Abstract
Reported lizard material from the Wapiti Formation (central-western Alberta, Canada) is limited to fragmentary remains of Kleskunsaurus grandeprairiensis and Socognathus unicuspis, a partial dentary attributed to Chamops cf. C. segnis, and a vertebra reportedly comparable to those of the much larger lizard Palaeosaniwa canadensis. P. canadensis is a Late Cretaceous North American member of Monstersauria, a Mesozoic and Cenozoic anguimorph group represented today by five species of Heloderma. Here, we document new squamate material from the DC Bonebed locality (Wapiti Unit 3; Campanian), including a right frontal identified as cf. P. canadensis and a taxonomically indeterminate squamate astragalocalcaneum. A partial skeleton from the Two Medicine Formation of Montana provisionally attributed to P. canadensis has a frontal resembling the corresponding element from the DC Bonebed in overall shape, in having narrowly separated facets for the prefrontal and postorbitofrontal, and in bearing osteoderms similar to the DC specimen’s in ornamentation and configuration. The Two Medicine and DC specimens differ from a roughly contemporaneous frontal from southern Alberta referred to the monstersaur Labrodioctes montanensis. The DC specimen confirms the presence of monstersaurian squamates in the Wapiti Formation, representing the northernmost record of any definitive Late Cretaceous monstersaur to date.
Keywords:
Squamata; Monstersauria; Palaeosaniwa; Wapiti Formation; Cretaceous; Campanian; Canada; Alberta 1. Introduction
Monstersaurs constitute an anguimorph squamate clade that includes a variety of Mesozoic and Cenozoic extinct taxa from North America, Europe, and Asia, together with five extant species of Heloderma [1,2,3]. Within Anguimorpha, some phylogenetic analyses have found Monstersauria to be closely related to Anguidae [4,5,6,7,8], and others have suggested that monstersaurs form a paraphyletic assemblage [9,10]. However, most analyses based on morphology have recovered monstersaurs as a monophyletic group close to Varanidae [2,3,6,11,12]. Following Conrad et al. [6], Monstersauria is defined herein as the set of taxa sharing a more recent common ancestor with Heloderma horridum than Varanus varius, Anguis fragilis, or Xenosaurus grandis, and Varanoidea as the set of taxa descended from the most recent common ancestor of H. horridum, V. varius and Lanthanotus borneensis. For convenience in using the term “varanoid”, we accept the taxonomic content of Varanoidea as limited to Varanus, Heloderma, Lanthanotus and their respective extinct stem-taxa, while recognizing that this assemblage may be non-monophyletic.
The name Monstersauria is highly appropriate given that the extant members of Heloderma are sizeable predators (maximum body length exceeding 50 cm for the Gila monster H. suspectum and 80 cm for the Rio Fuerte beaded lizard H. exasperatum [13,14]) armoured with distinctively thick, pitted osteoderms, and are recognized as the most venomous non-ophidian squamates [15]. The osteoderms are widely distributed over the dorsal and lateral surfaces of the body and the anterior surfaces of the limbs, as well as in the precloacal region, and are fused to the skull roof bones in adults [14]. Osteoderms likewise occur on the skull roof bones of nearly all known fossil monstersaurs [5,12,16,17,18,19,20], but for these taxa, no direct evidence is available to show whether they were present in the skin covering other parts of the body. Many fossil monstersaurs resemble extant Heloderma in having grooves in their teeth, suggesting specialization for venom delivery [18,19,21,22].
Three monstersaur taxa have previously been recovered from the Campanian and Maastrichtian of North America: Palaeosaniwa canadensis, Paraderma bogerti, and Labrodioctes montanensis [3,5,6,19,23,24,25]. A fourth taxon, Parasaniwa wyomingensis, may represent either a basal monstersaur [5,6] or a non-monstersaurian anguimorph [2,3,11,24]. Gilmore [[26]: pp. 84–85] erected P. canadensis based on isolated dorsal vertebrae from the Campanian Belly River Group of Alberta and “provisionally” referred a posterior dorsal vertebra from the Maastrichtian Lance Formation to this species. He regarded the vertebrae as “typically varanid” in most respects but distinguished from the Eocene varanid Saniwa by two “peculiarities”, namely “relatively large zygapophyses” and median grooves on the ventral surfaces of the dorsal centra. Similar median grooves occur in other squamates, including extant species of Varanus and some mosasauroids (e.g., Pannoniasaurus, IP pers. obs.; cf. Makádi et al. [27]), and grooves containing paired foramina occur on posterior dorsal vertebrae of the Late Cretaceous Mongolian monstersaur Estesia mongoliensis [2,11,22,25]. A dorsal vertebra (UALVP 33350) assigned to Ps. wyomingensis appears to bear a ventral groove [[19]: Figure 37G,H], but displays other differences from P. canadensis vertebrae, including small size, less well-developed diapophyses, and a weaker constriction at the base of the posterior condyle [19]. While the holotype specimen of P. canadensis lacks clear autapomorphies, Gilmore’s [26] implicit assumption that the species is represented by a dorsal vertebral morphotype that is unique in the Campanian-Maastrichtian of western North America has never been falsified, and we provisionally accept P. canadensis as a valid species on that basis.
Campanian specimens subsequently referred to P. canadensis include dentaries, tibiae, an incomplete maxilla, and additional vertebrae from the Belly River Group [19], and two vertebrae and a humerus from the Cerro del Pueblo Formation of Coahuila, Mexico [28]. Equally incomplete material from the Maastrichtian Lance and Hell Creek formations, and even the Palaeocene, was repeatedly referred to P. canadensis or Palaeosaniwa cf. P. canadensis in the second half of the last century [16,29,30]. However, among these fragmentary referred specimens, only dorsal vertebrae can be directly compared with the holotype of P. canadensis; other material has been attributed to the species based on the more general criteria of geological age, body size, and varanoid-like anatomy, and there is no clear evidence that the stratigraphic range of P. canadensis extends above the Campanian [19].
Palaeosaniwa canadensis may also be represented by a partial skeleton (MOR 792) from the Campanian Two Medicine Formation of Montana, which has ventrally grooved dorsal vertebrae and includes much of the skull [25]. This specimen has not yet been described in the peer-reviewed literature but was extensively discussed and identified as P. canadensis in an unpublished PhD thesis by Balsai [25] and was examined firsthand for the present study. A second partial skeleton (UCMP 118497) attributed to P. canadensis [21,25], from the Hell Creek Formation of Montana, lacks dorsal vertebrae and is much more fragmentary.
The other two undoubted North American Campanian–Maastrichtian monstersaur taxa are represented only by fragmentary material. Paraderma bogerti is known from isolated cranial and postcranial elements from Maastrichtian strata, including the Scollard Formation of Alberta, the Frenchman Formation of Saskatchewan, the Lance Formation of Wyoming, and the Hell Creek Formation of Montana [16,19]. Paraderma-like specimens reported from the Campanian are less diagnostic [23]. The holotype of Labrodioctes montanensis is an incomplete dentary from the Judith River Formation of Montana [19]. A partial frontal from the Belly River Group of Alberta was tentatively referred to this species on the basis that the frontal and the dentary are similar in geological age, come from the same general part of North America, and both show clear resemblances to corresponding bones in helodermatids [19].
Finally, the possible monstersaur Ps. wyomingensis was named by Gilmore [26], together with the putative congeneric species Ps. “obtusa”. Both were erected based on partial dentaries from the Lance Formation. However, their minor dental differences are partly artefactual and do not justify their taxonomic separation [16,19]. The former species is represented in the Frenchman and Lance formations by various isolated elements, and a single partial maxilla is known from the Scollard Formation [16,19,26]. Reports of Parasaniwa from the Eocene of the United States [31,32] remain unsubstantiated [19,33]. However, a second, unnamed species of Parasaniwa occurs in the Belly River Group of Alberta [19].
Farther north, the Campanian–Maastrichtian Wapiti Formation of central-western Alberta has been known as a source of fossil lizard specimens since the mid-20th century, when Sternberg [34] referred an isolated partial mandible from the Kleskun Hill locality to the borioteiioid Chamops cf. C. segnis. More recently, material from Kleskun Hill was assigned to another borioteiioid, namely Socognathus unicuspis, and to the enigmatic and potentially endemic Kleskunsaurus grandeprairiensis [35]. Furthermore, Sternberg’s [34] identification of the original Kleskun dentary was questioned [19,36]. Finally, Currie et al. [[37]: p. 11] briefly reported “a varanid (cf. Paleosaniwa [sic]) vertebra” from the Pipestone Creek Bonebed, another Wapiti Formation locality. Although the Pipestone vertebra may belong to Palaeosaniwa, the presence of monstersaurs in the Wapiti Formation has never been firmly established.
Among recently recovered material from the DC (Dinosaur–Chelonian) Bonebed locality in Unit 3 of the Wapiti Formation (Figure 1) is an isolated lizard frontal (UALVP 59503) identifiable as that of a monstersaur cf. P. canadensis based on comparisons to the intact frontals of the Two Medicine specimen (MOR 792). UALVP 59503 represents the first definitive monstersaur specimen from the Upper Cretaceous of central-western Alberta. This report aims to describe UALVP 59503 with reference to MOR 792, the frontal referred to L. montanensis (TMP 1978.018.0001), and other North American Cretaceous monstersaurian material. We consider the taxonomic status of both P. canadensis and L. montanensis and briefly explore the palaeoecology of the monstersaur represented by UALVP 59503. A taxonomically indeterminate lizard astragalocalcaneum (UALVP 59947) from the DC Bonebed is also briefly described.
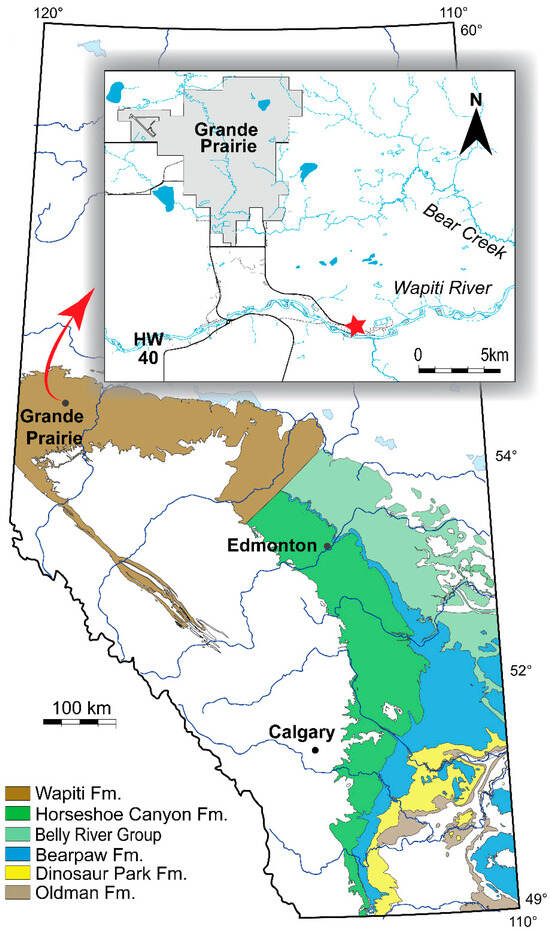
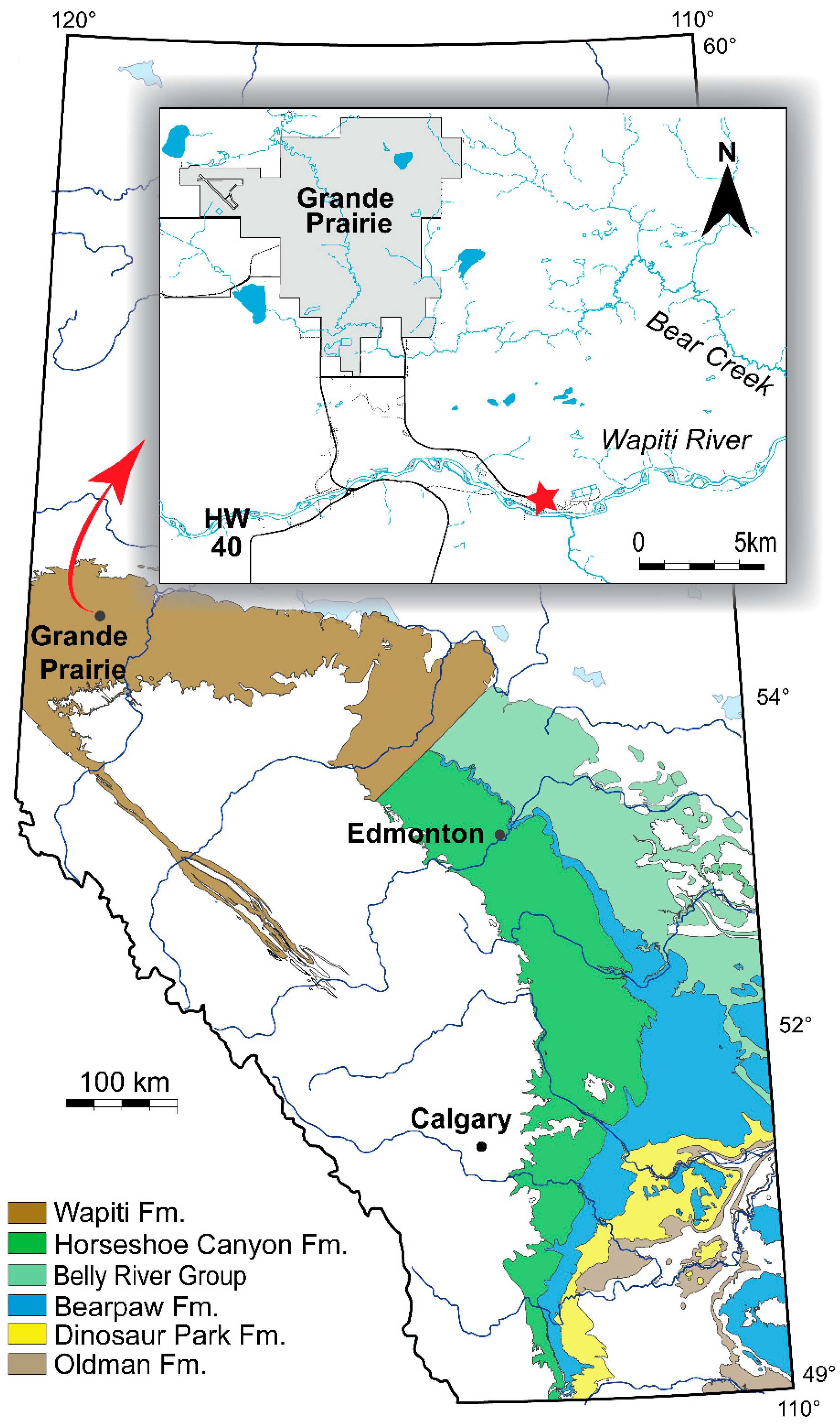
Figure 1.
