NTN1 Affects Porcine Intramuscular Fat Content by Affecting the Expression of Myogenic Regulatory Factors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Sample Collection
2.3. Western Blotting Analysis
2.4. Gene Dose of NTN1 in Individuals with Different Intramuscular Fat, and NTN1 RNA Expression in Different Tissues
2.5. NTN1 RNA Silencing in C2C12 Cells
2.6. RNA Silencing in 3T3-L1 Pre-Adipocytes Cells
2.7. RNA Over-Expression in C2C12 Cells
2.8. Real-Time Quantitative Polymerase Chain Reaction (qPCR) Assay
2.9. Statistical Analysis
3. Results
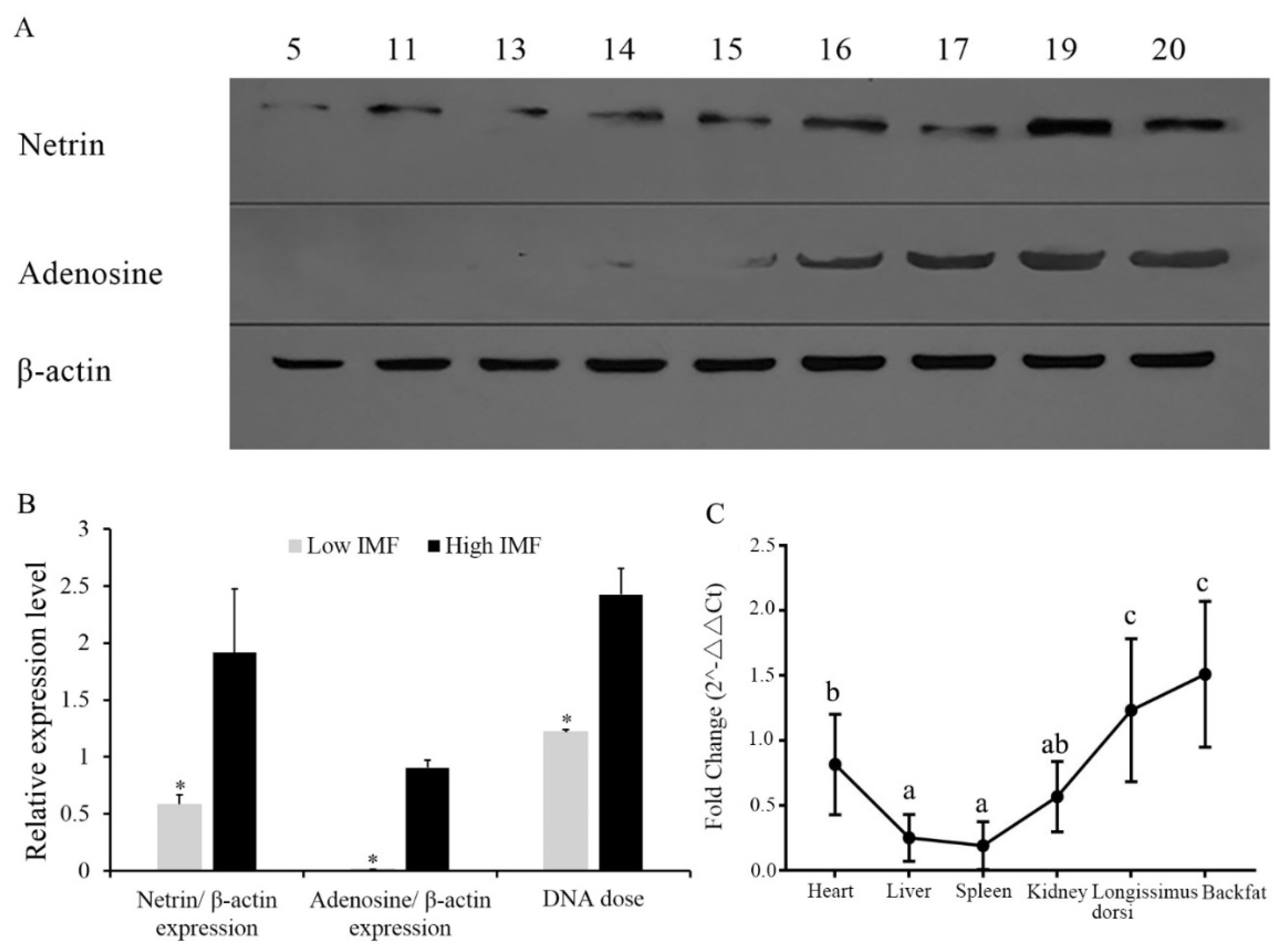
3.1. Expression of NTN1 Protein in Longissimus Dorsi with Different Intramuscular Fat
3.2. Gene Dose of NTN1 in Individuals with Different Intramuscular Fat, and NTN1 RNA Expression in Different Tissues
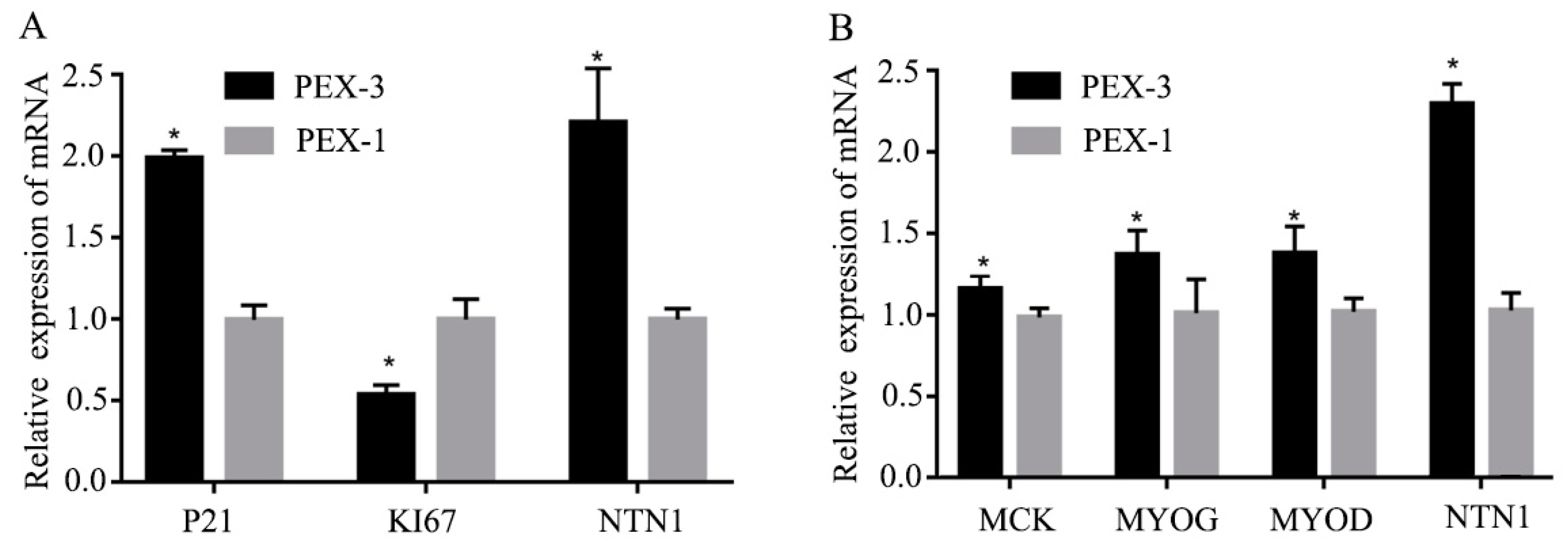
3.3. Effect of si-NTN1 on the Proliferation and Differentiation of C2C12 Cells
3.4. Effect of Over-Expression of NTN1 on the Proliferation and Differentiation of C2C12 Cells
3.5. Effect of si-NTN1 on the Proliferation and Differentiation of 3T3-L1 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biermann, A.D.; Yin, T.; König von Borstel, U.U.; Rübesam, K.; Kuhn, B.; König, S. From phenotyping towards breeding strategies: Using in vivo indicator traits and genetic markers to improve meat quality in an endangered pig breed. Animal 2015, 9, 919–927. [Google Scholar] [CrossRef]
- Fortin, A.; Robertson, W.M.; Tong, A.K. The eating quality of Canadian pork and its relationship with intramuscular fat. Meat Sci. 2005, 69, 297–305. [Google Scholar] [CrossRef]
- Manolio, T.A.; Collins, F.S.; Cox, N.J.; Goldstein, D.B.; Hindorff, L.A.; Hunter, D.J.; McCarthy, M.I.; Ramos, E.M.; Cardon, L.R.; Chakravarti, A.; et al. Finding the missing heritability of complex diseases. Nature 2009, 461, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Fadista, J.; Nygaard, M.; Holm, L.E.; Thomsen, B.; Bendixen, C. A snapshot of CNVs in the pig genome. PLoS ONE 2008, 3, e3916. [Google Scholar] [CrossRef]
