Administration of an Immune Stimulant during the Transition Period Improved Lipid Metabolism and Rumination without Affecting Inflammatory Status
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animal Management
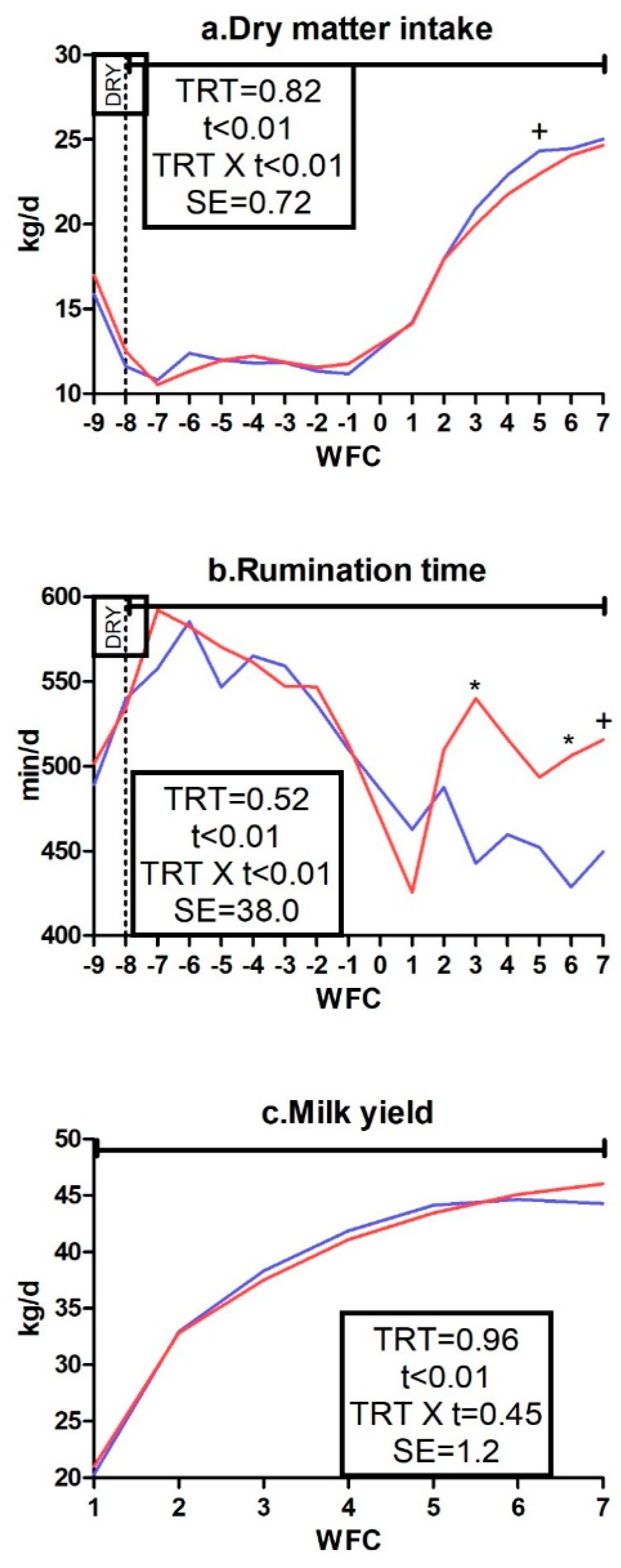
2.2. Body Weight, Body Condition Score, Dry Matter Intake, Rumination Time, and Milk Yield
2.3. Health Status
2.4. Blood Sample Collection
2.4.1. Metabolic Profile Assessment
2.4.2. Blood Cell Profile
2.4.3. Whole Blood Stimulation Assay
2.4.4. Interferon Gamma Release Assay
2.5. Milk Sample Collection and Analysis
2.6. Carrageenan Skin Test
2.7. Rumen Fluid Measurements
2.8. Statistical Analysis
3. Results
3.1. Body Weight, Body Condition Score, Dry Matter Intake, Rumination Time, and Milk Yield
3.2. Milk Quality and Rumen Fluid Measurements
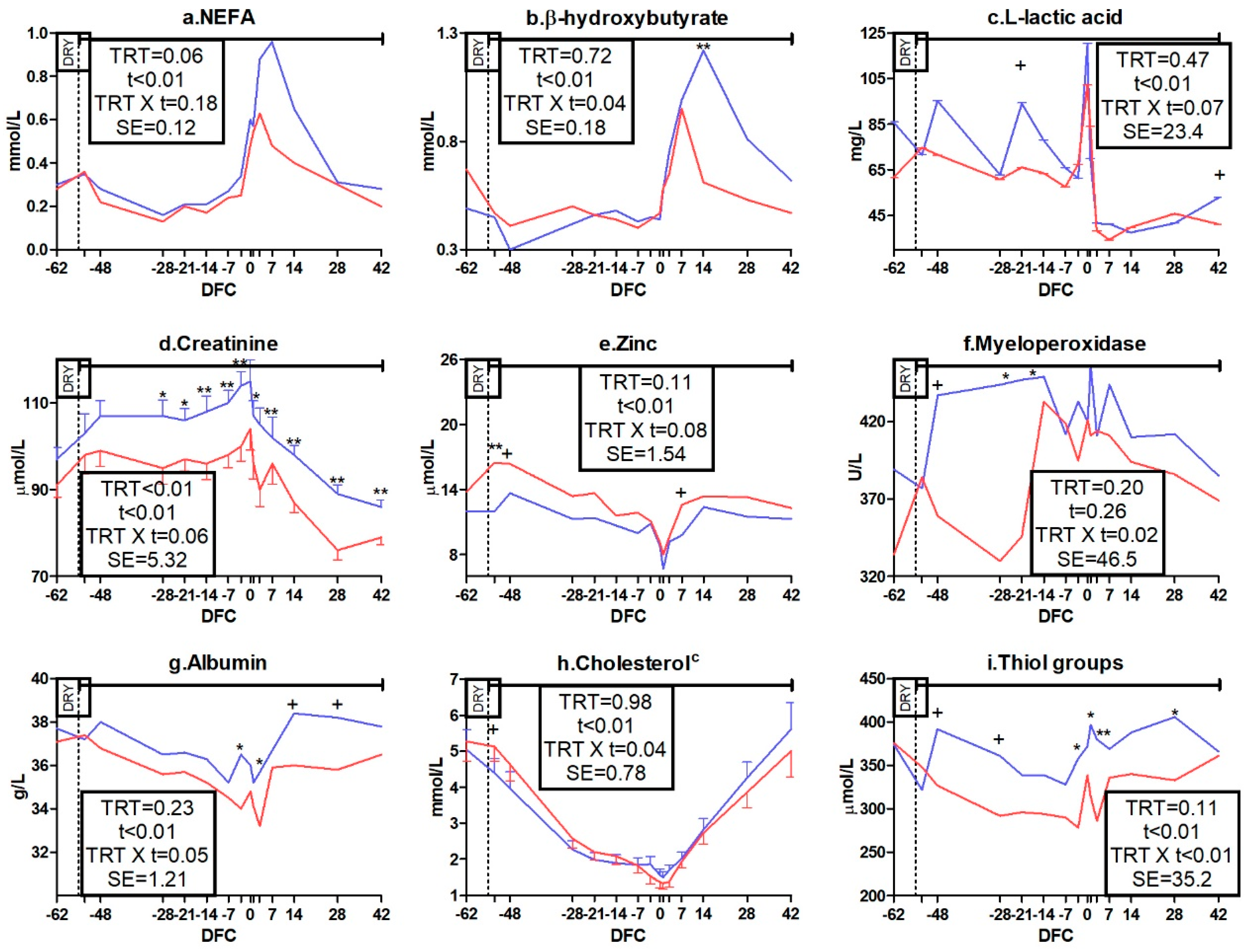
3.3. Metabolic Profile
3.3.1. Packed Cell Volume, Energy, Protein, and Mineral Metabolism Biomarkers
3.3.2. Liver Function and Inflammation Biomarkers
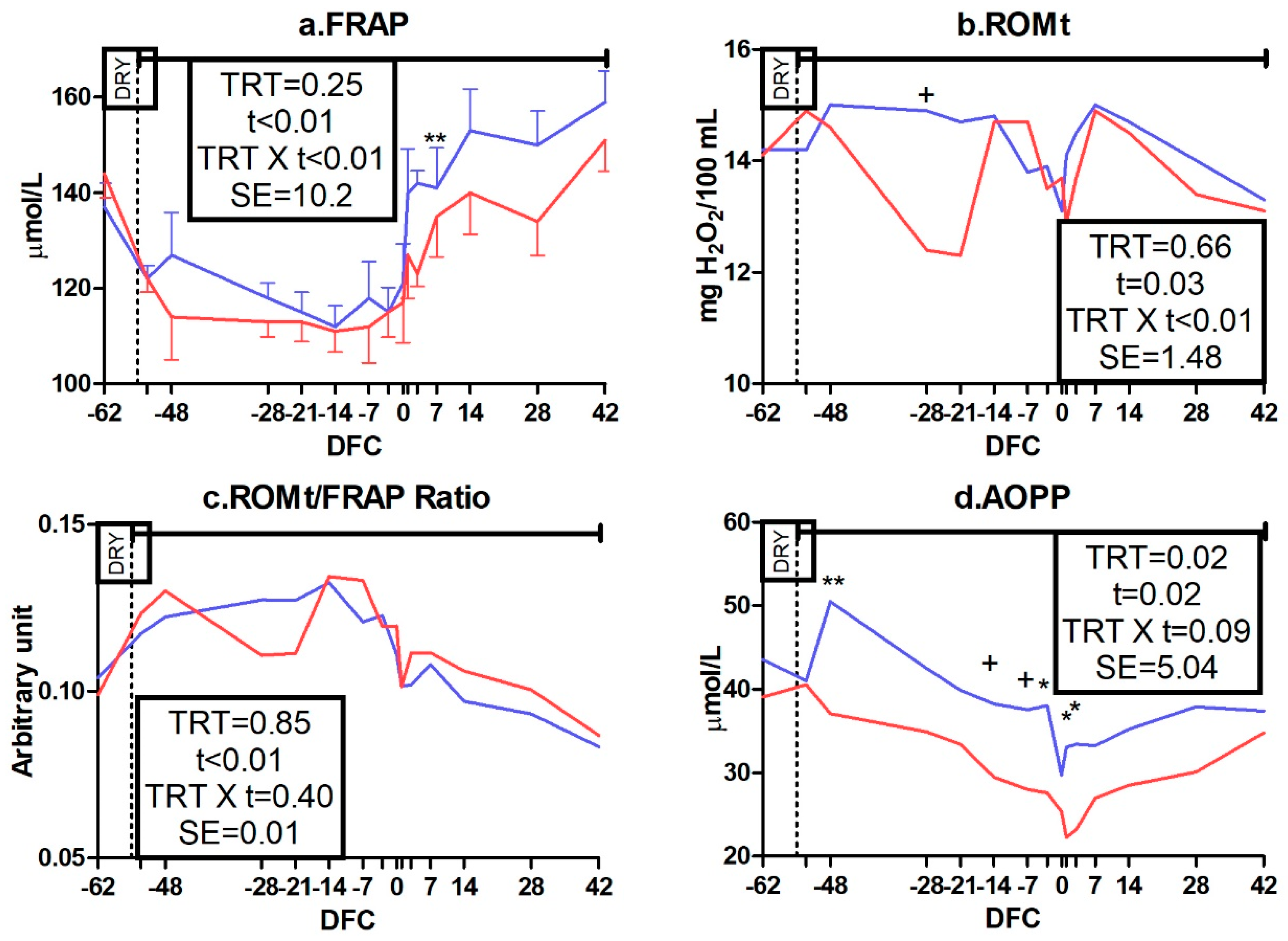
3.3.3. Oxidative Status Biomarkers
3.4. Blood Cell Profile
3.5. Whole Blood Stimulation Assay, Interferon Gamma Release Assay, and Carrageenan Skin Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sordillo, L.M. Nutritional strategies to optimize dairy cattle immunity. J. Dairy Sci. 2016, 99, 4967–4982. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A.; Travers, P.; Walport, M.; Shlomchik, M. Immunobiology: The Immune System in Health and Disease, 6th ed.; Garland Science Publishing: New York, NY, USA, 2005. [Google Scholar]
- Paape, M.J.; Bannerman, D.D.; Zhao, X.; Lee, J.W. The bovine neutrophil: structure and function in blood and milk. Vet. Res. 2003, 34, 597–627. [Google Scholar] [PubMed]
- Forsberg, N.E. Recent insights into ruminant immune function: effects of stress and immunostimulatory nutritional products. In Proceedings 2004 Florida Ruminant Nutrition Symposium; University of Florida: Gainesville, FL, USA, 2004; pp. 81–92. [Google Scholar]