Map showing the location of the DC Bonebed site within the Grande Prairie area (inset). Modified from Fanti et al. [38].
Institutional Abbreviations AMNH, American Museum of Natural History, New York, NY, USA; ANSP, Academy of Natural Sciences, Philadelphia, PA, USA; IGM, Institute of Geology, Mongolian Academy of Sciences, Ulaan Baatar, Mongolia; MOR, Museum of the Rockies, Bozeman, MT, USA; PIN, Paleontological Institute, Russian Academy of Sciences, Moscow, Russia; TMP, Royal Tyrrell Museum of Palaeontology, Drumheller, AB, Canada; UALVP, University of Alberta Laboratory for Vertebrate Palaeontology, Edmonton, AB, Canada; UAMZ, University of Alberta Museum of Zoology, Edmonton, AB, Canada; USNM, Smithsonian Institution, National Museum of Natural History, Washington, DC, USA; ZPAL, Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland.
2. Geological Setting
The specimens described herein (UALVP 59503 and 59947) were recovered from the Dinosaur–Chelonian (DC) Bonebed [38], a series of stacked mudstone and siltstone point bars preserved in a fluvial channel deposit within the Wapiti Formation of northern Alberta (Figure 1). The DC Bonebed is located on the northern bank of the Wapiti River, approximately 10 km southeast of the city of Grande Prairie, and preserves a diverse assemblage of isolated skeletal elements of predominantly small to medium-sized vertebrates, particularly fish, Champsosaurus, turtles, and dinosaurs. Composite sections along the Wapiti River [[38]: Figure 3] indicate that the bonebed belongs to Wapiti Formation Unit 3 (sensu Fanti and Catuneanu [39]), a stratigraphic interval approximately correlative with the Bearpaw Formation of southern Alberta [[38]: Figure 2]. New U/Pb ages from bentonitic layers sampled near the DC Bonebed, together with palynological evidence, suggest an age of about 73.5 Ma for the lizard material discussed here [38].
3. Materials and Methods
This study is based primarily on examination of UALVP 59503 and 59947. The former specimen was compared, based on detailed firsthand observations, to the frontals of the partial skeleton (MOR 792) attributed to Palaeosaniwa canadensis in an unpublished PhD dissertation [25] and to the fragmentary right frontal (TMP 1978.018.0001) referred to Labrodioctes montanensis by Gao and Fox [19]. A range of other fossil and extant lizard specimens were more cursorily examined and compared with UALVP 59503 and the less diagnostic UALVP 59947. A µCT-scan of UALVP 59503 was carried out using a 1076 ICT scanner (Bruker–Skyscan, Kontich, Belgium) at the Pharmaceutical Orthopaedic Research Lab of the University of Alberta, with a voltage of 100 kV, a current of 100 µA, and a voxel edge length of 8.6 µm. The CT slices were examined in order to provide insights into the internal morphology of the specimen.
4. Systematic Palaeontology
SQUAMATA [40]
ANGUIMORPHA [41]
MONSTERSAURIA [2]
Provisional diagnosis: Anguimorph lizards typically with thick, polygonal, non-imbricating, non-compound osteoderms bearing ridge-and-pit ornamentation but not keels or tubercles, fused to the frontal and other bones of the skull roof. Frontals remain paired in adults. Cristae cranii are well-developed but typically do not meet ventrally to enclose the olfactory canal.
Remarks: The diagnosis given here pertains to the frontal because the only specimen referred to Monstersauria in this paper is an isolated right frontal element.
cf. Palaeosaniwa canadensis
Referred Material: UALVP 59503, a right frontal.
Locality and Horizon―DC Bonebed, ~10 km southeast of Grande Prairie, Alberta, Canada (55°4′53″ N, 118°43′59″ W); middle part of Unit 3 of Wapiti Formation, upper Campanian [38].
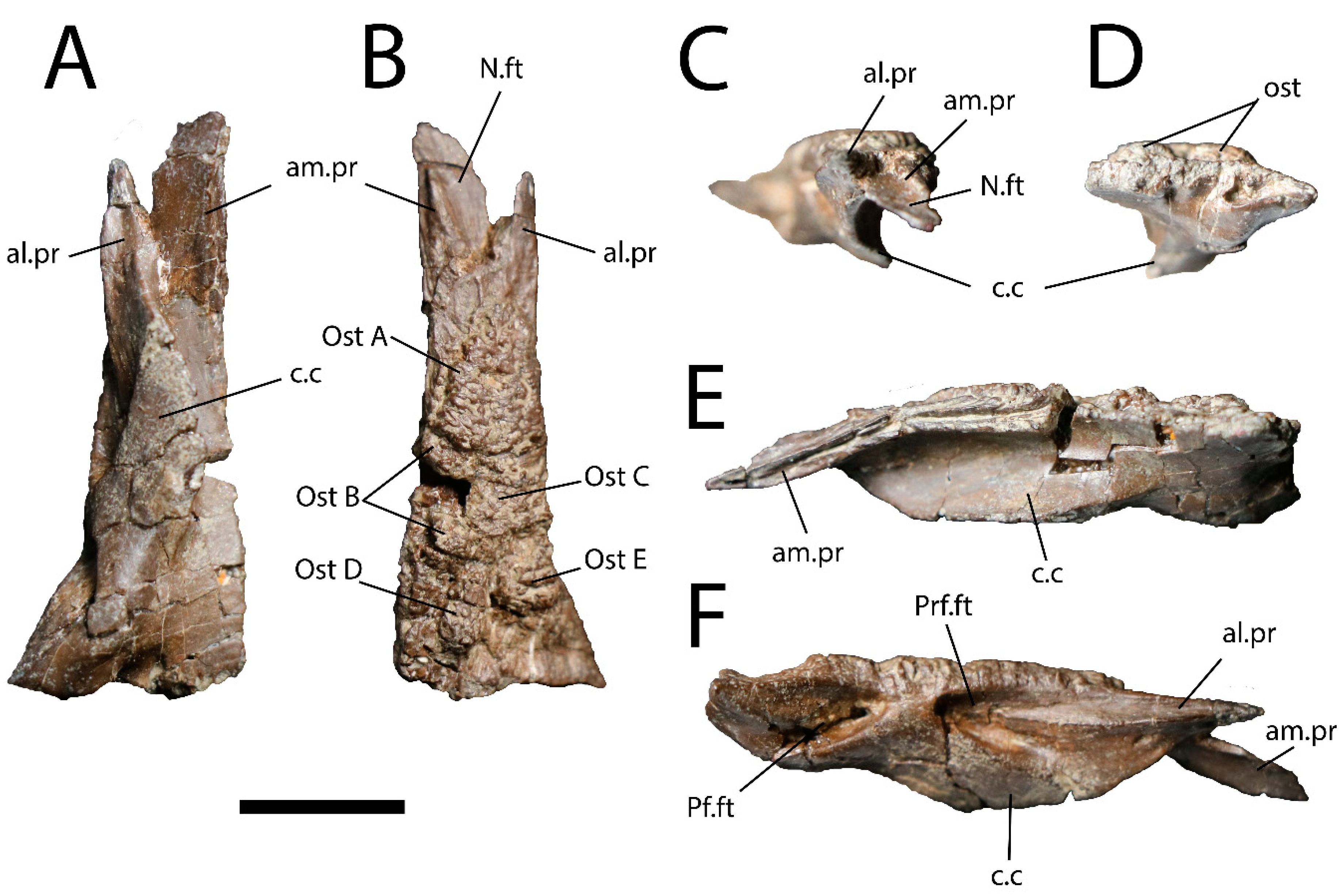
Description: UALVP 59503 (Figure 2) is a complete right frontal measuring 35.1 mm in length and 14.1 mm in maximum width, with several osteoderms rising from the dorsal surface of the bone. Most of the medial margin of UALVP 59503 is well-preserved, and the anterior part of the margin bears a deep longitudinal groove to accommodate the medial edge of the opposite frontal. More posteriorly, the medial margin is slightly damaged but does not appear to have been grooved. The condition of the margin shows that UALVP 59503 was not fused to its counterpart.
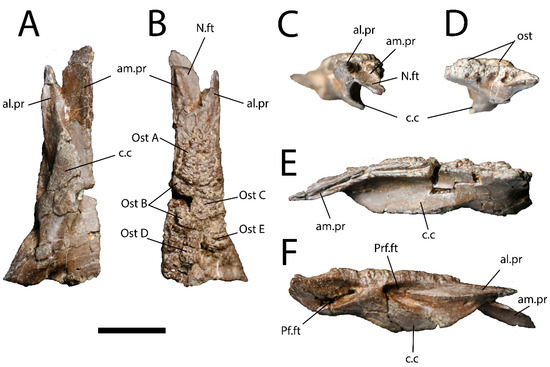
Figure 2.
Right frontal (UALVP 59503) in (A) ventral; (B) dorsal; (C) anterior; (D) posterior; (E) medial; and (F) lateral views. Scale bar = 1 cm. Abbreviations: al.pr, anterolateral process; am.pr, anteromedial process; c.c, crista cranii; N.ft, nasal facet; Ost, osteoderm: Pf.ft, facet for postfrontal portion of postorbitofrontal; Prf.ft, prefrontal facet. Letters identify individual osteoderms.
UALVP 59503 is roughly rectangular in outline for most of its length, with medial and lateral margins running parallel, but the posterior part of the lateral margin flares outward (Figure 2A,B) so that the posterior end of the frontal is nearly double the width of the narrower anterior portion (7.4 mm), as measured posterior to the nasal facet. The flaring of the lateral margin in UALVP 59503 is rather abrupt, forming a distinct angle near the anterior apex of a facet on the lateral surface of the frontal (Figure 2F; Pf.ft). The facet likely contacted the postfrontal portion of a single postorbitofrontal comprising fused postorbital and postfrontal ossifications, given that a fused postorbitofrontal is present in Varanus, Heloderma, Estesia mongoliensis, and most other varanoids [3,5,22,42,43]. Alternatively, the two bones may instead have remained separate, as described in Gobiderma pulchrum and the Cretaceous lanthanotine Cherminotus longifrons [5,17,43], or the postorbital may have been lost, as appears to be the case in Lanthanotus [43]. The presence of a fused postorbitofrontal is assumed in the remainder of this description.
The broad, flat, triangular facet for the nasal (Figure 2B,C; N.ft) is situated on the anteromedial process (am.pr) of the frontal, which extends farther anteriorly than the more pointed anterolateral process (al.pr). In other monstersaurs, the latter process usually intrudes between the nasal and prefrontal [2,5,6,22,24]. The nasal facet occupies most of the dorsal surface of the anteromedial process, but the posterior two-thirds of the medial edge of the process is elevated a few millimetres above the nasal facet as a narrow triangular ridge with a sharp anterior apex.
The prefrontal facet (Figure 2F; Prf.ft) is a complex articular surface that occupies approximately 50% of the total length of the lateral margin of the frontal, from about 3 mm posterior to the tip of the anterolateral process to a level slightly posterior to that of the apex of the crista cranii. The anterodorsal portion of the facet is a shallow, dorsoventrally narrow longitudinal groove that tapers anteriorly to a point. Posterior to the groove is the deeper part of the facet, which is subtriangular with a posteriorly directed and dorsally situated apex. The base of this triangular fossa is dorsoventrally broader than the anterior groove, but the prominent ventral margin of the groove continues posteriorly a short distance into the fossa. Ventral to the groove and anterior to the triangular fossa, the lateral surface of the frontal forms a subdued non-articular area that bears a small, round foramen.
The facet on the frontal for the postorbitofrontal is deep and triangular (Figure 2F). The facets for the prefrontal and postorbitofrontal mirror each other and are separated by about 3 mm of smooth bone, likely indicating a small gap between the prefrontal and postorbitofrontal bones and a small contribution of the frontal to the orbital margin.
The ventral surface of the frontal bears a large, thick, and well-preserved crista cranii (Figure 2; cc; also called the subolfactory process), whose ventral margin curls slightly medially. The ventral margin would nevertheless have been far from the midline of the intact skull, so that the crista would not have contacted its left counterpart medially to form an enclosed olfactory canal beneath the skull roof. The crista arises below the anterior end of the postorbitofrontal facet and recedes into the ventral surface of the frontal just posterior to the notch between the anteromedial and anterolateral processes. The crista’s apex is situated at the level of the anteroposterior midpoint of the deep triangular part of the prefrontal facet, posterior to the midpoint of the crista’s length. The crista is thicker posteriorly than anteriorly, and its posterior base bears a slight posteroventrally facing depression. The depression is bordered medially by the posteriormost part of the crista, a thin, subdued ridge, and bordered laterally by a low convexity that is elongated along a posterolateral-anteromedial axis.