- Revilla, M.; Puig-Oliveras, A.; Castelló, A.; Crespo-Piazuelo, D.; Paludo, E.; Fernández, A.I.; Ballester, M.; Folch, J.M. A global analysis of CNVs in swine using whole genome sequence data and association analysis with fatty acid composition and growth traits. PLoS ONE 2017, 12, e0177014. [Google Scholar] [CrossRef]
- Ran, X.Q.; Pan, H.; Huang, S.H.; Liu, C.; Niu, X.; Li, S.; Wang, J.F. Copy number variations of MTHFSD gene across pig breeds and its association with litter size traits in Chinese indigenous Xiang pig. J. Anim. Physiol. Anim. Nutr. (Berl) 2018, 102, 1320–1327. [Google Scholar] [CrossRef]
- Stafuzza, N.B.; Silva, R.M.; Please, O.; Fragomeni, B.O.; Masuda, Y.; Huang, Y.; Gray, K.; Lourenco, D.A.L. A genome-wide single nucleotide polymorphism and copy number variation analysis for number of piglets born alive. BMC Genomics 2019, 20, 321. [Google Scholar] [CrossRef]
- Wang, L.; Xu, L.; Liu, X.; Zhang, T.; Li, N.; Hay, E.I.; Zhang, Y.; Zhao, K.; Yan, H.; Liu, G.E.; et al. Copy number variation-based genome wide association study reveals additional variants contributing to meat quality in Swine. Sci. Rep. 2015, 5, 12535. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Wang, J.; Wang, H.; Zhang, Y.; Kang, H.; Feng, X.; Wang, J.; Yin, Z.; Bao, W.; Zhang, Q.; et al. Global copy number analyses by next generation sequencing provide insight into pig genome variation. BMC Genomics 2014, 15, 593. [Google Scholar] [CrossRef]
- Forcet, C.; Stein, E.; Pays, L.; Corset, V.; Llambi, F.; Tessier-Lavigne, M.; Mehlen, P. Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature 2002, 417, 443–447. [Google Scholar] [CrossRef]
- Furne, C.; Rama, N.; Corset, V.; Chédotal, A.; Mehlen, P. Netrin-1 is a survival factor during commissural neuron navigation. Proc. Natl. Acad. Sci. USA 2008, 105, 14465–14470. [Google Scholar] [CrossRef] [Green Version]
- Ramkhelawon, B.; Hennessy, E.J.; Ménager, M.; Ray, T.D.; Sheedy, F.J.; Hutchison, S.; Wanschel, A.; Oldebeken, S.; Geoffrion, M.; Spiro, W.; et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat. Med. 2014, 20, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Llambi, F.; Causeret, F.; Bloch-Gallego, E.; Mehlen, P. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J. 2001, 20, 2715–2722. [Google Scholar] [CrossRef] [Green Version]