- Sordillo, L.M.; Streicher, K.L. Mammary gland immunity and mastitis susceptibility. J. Mammary Gland Biol. Neoplasia 2002, 7, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Nace, E.L.; Nickerson, S.C.; Kautz, F.M.; Breidling, S.; Wochele, D.; Ely, L.O.; Hurley, D.J. Modulation of innate immune function and phenotype in bred dairy heifers during the periparturient period induced by feeding an immunostimulant for 60 days prior to delivery. Vet. Immunol. Immunopathol. 2014, 161, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Jahan, N.; Minuti, A.; Trevisi, E. Assessment of immune response in periparturient dairy cows using ex vivo whole blood stimulation assay with lipopolysaccharides and carrageenan skin test. Vet. Immunol. Immunopathol. 2015, 165, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Shuster, D.E.; Lee, E.K.; Kehrli, M.E. Bacterial growth, inflammatory cytokine production, and neutrophil recruitment during coliform mastitis in cows within ten days after calving, compared with cows at midlactation. Am. J. Vet. Res. 1996, 57, 1569–1575. [Google Scholar] [PubMed]
- Mallard, B.A.; Dekkers, J.C.; Ireland, M.J.; Leslie, K.E.; Sharif, S.; Lacey Vankampen, C.; Wagter, L.; Wilkie, B.N. Alteration in immune responsiveness during the peripartum period and its ramification on dairy cow and calf health. J. Dairy Sci. 1998, 81, 585–595. [Google Scholar] [CrossRef]
- Trevisi, E.; Jahan, N.; Bertoni, G.; Ferrari, A.; Minuti, A. Pro-inflammatory cytokine profile in dairy cows: consequences for new lactation. Ital. J. Anim. Sci. 2015, 14, 285–292. [Google Scholar] [CrossRef]
- Putman, A.K.; Brown, J.L.; Gandy, J.C.; Wisnieski, L.; Sordillo, L.M. Changes in biomarkers of nutrient metabolism, inflammation, and oxidative stress in dairy cows during the transition into the early dry period. J. Dairy Sci. 2018, 101, 9350–9359. [Google Scholar] [CrossRef] [Green Version]
- Bionaz, M.; Trevisi, E.; Calamari, L.; Librandi, F.; Ferrari, A.; Bertoni, G. Plasma paraoxonase, health, inflammatory conditions, and liver function in transition dairy cows. J. Dairy Sci. 2007, 90, 1740–1750. [Google Scholar] [CrossRef]
- Trevisi, E.; Amadori, M.; Cogrossi, S.; Razzuoli, E.; Bertoni, G. Metabolic stress and inflammatory response in high-yielding, periparturient dairy cows. Res. Vet. Sci. 2012, 93, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Drackley, J.K. Biology of dairy cows during the transition period: the final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Celi, P. Oxidative stress in ruminants. In Studies on Veterinary Medicine. Oxidative Stress in Applied Basic Research and Clinical Practice; Mandelker, L., Vajdovich, P., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 191–231. ISBN 978-1-61779-070-6. [Google Scholar]