The medial portion of the sutural surface for contact with the parietal is dorsoventrally narrow, whereas the lateral portion is ventrally expanded into a triangular hollow with a laterally directed apex (Figure 2D). The posterior margin of the frontal is sinuous in dorsal or ventral view (Figure 2A,B).
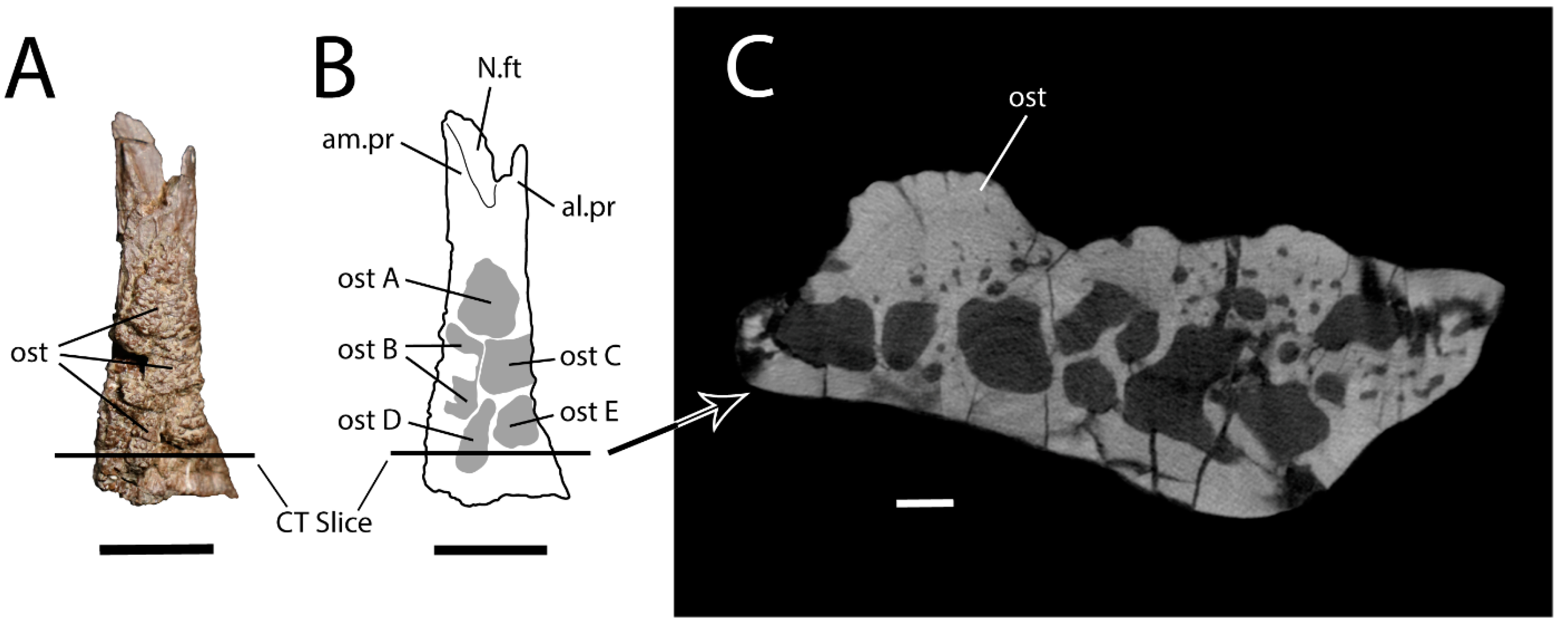
As in most other monstersaurs, UALVP 59503 has osteoderms that are non-overlapping, approximately polygonal, and without keels (Figure 2A and Figure 3). They are fused to the dorsal surface of the frontal, as a µCT scan (Figure 3C) reveals no plane of separation between the osteoderms and the underlying bone. The osteoderms show differing numbers of sides and relative side lengths and are also variable in size and thickness, though they account for only a small part of the total dorsoventral depth of the frontal (Figure 3C). They bear ornamentation comprising an irregular pattern of pits separated by narrow ridges.
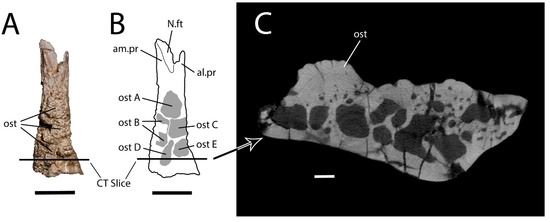
Figure 3.
Right frontal (UALVP 59503). (A) Dorsal view. Scale bar = 1 cm. (B) Line drawing of (A) with osteoderms shaded in grey. Scale bar = 1 cm. (C) Transverse CT slice through UALVP 59503 at location indicated in (A,B), oriented as if in posterior view (i.e., medial is to the left). Scale bar = 1 mm. Abbreviations: al.pr, anterolateral process; am.pr, anteromedial process; N.ft, nasal facet; ost, osteoderm. Letters identify individual osteoderms.
Five individual osteoderms appear to be present (Figure 2A and Figure 3). The posteriormost two are separated from the others by small patches of subdued but rugose bone. These subdued areas represent the dorsal surface of the frontal, which is sculptured as in some extant lizards (Figure 4A,B). The three more anteriorly positioned osteoderms are integrated into a continuous osteodermal pavement that extends over most of the frontal, with the bases of adjacent osteoderms appearing to coalesce. Nevertheless, the three anterior, confluent osteoderms are demarcated by shallow grooves. For convenience, the five osteoderms are assigned the letters A–E. Ost (osteoderm) A is the largest and anteriormost osteoderm on the frontal. The base of this subtriangular osteoderm occupies the full width of the frontal at a level slightly anterior to the posterior end of the prefrontal facet, and the osteoderm tapers anteriorly and terminates about 5 mm posterior to the notch between the anteromedial and anterolateral processes. Ost B is largely broken away but occupies a subrectangular area on the medial part of the frontal, posterior to Ost A. As the medial surface of Ost B is missing, it is possible that this osteoderm originally extended across the skull midline. Ost C is intact, lateral to Ost B, and subrectangular, being mediolaterally wider than anteroposteriorly long. The posteromedial corner of Ost C gives rise to a subdued, convex posterior extension that contacts the anterior tip of Ost D. The latter osteoderm is teardrop-shaped and elongated, with a long axis that trends anteriorly and slightly laterally. The anterior end of Ost D tapers to a point, whereas the posterior end is rounded. Ost D is situated posterior to the lateral part of Ost B and the medial part of Ost C and approaches but does not reach the posterior and medial margins of the frontal. Ost E is subtriangular, situated posterior to Ost C on the lateral part of the frontal, and separated from Osts C and D by deep furrows. Anterior to Ost A and posterior to Osts D and E, the dorsal surface of the frontal is free of osteoderms, and the posterolateral corner of the dorsal surface is smooth rather than rugose.
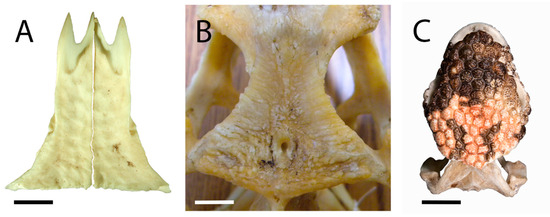
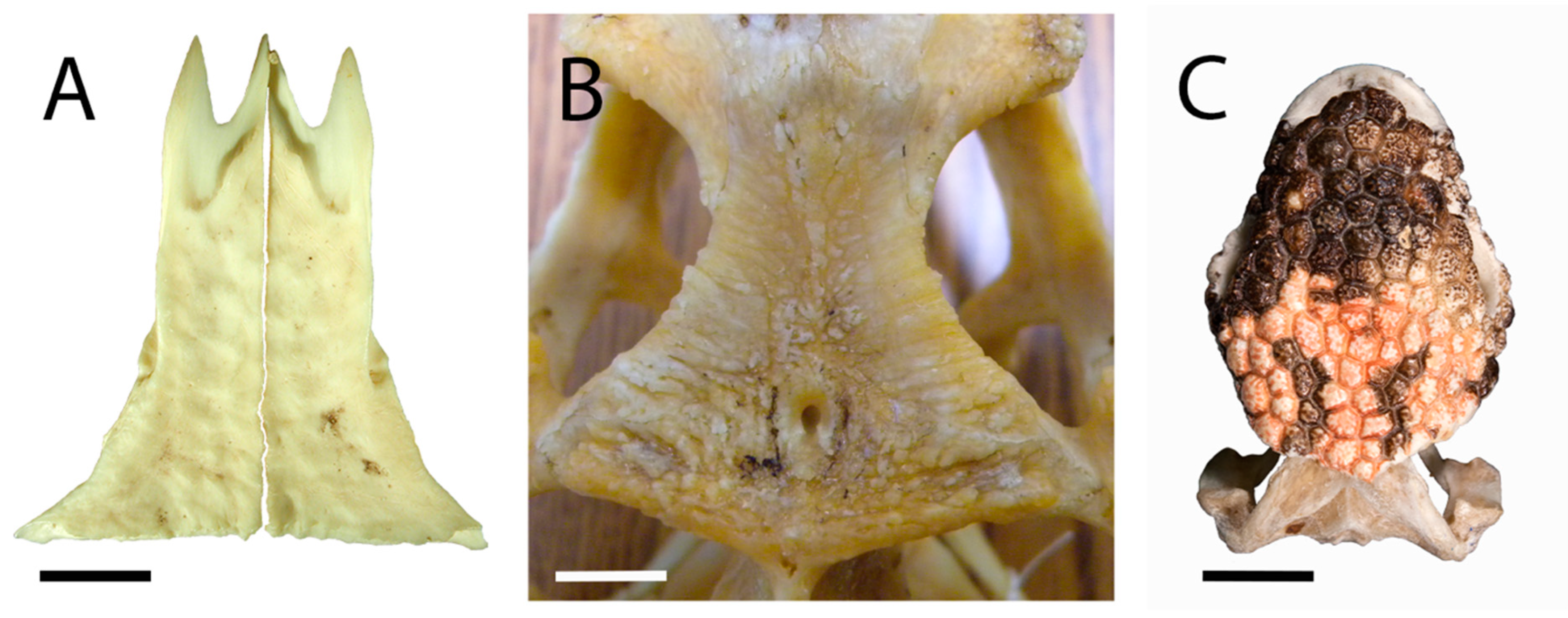
Figure 4.
Dorsal views of extant lizard frontals. (A) Varanus rudicollis (UAMZ 145710) displays a slight hummocky ornamentation on the otherwise smooth surface of the paired frontals. Scale bar = 1.5 cm. (B) Cyclura cornuta (AMNH 93182) displays sculpturing on the single frontal. Scale bar = 1.5 cm. (C) Heloderma suspectum (TMP 1990.007.0357) displays fully formed, skin-covered osteoderms on the dorsal surface of the skull, obscuring the paired frontals. Scale bar = 1 cm.
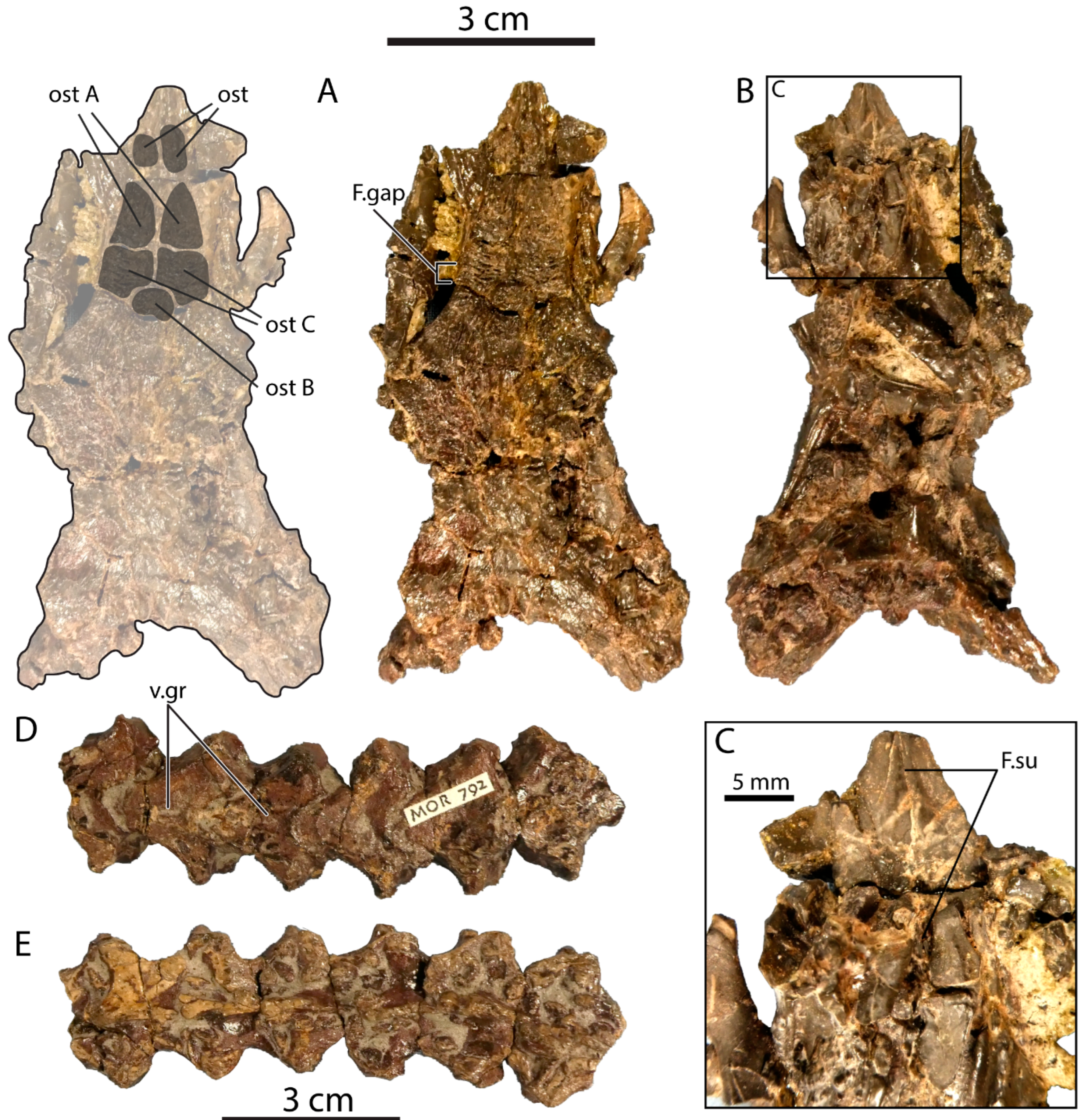
Comparisons: The frontals of P. canadensis MOR 792 (Figure 5A–C) are nearly complete and slightly larger than those of UALVP 59503, with a preserved length of about 40 mm. The frontals are tightly articulated with one another, but a midline suture is apparent in ventral view, implying at least a partial lack of fusion (Figure 5C; F.su).