- Corset, V.; Nguyen-Ba-Charvet, K.T.; Forcet, C.; Moyse, E.; Chédotal, A.; Mehlen, P. Netrin-1-mediated axon outgrowth and cAMP production requires interaction with adenosine A2b receptor. Nature 2000, 407, 747–750. [Google Scholar] [CrossRef]
- Cui, H.; Zheng, M.; Zhao, G.; Liu, R.; Wen, J. Identification of differentially expressed genes and pathways for intramuscular fat metabolism between breast and thigh tissues of chickens. BMC Genomics 2018, 19, 55. [Google Scholar] [CrossRef]
- Hocquette, J.F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development genetic and nutritional control.; and identification of putative markers. Animal 2010, 4, 303–319. [Google Scholar] [CrossRef]
- Cui, H.; Liu, R.; Zhao, G.; Zheng, M.; Chen, J.; Wen, J. Identification of differentially expressed genes and pathways for intramuscular fat deposition in pectoralis major tissues of fast-and slow-growing chickens. BMC Genomics 2012, 13, 213. [Google Scholar] [CrossRef]
- Gharibi, B.; Abraham, A.A.; Ham, J.; Evans, B.A. Contrasting effects of A1 and A2b adenosine receptors on adipogenesis. Int. J. Obes. (Lond.) 2012, 3, 397–406. [Google Scholar] [CrossRef]
- Won, S.; Jung, J.; Park, E.; Kim, H. Identification of genes related to intramuscular fat content of pigs using genome-wide association study. Asian Australas J. Anim. Sci. 2018, 31, 157–162. [Google Scholar] [CrossRef]
- Duarte, M.S.; Paulino, P.V.; Das, A.K.; Wei, S.; Serão, N.V.; Fu, X.; Harris, S.M.; Dodson, M.V.; Du, M. Enhancement of adipogenesis and fibrogenesis in skeletal muscle of Wagyu compared with Angus cattle. J. Anim. Sci. 2013, 91, 2938–2946. [Google Scholar] [CrossRef]
- Endesfelder, S.; Kliche, A.; Lochmüller, H.; von Moers, A.; Speer, A. Antisense oligonucleotides and short interfering RNAs silencing the cyclin-dependent kinase inhibitor p21 improve proliferation of Duchenne muscular dystrophy patients’ primary skeletal myoblasts. J. Mol. Med. (Berl) 2005, 83, 64–71. [Google Scholar] [CrossRef]