- Curtis, C.R.; Erb, H.N.; Sniffen, C.J.; Smith, R.D.; Kronfeld, D.S. Path analysis of dry period nutrition, postpartum metabolic and reproductive disorders, and mastitis in Holstein cows. J. Dairy Sci. 1985, 68, 2347–2360. [Google Scholar] [CrossRef]
- Suriyasathaporn, W.; Daemen, A.J.J.M.; Noordhuizen-Stassen, E.N.; Dieleman, S.J.; Nielen, M.; Schukken, Y.H. β-hydroxybutyrate levels in peripheral blood and ketone bodies supplemented in culture media affect the in vitro chemotaxis of bovine leukocytes. Vet. Immunol. Immunopathol. 1999, 68, 177–186. [Google Scholar] [CrossRef]
- Wang, Y.; Puntenney, S.B.; Burton, J.L.; Forsberg, N. Ability of a commercial feed additive to modulate expression of innate immunity in sheep immunosuppressed with dexamethasone. Animal 2007, 1, 945. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Puntenney, S.B.; Burton, J.L.; Forsberg, N.E. Use of gene profiling to evaluate the effects of a feed additive on immune function in periparturient dairy cattle. J. Anim. Physiol. Anim. Nutr. 2009, 93, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Ryman, V.E.; Nickerson, S.C.; Kautz, F.M.; Hurley, D.J.; Ely, L.O.; Wang, Y.Q.; Forsberg, N.E. Effect of dietary supplementation on the antimicrobial activity of blood leukocytes isolated from Holstein heifers. Res. Vet. Sci. 2013, 95, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H. Intestinal M cells. J. Biochem. 2016, 159, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Puntenney, S.B.; Forsberg, N.E. Identification of the mechanisms by which OmniGen-AF®, a nutritional supplement, augments immune function in ruminant livestock. Am. Soc. Anim. Sci. West. Sect. 2004, 55, 349–352. [Google Scholar]
- Revelo, X.S.; Davis, J.W.; Schnabel, R.D.; Kenny, A.L.; Barkley, N.M.; Waldron, M.R. Effects of an immunomodulatory dietary supplement on the global gene expression profile of neutrophils from periparturient dairy cows. J. Dairy Sci. 2013, 96, 485. [Google Scholar]
- Agricultural Development and Advisory Service Condition scoring of dairy cows. In Proceedings of the Pubblication 612; Ministry of Agriculture, Fisheries Food: Northumberland, UK, 1986.