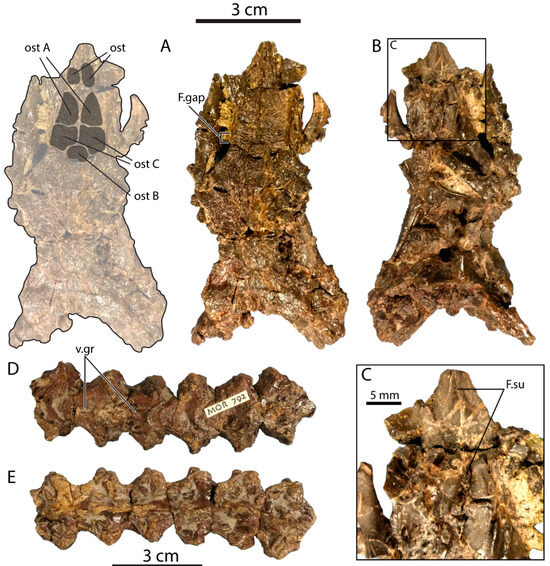
Figure 5.
Portions of a partial monstersaur skeleton from the Two Medicine Formation of Montana (MOR 792), attributed to Palaeosaniwa canadensis in an unpublished PhD dissertation [25]. Skull in (A) dorsal; (B) ventral and (C) close-up ventral views, with overlay showing outline and individual osteoderms at left in (A). Five articulated dorsal vertebrae in (D) ventral and (E) dorsal views. Abbreviations: F.gap, gap between articular facets for prefrontal and postorbitofrontal; F.su, interfrontal suture; ost, osteoderm; v.gr, ventral groove on dorsal vertebra. Letters identify individual osteoderms.
As in UALVP 59503 and the basal monstersaur Gobiderma pulchrum [5], the posterior end of the frontal is much wider than the anterior portion of the bone, whereas in Heloderma, the frontal has a more consistent width throughout its length [18]. The posterior part of the frontal widens gradually so that the lateral margin forms a gentle curve as in G. pulchrum and Estesia mongoliensis [5,22], rather than an abrupt angle as in UALVP 59503.
The nasal facet and associated medial ridge are similar in form to their counterparts in UALVP 59503, but the anteromedial processes of both frontals are incomplete, and the anterolateral processes are almost entirely broken away. The prefrontal and postorbitofrontal facets closely approach one another, being separated by a gap (Figure 5A: F.gap) comparable in magnitude to that seen in UALVP 59503 and the Cenozoic helodermatid Lowesaurus matthewi [18]. By comparison, the prefrontal and postorbitofrontal have extensive sutural contact in Heloderma and a slight contact in E. mongoliensis, whereas the prefrontal and postfrontal are widely separated in G. pulchrum [5,17,18,22,44]. The posterior margin of the frontal is sinuous, as in UALVP 59503.
The frontal bears four well-developed osteoderms (Figure 5A), which resemble those of UALVP 59503 in being approximately polygonal and having irregular ridge-and-pit ornamentation. Three of the osteoderms are clustered together in the central part of the frontal, forming a pavement, and the anteriormost osteoderm in the central cluster corresponds closely to Ost A of UALVP 59503 (Figure 3A,B) in shape and position. A small osteoderm, possibly equivalent to Ost B, is present on the midline and is transversely wider than anteroposteriorly long. Directly posterior to “Ost A” of MOR 792 is what may be the equivalent of Ost C, an osteoderm that would be quadrangular except that its posteromedial corner is recessed to accommodate “Ost B”. Unlike in UALVP 59503, “Ost C” contacts its counterpart across the midline in MOR 792. However, it should be noted that what we interpret as an anterior fragment of Ost B in UALVP 59503 may instead be a medial extension of Ost C, which would make the osteoderm arrangement more similar to that observed in MOR 792. In the latter specimen, the grooves on “Ost C” are long and transversely oriented, a pattern not seen in UALVP 59503. The frontal of MOR 792 also bears an osteoderm well anterior to “Ost A”, at the base of the medial ridge on the anteromedial process and lacks equivalents to Osts D and E.
The frontal osteoderms of Gobiderma resemble those of MOR 792 and UALVP 59503 in forming a continuous pavement and being larger anteriorly than posteriorly [5,17]. However, the osteoderms of Gobiderma are more uniform in size, and the osteoderm pavement covers the entire frontal. Osteoderms that may be individually equivalent to those seen in MOR 792 and UALVP 59503 are difficult, at best, to recognize.
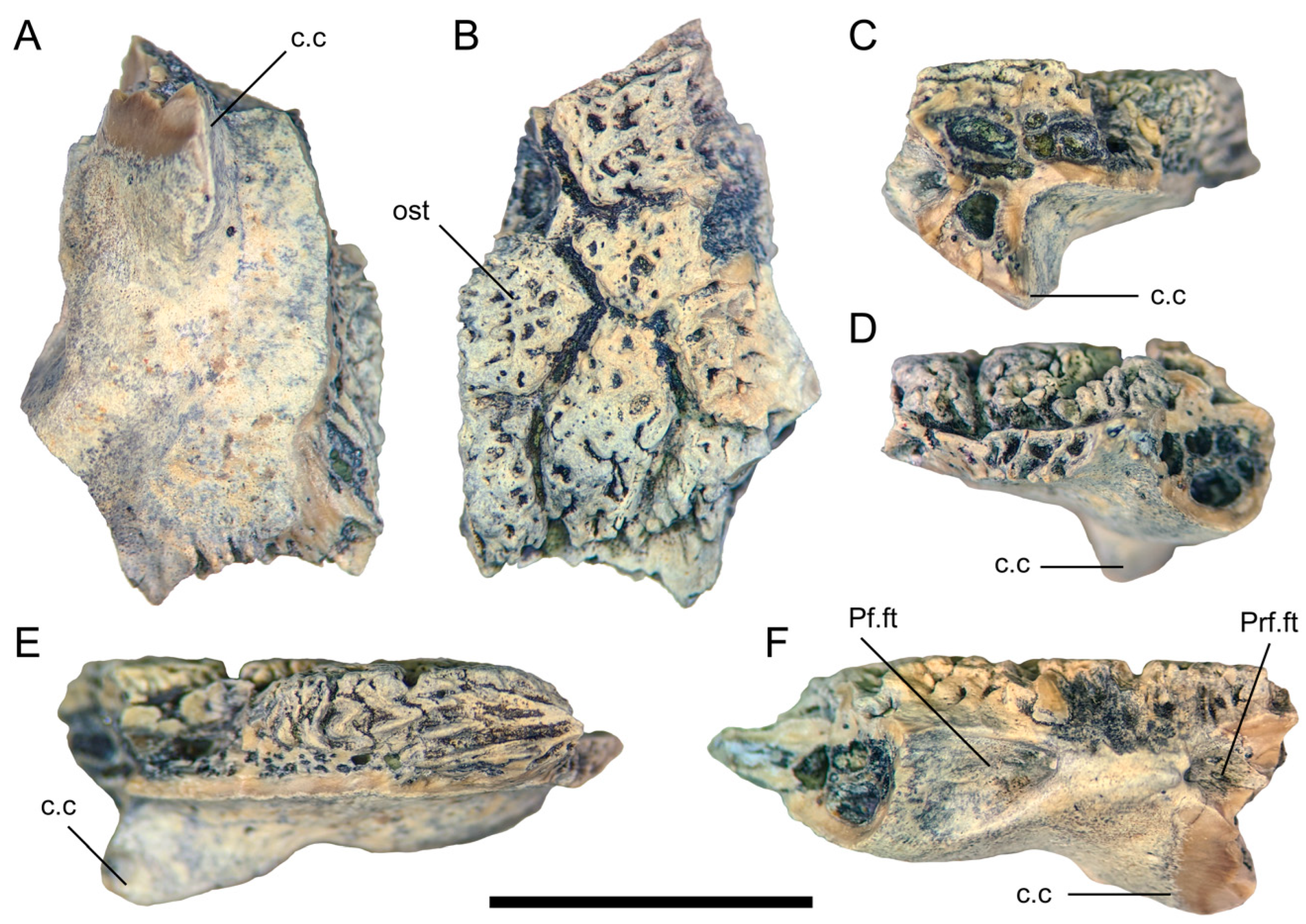
In TMP 1978.018.0001, the frontal fragment (Figure 6) referred to Labrodioctes montanensis by Gao and Fox [19], a posterior portion of the medial margin is present (Figure 6E). The preserved section of the medial margin is much dorsoventrally thicker than its equivalent in UALVP 59503, with well-developed, irregular grooves and ridges that would have formed an interdigitating contact with the opposite frontal.
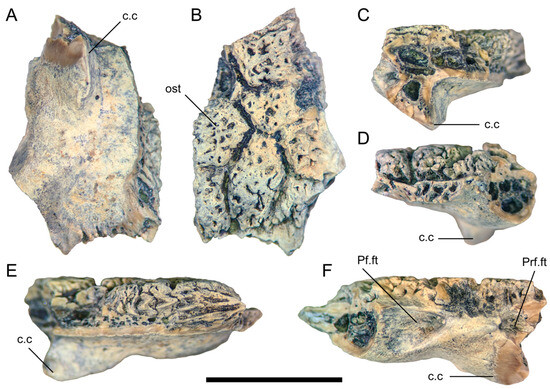
Figure 6.
Partial right frontal (TMP 1978.018.0001) in (A) ventral; (B) dorsal; (C) anterior; (D) posterior; (E) medial; and (F) lateral views. Scale bar = 1 cm. Abbreviations: c.c, crista cranii; ost, osteoderm: Pf.ft, facet for the postfrontal portion of the postorbitofrontal; Prf.ft, prefrontal facet.
Only a small portion of the lateral margin of the frontal is preserved in TMP 1978.018.0001 (Figure 6F), including the anterior part of the postorbitofrontal facet and the contribution of the frontal to the orbital margin. A distinct angle formed by the posterolateral flaring of the frontal is also evident (Figure 6A,B), as in UALVP 59503. Gao and Fox [19] regarded TMP 1978.018.0001 as a left frontal, but it must be a right frontal given the position and alignment of the crista cranii relative to the angled part of the lateral margin and the articular facets for the prefrontal and postorbitofrontal. The preserved portion of the postorbitofrontal facet is similar in form to its counterpart in UALVP 59503, but deeper, excavating anteromedially into the frontal. The prefrontal and postorbitofrontal facets closely approach one another, as in UALVP 59503.
The crista cranii is too incompletely preserved to determine whether this structure would have contacted its counterpart on the left side of the skull or remained well lateral to the midline as in UALVP 59503. Among anguimorphs, contact between the cristae cranii occurs in Varanus and Heloderma but not in anguids, Shinisaurus, Xenosaurus, Lanthanotus, G. pulchrum, or E. mongoliensis [[5,18]: Figures 2, 22, and 44]. The posterior base of the crista lacks the posteroventrally directed depression seen in UALVP 59503, and the convexity situated lateral to the base of the crista is less well-developed than in that specimen (Figure 6A). Heloderma lacks a swelling in this area, and the crista cranii instead arises from the posterolateral corner of the ventral surface of the frontal as a distinct crest [[18]: Figure 2].
Some areas on the osteoderms attached to the frontal have only scattered pitting, as in the few cephalic osteoderms preserved in the only known specimen of the Late Cretaceous Chinese monstersaur Chianghsia nankangensis [45], whereas others show ridge-and-pit ornamentation (Figure 6B) as in UALVP 59503, Heloderma (Figure 4C), and large frontal osteoderms of Lo. matthewi [20]. Adjacent osteoderms are separated by distinct grooves, as in the helodermatids Heloderma and Lo. matthewi [18,20,44,46], but in contrast to the condition in UALVP 59503. The osteoderms broadly resemble those of UALVP 59503, and differ from those of Heloderma [20,44], in being variably polygonal rather than uniformly hexagonal.
Although TMP 1978.018.0001 is highly incomplete, eight osteoderms are at least partially preserved, occupying the entire dorsal surface of the preserved part of the bone. An elongate hexagonal osteoderm is similar in orientation and position to Ost D of UALVP 59503, but the arrangement of the osteoderms is otherwise dissimilar between the two specimens. The osteoderms of TMP 1978.018.0001 have a better-defined polygonal character than those of UALVP 59503, with straighter edges and sharper corners.
Squamata indet.
Referred Material: UALVP 59947, a right astragalocalcaneum.
Locality and Horizon: DC Bonebed, ~10 km southeast of Grande Prairie, Alberta, Canada (55°4′53″ N, 118°43′59″ W); middle part of Unit 3 of Wapiti Formation, upper Campanian [38].
