal geochemistry of platinum and palladium: II. The solubility of PtS and PdS in aqueous sulfide solutions. Geochim. Cosmochim. Acta 1993, 57, 2451–2467. [Google Scholar] [CrossRef]
- Polovina, J.S.; Hudson, M.D.; Jones, R.E. Petrographic and geochemical characteristics of postmagmatic hydrothermal alteration and mineralization in the JM Reef, Stillwater Complex, Montana. Can. Mineral. 2004, 42, 261–277. [Google Scholar] [CrossRef]
- Pan, P.; Wood, S.A. Solubility of Pt and Pd sulphides and Au metal in aqueous bisulphide solutions. II. Results at 200° to 350 °C and saturated vapour pressure. Miner. Deposita 1994, 29, 373–390. [Google Scholar] [CrossRef]












| Deposit/Drill Core | Spruce Road/WM-002 | Dunka Pit Deposit | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Z2-29 | Z2-29 | Z2-60 | Z2-60 | Z2-60 | Z-162 | Z-162 | Z-162 | Z-162 | Z-169 | Z-169 | Z-169 | Z-169 |
| Depth (m) | 126 | 196 | 293 | 293 | 293 | 68 | 68 | 68 | 68 | 120 | 120 | 120 | 120 |
| Mineral | cpx | opx | cpx | opx | opx | cpx | cpx | opx | opx | cpx | cpx | opx | opx |
| wt% | |||||||||||||
| SiO2 | 52.76 | 55.24 | 53.34 | 52.93 | 53.93 | 53.03 | 52.36 | 52.55 | 52.30 | 53.15 | 53.28 | 53.78 | 53.46 |
| TiO2 | 0.23 | 0.05 | 0.37 | 0.00 | 0.00 | 0.01 | 0.08 | 0.10 | 0.00 | 0.13 | 0.19 | 0.07 | 0.10 |
| Al2O3 | 0.51 | 0.79 | 1.12 | 0.89 | 0.83 | 0.86 | 0.66 | 0.29 | 0.48 | 0.71 | 0.51 | 0.34 | 0.21 |
| Fe2O3 | 2.79 | 0.21 | 1.20 | 0.00 | 0.00 | 1.69 | 3.06 | 0.00 | 0.07 | 3.13 | 2.06 | 0.58 | 1.01 |
| FeO | 5.48 | 15.82 | 5.93 | 17.16 | 18.78 | 7.44 | 5.67 | 22.73 | 22.46 | 5.31 | 6.08 | 21.44 | 20.70 |
| MnO | 0.43 | 1.03 | 0.39 | 0.63 | 0.71 | 0.45 | 0.54 | 1.05 | 1.00 | 0.36 | 0.33 | 1.03 | 0.92 |
| MgO | 16.14 | 23.75 | 15.32 | 28.32 | 27.01 | 14.74 | 14.86 | 22.10 | 22.10 | 15.47 | 15.02 | 23.01 | 23.26 |
| CaO | 19.85 | 1.77 | 21.44 | 0.31 | 0.28 | 21.37 | 22.03 | 1.13 | 1.04 | 22.14 | 22.38 | 0.60 | 0.62 |
| Na2O | 1.23 | 0.12 | 0.75 | 0.00 | 0.00 | 0.41 | 0.38 | 0.02 | 0.00 | 0.45 | 0.43 | 0.01 | 0.01 |
| K2O | 0.05 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.04 | 0.00 | 0.00 | 0.01 | 0.02 |
| Total | 99.47 | 98.78 | 99.88 | 100.24 | 101.55 | 100.02 | 99.63 | 99.98 | 99.50 | 100.94 | 100.41 | 100.88 | 100.32 |
| Cations per formula unit | |||||||||||||
| Si | 1.97 | 2.03 | 1.98 | 1.93 | 1.95 | 1.97 | 1.95 | 1.97 | 1.97 | 1.95 | 1.97 | 1.98 | 1.98 |
| Ti | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 |
| Al | 0.02 | 0.03 | 0.05 | 0.04 | 0.04 | 0.04 | 0.03 | 0.01 | 0.02 | 0.03 | 0.02 | 0.01 | 0.01 |
| Fe3+ | 0.08 | 0.01 | 0.03 | 0.00 | 0.00 | 0.05 | 0.09 | 0.00 | 0.00 | 0.09 | 0.06 | 0.02 | 0.03 |
| Fe2+ | 0.17 | 0.49 | 0.18 | 0.52 | 0.57 | 0.23 | 0.18 | 0.71 | 0.71 | 0.16 | 0.19 | 0.66 | 0.64 |