- Soriani, N.; Trevisi, E.; Calamari, L. Relationships between rumination time, metabolic conditions, and health status in dairy cows during the transition period. J. Anim. Sci. 2012, 90, 4544–4554. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining postpartum uterine disease in cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef]
- Ireland-Perry, R.L.; Stallings, C.C. Fecal consistency as related to dietary composition in lactating holstein cows. J. Dairy Sci. 1993, 76, 1074–1082. [Google Scholar] [CrossRef]
- Duffield, T.F. Subclinical ketosis in lactating dairy cattle. Vet. Clin. North Am. Food Anim. Pract. 2000, 16, 231–253. [Google Scholar] [CrossRef]
- Calamari, L.; Ferrari, A.; Minuti, A.; Trevisi, E. Assessment of the main plasma parameters included in a metabolic profile of dairy cow based on Fourier Transform mid-infrared spectroscopy: preliminary results. BMC Vet. Res. 2016, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Jacometo, C.B.; Osorio, J.S.; Socha, M.; Corrêa, M.N.; Piccioli-Capelli, F.; Trevisi, E.; Loor, J.J. Maternal consumption of organic trace minerals (4-Plex) alters calf systemic and neutrophil mRNA and microRNA indicators of inflammation and oxidative stress. J. Dairy Sci. 2015, 98, 7717–7729. [Google Scholar] [CrossRef] [PubMed]
- Minuti, A.; Ahmed, S.; Trevisi, E.; Piccioli-Cappelli, F.; Bertoni, G.; Jahan, N.; Bani, P. Experimental acute rumen acidosis in sheep: consequences on clinical, rumen, and gastrointestinal permeability conditions and blood chemistry. J. Anim. Sci. 2014, 92, 3966–3977. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothsein, G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef]
- Hanasand, M.; Omdal, R.; Norheim, K.B.; Gøransson, L.G.; Brede, C.; Jonsson, G. Improved detection of advanced oxidation protein products in plasma. Clin. Chim. Acta 2012, 413, 901–906. [Google Scholar] [CrossRef]
- Ling, T.; Hernandez-Jover, M.; Sordillo, L.M.; Abuelo, A. Maternal late-gestation metabolic stress is associated with changes in immune and metabolic responses of dairy calves. J. Dairy Sci. 2018, 101, 6568–6580. [Google Scholar] [CrossRef]
- Trevisi, E.; Amadori, M.; Riva, F.; Bertoni, G.; Bani, P. Evaluation of innate immune responses in bovine forestomachs. Res. Vet. Sci. 2014, 96, 69–78. [Google Scholar] [CrossRef]
- Calamari, L.; Petrera, F.; Bertin, G. Effects of either sodium selenite or Se yeast (Sc CNCM I-3060) supplementation on selenium status and milk characteristics in dairy cows. Livest. Sci. 2010, 128, 154–165. [Google Scholar] [CrossRef]
- Chessa, S.; Bulgari, O.; Rizzi, R.; Calamari, L.; Bani, P.; Biffani, S.; Caroli, A.M. Selection for milk coagulation properties predicted by Fourier transform infrared spectroscopy in the Italian Holstein-Friesian breed. J. Dairy Sci. 2014, 97, 4512–4521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Littell, R.C.; Henry, P.R.; Ammerman, C.B. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998, 76, 1216. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Bulgari, O.; Vailati-Riboni, M.; Trevisi, E.; Ballou, M.A.; Cardoso, F.C.; Luchini, D.N.; Loor, J.J. Rumen-protected methionine compared with rumen-protected choline improves immunometabolic status in dairy cows during the peripartal period. J. Dairy Sci. 2016, 99, 8956–8969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morey, S.D.; Mamedova, L.K.; Anderson, D.E.; Armendariz, C.K.; Titgemeyer, E.C.; Bradford, B.J. Effects of encapsulated niacin on metabolism and production of periparturient dairy cows. J. Dairy Sci. 2011, 94, 5090–5104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevisi, E.; Grossi, P.; Piccioli-Cappelli, F.; Cogrossi, S.; Bertoni, G. Attenuation of inflammatory response phenomena in periparturient dairy cows by the administration of an omega-3 rumen protected supplement containing vitamin E. Ital. J. Anim. Sci. 2011, 10, 277–285. [Google Scholar] [CrossRef]
- Van Knegsel, A.T.M.; Hammon, H.M.; Bernabucci, U.; Bertoni, G.; Bruckmaier, R.M.; Goselink, R.M.A.; Gross, J.J.; Kuhla, B.; Metges, C.C.; Parmentier, H.K.; et al. Metabolic adaptation during early lactation: key to cow health, longevity and a sustainable dairy production chain. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2014, 9, 1–15. [Google Scholar] [CrossRef]