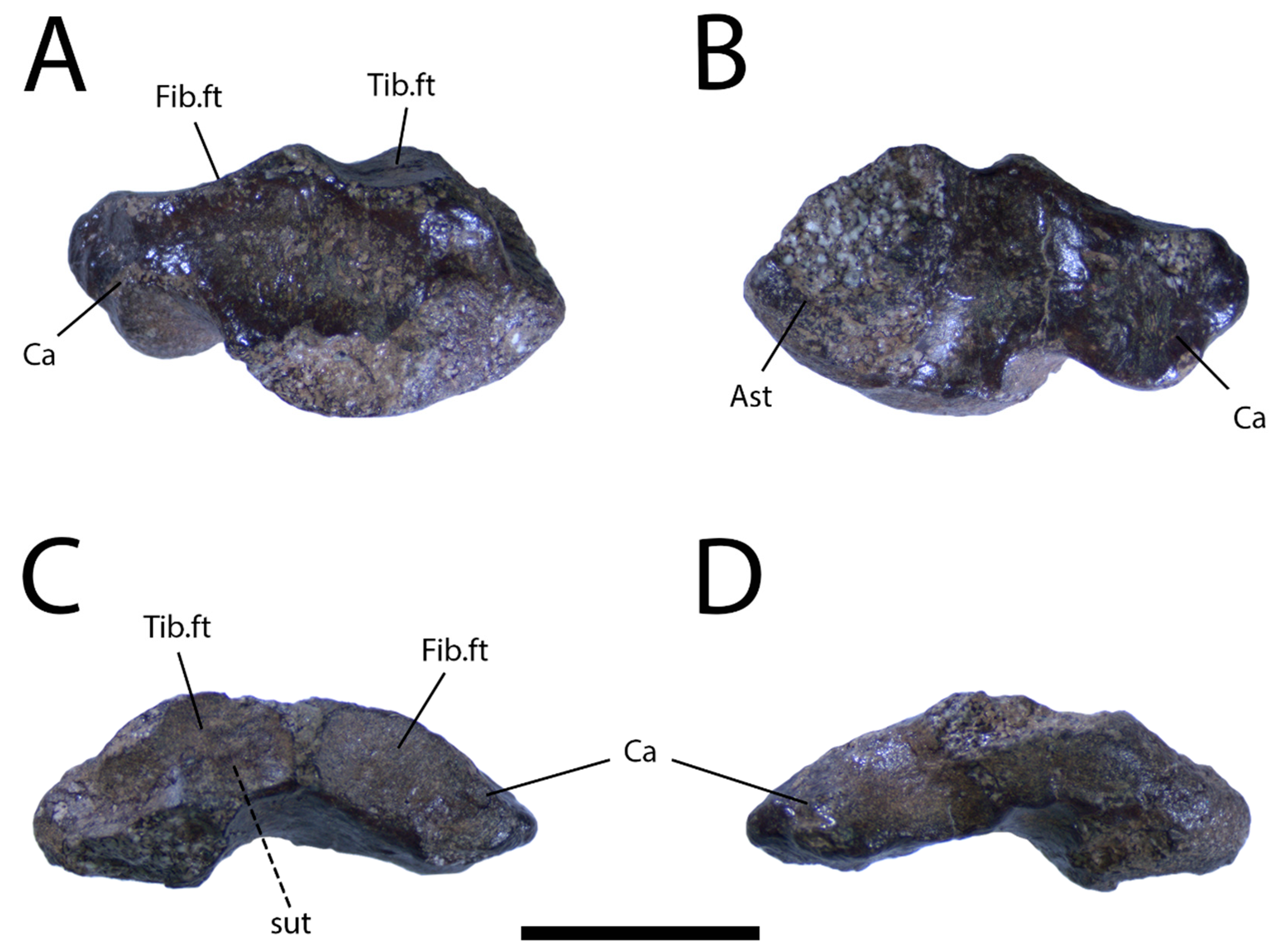
Description: The squamate right astragalocalcaneum (UALVP 59947) from the DC Bonebed (Figure 7) was not collected in close association with the frontal UALVP 59503, but belongs to either the same individual or another large lizard. The astragalus and calcalcaneum appear to have fully fused, an event that takes place after sexual maturity in most modern lizard taxa [47]. The articular facet for the tibia is interrupted by a saddle-shaped ridge, as is typical in non-iguanian lizards [9], and is separated from the facet for the fibula only by a narrow, proximally prominent strip of intervening bone. The lateral calcaneal process of UALVP 59947 is short and distinctly proximal to the articular surface for the fourth distal tarsal, which is damaged (Figure 7A). UALVP 59947 tapers medially to a narrow point (Figure 7A,B), lacking the type of proximodistally broad medial surface present in both Gobiderma pulchrum [[5]: Figure 51A]] and Heloderma suspectum (TMP 1990.007.0357).
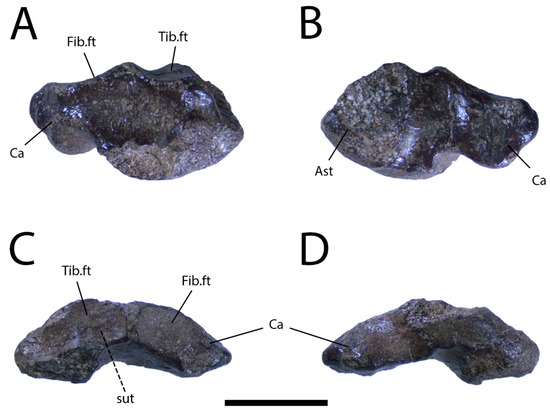
Figure 7.
Right astragalocalcaneum (UALVP 59947) in (A) dorsal, (B) ventral, (C) proximal, and (D) distal views. Scale bar = 1 cm. Abbreviations: Ast, Astragalus; Ca, calcaneum; Fib.ft, fibular facet; Tib.ft, tibial facet.
5. Discussion
5.1. Taxonomic Identification and Ontogenetic Assessment of UALVP 59503
Monstersaur skull material is rare in the Upper Cretaceous of North America, and most known specimens are fragmentary [12,16,18,19,46]. However, UALVP 59503 resembles the frontals of the Mongolian Late Cretaceous monstersaurs Gobiderma pulchrum and Estesia mongoliensis in showing no indication of fusion with the opposite element, being oblong with a transversely flared posterior end, and having a crista cranii that is large but would not have contacted its counterpart at the midline [2,5,17,22]. UALVP 59503 further resembles most previously known monstersaur frontals in that the dorsal surface bears non-keeled, non-imbricating osteoderms that are fused to the underlying bone, are not studded with tubercles, and each constitute a single subpolygonal ossification [5,17,18,44,46]. E. mongoliensis may be exceptional among monstersaurs in lacking osteoderms entirely, although a scar on the right supratemporal process of the holotype specimen (IGM 3/14) suggests that unfused osteoderms were possibly present [2,22].
Although cephalic osteoderms occur in a range of non-monstersaurian squamates, and are prevalent in Scincoidea and various anguimorph groups other than Monstersauria, monstersaurian osteoderms are morphologically distinctive [44,48,49,50]. Non-monstersaurian osteoderms tend to be flatter, thinner and more plate-like, and may be keeled, overlapping, relatively small in area, unfused to the skull roof, and/or composed of multiple smaller ossifications [17,20,49,51,52,53,54,55,56,57]. The cephalic osteoderms of Cenozoic glyptosaurine anguids bear discrete tubercles rather than the ridge-and-pit ornamentation typical of monstersaurs, and in some glyptosaurines, the cephalic osteoderms are proportionally very large [58,59,60,61]. The cephalic osteoderms seen in some varanids remain unfused to the underlying skull bones throughout ontogeny, unlike in typical monstersaurs, and multiple osteoderm morphotypes may be present in a single individual [44,62]. Those varanid osteoderms that are polygonal differ from monstersaur osteoderms in being thin and plate-like [63,64].
In UALVP 59503, the osteoderms are thick, polygonal, non-imbricating, non-compound, and fused to the frontal. These osteoderms bear ridge-and-pit ornamentation but not keels or tubercles, all of which is consistent with a referral to Monstersauria but not with the condition in other squamate clades. The lack of interfrontal fusion is also typical of monstersaurs, but is less distinctive because this feature is widespread among anguimorphs, although the frontals are fused in a few non-monstersaurian taxa such as Shinisaurus crocodilurus [44,65]. Fusion of the frontals is the typical adult condition in many lizard clades, including iguanians, gekkotans, teiids, gymnophthalmids and xenosaurids [43], corroborating the hypothesis that UALVP 59503 does not belong to any of these groups. Similarly, the deep but ventrally free crista cranii of UALVP 59503 distinguishes this specimen from Varanus, in which the crista meets its opposite counterpart to ventrally enclose the olfactory canal [43,44]. However, well-developed cristae that stop short of mutual contact also occur in anguids, S. crocodilurus, Xenosaurus, and some non-anguimorphs [43]. The character of the osteoderms thus represents the strongest evidence for referring UALVP 59503 to Monstersauria, while the unfused condition of the frontals and the ventrally free crista cranii are consistent with this referral and confirm that some alternative identifications are untenable.
The only named suprageneric clade within Monstersauria, Helodermatidae, may be defined as the most recent common ancestor of Heloderma horridum, Lowesaurus matthewi, Eurheloderma gallicum, and all of its descendants [3]. This clade varies widely in taxonomic content across phylogenetic studies [2,3,5,6,24], but UALVP 59503 differs from the frontals of the unambiguous helodermatids Heloderma and Lowesaurus in being oblong and posteriorly flared rather than subtriangular, and in that distinct grooves do not separate the osteoderms. UALVP 59503 further differs from Heloderma in that the crista cranii would not have contacted its counterpart across the midline, and the prefrontal and postorbitofrontal would not have excluded the frontal from the orbital margin. Accordingly, the taxon represented by UALVP 59503 is best regarded as a Campanian monstersaur lacking the derived features of the frontal seen in Heloderma, some of which also occur in Lo. matthewi.
Among North American Cretaceous monstersaurs, UALVP 59503 particularly resembles the frontals of MOR 792, the partial skeleton from the Two Medicine Formation provisionally assigned to Palaeosaniwa canadensis in an unpublished doctoral dissertation [25]. The key similarities include the slight separation between the prefrontal and postorbitofrontal articular facets, and the general ornamentation and arrangement of the osteoderms. Differences in morphological detail between the two specimens exist but are minor enough that UALVP 59503 can be considered conspecific with MOR 792. Balsai [25] assigned the latter specimen to P. canadensis based primarily on the presence of a median ventral groove on the dorsal vertebrae, a feature that Gilmore [26] regarded as a morphological peculiarity distinguishing P. canadensis from the Eocene varanid Saniwa. The ventral groove is indeed present in MOR 792 (Figure 5D,F), but is not unique to P. canadensis, as noted above (see Introduction). We identify UALVP 59503 as a monstersaur cf. P. canadensis, a designation that would also be justifiable for MOR 792 pending a description of the latter specimen in the peer-reviewed literature and a thorough re-evaluation of the taxonomic distinctness of the P. canadensis holotype vertebra. In sum, the use of “cf.” acknowledges that, while we regard UALVP 59503 and MOR 792 as belonging to one species based on the evidence available, and the dorsal vertebrae of MOR 792 as comparable to the P. canadensis holotype, we are unsure whether the shared vertebral features are diagnostic enough to justify actual referral of UALVP 59503 and MOR 792 to P. canadensis. We consider both UALVP 59503 and MOR 792 to represent a single species of monstersaur cf. P. canadensis, but not necessarily P. canadensis itself.
The fact that UALVP 59503 is slightly smaller than the frontals of MOR 792 could reflect either an ontogenetic age difference or the body size variation among individuals expected within any species. In helodermatids, fusion of the cranial osteoderms to the underlying bone tends to occur late in ontogeny [20,62,64,66,67,68]. The fusion of the overlying osteoderms to the frontal in UALVP 59503, as indicated by the µCT scan (Figure 3C), accordingly suggests skeletal maturity.
The frontal of UALVP 59503 is nearly three times longer than that of G. pulchrum ZPAL MgR-III/66 (sagittal length = 12 mm) [17] and only slightly smaller than that of the holotype of E. mongoliensis IGM 3/14 (sagittal length = 39 mm) [22]. Assuming the sagittal length of the frontal and the total length of the skull to scale isometrically relative to E. mongoliensis (maximum skull length = 150 mm) [24], UALVP 59503 is estimated to be from an individual with a skull length of ~130 mm, and MOR 792 to have had an intact skull length of ~150 mm. By comparison, Moreno et al. [[69]: p. 737] reported that a “young adult male” Varanus komodoensis had a maximum skull length of 142 mm and a total (“snout-tail”) body length of 1.6 m. Therefore, UALVP 59503 and MOR 792 are likely from individuals on the scale of smaller adult specimens of the largest extant lizard, which can reach about 3 m in total length [70].
5.2. Identification of UALVP 59947
UALVP 59947 has the characteristic form of a fused lepidosaurian astragalocalcaneum, showing the expected mediolaterally wide proportions, broad facets for the tibia and fibula, and concave surface for articulation with distal tarsal IV [71,72], although the last of those features is damaged. UALVP 59947 presumably belongs to a squamate, given that the northern continents were evidently free of other lepidosaurs by the Late Cretaceous [73], but cannot be confidently referred to any narrower clade due to the lack of clear diagnostic features. However, the saddle-shaped ridge on the articular facet for the tibia indicates the specimen is not of iguanian origin [9], while the contrast between the tapered shape of the medial margin of UALVP 59947 and the broad margin seen in Gobiderma pulchrum and Heloderma suspectum suggests that the astragalocalcaneum is not monstersaurian. Further comparisons and more complete lizard specimens from the Wapiti Formation will be necessary to establish a more precise taxonomic identification. At present, we cannot conclusively rule out the possibility that UALVP 59947 is from a monstersaur, or even the possibility that it represents the same individual as UALVP 59503.