- Wang, N.; Cao, Y.; Zhu, Y. Netrin-1 prevents the development of cardiac hypertrophy and heart failure. Mol. Med. Rep. 2016, 13, 2175–2181. [Google Scholar] [CrossRef] [Green Version]
- Bechshøft, C.J.L.; Schjerling, P.; Kjaer, M.; Mackey, A.L. The influence of direct and indirect fibroblast cell contact on human myogenic cell behaviour and gene expression in vitro. J. Appl. Physiol. 2019, 127, 342–355. [Google Scholar] [CrossRef]
- Borselli, C.; Storrie, H.; Benesch-Lee, F.; Shvartsman, D.; Cezar, C.; Lichtman, J.W.; Vandenburgh, H.H.; Mooney, D.J. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc. Nat Acad. Sci. USA 2010, 107, 3287–3292. [Google Scholar] [CrossRef]
- Okamoto, S.; Asgar, N.F.; Yokota, S.; Saito, K.; Minokoshi, Y. Role of the α2 subunit of AMP-activated protein kinase and its nuclear localization in mitochondria and energy metabolism-related gene expressions in C2C12 cells. Metabolism 2019, 90, 52–68. [Google Scholar] [CrossRef]
- Qi, H.; Liu, Y.; Li, S.; Chen, Y.; Li, L.; Cao, Y.; Shi, P.; Song, C.; Li, B.; Sun, H.; et al. Activation of AMPK Attenuated Cardiac Fibrosis by Inhibiting CDK2 via p21/p27 and miR-29 Family Pathways in Rats. Mol. Ther. Nucleic Acids 2017, 8, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Li, X.; Xie, X.; Ye, F.; Chen, B.; Song, C.; Tang, H.; Xie, X. High expressions of LDHA and AMPK as prognostic biomarkers for breast cancer. Breast 2016, 30, 39–46. [Google Scholar] [CrossRef]
- Dial, A.G.; Rooprai, P.; Lally, J.S.; Bujak, A.L.; Steinberg, G.R.; Ljubicic, V. The role of AMP-activated protein kinase in the expression of the dystrophin-associated protein complex in skeletal muscle. FASEB J. 2018, 32, 2950–2965. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, X.; Liu, J.; Ye, H.; Zhu, L.; Wu, J.Y. AMPK interacts with DSCAM and plays an important role in netrin-1 induced neurite outgrowth. Protein Cell 2013, 4, 155–161. [Google Scholar] [CrossRef]
- Yao, C.; Pang, D.; Lu, C.; Xu, A.; Huang, P.; Ouyang, H.; Yu, H. Data Mining and Validation of AMPK Pathway as a Novel Candidate Role Affecting Intramuscular Fat Content in Pigs. Animals (Basel) 2019, 9, 137. [Google Scholar] [CrossRef]