- Kaufman, E.I.; LeBlanc, S.J.; McBride, B.W.; Duffield, T.F.; DeVries, T.J. Association of rumination time with subclinical ketosis in transition dairy cows. J. Dairy Sci. 2016, 99, 5604–5618. [Google Scholar] [CrossRef] [Green Version]
- Burton, J.L.; Kehrli, M.E.; Kapil, S.; Horst, R.L. Regulation of L-selectin and CD18 on bovine neutrophils by glucocorticoids: effects of cortisol and dexamethasone. J. Leukoc. Biol. 1995, 57, 317–325. [Google Scholar] [CrossRef]
- Rowson, A.D.; Wang, Y.Q.; Aalseth, E.; Forsberg, N.E.; Puntenney, S.B. Effects of an immunomodulatory feed additive on the development of mastitis in a mouse infection model using four bovine-origin isolates. Animal 2011, 5, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.W.; Rivera, F.A.; Villar, F.; Chapman, J.D.; Long, N.M.; Collier, R.J. Evaluation of OmniGen-AF® in lactating heat-stressed Holstein cows. In Proceedings of the 25th Annual Florida Ruminant Nutrition Symposium; University of Florida: Gainesville, FL, USA, 2014; pp. 16–26. [Google Scholar]
- Stevens, M.G.H.; Peelman, L.J.; De Spiegeleer, B.; Pezeshki, A.; Van De Walle, G.R.; Duchateau, L.; Burvenich, C. Differential gene expression of the toll-like receptor-4 cascade and neutrophil function in early- and mid-lactating dairy cows. J. Dairy Sci. 2011, 94, 1277–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Feeding the immune system. Proc. Nutr. Soc. 2013, 72, 299–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Hailemariam, D.; Dervishi, E.; Goldansaz, S.A.; Deng, Q.; Dunn, S.M.; Ametaj, B.N. Dairy cows affected by ketosis show alterations in innate immunity and lipid and carbohydrate metabolism during the dry off period and postpartum. Res. Vet. Sci. 2016, 107, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Castell, J.V.; Gómez-Lechón, M.J.; David, M.; Andus, T.; Geiger, T.; Trullenque, R.; Fabra, R.; Heinrich, P.C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989, 242, 237–239. [Google Scholar] [CrossRef] [Green Version]
- Ceciliani, F.; Ceron, J.J.; Eckersall, P.D.; Sauerwein, H. Acute phase proteins in ruminants. J. Proteomics 2012, 75, 4207–4231. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, G.; Trevisi, E.; Han, X.; Bionaz, M. Effects of inflammatory conditions on liver activity in puerperium period and consequences for performance in dairy cows. J. Dairy Sci. 2008, 91, 3300–3310. [Google Scholar] [CrossRef] [PubMed]
- Gruys, E.; Toussaint, M.J.M.; Niewold, T.A.; Koopmans, S.J. Acute phase reaction and acute phase proteins. J. Zhejiang Univ. Sci. 2005, 6, 1045–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graulet, B.; Matte, J.J.; Desrochers, A.; Doepel, L.; Palin, M.-F.; Girard, C.L. Effects of Dietary Supplements of Folic Acid and Vitamin B12 on Metabolism of Dairy Cows in Early Lactation. J. Dairy Sci. 2007, 90, 3442–3455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faith, M.; Sukumaran, A.; Pulimood, A.B.; Jacob, M. How reliable an indicator of inflammation is myeloperoxidase activity? Clin. Chim. Acta 2008, 396, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Celi, P.; Gabai, G. Oxidant/antioxidant balance in animal nutrition and health: the role of protein oxidation. Front. Vet. Sci. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Jimenez, S.; Haerr, K.J.; Trevisi, E.; Loor, J.J.; Cardoso, F.C.; Osorio, J.S. Prepartal standing behavior as a parameter for early detection of postpartal subclinical ketosis associated with inflammation and liver function biomarkers in peripartal dairy cows. J. Dairy Sci. 2018, 101, 8224–8235. [Google Scholar] [CrossRef] [PubMed]
- Gesslbauer, B.; Bochkov, V. Biochemical targets of drugs mitigating oxidative stress via redox-independent mechanisms. Biochem. Soc. Trans. 2017, 45, 1225–1252. [Google Scholar] [CrossRef] [PubMed]
 ), milk sample collection (
), milk sample collection (  ), and blood sample collection for the interferon gamma release assay (
), and blood sample collection for the interferon gamma release assay (  ), carrageenan skin test performance and blood cell profile and whole blood stimulation assay (