5.3. Taxonomic Status of Labrodioctes montanensis and Palaeosaniwa canadensis
Labrodioctes montanensis was erected by Gao and Fox [19] based on a partial left dentary (ANSP 18664) from the Campanian Judith River Formation of eastern Montana. In addition, these authors tentatively referred the partial right frontal TMP 1978.018.0001 from the Belly River Group of White Rock Coulee, southern Alberta, to L. montanensis, noting that this specimen was similar in geological age to the holotype and appeared to be from a “helodermatid-like” [[19]: p. 88] lizard of about the same size (Figure 6).
Balsai [25] suggested, based on dentary characters, that L. montanensis was a junior synonym of Palaeosaniwa canadensis. However, TMP 1978.018.0001 merits independent comparison to UALVP 59503, MOR 792 and other specimens that preserve potentially diagnostic frontal features, particularly given the rather loose basis for referring TMP 1978.018.0001 to L. montanensis in the first place. TMP 1978.018.0001 is broadly proportionate in size to UALVP 59503 and the frontals of MOR 792, but differs from both these specimens in having sharply polygonal osteoderms that show patchy rather than consistent ridge-and-pit ornamentation and are demarcated by distinct grooves. TMP 1978.018.0001 further differs from UALVP 59503 in that the short segment of orbital rim formed by the frontal is more laterally prominent in the former specimen, the posterior part of the medial margin of the bone is thicker, the facet for the postorbitofrontal is more deeply excavated, and the convexity posterolateral to the posterior end of the crista cranii is lower and not associated with a small depression. These differences strongly indicate that TMP 1978.018.0001 is not conspecific with UALVP 59503 and MOR 792 and that at least two species of large monstersaurs were, therefore, present during the Campanian in western North America. Whether TMP 1978.018.0001 should be assigned to L. montanensis or another species will depend on the distinctness of ANSP 18664 relative to other Campanian monstersaur dentaries from western North America, and on whether future specimens that combine intact frontals with dentaries resembling ANSP 18664 display the diagnostic features seen in TMP 1978.018.0001.
The taxonomic status of P. canadensis is also questionable in that the species rests on a highly incomplete holotype, namely the dorsal vertebra USNM 10864 [26]. This specimen resembles the dorsal vertebrae of MOR 792 in general morphology and in having a ventral median groove, but such a groove is not unique among varanoids and is even found in some mosasaurids (e.g., Pannoniasaurus [27]) and some modern varanids. However, there is no evidence that multiple taxa from the Cretaceous of North America possess the vertebral morphology currently considered diagnostic of P. canadensis, which combines the ventral groove with more typical varanoid features [26]. Dorsal vertebrae attributed to other taxa have either lacked the ventral groove, as in Paraderma bogerti UALVP 29903, or borne an apparent ventral groove but differed from the P. canadensis holotype in other respects, as in Parasaniwa wyomingensis UALVP 33350 [19]. Nevertheless, P. canadensis is taxonomically precarious in the sense that if a North American Cretaceous monstersaur diagnostically separable from MOR 792 is ever discovered to have dorsal vertebrae closely resembling USNM 10864, P. canadensis will either become a nomen dubium or need to be provided with a neotype, as per article 75.5 of the International Code of Zoological Nomenclature [74].
5.4. Implications of the Presence of a Monstersaur cf. Palaeosaniwa canadensis in Unit 3 of the Wapiti Formation
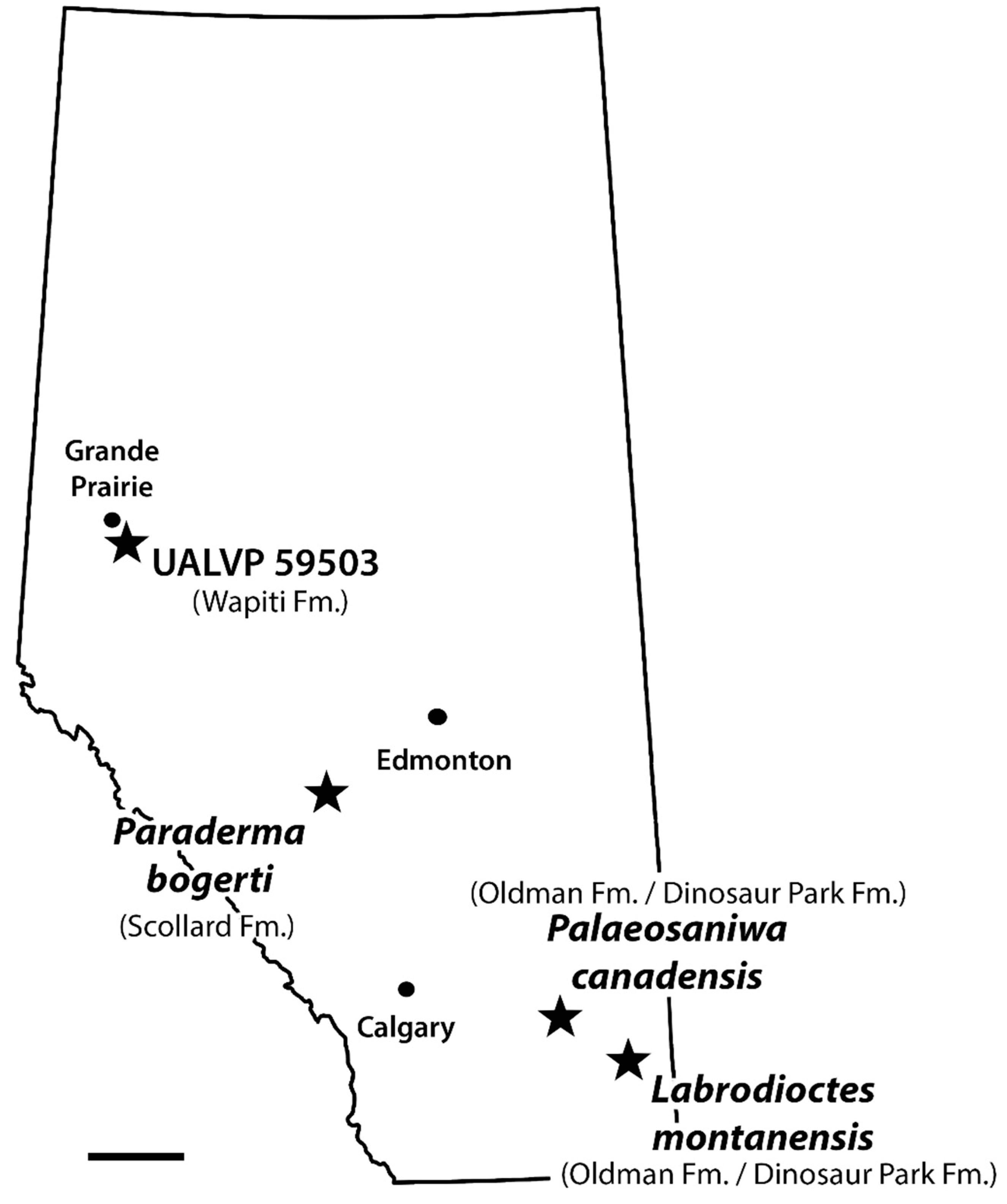
The discovery of UALVP 59503 confirms the presence of monstersaurs in the Wapiti Formation, which was previously suggested by the presence in the Pipestone Creek Bonebed of a vertebra comparable to Palaeosaniwa [37]. UALVP 59503 dates to about 73.5 Ma and is from Unit 3 of the Wapiti Formation, whereas the Pipestone Creek Bonebed is likely situated in the lower part of Unit 4, deposited at least several hundred thousand years later [38]. UALVP 50593 is slightly geologically younger than reported occurrences of P. canadensis in the Belly River Group of southern Alberta [19,26], but older than reported post-Campanian occurrences [16,29,30]. A critical reevaluation of the post-Campanian occurrences is needed, but if they are valid, then UALVP 59503 represents a temporally unremarkable occurrence of a monstersaur cf. P. canadensis. However, the specimen also constitutes the northernmost definitive record in the Upper Cretaceous of North America for the Monstersauria (Figure 8), extending their known distribution far north and west of the southern Alberta monstersaur sites [19,26]. At 73.5 Ma, the palaeolatitude of the DC Bonebed would have been 63.0° N, close to the palaeoarctic, according to the online palaeolatitude calculator of van Hinsbergen et al. [75].
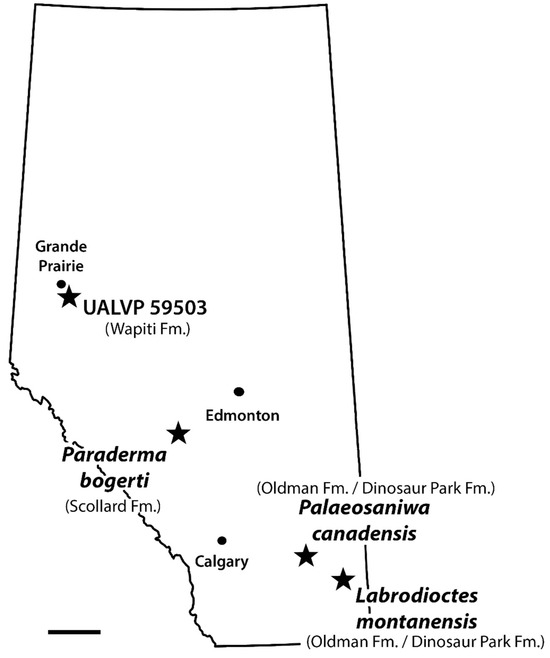
Figure 8.
Map of Alberta, Canada, indicating the rock units and approximate geographic locations from which monstersaur specimens have been recovered in the province. Scale bar = 100 km.
The extant species of Heloderma occupy habitats that are hot and arid, at least seasonally [1,13,20,76]. H. suspectum primarily inhabits dry scrublands and desert grasslands in the southwestern United States and northwestern Mexico, whereas H. horridum and other beaded lizards occur primarily in seasonally dry tropical forests in Mexico, or in Guatemala in the case of H. charlesbogerti. However, H. suspectum avoids the harshest desert habitats within its geographic range, and it is likely that extreme aridity and/or excessively cold or prolonged winters render environments inhospitable to Heloderma [13]. Presumably, sensitivity to winter conditions has prevented Heloderma from extending its range into the northwestern United States. Beck [[13]: p. 29] further suggested that Heloderma is well adapted to strongly seasonal environments in having a “conservative energy-use strategy” conducive to survival during periods when food is scarce, conferring an advantage over competitors that are less tolerant of seasonally harsh conditions.
The Mongolian Cretaceous monstersaurs Gobiderma pulchrum and Estesia mongoliensis are known from the Djadokhta Formation, thought to have been deposited in a semiarid to arid environment, albeit one wet enough for ponds and potentially small lakes to have been ephemerally present [77]. The palaeolatitude was about 40° N, and summer mean temperatures are estimated at ~26 °C, based on isotopic evidence from nodular carbonates [78,79]. By comparison, St. George, UT, USA, a Mojave Desert city in the northern part of the modern range of H. suspectum [13], is at a latitude of 37.1° N and has a summer mean temperature of 28.9 °C (and a mean annual temperature [MAT] of 17.1 °C).
By contrast, Wapiti Unit 3 (WU3) was laid down in a waterlogged, near-coastal setting with abundant lakes and marshes [38]. Cockx et al. [80] estimated that the temperature in this environment was 26.6 °C based on isotopic analysis of amber samples from the Pipestone Creek Bonebed, which was probably low in WU4 [38], suggesting at least seasonally warm conditions [80]. Based on palaeosol composition, Quinney et al. [81] estimated that MAT fluctuated between about 9 °C and 11 °C during the deposition of the Horseshoe Canyon Formation of southern Alberta, which occurred during the late Campanian to mid-Maastrichtian [81,82]. Amiot et al. [83] used isotopic analysis of vertebrate skeletal elements to construct a latitudinal temperature gradient for Campanian-Maastrichtian time. Most of the data they employed were from western North America, and their plotted gradient indicates an MAT of about 9 °C at 63° N. Evidence of relatively low palaeotemperatures from the Campanian–Maastrichtian of northwestern North America in general, and evidence of waterlogged conditions from WU3 in particular, suggest that the DC Monster could tolerate a wet environment consistently cooler than those inhabited by extant Heloderma. The seasonally dry tropical forests inhabited by some Heloderma populations can also be wet on a seasonal basis, as they may receive abundant rainfall for part of the year [13]. However, such forests are subject to seasonal drought, and whether this was true of the WU3 palaeoenvironment is presently uncertain.
Nevertheless, the high-latitude WU3 palaeoenvironment would have resembled the habitats favoured by Heloderma in displaying some form of strong seasonality, based at least in part on large differences in photoperiod between the summer and winter months. Under such conditions, the DC Monster may have thrived by limiting energy expenditure during the harsher portion of each year, like Heloderma. Much farther north, at a palaeolatitude of 80–85° N, the early Maastrichtian portion of the Prince Creek Formation of northern Alaska was deposited in an environment with an estimated MAT of 6–7 °C, and has not yielded fossils of squamates or other ectothermic non-dinosaurian tetrapods [84,85]. Presumably, the Prince Creek palaeoenvironment was too cold and dark in winter for monstersaurs and other lizards to survive. By contrast, the Two Medicine Formation of Montana was deposited in a substantially warmer climate, with an estimated MAT of 14–19 °C and a palaeolatitude of ~55° N [86], and likely underwent seasonal droughts in some years [87]. The monstersaur species cf. P. canadensis represented by UALVP 59503 and the Two Medicine specimen MOR 792 was accordingly capable of thriving under a range of climatic conditions.