- Fu, M.; Rao, M.; Bouras, T.; Wang, C.; Wu, K.; Zhang, X.; Li, Z.; Yao, T.P.; Pestell, R.G. Cyclin D1 inhibits peroxisome proliferator-activated receptor gamma-mediated adipogenesis through histone deacetylase recruitment. J. Biol. Chem. 2005, 280, 16934–16941. [Google Scholar] [CrossRef]
- Lagarrigue, S.; Lopez-Mejia, I.C.; Denechaud, P.D.; Escoté, X.; Castillo-Armengol, J.; Jimenez, V.; Chavey, C.; Giralt, A.; Lai, Q.; Zhang, L.; et al. CDK4 is an essential insulin effector in adipocytes. J. Clin. Investig. 2016, 12, 335–348. [Google Scholar] [CrossRef]
- Janani, C.; Ranjitha Kumari, B.D. PPAR gamma gene—A review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef]
- Mita, T.; Furuhashi, M.; Hiramitsu, S.; Ishii, J.; Hoshina, K.; Ishimura, S.; Fuseya, T.; Watanabe, Y.; Tanaka, M.; Ohno, K.; et al. FABP4 is secreted from adipocytes by adenyl cyclase-PKA- and guanylyl cyclase-PKG-dependent lipolytic mechanisms. Obesity (Silver Spring) 2015, 23, 359–367. [Google Scholar] [CrossRef]
- Cui, J.; Chen, W.; Liu, J.; Xu, T.; Zeng, Y. Study on quantitative expression of PPARγ and ADRP in muscle and its association with intramuscular fat deposition of pig. Springerplus 2016, 5, 1501. [Google Scholar] [CrossRef]
- Hosooka, T.; Ogawa, W. A novel role for the cell cycle regulatory complex cyclin D1-CDK4 in gluconeogenesis. J. Diabetes Investig. 2016, 7, 27–28. [Google Scholar] [CrossRef]
- Smith, A.; Yu, X.; Yin, L. Diazinon exposure activated transcriptional factors CCAAT-enhancer-binding proteins α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) and induced adipogenesis in 3T3-L1 preadipocytes. Pestic. Biochem. Physiol. 2018, 150, 48–58. [Google Scholar] [CrossRef]
- Haj-Yasein, N.N.; Berg, O.; Jernerén, F.; Refsum, H.; Nebb, H.I.; Dalen, K.T. Cysteine deprivation prevents induction of peroxisome proliferator-activated receptor gamma-2 and adipose differentiation of 3T3-L1 cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 623–635. [Google Scholar] [CrossRef]


| Gene Symbol * | Sense Strands (5′-3′) | Anti-Sense STRANDS (5′-3′) |
|---|---|---|