), carrageenan skin test performance and blood cell profile and whole blood stimulation assay (  ). Blood sample collection for metabolic profile (
). Blood sample collection for metabolic profile (  ). Milk yield measurement (
). Milk yield measurement (  ), body weight and body condition score determination (
), body weight and body condition score determination (  ), and dry matter intake and rumination time determination (
), and dry matter intake and rumination time determination (  ). Empty ticks indicate −55, −53, −3, 1 and 3 DFC, respectively.
). Empty ticks indicate −55, −53, −3, 1 and 3 DFC, respectively.
 ), milk sample collection (
), milk sample collection (  ), and blood sample collection for the interferon gamma release assay (
), and blood sample collection for the interferon gamma release assay (  ), carrageenan skin test performance and blood cell profile and whole blood stimulation assay (
), carrageenan skin test performance and blood cell profile and whole blood stimulation assay (  ). Blood sample collection for metabolic profile (
). Blood sample collection for metabolic profile (  ). Milk yield measurement (
). Milk yield measurement (  ), body weight and body condition score determination (
), body weight and body condition score determination (  ), and dry matter intake and rumination time determination (
), and dry matter intake and rumination time determination (  ). Empty ticks indicate −55, −53, −3, 1 and 3 DFC, respectively.
). Empty ticks indicate −55, −53, −3, 1 and 3 DFC, respectively.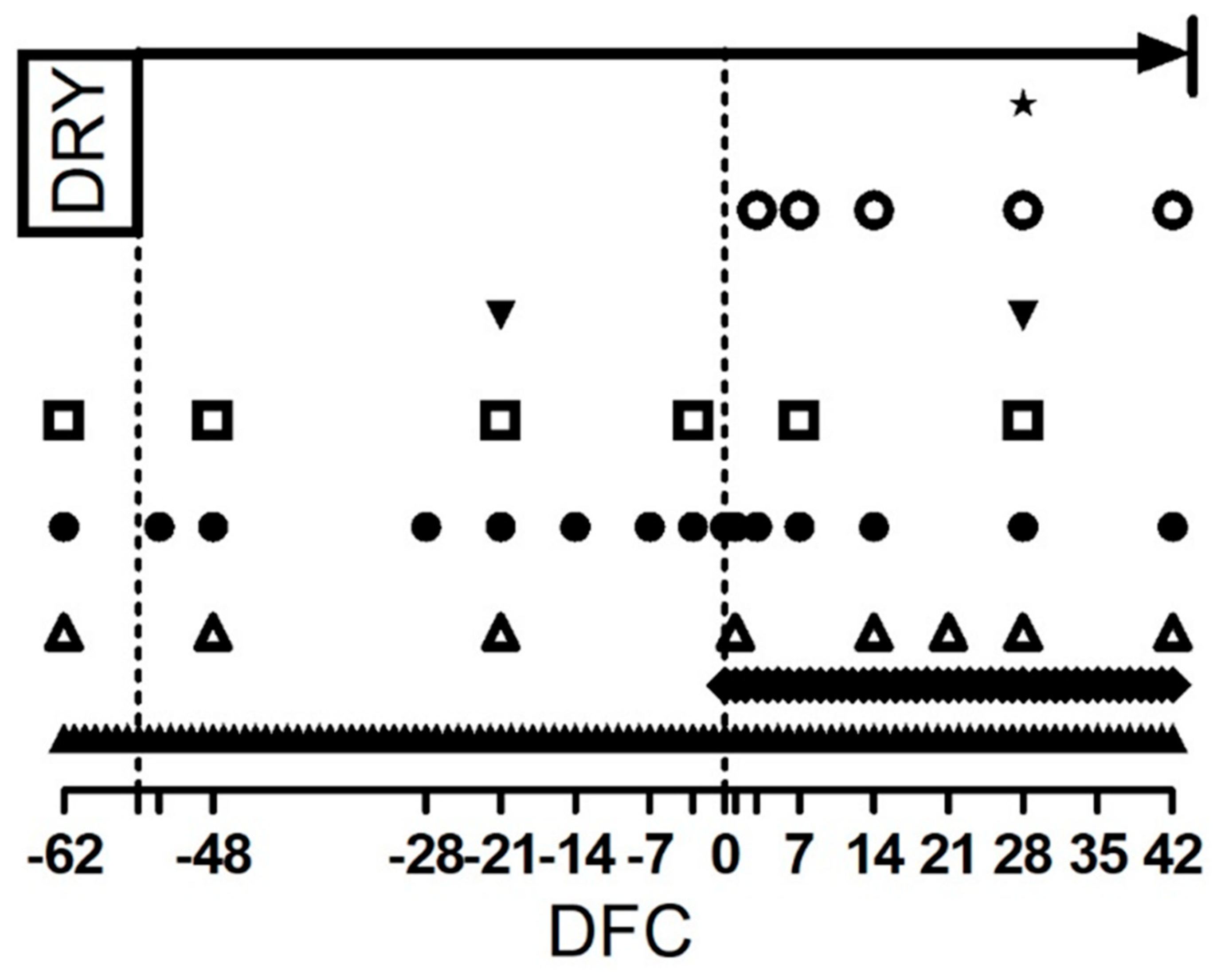
| Diet, % DM | Phase 1 4 | Phase 2 | Phase 3 | Phase 4 | Phase 5 |
|---|---|---|---|---|---|
| DFC 3 | −62 to −55 | −47 to −7 | −6 to 0 | 0 to 30 | 31 to 42 |
| Item | |||||
| Corn silage | 28.5 | 18.6 | 23.6 | 24.0 | 23.3 |
| Alfalfa hay | 16.4 | - | - | 13.7 | 11.1 |
| Grass hay | 23.4 | 71.4 | 58.0 | 18.1 | 11.9 |
| Concentrate (−62 to 0 DFC 3) | - | 10.0 | 10.1 | 5 | - |
| Concentrate (1 to 42 DFC 3) | 31.7 | - | 8.3 | 44.2 | 53.7 |
| Concentrate Composition, % DM | |||||
| Corn flour | - | - | - | 40.0 | 40.0 |
| Barley flour | - | - | - | 1.4 | 1.4 |
| Sorghum grain expanded | - | - | - | - | - |
| Soybean meal | 90.5 | 90.5 | 90.5 | 13.1 | 13.1 |
| Soybean dry rolled | - | - | - | - | - |
| Sunflower meal | - | - | - | 4.9 | 4.9 |
| Corn gluten feed | - | - | - | - | - |
| Beet pulp | - | - | - | 16.6 | 16.6 |
| Wheat bran | - | - | - | 9.8 | 9.8 |
| Beet molasse slops | - | - | - | 2.6 | 2.6 |
| Potato protein | - | - | - | 2.2 | 2.2 |
| Hydrogenated palm oil | - | - | - | 3.3 | 3.3 |
| Limestone | - | - | - | 1.39 | 1.39 |
| Dicalcium phosphate | - | - | - | 1.80 | 1.80 |
| Sodium bicarbonate | - | - | - | 0.98 | 0.98 |
| Magnesium oxide | 2.2 | 2.2 | 2.2 | 0.64 | 0.64 |
| Sodium Chloride | 1.4 | 1.4 | 1.4 | 0.32 | 0.32 |
| Supplement 1 | 5.9 | 5.9 | 5.9 | 1.07 | 1.07 |
| Chemical Composition | |||||
| NEL, Mcal kg of DM−1 | 1.59 | 1.45 | 1.53 | 1.60 | 1.63 |
| Crude protein, % DM | 14.9 | 13.6 | 14.5 | 16.2 | 17.2 |
| Starch + sugar, % DM | 23.7 | 16.8 | 19.3 | 26.0 | 18.3 |
| Ether extract, % DM | 3.80 | 1.80 | 2.40 | 4.48 | 5.08 |
| NDF, % DM | 39.4 | 49.3 | 45.5 | 35.7 | 32.6 |
| MP 2, % CP | 9.8 | 9.1 | 9.7 | 10.5 | 11.1 |
| RUP 2, % DM | 4.64 | 4.48 | 4.77 | 5.23 | 5.96 |
| Item, Unit | TRT 1 | Days from Calving | SE 5 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 7 | 14 | 28 | 42 | TRT 1 | t 2 | TRT × t 3 | |||
| Butterfat, mg 100 mL−1 | CTR | 4.69 | 4.24 | 4.68 | 3.67 | 0.35 | 0.21 | 0.01 | 0.78 |
| IMS | 4.33 | 4.14 | 3.82 | 3.36 | |||||
| Fat output, kg | CTR | 0.38 | 0.41 | 0.65 | 0.48 | 0.07 | 0.17 | 0.02 | 0.65 |
| IMS | 0.27 | 0.37 | 0.48 | 0.44 | |||||
| Total protein, mg 100 mL−1 | CTR | 3.84 | 3.30 | 3.07 | 3.09 | 0.10 | 0.49 | <0.01 | 0.66 |
| IMS | 3.81 | 3.34 | 3.25 | 3.19 | |||||
| Protein output, kg | CTR | 1.21 | 1.18 | 1.33 | 1.36 | 0.06 | 0.61 | <0.01 | 0.15 |
| IMS | 1.15 | 1.18 | 1.38 | 1.50 | |||||
| Fat/protein ratio, - | CTR | 1.22 | 1.28 | 1.54 | 1.19 | 0.12 | 0.11 | 0.11 | 0.64 |
| IMS | 1.14 | 1.24 | 1.19 | 1.05 | |||||