| Mn | 0.01 | 0.03 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.03 | 0.03 | 0.01 | 0.01 | 0.03 | 0.03 |
| Mg | 0.90 | 1.30 | 0.85 | 1.54 | 1.45 | 0.82 | 0.83 | 1.24 | 1.24 | 0.85 | 0.83 | 1.27 | 1.28 |
| Ca | 0.80 | 0.07 | 0.85 | 0.01 | 0.01 | 0.85 | 0.88 | 0.05 | 0.04 | 0.87 | 0.89 | 0.02 | 0.02 |
| Na | 0.09 | 0.01 | 0.05 | 0.00 | 0.00 | 0.03 | 0.03 | 0.00 | 0.00 | 0.03 | 0.03 | 0.00 | 0.00 |
| K | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| End members | |||||||||||||
| Wollastonite | 0.40 | 0.04 | 0.42 | 0.01 | 0.01 | 0.41 | 0.43 | 0.02 | 0.02 | 0.42 | 0.44 | 0.01 | 0.01 |
| Enstatite | 0.45 | 0.65 | 0.42 | 0.77 | 0.73 | 0.41 | 0.41 | 0.62 | 0.62 | 0.42 | 0.41 | 0.63 | 0.64 |
| Ferrosilite | 0.09 | 0.24 | 0.09 | 0.26 | 0.28 | 0.12 | 0.09 | 0.36 | 0.35 | 0.08 | 0.09 | 0.33 | 0.32 |
| Pyroxmangit | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.01 |
| Sample Name | Z-151 | Z-161 | Z-165 | Z-169 | |
|---|---|---|---|---|---|
| Depth (m) | 206 | 253 | 296 | 319 | |
| Petrographic position | Partial melt pool | Partial melt pool | Myrmekite | Myrmekite | Myrmekite |
| 1 | 360 | 151 | 205 | 161 | 161 |
| 2 | 360 | 174 | 190 | 189 | 189 |
| 3 | 409 | 159 | 204 | 173 | 173 |
| 4 | 350 | 164 | 150 | 176 | 176 |
| 5 | 370 | 151 | 142 | 184 | 181 |
| 6 | 360 | 189 | 135 | 176 | 200 |
| 7 | 410 | 141 | 143 | 168 | 176 |
| 8 | 360 | 63 | 134 | 129 | 168 |
| 9 | 422 | 206 | 184 | 170 | 127 |
| 10 | 260 | 190 | 87 | 170 | |
| 11 | 394 | 166 | 100 | 199 | |
| 12 | 404 | 16.5 | 199 | 158 | |
| 13 | 150 | 158 | 147 | ||
| 14 | 137 | 147 | 168 | ||
| 15 | 165 | 168 | 141 | ||
| 16 | 141 | ||||
| 17 | 81 | ||||
| S | 372 | 155 | 154 | 153 | 169 |
| σ | 43 | 38 | 28 | 29 | 18 |
| Min | 206 | 63 | 17 | 81 | 127 |
| Max | 422 | 206 | 205 | 199 | 200 |
| aTi | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| ST(°C) | 827.6 | 717.9 | 716.9 | 716.4 | 727.4 |
| σT(°C) | 17.4 | 36.0 | 56.0 | 29.0 | 14.0 |
| Deposit | Serpentine | Dunka Pit | Spruce Road | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drill hole | B1-178 | B1-178 | B1-178 | B1-178 | NM-57 | NM-57 | NM-57 | NM-57 | WM-002 | WM-002 | WM-002 | WM-002 |
| Depth (m) | 132.7 | 132.7 | 167.2 | 167.2 | 253.1 | 253.1 | 306.3 | 302.0 | 195.4 | 195.4 | 257.9 | 257.9 |
| Sample | Z-118 | Z-118 | Z-118 | Z-118 | Z-161 | Z-161 | Z-168 | Z-167 | Z2-28 | Z2-28 | Z2-54 | Z2-54 |
| Mineral | bt | bt | bt | bt | bt | bt | bt | bt | bt | bt | bt | bt |
| wt% | ||||||||||||
| SiO2 | 35.11 | 35.48 | 37.04 | 38.15 | 38.24 | 37.77 | 37.80 | 38.12 | 38.96 | 38.28 | 37.37 | 37.35 |
| TiO2 | 3.79 | 3.80 | 5.03 | 4.75 | 4.62 | 4.54 | 4.59 | 4.73 | 2.89 | 3.75 | 5.07 | 4.84 |