| NTN1 | TTGCAAAGCCTGTGATTGCC | AATCTTGATGCAAGGGGCAG |
| GCG | AAGCTTCAAACAGGGGTACAAT | CCACTTGGAATGTTACCCTAATG |
| GAPDH | CACUCAAGAUUGUCAGCAATT | UUGCUGACAAUCUUGAGUGAG |
| KI67 | AGCTAACTTGCGCTGACTGG | ATATTGCCTCTTGCTCTTTGACT |
| P21 | GTACTTCCTCTGCCCTGCTGCA | CCAATCTGCGCTTGGAGTGATAG |
| MYOD | GGCAGAATGGCTACGACAC | GGGTCTGGGTTCCCTGTTCT |
| MYOG | CGGTGGAGGATATGTCTGTTG | GGTGTTAGCCTTATGTGAATGG |
| MCK | AGGAGTACCCAGACCTCAGCAA | GACCGTGTAGGACTCCTCATCG |
| CyclinD1 | CAGTAACGTCACACGGACTACAGG | CGTTGAGGAGATTGGTGTCAGG |
| PPARγ | AAGAGCTGACCCAATGGTTG | ACCCTTGCATCCTTCACAAG |
| FABP4 | TAAAAACACCGAGATTTCCTTCA | CCTTTCATAACACATTCCACCA |
| CDCK4 | CTACATACGCAACACCCG | TCAAAGATTTTCCCCAACT |
| si-NTN1 | Sense Strands (5′-3′) | Anti-Sense STRANDS (5′-3′) |
|---|---|---|
| si-1085 | GCGCGACUCCUAUUACUAUTT | AUAGUAAUAGGAGUCGCGCTT |
| si-1225 | GCGACCGUUGCAAGCCCUUTT | AAGGGCUUGCAACGGUCGCTT |
| si-562 | GCAACUCUUCGGAUCCCAATT | UUGGGAUCCGAAGAGUUGCTT |
| NC | UUCUCCGAACGUGUCACGUTT | UUCUCCGAACGUGUCACGUTT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhao, L.; Zhang, L.; Liu, X.; Hou, X.; Gao, H.; Yan, H.; Zhao, F.; Wang, L. NTN1 Affects Porcine Intramuscular Fat Content by Affecting the Expression of Myogenic Regulatory Factors. Animals 2019, 9, 609. https://doi.org/10.3390/ani9090609
Wang L, Zhao L, Zhang L, Liu X, Hou X, Gao H, Yan H, Zhao F, Wang L. NTN1 Affects Porcine Intramuscular Fat Content by Affecting the Expression of Myogenic Regulatory Factors. Animals. 2019; 9(9):609. https://doi.org/10.3390/ani9090609
Chicago/Turabian StyleWang, Ligang, Lingling Zhao, Longchao Zhang, Xin Liu, Xinhua Hou, Hongmei Gao, Hua Yan, Fuping Zhao, and Lixian Wang. 2019. "NTN1 Affects Porcine Intramuscular Fat Content by Affecting the Expression of Myogenic Regulatory Factors" Animals 9, no. 9: 609. https://doi.org/10.3390/ani9090609
APA StyleWang, L., Zhao, L., Zhang, L., Liu, X., Hou, X., Gao, H., Yan, H., Zhao, F., & Wang, L. (2019). NTN1 Affects Porcine Intramuscular Fat Content by Affecting the Expression of Myogenic Regulatory Factors. Animals, 9(9), 609. https://doi.org/10.3390/ani9090609