| Lactose, mg 100 mL−1 | CTR | 4.79 | 5.25 | 5.06 | 5.17 | 0.07 | 0.73 | <0.01 | 0.03 |
| IMS | 4.96 | 5.09 | 5.16 | 5.14 | |||||
| + | |||||||||
| Caseins, mg 100 mL−1 | CTR | 2.84 | 2.50 | 2.28 | 2.33 | 0.08 | 0.42 | <0.01 | 0.59 |
| IMS | 2.85 | 2.51 | 2.45 | 2.40 | |||||
| Titratable acidity, °SH 50 mL−1 | CTR | 3.96 | 3.33 | 3.23 | 3.12 | 0.17 | 0.77 | <0.01 | 0.93 |
| IMS | 3.86 | 3.19 | 3.20 | 3.12 | |||||
| Urea-N, mg dL−1 | CTR | 31.2 | 34.7 | 26.8 | 31.2 | 3.11 | 0.64 | 0.08 | 0.66 |
| IMS | 33.7 | 33.3 | 30.2 | 33.0 | |||||
| Coagulation time (rCT), min | CTR | 12.3 | 20.0 | 14.0 | 14.5 | 2.03 | 0.77 | <0.01 | 0.68 |
| IMS | 11.5 | 16.2 | 14.8 | 16.1 | |||||
| Curd firmness (a30), mm | CTR | 37.7 | 23.2 | 35.3 | 32.8 | 4.59 | 0.27 | 0.31 | 0.40 |
| IMS | 43.4 | 33.6 | 35.6 | 34.7 | |||||
| SCC 4, 103 mL−1 | CTR | 293.4 | - | 37.7 | - | 91.4 | 0.47 | 0.18 | 0.31 |
| IMS | 72.4 | - | 54.9 | - | |||||
| Item | Unit | TRT 1 | SE 3 | p-Value | |
|---|---|---|---|---|---|
| CTR | IMS | ||||
| pH | - | 6.78 | 6.54 | 0.15 | 0.14 |
| Ammonia-N | mg L−1 | 4.65 | 4.47 | 0.10 | 0.96 |
| Total VFA 2 | mM L−1 | 103.8 | 109.0 | 8.72 | 0.52 |
| Individual VFA | mol 100 mol−1 total VFA | ||||
| Acetic acid | 66.2 | 67.0 | 5.08 | 0.79 | |
| Propionic acid | 21.8 | 25.4 | 3.81 | 0.38 | |
| Butyric acid | 11.4 | 12.3 | 1.17 | 0.47 | |
| Isobutyric acid | 0.89 | 0.80 | 0.08 | 0.39 | |
| Valeric acid | 1.26 | 1.42 | 0.21 | 0.41 | |
| Isovaleric acid | 1.55 | 1.57 | 0.14 | 0.77 | |
| Caproic acid | 0.55 | 0.46 | 0.14 | 0.61 | |
| Enanthic acid | 0.04 | 0.03 | 0.01 | 0.35 | |
| L-lactic acid | mM L−1 | 0.39 | 0.37 | 0.11 | 0.88 |
| D-lactic acid | mM L−1 | 0.39 | 0.37 | 0.11 | 0.91 |
| Total lactic acid | mM L−1 | 0.78 | 0.75 | 0.21 | 0.89 |
| Item, Unit | TRT 1 | Days from Calving | SE 2 | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −62 | −48 | −21 | −3 | 7 | 28 | TRT1 | t 3 | TRT × t 4 | |||
| WBC 5, 103 µL−1 | CTR | 6.4 | 5.8 | 6.3 | 7.7 | 6.0 | 5.9 | 0.77 | 0.54 | <0.01 | 0.59 |
| IMS | 7.5 | 6.6 | 6.8 | 8.4 | 6.8 | 5.7 | 0.77 | ||||
| Neu 6, 103 µL−1 | CTR | 3.3 | 3.0 | 3.2 | 4.7 | 3.6 | 3.2 | 0.61 | 0.52 | <0.01 | 0.69 |
| IMS | 4.0 | 3.3 | 3.5 | 5.1 | 3.9 | 3.0 | 0.61 | ||||
| Lym 7, 103 µL−1 | CTR | 2.2 | 2.0 | 2.2 | 2.2 | 1.7 | 1.9 | 0.59 | 0.56 | <0.01 | 0.04 |
| IMS | 2.7 | 2.6 | 2.6 | 2.3 | 2.2 | 2.0 | 0.61 | ||||
| HGB 8, g dL−1 | CTR | 10.8 | 11.4 | 10.6 | 11.0 | 10.7 | 9.8 | 0.40 | 0.20 | <0.01 | 0.42 |
| IMS | 10.0 | 10.5 | 10.0 | 10.2 | 10.4 | 9.4 | 0.40 | ||||
| MCV 9, fL | CTR | 48.3 | 48.6 | 48.6 | 51.0 | 49.6 | 48.7 | 1.4 | 0.08 | <0.01 | 0.78 |
| IMS | 45.8 | 45.5 | 45.9 | 47.5 | 47.6 | 46.8 | 1.4 | ||||
| MCH 10, pg | CTR | 16.5 | 16.9 | 16.9 | 17.2 | 17.1 | 16.9 | 0.47 | 0.08 | 0.06 | 0.69 |
| IMS | 15.7 | 15.7 | 16.0 | 16.5 | 16.4 | 16.4 | 0.47 | ||||
| PLT 11, 103 µL−1 | CTR | 323.8 | 273.6 | 280.0 | 305.8 | 313.8 | 420.4 | 38.2 | 0.07 | 0.02 | 0.84 |
| IMS | 354.6 | 348.8 | 345.8 | 412.8 | 357.2 | 437.6 | 38.2 | ||||
| MPV 12, fL | CTR | 6.9 | 8.3 | 7.6 | 6.9 | 6.8 | 5.8 | 0.36 | 0.01 | 0.01 | 0.01 |
| IMS | 6.1 | 5.7 | 6.3 | 6.5 | 5.9 | 5.5 | 0.45 | ||||
| ** | * | + | |||||||||
| Item | D 2 | TRT 1 | Days from Calving | SE 3 | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −62 | −45 | −21 | −3 | 7 | 28 | TRT 1 | t 4 | D 2 | TRT × t 5 | TRT × t × D 6 | ||||
| IL-1 7 | Low | CTR | 3.5 | 9.9 | 10.0 | 20.2 | 18.7 | 6.9 | 11.3 | 0.66 | <0.01 | <0.01 | 0.13 | 0.87 |
| IMS | 5.2 | 9.9 | 13.1 | 10.2 | 9.8 | 9.7 | ||||||||
| High | CTR | 13.1 | 21.2 | 33.6 | 52.1 | 55.3 | 23.7 | 11.3 | ||||||
| IMS | 20.0 | 40.9 | 39.9 | 51.8 | 59.6 | 32.3 | ||||||||
| Total | CTR | 8.3 | 15.5 | 21.8 | 36.1 | 37.0 | 15.3 | 9.3 | ||||||
| IMS | 12.6 | 25.4 | 26.5 | 31.0 | 34.7 | 21.0 | ||||||||
| IL-6 8 | Low | CTR | 1.34 | 1.61 | 1.27 | 1.92 | 1.57 | 1.91 | 0.39 | 0.97 | 0.06 | <0.01 | 0.10 | 0.95 |
| IMS | 1.69 | 1.65 | 1.56 | 1.33 | 1.78 | 1.60 | ||||||||
| High | CTR | 1.90 | 2.19 | 1.99 | 2.10 | 2.40 | 3.07 | 0.39 | ||||||
| IMS | 2.20 | 2.38 | 1.77 | 1.71 | 2.87 | 2.38 | ||||||||
| Total | CTR | 1.62 | 1.90 | 1.63 | 2.01 | 1.98 | 2.49 | 0.31 | ||||||
| IMS | 1.95 | 2.01 | 1.67 | 1.52 | 2.32 | 1.99 | ||||||||