This monstersaur species is also the third, and by far the largest, lizard to have been securely documented in WU3, alongside Socognathus unicuspis and Kleskunsaurus grandeprairiensis [30]. The relatively high diversity and body size disparity of squamates in this stratigraphic unit contrast with the paucity of crocodyliforms, which are represented only by three small teeth from the DC Bonebed locality, whereas Champsosaurus and turtle specimens are abundant in the Bonebed [38]. Amiot et al. [83] inferred that 60° N was the palaeolatitudinal northward limit on the distribution of both turtles and crocodilians during the Campanian–Maastrichtian. The crocodyliform teeth from the DC Bonebed are from the slightly higher palaeolatitude of 63° N, but the rarity and small size of crocodyliforms in the Wapiti Formation suggest they were only minor components of the WU3 fauna and indeed at or near the northern extreme of their range. However, turtles were evidently thriving in the WU3 ecosystem, as was Champsosaurus, which has a largely northern distribution and may have been better adapted than crocodyliforms to high-latitude environments [38]. The hypothesis that squamates were also less constrained than crocodyliforms in their North American latitudinal distribution during the Late Cretaceous is supported by available evidence from WU3 and consistent with the fact that extant squamates and turtles range farther north than crocodilians, but remains to be tested further as data from the Wapiti Formation continue to accumulate.
6. Conclusions
The frontal element UALVP 59503 adds to the diversity of lizards known from WU3 and is closely similar to the frontals of MOR 792, a partial monstersaur skeleton previously referred to Palaeosaniwa canadensis on the basis of vertebral features whose diagnostic value requires re-evaluation. Given their similarities in frontal morphology, we consider UALVP 59503 and MOR 792 to represent a single monstersaur species cf. P. canadensis. UALVP 59503 then corroborates the presence, already suggested by a vertebra from the Pipestone Creek Bonebed [37], of a monstersaur similar to Palaeosaniwa canadensis in the Wapiti Formation. Furthermore, UALVP 59503 represents the northernmost definitive occurrence of Monstersauria in the Upper Cretaceous of North America. The frontal TMP 1978.018.0001, referred to Labrodioctes montanensis, clearly differs from UALVP 59503, implying that at least two monstersaur species coexisted in Alberta during the Campanian. The presence of UALVP 59503 in WU3 implies that this monstersaur was tolerant of a habitat that was at least seasonally wet, and considerably cooler than any environment inhabited by extant Heloderma.
Author Contributions
Conceptualization, S.M.H. and C.S.; Methodology, N.E.C., S.M.H., I.P. and C.S.; Investigation, N.E.C., S.M.H., I.P., R.L.S. and C.S.; Resources, C.S.; Data Curation, N.E.C., S.M.H., I.P., R.L.S. and C.S.; Writing—Original Draft Preparation, S.M.H., I.P. and C.S.; Writing—Review and Editing, S.M.H., I.P., P.R.B., N.E.C., F.F., D.W.L., R.L.S., M.J.V., M.J.B. and C.S.; Validation—M.J.B., P.R.B., N.E.C., S.M.H., D.W.L., I.P., R.L.S. and C.S.; Visualization, N.E.C., S.M.H., I.P. and R.L.S.; Supervision, C.S.; Project Administration, C.S.; Funding Acquisition, N.E.C., M.J.V. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an NSERC Discovery Grant to C.S. (RGPIN-2017-06246), an endowment associated with the Philip J. Currie Professorship at the University of Alberta, and Dinosaur Research Institute grants to N.E.C. and M.J.V.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank Michael Caldwell, Randall Nydam, and Braden Barr for valuable discussions. For access to specimens and comparative material, we are deeply thankful to: Clive Coy and Howard Gibbins (UALVP); Michael Caldwell and Braden Barr (UAMZ); Brandon Strilisky (RTMP); Lauren Vonnahme and David Kizirian (AMNH); Vladimir Alifanov and Andrey Sennikov (PIN); John Scannella and Eric Metz (MOR); and Jolanta Kobylinska (ZPAL). We also thank Greg Funston for helpful discussion and for capturing some of the images used in this study, and Emily Bamforth for pointing us to useful palaeoclimatic information. We are grateful to Northwestern Polytechnic for generously facilitating fieldwork in the Peace Region of northern Alberta, and to many individuals who have assisted us in the field. We thank Michael R. Doschak (Faculty of Pharmacy and Pharmaceutical Sciences and Department of Biomedical Engineering, University of Alberta) for facilitating µCT scanning of UALVP 50593. Comments from two anonymous reviewers greatly improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reiserer, R.S.; Schuett, G.W.; Beck, D.D. Taxonomic reassessment and conservation status of the beaded lizard, Heloderma horridum (Squamata: Helodermatidae). Amphib. Reptile Conserv. 2013, 7, 74–96. [Google Scholar]
- Norell, M.A.; Gao, K. Braincase and phylogenetic relationships of Estesia mongoliensis from the Late Cretaceous of the Gobi Desert and the recognition of a new clade of lizards. Am. Mus. Novit. 1997, 3211, 1–25. [Google Scholar]
- Conrad, J. Phylogeny and systematics of Squamata (Reptilia) based on morphology. Bull. Am. Mus. Nat. Hist. 2008, 310, 1–182. [Google Scholar] [CrossRef]
- Burbrink, F.T.; Grazziotin, F.G.; Pyron, R.A.; Cundall, D.; Donnellan, S.; Irish, F.; Keogh, J.S.; Kraus, F.; Murphy, R.W.; Noonan, B.; et al. Interrogating genomic-scale data for Squamata (lizards, snakes and amphisbaenians) shows no support for key traditional morphological relationships. Syst. Biol. 2020, 69, 502–520. [Google Scholar] [CrossRef]
- Conrad, J.L.; Rieppel, O.; Gautier, J.A.; Norell, M.A. Osteology of Gobiderma pulchrum (Monstersauria, Lepidosauria, Reptilia). Bull. Am. Mus. Nat. Hist. 2011, 362, 1–88. [Google Scholar] [CrossRef]
- Conrad, J.L.; Ast, J.C.; Montanari, S.; Norell, M.A. A combined evidence phylogenetic analysis of Anguimorpha (Reptilia: Squamata). Cladistics 2011, 27, 230–277. [Google Scholar] [CrossRef]
- Reeder, T.W.; Townsend, T.M.; Mulcahy, D.G.; Noonan, B.P.; Wood, P.L., Jr.; Sites, J.W., Jr.; Wiens, J.J. Integrated analyses resolve conflicts over squamate reptile phylogeny and reveal unexpected placements for fossil taxa. PLoS ONE 2015, 10, e0118199. [Google Scholar] [CrossRef]
- Simões, T.R.; Caldwell, M.W.; Tałanda, M.; Bernardi, M.; Palci, A.; Vernygora, O.; Bernardini, F.; Mancini, L.; Nydam, R.L. The origin of squamates revealed by a Middle Triassic lizard from the Italian Alps. Nature 2018, 557, 706–709. [Google Scholar] [CrossRef]
- Gauthier, J.A.; Kearney, M.; Maisano, J.A.; Rieppel, O.; Behlke, A.D.B. Assembling the squamate tree of life: Perspectives from the phenotype and the fossil record. Bull. Peabody Mus. Nat. Hist. 2012, 53, 3–308. [Google Scholar] [CrossRef]
- Longrich, N.R.; Bhullar, B.-A.S.; Gauthier, J.A. Mass extinction of lizards and snakes at the Cretaceous-Paleogene boundary. Proc. Acad. Natl. Sci. USA 2012, 109, 21396–21401. [Google Scholar] [CrossRef]
- Gao, K.; Norell, M.A. Taxonomic revision of Carusia (Reptilia: Squamata) from the Late Cretaceous of the Gobi desert and phylogenetic relationships of anguimorphan lizards. Am. Mus. Novit. 1998, 3230, 1–51. [Google Scholar]
- Nydam, R.L. A new taxon of helodermatid-like lizard from the Albian–Cenomanian of Utah. J. Vertebr. Paleontol. 2000, 20, 285–294. [Google Scholar] [CrossRef]
- Beck, D.D. Biology of Gila Monsters and Beaded Lizards; University of California Press: Berkeley, CA, USA, 2005; pp. 1–211. ISBN 978-0-520-25987-4. [Google Scholar]
- Bogert, C.; del Campo, M. The Gila monster and its allies. Bull. Am. Mus. Nat. Hist. 1956, 109, 1–238. [Google Scholar]
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, S.F.R.; Kuruppu, S.; Fung, K.; Hedges, S.B.; Richardson, M.K.; et al. Early evolution of the venom system in lizards and snakes. Nature 2006, 439, 584–588. [Google Scholar] [CrossRef]
- Estes, R. Fossil vertebrates from the Late Cretaceous Lance Formation eastern Wyoming. Univ. Calif. Pub. Geol. Sci. 1964, 49, 1–180. [Google Scholar]
- Borsuk-Białynicka, M. Anguimorphans and related lizards from the Late Cretaceous of the Gobi Desert, Mongolia. Palaeontol. Pol. 1984, 46, 5–105. [Google Scholar]
- Pregill, G.K.; Gauthier, J.A.; Greene, H.W. The evolution of helodermatid squamates, with description of a new taxon and an overview of Varanoidea. San. Dieg. Soc. Nat. Hist. 1986, 21, 167–202. [Google Scholar]
- Gao, K.; Fox, R.C. Taxonomy and evolution of Late Cretaceous lizards (Reptilia: Squamata) from western Canada. Bull. Carn. Mus. Nat. Hist. 1996, 3, 1–107. [Google Scholar] [CrossRef]
- Mead, J.I.; Schubert, B.W.; Wallace, S.C.; Swift, S.L. Helodermatid lizard from the Mio-Pliocene oak-hickory forest of Tennessee, eastern USA, and a review of monstersaurian osteoderms. Acta Palaeontol. Pol. 2012, 57, 111–121. [Google Scholar] [CrossRef]
- Estes, R. Sauria Terrestria, Amphisbaenia. Handbuch der Paläoherpetologie, Part 10A; Gustav Fisher Verlag: Stuttgart, Germany, 1983; ISBN 9783437303913. [Google Scholar]
- Norell, M.A.; McKenna, M.C.; Novacek, M.J. Estesia mongoliensis, a new fossil varanoid from the Late Cretaceous Barun Goyot Formation of Mongolia. Am. Mus. Novit. 1992, 3025, 1–24. [Google Scholar]
- Nydam, R.L. Squamates from the Jurassic and Cretaceous of North America. Paleobio. Palaeoenv. 2013, 93, 535–565. [Google Scholar] [CrossRef]
- Yi, H.Y.; Norell, M.A. New materials of Estesia mongoliensis (Squamata: Anguimorpha) and the evolution of venom grooves in lizards. Am. Mus. Novit. 2013, 3767, 1–31. [Google Scholar] [CrossRef]
- Balsai, M.J. The Phylogenetic Position of Palaeosaniwa and the Early Evolution of the Platynotan (Varanoid) Anguimorphs. Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 2001; pp. 1–359. [Google Scholar]
- Gilmore, C.W. Fossil lizards of North America. Natl. Acad. Sci. Mem. 1928, 22, 1–201. [Google Scholar]
- Makádi, L.; Caldwell, M.W.; Ősi, A. The first freshwater mosasauroid (Upper Cretaceous, Hungary) and a new clade of basal mosasauroids. PLoS ONE 2012, 7, e51781. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.C.A. Fossil Vertebrates from the Cerro del Pueblo Formation, Coahuila, Mexico, and the Distribution of Late Campanian (Cretaceous) Terrestrial Vertebrate Faunas. Master’s Thesis, Southern Methodist University, Dallas, TX, USA, 2010; pp. 1–135. [Google Scholar]
- Estes, R.; Berberian, P.; Meszoely, C.A.M. Lower vertebrates from the Late Cretaceous Hell Creek Formation, McCone County, Montana. Breviora 1969, 337, 1–33. [Google Scholar]
- Sullivan, R.M. Fossil lizards from Swain Quarry “Fort Union Formation,” middle Paleocene (Torrejonian), Carbon County, Wyoming. J. Paleont. 1982, 56, 996–1010. [Google Scholar]
- McGrew, P.O.; Berman, J.E.; Hecht, M.K.; Hummel, J.M.; Simpson, G.G.; Wood, A.E. The geology and paleontology of the Elk Mountain and Tabernacle Butte area, Wyoming. Bull. Am. Nat. Hist. 1959, 117, 117–176. [Google Scholar]
- McKenna, M.C. Fossil Mammalia from the early Wasatchian Four Mile fauna, Eocene of northwest Colorado. Univ. Calif. Pub. Geol. Sci. 1960, 37, 1–130. [Google Scholar]
- Smith, K.T.; Gauthier, J.A. Early Eocene lizards of the Wasatch Formation near Bitter Creek, Wyoming: Diversity and paleoenvironment during an interval of global warming. Bull. Peabody Mus. Nat. Hist. 2013, 54, 135–230. [Google Scholar] [CrossRef]
- Sternberg, C.M. The lizard Chamops from the Wapiti Formation of northern Alberta: Polyodontosaurus grandis not a lizard. Bull. Nat. Mus. Can. 1951, 123, 256–258. [Google Scholar]