| Al2O3 | 16.37 | 16.80 | 14.16 | 14.23 | 14.44 | 15.19 | 14.21 | 14.63 | 13.12 | 12.55 | 12.86 | 12.72 |
| Cr2O3 | 0.13 | 0.12 | 0.41 | 0.38 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| FeO | 23.57 | 23.49 | 17.37 | 16.19 | 14.65 | 17.13 | 13.67 | 13.47 | 15.14 | 15.59 | 13.61 | 13.92 |
| MnO | 0.11 | 0.05 | 0.02 | 0.03 | 0.00 | 0.44 | 0.32 | 0.26 | 0.32 | 0.39 | 0.20 | 0.18 |
| MgO | 7.79 | 7.66 | 12.32 | 12.75 | 13.99 | 12.34 | 14.36 | 14.03 | 15.55 | 15.59 | 15.16 | 15.55 |
| CaO | 0.04 | 0.00 | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.10 | 0.05 | 0.00 | 0.00 |
| Na2O | 0.13 | 0.10 | 0.04 | 0.02 | 0.00 | 0.10 | 0.06 | 0.10 | 0.06 | 0.11 | 0.19 | 0.14 |
| K2O | 9.33 | 9.16 | 9.70 | 9.93 | 10.16 | 9.99 | 9.94 | 10.03 | 9.41 | 9.69 | 9.53 | 9.62 |
| F | 0.37 | 0.41 | 0.57 | 0.60 | 0.79 | 0.77 | 1.28 | 1.34 | 0.36 | 0.34 | 0.54 | 0.55 |
| Cl | 0.08 | 0.08 | 0.05 | 0.02 | 0.06 | 0.11 | 0.00 | 0.00 | 0.04 | 0.03 | 0.26 | 0.27 |
| H2O | 3.61 | 3.62 | 3.61 | 3.64 | 3.57 | 3.57 | 3.31 | 3.31 | 3.79 | 3.79 | 3.59 | 3.63 |
| Subtotal | 100.44 | 100.76 | 100.31 | 100.81 | 100.53 | 101.95 | 99.56 | 100.01 | 99.74 | 100.16 | 99.61 | 100.53 |
| F,Cl=O | 0.17 | 0.19 | 0.25 | 0.26 | 0.35 | 0.35 | 0.54 | 0.57 | 0.16 | 0.15 | 0.29 | 0.29 |
| Total | 100.27 | 100.57 | 100.06 | 100.55 | 100.18 | 101.61 | 99.02 | 99.45 | 99.58 | 100.01 | 99.33 | 100.23 |
| Calculated formula based on 12 (O,OH,F,Cl) | ||||||||||||
| Si | 2.77 | 2.77 | 2.86 | 2.91 | 2.90 | 2.86 | 2.89 | 2.90 | 2.94 | 2.90 | 2.86 | 2.83 |
| Ti | 0.22 | 0.22 | 0.29 | 0.27 | 0.26 | 0.26 | 0.26 | 0.27 | 0.16 | 0.21 | 0.29 | 0.28 |
| Al | 1.52 | 1.55 | 1.29 | 1.28 | 1.29 | 1.35 | 1.28 | 1.31 | 1.17 | 1.12 | 1.16 | 1.14 |
| Cr | 0.01 | 0.01 | 0.02 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fe2+ | 1.55 | 1.54 | 1.12 | 1.03 | 0.93 | 1.08 | 0.87 | 0.86 | 0.96 | 0.99 | 0.87 | 0.88 |
| Mn | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.01 | 0.01 |
| Mg | 0.91 | 0.89 | 1.42 | 1.45 | 1.58 | 1.39 | 1.64 | 1.59 | 1.75 | 1.76 | 1.73 | 1.76 |
| Ca | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 |
| Na | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.03 | 0.02 |
| K | 0.94 | 0.91 | 0.95 | 0.97 | 0.98 | 0.96 | 0.97 | 0.97 | 0.91 | 0.94 | 0.93 | 0.93 |
| F | 0.09 | 0.10 | 0.14 | 0.15 | 0.19 | 0.18 | 0.31 | 0.32 | 0.09 | 0.08 | 0.13 | 0.13 |
| Cl | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.03 |
| H | 1.90 | 1.89 | 1.86 | 1.85 | 1.80 | 1.80 | 1.69 | 1.68 | 1.91 | 1.92 | 1.84 | 1.83 |
| Mg# (Fetot) | 0.37 | 0.37 | 0.56 | 0.58 | 0.63 | 0.56 | 0.65 | 0.65 | 0.65 | 0.64 | 0.67 | 0.67 |
| Al (IV) | 1.23 | 1.23 | 1.14 | 1.09 | 1.10 | 1.14 | 1.11 | 1.10 | 1.06 | 1.10 | 1.14 | 1.14 |