| Item | D 2 | TRT 1 | Days from Calving | SE 3 | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −62 | −45 | −21 | −3 | 7 | 28 | TRT 1 | t 4 | D 2 | TRT × t 5 | TRT × t × D 6 | ||||
| Glu 7 | Low | CTR | 0.96 | 0.96 | 0.94 | 0.96 | 0.93 | 0.97 | 0.04 | 0.13 | 0.41 | 0.02 | 0.06 | 1.00 |
| IMS | 0.98 | 0.98 | 0.99 | 0.91 | 1.08 | 0.97 | ||||||||
| High | CTR | 0.95 | 0.94 | 0.89 | 0.91 | 0.88 | 0.93 | 0.04 | ||||||
| IMS | 0.92 | 0.93 | 0.96 | 0.85 | 1.00 | 0.91 | ||||||||
| Total | CTR | 0.95 | 0.95 | 0.91 | 0.93 | 0.91 | 0.95 | 0.03 | ||||||
| IMS | 0.95 | 0.95 | 0.98 | 0.88 | 1.04 | 0.94 | ||||||||
| + | ** | |||||||||||||
| DLA 8 | Low | CTR | 0.97 | 1.00 | 1.05 | 1.12 | 1.08 | 1.09 | 0.04 | 0.62 | 0.01 | 0.04 | 0.08 | 0.99 |
| IMS | 1.02 | 1.03 | 0.99 | 1.07 | 1.01 | 0.99 | ||||||||
| High | CTR | 0.98 | 1.00 | 1.09 | 1.13 | 1.11 | 1.15 | 0.04 | ||||||
| IMS | 1.06 | 1.06 | 1.05 | 1.12 | 1.09 | 1.07 | ||||||||
| Total | CTR | 0.98 | 1.00 | 1.07 | 1.12 | 1.10 | 1.12 | 0.03 | ||||||
| IMS | 1.04 | 1.05 | 1.02 | 1.10 | 1.05 | 1.03 | ||||||||
| + | ||||||||||||||
| LLA 9 | Low | CTR | 1.02 | 1.02 | 1.02 | 1.04 | 1.06 | 1.06 | 0.02 | 0.35 | 0.10 | <0.01 | 0.10 | 0.84 |
| IMS | 1.04 | 1.02 | 1.03 | 1.04 | 1.02 | 1.00 | ||||||||
| High | CTR | 1.03 | 1.03 | 1.08 | 1.07 | 1.10 | 1.14 | 0.02 | ||||||
| IMS | 1.07 | 1.03 | 1.04 | 1.06 | 1.05 | 1.08 | ||||||||
| Total | CTR | 1.02 | 1.02 | 1.05 | 1.06 | 1.08 | 1.10 | 0.02 | ||||||
| IMS | 1.05 | 1.02 | 1.04 | 1.05 | 1.04 | 1.04 | ||||||||
| * | ||||||||||||||
| Item | D 2 | TRT 1 | Days from Calving | SE 3 | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −62 | −45 | −21 | −3 | 7 | 28 | TRT 1 | t 4 | D 2 | TRT × t 5 | TRT × t × D 6 | ||||
| NO2 7 | Low | CTR | 1.02 | 0.99 | 1.03 | 1.11 | 0.96 | 1.13 | 0.07 | 0.96 | <0.01 | 0.74 | 0.16 | 0.70 |
| IMS | 0.97 | 0.97 | 1.23 | 1.23 | 1.03 | 0.97 | ||||||||
| High | CTR | 1.04 | 1.00 | 1.03 | 1.18 | 0.99 | 1.06 | 0.07 | ||||||
| IMS | 1.05 | 1.01 | 1.01 | 1.14 | 1.02 | 0.95 | ||||||||
| Total | CTR | 1.03 | 1.00 | 1.03 | 1.15 | 0.97 | 1.10 | 0.05 | ||||||
| IMS | 1.01 | 0.99 | 1.12 | 1.18 | 1.03 | 0.96 | ||||||||
| NO3 8 | Low | CTR | 0.95 | 1.07 | 1.05 | 1.06 | 1.04 | 1.01 | 0.12 | 0.96 | 0.99 | 0.03 | 0.94 | 0.78 |
| IMS | 0.99 | 1.01 | 0.98 | 1.02 | 1.02 | 0.96 | ||||||||
| High | CTR | 1.20 | 1.05 | 1.20 | 1.05 | 1.04 | 1.05 | 0.12 | ||||||
| IMS | 1.14 | 1.40 | 1.13 | 1.02 | 1.11 | 1.23 | ||||||||
| Total | CTR | 1.07 | 1.06 | 1.12 | 1.05 | 1.04 | 1.03 | 0.08 | ||||||
| IMS | 1.06 | 1.20 | 1.05 | 1.02 | 1.07 | 1.09 | ||||||||
| NOx 9 | Low | CTR | 0.98 | 1.05 | 1.05 | 1.07 | 1.02 | 1.05 | 0.07 | 0.73 | 0.97 | 0.03 | 0.94 | 0.79 |
| IMS | 0.98 | 1.01 | 1.03 | 1.05 | 1.03 | 0.97 | ||||||||
| High | CTR | 1.14 | 1.08 | 1.15 | 1.08 | 1.02 | 1.05 | 0.07 | ||||||
| IMS | 1.11 | 1.17 | 1.07 | 1.04 | 1.09 | 1.14 | ||||||||
| Total | CTR | 1.06 | 1.06 | 1.10 | 1.08 | 1.02 | 1.05 | 0.05 | ||||||
| IMS | 1.05 | 1.09 | 1.05 | 1.04 | 1.06 | 1.06 | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezzetti, M.; Minuti, A.; Piccioli-Cappelli, F.; Gabai, G.; Trevisi, E. Administration of an Immune Stimulant during the Transition Period Improved Lipid Metabolism and Rumination without Affecting Inflammatory Status. Animals 2019, 9, 619. https://doi.org/10.3390/ani9090619
Mezzetti M, Minuti A, Piccioli-Cappelli F, Gabai G, Trevisi E. Administration of an Immune Stimulant during the Transition Period Improved Lipid Metabolism and Rumination without Affecting Inflammatory Status. Animals. 2019; 9(9):619. https://doi.org/10.3390/ani9090619
Chicago/Turabian StyleMezzetti, Matteo, Andrea Minuti, Fiorenzo Piccioli-Cappelli, Gianfranco Gabai, and Erminio Trevisi. 2019. "Administration of an Immune Stimulant during the Transition Period Improved Lipid Metabolism and Rumination without Affecting Inflammatory Status" Animals 9, no. 9: 619. https://doi.org/10.3390/ani9090619
APA StyleMezzetti, M., Minuti, A., Piccioli-Cappelli, F., Gabai, G., & Trevisi, E. (2019). Administration of an Immune Stimulant during the Transition Period Improved Lipid Metabolism and Rumination without Affecting Inflammatory Status. Animals, 9(9), 619. https://doi.org/10.3390/ani9090619