- Nydam, R.L.; Caldwell, M.W.; Fanti, F. Borioteiioidean lizard skulls from Kleskun Hill (Wapiti Formation; Upper Campanian), west-central Alberta, Canada. J. Vertebr. Paleontol. 2010, 30, 1090–1099. [Google Scholar] [CrossRef]
- Nydam, R.L. Lizards and snakes from the Cenomanian through Campanian of southern Utah: Filling the gap in the fossil record of Squamata from the Late Cretaceous of the Western Interior of North America. In At the Top of the Grand Staircase: The Late Cretaceous of Southern Utah; Titus, A.L., Loewen, M.A., Eds.; Indiana University Press: Bloomington, IN, USA, 2013; pp. 370–423. ISBN 978-0253008831. [Google Scholar]
- Currie, P.J.; Langston, W., Jr.; Tanke, D.H. A New Horned Dinosaur from an Upper Cretaceous Bone Bed in Alberta; NRC Research Press: Ottawa, ON, Canada, 2008; pp. 1–144. ISBN 978-0-660-19820-0. [Google Scholar]
- Fanti, F.; Bell, P.R.; Vavrek, M.; Larson, D.; Koppelhus, E.; Sissons, R.L.; Langone, A.; Campione, N.E.; Sullivan, C. Filling the Bearpaw gap: Evidence for palaeoenvironment-driven taxon distribution in a diverse, non-marine ecosystem from the late Campanian of west-Central Alberta, Canada. Palaeogeogr. Palaeocl. Palaeoecol. 2022, 592, 1–18. [Google Scholar] [CrossRef]
- Fanti, F.; Catuneanu, O. Fluvial sequence stratigraphy: The Wapiti Formation, west-central Alberta, Canada. J. Sediment. Res. 2010, 80, 320–338. [Google Scholar] [CrossRef]
- Oppel, M. Die Ordnungen, Familien und Gattungen der Reptilien als Prodrom Einer Naturgeschichte Derselben; Joseph Lindauer Verlag: Munich, Germany, 1811; pp. 1–86. [Google Scholar]
- Fürbringer, M. Zur vergleichenden Anatomie des Brustschulterapparates und der Schultermuskeln. Jan. Zeitschr. 1900, 34, 215–718. [Google Scholar]
- Lee, M.S.Y. The phylogeny of varanoid lizards and the affinities of snakes. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1997, 352, 53–91. [Google Scholar] [CrossRef]
- Evans, S.E. The skull of lizards and tuatara. In Biology of the Reptilia; Gans, C., Gaunt, A.S., Adler, K., Eds.; Society for the Study of Amphibians and Reptiles: Ithaca, NY, USA, 2008; Volume 20, pp. 1–347. ISBN 978-0-91698-476-2. [Google Scholar]
- McDowell, S.B.; Bogert, C.M. The systematic position of Lanthanotus and the affinities of the anguinomorphan lizards. B. Am. Mus. Nat. Hist. 1954, 105, 1–142. [Google Scholar]
- Mo, J.-y.; Xu, X.; Evans, S.E. A large predatory lizard (Platynota, Squamata) from the Late Cretaceous of South China. J. Syst. Palaeont. 2012, 10, 333–339. [Google Scholar] [CrossRef]
- Yatkola, D.A. Fossil Heloderma (Reptilia, Helodermatidae). Occas. Pap. Mus. Nat. Hist. Univ. Kans. 1976, 51, 1–14. [Google Scholar]
- Maisano, J.A. Terminal fusions of skeletal elements as indicators of maturity in squamates. J. Vertebr. Paleontol. 2002, 22, 268–275. [Google Scholar] [CrossRef]
- Estes, R.; de Queiroz, K.; Gauthier, J.A. Phylogenetic relationships within Squamata. In Phylogenetic Relationships of the Lizard Families; Estes, R., Pregill, G., Eds.; Stanford University Press: Stanford, CA, USA, 1988; pp. 119–291. ISBN 9780804714358, 0804714355. [Google Scholar]
- Richter, A. Lacertilier aus der Unteren Kreide von Uña und Galve (Spanien) und Anoual (Marokko). Berl. Geowiss. Abh. 1994, 14, 1–147. [Google Scholar]
- Vickaryous, M.K.; Sire, J.Y. The integumentary skeleton of tetrapods: Origin, evolution, and development. J. Anat. 2009, 214, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Bever, G.S.; Bell, C.J.; Maisano, J.A. The ossified braincase and cephalic osteoderms of Shinisaurus crocodilurus (Squamata, Shinisauridae). Palaeont. Electr. 2005, 8, 4A. [Google Scholar]
- Bhullar, B.-A.S. Cranial osteology of Exostinus serratus (Squamata: Anguimorpha), fossil sister taxon to the enigmatic clade Xenosaurus. Zool. J. Linn. Soc. 2010, 159, 921–953. [Google Scholar] [CrossRef]
- Bhullar, B.-A.S. The power and utility of morphological characters in systematics: A fully resolved phylogeny of Xenosaurus and its fossil relatives (Squamata: Anguimorpha). Bull. Mus. Comp. Zool. 2011, 160, 65–181. [Google Scholar] [CrossRef]
- Dong, L.; Xu, X.; Wang, Y.; Evans, S.E. The lizard genera Bainguis and Parmeosaurus from the Upper Cretaceous of China and Mongolia. Cret. Res. 2018, 85, 95–108. [Google Scholar] [CrossRef]
- Camp, C.L. Classification of the lizards. Bull. Am. Mus. Nat. Hist. 1923, 48, 289–481. [Google Scholar]
- Mead, J.I.; Arroyo-Cabrales, J.; Johnson, E. Pleistocene lizards (Reptilia: Squamata) from San Josecito Cave, Nuevo León, Mexico. Copeia 1999, 1999, 163–173. [Google Scholar] [CrossRef]
- Thorn, K.M.; Fusco, D.A.; Hutchinson, M.N.; Gardner, M.G.; Clayton, J.L.; Prideaux, G.J.; Lee, M.S.Y. A giant armoured skink from Australia expands lizard morphospace and the scope of the Pleistocene extinctions. Proc. R. Soc. B 2023, 290, 20230704. [Google Scholar] [CrossRef]
- Bauer, A.M.; Russell, A.P. Supraorbital ossifications in geckos. Can. J. Zool. 1989, 67, 678–684. [Google Scholar] [CrossRef]
- de Buffrénil, V.; Sire, J.Y.; Rage, J.C. The histological structure of glyptosaurine osteoderms (Squamata: Anguidae), and the problem of osteoderm development in squamates. J. Morphol. 2010, 271, 729–737. [Google Scholar] [CrossRef]
- Paluh, D.J.; Griffing, A.H.; Bauer, A.M. Sheddable armour: Identification of osteoderms in the integument of Geckolepis maculata (Gekkota). Afr. J. Herpet. 2017, 66, 12–24. [Google Scholar] [CrossRef]
- Scarpetta, S.G. Peltosaurus granulosus (Squamata, Anguidae) from the middle Oligocene of Sharps Corner, South Dakota, and the youngest known chronostratigraphic occurrence of Glyptosaurinae. J. Vertebr. Paleont. 2019, 39, e1622129. [Google Scholar] [CrossRef]
- Maisano, J.A.; Bell, C.J.; Gauthier, J.A.; Rowe, T. The osteoderms and palpebral in Lanthanotus borneensis (Squamata: Anguimorpha). J. Herpetol. 2002, 36, 678–682. [Google Scholar] [CrossRef]
- Erickson, G.M.; de Ricqles, A.; de Buffrénil, V.; Molnar, R.E.; Bayless, M.K. Vermiform bones and the evolution of gigantism in Megalania–how a reptilian fox became a lion. J. Vertebr. Paleont. 2003, 23, 966–970. [Google Scholar] [CrossRef]
- Maisano, J.A.; Laduc, T.J.; Bell, C.J.; Barber, D. The cephalic osteoderms of Varanus komodoensis as revealed by high-resolution X-ray computed tomography. Anat. Rec. 2019, 147, 1–6. [Google Scholar] [CrossRef]
- Rieppel, O. The Phylogeny of Anguimorph Lizards; Birkhäuser Verlag: Basel, Switzerland, 1980; pp. 1–86. ISBN 978-3-7643-1224-4. [Google Scholar]
- Boulenger, G.A. Notes on the osteology of Heloderma horridum and H. suspectum, with remarks on the systematic position of the Helodermatidae and on the vertebrae of the Lacertilia. J. Zool. 1891, 59, 109–118. [Google Scholar] [CrossRef]
- Hoffstetter, R. Un saurien hélodematidé (Eurheloderma gallicum nov. gen. et sp.) dans la faune fossile des phosphorites du Quercy. Bull. Soc. Géo. Fr. 1957, 7, 775–786. [Google Scholar] [CrossRef]
- Stevens, M.S. Further study of Castolon local fauna (early Miocene) Big Bend National Park, Texas. Pearche-Sellards Ser. Tex. Mem. Mus. 1977, 28, 1–69. [Google Scholar]
- Moreno, K.; Wroe, S.; Clausen, P.; McHenry, C.; D’Amore, D.C.; Rayfield, E.J.; Cunningham, E. Cranial performance in the Komodo dragon (Varanus komodoensis) as revealed by high-resolution 3-D finite element analysis. J. Anat. 2008, 212, 736–746. [Google Scholar] [CrossRef]
- Collar, D.C.; Schulte, J.A., II; Losos, J.B. Evolution of extreme body size disparity in monitor lizards (Varanus). Evolution 2011, 65, 2664–2680. [Google Scholar] [CrossRef]
- Brinkman, D. Structural correlates of tarsal and metatarsal functioning in Iguana (Lacertilia; Iguanidae) and other lizards. Can. J. Zool. 1980, 58, 277–289. [Google Scholar] [CrossRef]
- Evans, S.E. At the feet of the dinosaurs: The early history and radiation of lizards. Biol. Rev. 2003, 78, 513–551. [Google Scholar] [CrossRef]
- Evans, S.E.; Jones, M.E.H. The origin, early history and diversification of lepidosauromorph reptiles. In New Aspects of Mesozoic Biodiversity; Bandyopadhyay, Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 27–44. ISBN 978-3-642-10310-0. [Google Scholar]
- International Commission on Zoological Nomenclature (ICZN). International Code of Zoological Nomenclature, 4th ed.; International Trust for Zoological Nomenclature: London, UK, 1999; pp. 1–306. [Google Scholar]
- van Hinsbergen, D.J.J.; de Groot, L.V.; van Schaik, S.J.; Spakman, W.; Bijl, P.K.; Sluijs, A.; Langereis, C.G.; Brinkhuis, H. A paleolatitude calculator for paleoclimate studies. PLoS ONE 2015, 10, e0126946. [Google Scholar] [CrossRef]
- Beck, D.D.; Lowe, C.H. Ecology of the beaded lizard, Heloderma horridum, in a tropical dry forest in Jalisco, Mexico. J. Herpet. 1991, 25, 395–406. [Google Scholar] [CrossRef]
- Jerzykiewicz, T.; Currie, P.J.; Fanti, F.; Lefeld, J. Lithobiotopes of the Nemegt Gobi Basin. Can. J. Earth Sci. 2021, 58, 829–851. [Google Scholar] [CrossRef]
- Eagle, R.A.; Enriquez, M.; Grellet-Tinner, G.; Pérez-Huerta, A.; Hu, D.; Tütken, T.; Montanari, S.; Loyd, S.J.; Ramirez, P.; Tripati, A.K.; et al. Isotopic ordering in eggshells reflects body temperatures and suggests differing thermophysiology in two Cretaceous dinosaurs. Nat. Commun. 2015, 6, 8296. [Google Scholar] [CrossRef]
- Naugolnykh, S.V.; Kuleshov, V.N. New palaeoclimatic insights on Late Cretaceous environments of Mongolia based on the isotope data (δ13C, δ18O) of dinosaur eggshells and pedogenic carbonates from Bayn Dzak section. Glob. Geol. 2020, 23, 199–213. [Google Scholar] [CrossRef]
- Cockx, P.; McKellar, R.; Tappert, R.; Vavrek, M.; Muehlenbachs, K. Bonebed amber as a new source of paleontological data: The case of the Pipestone Creek deposit (Upper Cretaceous), Alberta, Canada. Gond. Res. 2020, 81, 378–389. [Google Scholar] [CrossRef]
- Quinney, A.; Therrien, F.; Zelenitsky, D.; Eberth, D.A. Palaeoenvironmental and palaeoclimatic reconstruction of the Upper Cretaceous (late Campanian-early Maastrichtian) Horseshoe Canyon Formation, Alberta, Canada. Palaeogeogr. Palaeocl. Palaeoecol. 2013, 371, 26–44. [Google Scholar] [CrossRef]
- Eberth, D.A.; Kamo, S.L. High-precision U-Pb CA-ID-TIMS dating and chronostratigraphy of the dinosaur-rich Horseshoe Canyon Formation (Upper Cretaceous, Campanian-Maastrichtian), Red Deer River valley, Alberta, Canada. Can. J. Earth Sci. 2020, 57, 1220–1237. [Google Scholar] [CrossRef]
- Amiot, R.; Lécuyer, C.; Buffetaut, E.; Fluteau, F.; Legendre, S.; Martineau, F. Latitudinal temperature gradient during the Cretaceous Upper Campanian-Middle Maastrichtian: δ18O record of continental vertebrates. Earth Plan. Sci. Let. 2004, 226, 255–272. [Google Scholar] [CrossRef]
- Druckenmiller, P.S.; Erickson, G.M.; Brinkman, D.; Brown, C.M.; Eberle, J.J. Nesting at extreme polar latitudes by non-avian dinosaurs. Curr. Biol. 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Salazar-Jaramillo, S.; McCarthy, P.J.; Ochoa, A.; Fowell, S.J.; Longstaffe, F.J. Paleoclimate reconstruction of the Prince Creek Formation, Arctic Alaska, during Maastrichtian global warming. Palaeogeogr. Palaeocl. Palaeoecol. 2019, 532, 109265. [Google Scholar] [CrossRef]
- Burgener, L.; Hyland, E.; Huntington, K.W.; Kelson, J.R.; Sewall, J.O. Revisiting the equable climate problem during the Late Cretaceous greenhouse using paleosol carbonate clumped isotope temperatures from the Campanian of the Western Interior Basin, USA. Palaeogeogr. Palaeocl. Palaeoecol. 2019, 516, 244–267. [Google Scholar] [CrossRef]
- Falcon-Lang, H.J. Growth interruptions in silicified conifer woods from the Upper Cretaceous Two Medicine Formation, Montana, USA: Implications for palaeoclimate and dinosaur palaeoecology. Palaeogeogr. Palaeocl. Palaeoecol. 2003, 199, 299–314. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).