| Al (VI) | 0.29 | 0.32 | 0.15 | 0.19 | 0.19 | 0.21 | 0.17 | 0.21 | 0.11 | 0.02 | 0.02 | 0.00 |
| # Cations | 7.96 | 7.92 | 7.96 | 7.94 | 7.95 | 7.95 | 7.95 | 7.93 | 7.92 | 7.96 | 7.96 | 7.95 |
| Deposit | Giants Range Batholite | Serpentine | Dunka Pit | Spruce Road | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drill hole | B1-178 | B1-178 | B1-178 | B1-178 | NM-57 | NM-57 | NM-57 | NM-57 | WM-002 | WM-002 | WM-002 | WM-002 | ||
| Depth (m) | 132.7 | 132.7 | 167.2 | 167.2 | 253.4 | 253.4 | 306.3 | 302.0 | 192.0 | 192.0 | 293.5 | 293.5 | ||
| Sample | Z-118 | Z-118 | Z-131 | Z-131 | Z-161 | Z-161 | Z-165 | Z-165 | Z2-26 | Z2-26 | Z2-60 | Z2-60 | ||
| Mineral | ap | ap | ap | ap | ap | ap | ap | ap | ap | ap | ap | ap | ||
| wt% | ||||||||||||||
| SiO2 | 0.22 | 0.07 | 0.00 | 0.05 | 0.00 | 0.09 | 0.38 | 0.03 | 0.50 | 0.36 | 0.19 | 0.26 | 0.05 | 0.26 |
| Al2O3 | 0.02 | 0.00 | 0.11 | 0.15 | 0.09 | 0.24 | 0.08 | 0.16 | 0.18 | 0.00 | 0.18 | 0.02 | 0.13 | 0.06 |
| P2O5 | 41.63 | 41.62 | 41.77 | 41.60 | 41.89 | 41.89 | 41.05 | 41.85 | 40.85 | 41.02 | 42.70 | 43.00 | 40.82 | 40.36 |
| FeO | 0.00 | 0.00 | 1.39 | 0.85 | 0.17 | 0.10 | 0.33 | 0.00 | 0.09 | 0.13 | 0.21 | 0.26 | 0.00 | 0.06 |
| MgO | 0.00 | 0.00 | 0.42 | 0.25 | 0.00 | 0.00 | 0.05 | 0.08 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.04 |
| CaO | 55.65 | 55.48 | 52.66 | 53.37 | 54.21 | 54.18 | 55.01 | 54.86 | 54.15 | 54.65 | 51.98 | 52.71 | 54.26 | 54.25 |
| K2O | 0.00 | 0.00 | 0.34 | 0.37 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| F | 3.48 | 3.31 | 2.99 | 3.25 | 2.67 | 2.45 | 3.53 | 3.38 | 2.50 | 2.75 | 2.57 | 2.89 | 2.18 | 2.04 |
| Cl | 0.00 | 0.00 | 0.15 | 0.11 | 0.35 | 0.31 | 0.16 | 0.20 | 0.28 | 0.27 | 0.26 | 0.38 | 1.27 | 1.27 |
| H2O | 0.05 | 0.10 | 0.27 | 0.17 | 0.39 | 0.50 | 0.05 | 0.11 | 0.48 | 0.38 | 0.39 | 0.23 | 0.39 | 0.46 |
| Total | 101.05 | 100.58 | 100.09 | 100.18 | 99.80 | 99.76 | 100.78 | 100.68 | 99.02 | 99.61 | 98.47 | 99.75 | 99.25 | 99.10 |
| F,Cl=O | 1.28 | 1.62 | 1.29 | 1.39 | 1.20 | 1.10 | 1.52 | 1.47 | 1.12 | 1.22 | 1.14 | 1.30 | 1.21 | 1.14 |
| Cation numbers based on 26 (O, OH, F, Cl) | ||||||||||||||
| Si | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.02 | 0.06 | 0.01 | 0.09 | 0.06 | 0.03 | 0.05 | 0.01 | 0.04 |
| Al | 0.00 | 0.00 | 0.02 | 0.03 | 0.02 | 0.05 | 0.02 | 0.03 | 0.04 | 0.00 | 0.04 | 0.00 | 0.03 | 0.01 |
| P | 5.75 | 5.61 | 6.15 | 6.07 | 6.10 | 6.11 | 5.89 | 6.02 | 5.96 | 5.93 | 6.49 | 6.45 | 5.94 | 5.87 |
| Fe2+ | 0.00 | 0.00 | 0.20 | 0.12 | 0.02 | 0.01 | 0.05 | 0.00 | 0.01 | 0.02 | 0.03 | 0.04 | 0.00 | 0.01 |
| Mg | 0.00 | 0.00 | 0.11 | 0.06 | 0.00 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 |
| Ca | 9.73 | 9.66 | 9.82 | 9.86 | 9.99 | 10.00 | 9.99 | 9.98 | 10.00 | 10.00 | 10.00 | 10.00 | 9.99 | 9.99 |
| K | 0.00 | 0.00 | 0.07 | 0.08 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| F | 1.79 | 2.07 | 1.64 | 1.77 | 1.45 | 1.33 | 1.89 | 1.81 | 1.36 | 1.49 | 0.46 | 1.62 | 1.19 | 1.11 |
| Cl | 0.00 | 0.01 | 0.04 | 0.03 | 0.10 | 0.09 | 0.05 | 0.06 | 0.08 | 0.08 | 0.08 | 0.11 | 0.37 | 0.37 |
| H | 0.20 | 0.26 | 0.31 | 0.20 | 0.45 | 0.58 | 0.06 | 0.13 | 0.55 | 0.43 | 0.46 | 0.27 | 0.44 | 0.52 |
| XOH | 0.10 | 0.14 | 0.16 | 0.10 | 0.22 | 0.29 | 0.03 | 0.06 | 0.28 | 0.22 | 0.23 | 0.13 | 0.22 | 0.26 |
| XF | 0.90 | 0.86 | 0.82 | 0.89 | 0.73 | 0.67 | 0.95 | 0.91 | 0.68 | 0.74 | 0.73 | 0.81 | 0.59 | 0.55 |
| XCl | 0.00 | 0.00 | 0.02 | 0.02 | 0.05 | 0.05 | 0.02 | 0.03 | 0.04 | 0.04 | 0.04 | 0.06 | 0.19 | 0.19 |
| Giants Range Batholith | Synthetic Granite Compositions [25] | |||
|---|---|---|---|---|
| wt% | Granite (Embarrass) | Hornblende-biotite granite | ||
| SiO2 | 72.39 | 66.31 | 70.77 | 67.51 |
| TiO2 | 0.49 | 0.49 | - | - |
| Al2O3 | 13.39 | 12.70 | 14.83 | 17.45 |
| Fe2O3 | 0.18 | 1.98 | 2.44 | 2.44 |
| FeO | 1.44 | 2.66 | - | - |
| MnO | 0.01 | 0.04 | - | - |
| MgO | 1.61 | 3.01 | 1.19 | 1.19 |
| CaO | 0.92 | 4.77 | 1.34 | 3.52 |
| Na2O | 5.35 | 5.03 | 3.33 | 3.90 |
| K2O | 2.42 | 2.33 | 6.10 | 3.99 |
| P2O5 | 0.10 | 0.13 | - | - |
| ZrO2 | 0.15 | 0.13 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benkó, Z.; Mogessie, A.; Molnár, F.; Hauck, S.A.; Severson, M.J.; Ettinger, K. The Influence of Thermal Differences and Variation of Cl–F–OH Ratios on Cu-Ni-PGE Mineralization in the Contact Aureole of the South Kawishiwi Intrusion, Duluth Complex. Geosciences 2018, 8, 474. https://doi.org/10.3390/geosciences8120474
Benkó Z, Mogessie A, Molnár F, Hauck SA, Severson MJ, Ettinger K. The Influence of Thermal Differences and Variation of Cl–F–OH Ratios on Cu-Ni-PGE Mineralization in the Contact Aureole of the South Kawishiwi Intrusion, Duluth Complex. Geosciences. 2018; 8(12):474. https://doi.org/10.3390/geosciences8120474
Chicago/Turabian StyleBenkó, Zsolt, Aberra Mogessie, Ferenc Molnár, Steven A. Hauck, Mark J. Severson, and Karl Ettinger. 2018. "The Influence of Thermal Differences and Variation of Cl–F–OH Ratios on Cu-Ni-PGE Mineralization in the Contact Aureole of the South Kawishiwi Intrusion, Duluth Complex" Geosciences 8, no. 12: 474. https://doi.org/10.3390/geosciences8120474
APA StyleBenkó, Z., Mogessie, A., Molnár, F., Hauck, S. A., Severson, M. J., & Ettinger, K. (2018). The Influence of Thermal Differences and Variation of Cl–F–OH Ratios on Cu-Ni-PGE Mineralization in the Contact Aureole of the South Kawishiwi Intrusion, Duluth Complex. Geosciences, 8(12), 474. https://doi.org/10.3390/geosciences8120474






